Summary
This study examined the contribution of cytochrome P450 metabolites of arachidonic acid in mediating ischaemia/reperfusion (I/R)-induced cardiac dysfunction in normal and diabetic rats.
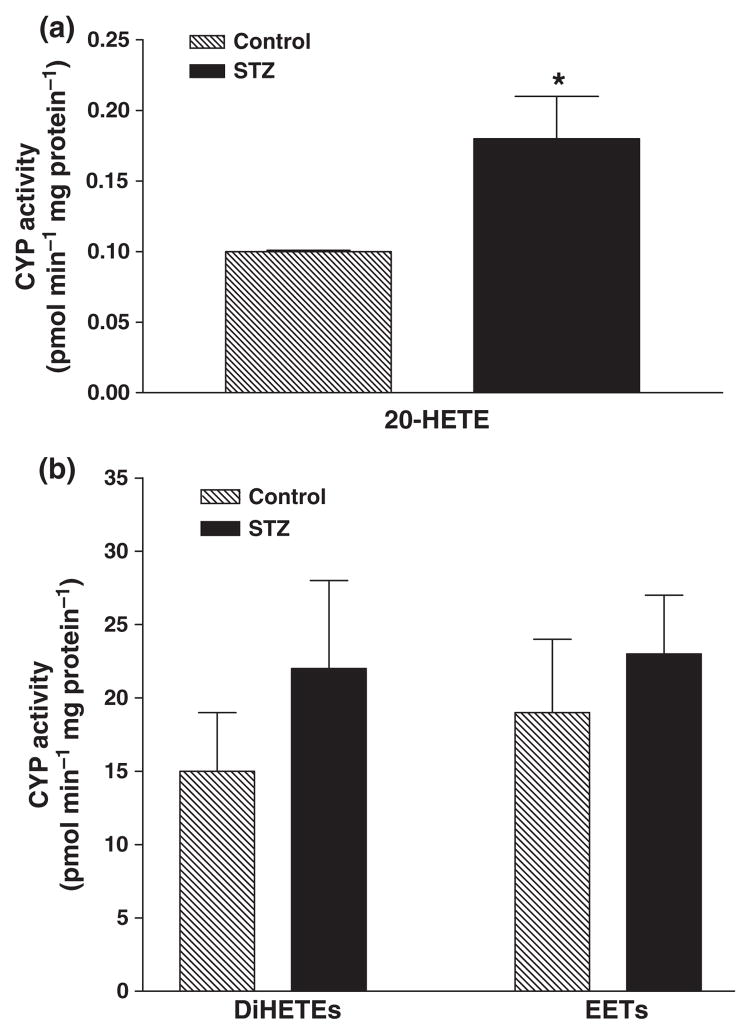
We first compared the metabolism of arachidonic acid in microsomes prepared from the hearts of control rats and rats treated with streptozotocin (55 mg kg−1) to induce diabetes. The production of dihydroxyeicosatrienoic acids and epoxyeicosatrienoic acids (EETs) were similar in microsomes prepared from the hearts of control and diabetic rats, but the production of 20-hydroxyeicosatetraenoic acid (20-HETE) was two-fold higher in diabetic hearts than in control animals.
We then compared the change in left ventricular pressure (Pmax), left ventricular end-diastolic pressure, coronary flow and coronary vascular resistance in isolated perfused hearts obtained from control and diabetic animals after 40 min of global ischaemia (I) followed by 30 min of reperfusion (R). The decline in cardiac function was three- to five-fold greater in the hearts obtained from diabetic vs. control animals.
Pretreatment of the hearts with N-hydroxy-N′-(4-butyl-2-methyl-phenyl)-formamidine (HET0016,1 μM), a selective inhibitor of the synthesis of 20-HETE, for 30 min before I/R resulted in significant improvement in the recovery of cardiac function in the hearts obtained from diabetic but not in control rats. Perfusion with an inhibitor of soluble epoxide hydrolase, 1-cyclohexyl-3-dodecyl urea (CDU), before I/R improved the recovery of cardiac function in hearts obtained from both control and diabetic animals. Perfusion with both HET0016 and CDU resulted in significantly better recovery of cardiac function of diabetic hearts following I/R than that seen using either drug alone. Pretreatment of the hearts with glibenclamide (1 μM), an inhibitor of ATP-sensitive potassium channels, attenuated the cardioprotective effects of both CDU and HET0016.
This is the first study to suggest that acute blockade of the formation of 20-HETE and/or reduced inactivation of EETs could be an important strategy to reduce cardiac dysfunction following I/R events in diabetes.
Keywords: diabetes, ischaemia, reperfusion, CDU, HET0016, glibenclamide
Introduction
Arachidonic acid (AA) is metabolized by cytochrome P450 (CYP450) enzymes to 20-hydroxyeicosatetraenoic acid (20-HETE) and epoxyeicosatrienoic acids (EETs) in the heart, brain, lung, kidney and peripheral blood vessels (Campbell et al., 1996; Roman, 2002; Kroetz & Xu, 2005; Larsen et al., 2006; Campbell & Falck, 2007; Gross et al., 2007). 20-HETE is a potent constrictor of renal, cerebral and mesenteric arterioles and small coronary arteries and it plays an important role in the regulation of vascular tone in these vascular beds (Roman, 2002; Kroetz & Xu, 2005; Larsen et al., 2006). Increased production of 20-HETE has been reported following ischaemia-reperfusion (I/R) injury in the heart and in rat models of hypertension and diabetes (Roman, 2002; Nithipatikom et al., 2004, 2006a; Benter et al., 2005a; Wang et al., 2006). Moreover, exogenous administration of 20-HETE increases I/R-induced cardiac injury, whereas inhibition of the formation of 20-HETE is cardio-protective (Nithipatikom et al., 2004, 2006b). Similarly, inhibitors of the formation of 20-HETE markedly reduce infarct size in the brain following transient occlusion and reperfusion of the middle cerebral artery (Omura et al., 2006). Previous studies have indicated that the degree of ischaemic injury and cardiac dysfunction is markedly enhanced in hearts of diabetic animals and there is evidence that the expression of CYP450 enzymes and the production of 20-HETE is elevated at least in the liver and kidney of diabetic animals (Shimojo et al., 1993; Han et al., 2007; Muniyappa et al., 2007). Inhibitors of the formation of 20-HETE also attenuate diabetes-induced vascular dysfunction in the rat carotid artery (Benter et al., 2005b). However, potential contribution of 20-HETE to the enhanced susceptibility of the diabetic heart to I/R injury has yet to be explored.
Cytochrome P450 enzymes of the CYP4502J2, CYP4502C8 and CYP4502C9 families metabolize AA to EETs and are expressed in human heart, aorta and coronary artery (DeLozier et al., 2007). There is evidence that EETs exert a cardioprotective role against myocardial infarction (Seubert et al., 2004, 2007; Larsen et al., 2006; Nithipatikom et al., 2006b; Gauthier et al., 2007; Gross et al., 2007). For example hearts from transgenic mice overexpressing human CYP2J2 have improved functional recovery following I/R and exogenous administration of 11,12-EET or 14,15-EET reduces infarct size in canine hearts subjected to I/R injury (Seubert et al., 2004, 2007; Nithipatikom et al., 2006a,b). EETs are hydrolysed by soluble epoxide hydrolase (sEH) to less active dihydroxyeicosatrienoic acids (DiHETEs) (Yu et al., 2000; Fang et al., 2004; Spector et al., 2004). Inhibition of sEH enhances the bioavailability of EETs and has been suggested as a possible means to improve myocardial perfusion in cardiovascular disease states characterized by increased oxidative stress and endothelial dysfunction such as hypertension and diabetes (Davis et al., 2002; Imig et al., 2002; Zhao et al., 2004; Schmelzer et al., 2005). Thus, this study compared the metabolism of AA by CYP450 enzymes in the hearts of diabetic and control animals and examined the effects of an inhibitor of the formation of 20-HETE, N-hydroxy-N′-(4-butyl-2-methyl-phenyl)-formamidine (HET0016), vs. a sEH inhibitor, 1-cyclohexyl-3-dodecyl urea (CDU), on the recovery of cardiac function following global ischaemia of hearts obtained from diabetic and control rats. Furthermore, several studies have suggested that the EETs have been shown to activate mitochondrial KATP channels (mitoKATP) in mouse hearts (Seubert et al., 2004). We therefore tested the importance of mitoKATP in mediating the effects of HET0016 and CDU.
Materials and methods
General
Experiments were performed on male Wistar rats weighing approximately 300 g. The rats had free access to food and water throughout the study. Diabetes was induced by a single i.p. injection (55 mg kg−1, body weight) of streptozotocin (STZ). Age-matched controls were injected with vehicle alone. Body weight and basal glucose levels were determined before and 48 h after injection of STZ. Rats with fasting blood glucose levels above 250 mg dl−1 were considered diabetic and included in the study. The body weights and glucose levels of the rats were determined 4 weeks later prior to killing the animals. The use of animals in these studies was approved by Kuwait University Research Administration as the studies were performed in accordance with National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
Metabolism of arachidonic acid
Cardiac tissue obtained from diabetic and non-diabetic rats was washed and homogenized in a 10 mM KPO4 buffer (pH 7.7) containing 250 mM sucrose, 1 mM EDTA, 10 mM MgCl2 and 0.1 mM PMSF. Microsomes were prepared by differential\fugation as previously described (Imig et al., 2002; Gross et al., 2007). The metabolism of AA to EETs, DiHETEs and 20-HETE was determined by incubating the microsomes (0.5 mg of protein) with a saturating concentration of 14C-AA (0.1 μCi, 42 μM) for 30 min at 37 °C in 1 ml of a 100 mM KPO4 buffer (pH 7.4) containing 10 mM MgCl2, 1 mM EDTA, 1 mM NADPH and a NADPH-regenerating system (10 mM isocitrate and isocitrate dehydrogenase, 0.4 U ml−1). The reactions were terminated by acidification to pH 3.5 using 1 M formic acid and the samples were extracted with ethyl acetate. The metabolites formed were separated using a 2 mm × 25 cm C18-reverse phase HPLC column and a linear elution gradient ranging from acetonitrile: water:acetic acid (50/50/0.1) to acetonitrile: acetic acid (100/0.1) over 40 min. The products formed were monitored using a radioactive flow detector Model 120 (Radiomatic Instrument Co., Tampa, FL, USA). Values are expressed as picomoles formed per minute per milligram of protein.
Ischaemia reperfusion studies
Rats were anaesthetized with thiopentone sodium (40 mg kg−1 body weight) and their hearts were removed after intravenous heparinization (1000 U kg−1 body weight). The hearts were mounted on the Langendorff apparatus (ML870B2 Langendorff System; ADI Instruments, Panlab, Spain), and were perfused at a constant pressure of 50 mmHg with an oxygenated (95% O2 + 5% CO2) Krebs–Henseleit (KH) buffer (37 °C) of the following composition (in mM): NaCl 117; KCl 3.75; CaCl2 2.5; NaHCO3 20.0; KH2PO4 1.21; MgCl2.6H2O 1.2; glucose 12.0; pH 7.35. A water-filled balloon was introduced into the left ventricle and connected to a Statham pressure transducer (P23Db) and baseline end-diastolic pressure was adjusted to 5 mmHg. Left ventricular systolic (Pmax) and end-diastolic pressures (LVEDP) were continuously monitored. Coronary flow (CF) was also measured using an electromagnetic flow probe on tubing leading to the perfusion cannula so that coronary vascular resistance (CVR) could be calculated. Perfusion pressure was measured from a side port near the tip of the aortic cannula using a Statham pressure transducer and was maintained constant at 50 mmHg by means of a perfusion pressure control module. The hearts were perfused for 30 min and then subjected to 40 min of no flow global ischaemia (I) followed by a 30 min of reperfusion (R). Post-I/R recovery of left ventricular function and haemodynamics was measured. The effects of CDU and/or HET0016 on cardiac function were determined by adding various concentrations of these inhibitor(s) to the KH perfusion solution during the 30-min perfusion period before I/R.
Sixteen groups of animals were studied: Group 1 consisted of hearts obtained from non-diabetic animals that was perfused with KH alone. In groups 2–4, the hearts obtained from non-diabetic rats were pretreated with 0.01, 0.1 and 1.0 μM CDU, respectively. In groups 5–7, the hearts obtained from non-diabetic animals were pretreated with 0.01, 0.1 and 1.0 μM HET0016, respectively. In Group 8, hearts obtained from non-diabetic rats were perfused with both HET0016 (0.01 μM) and CDU (0.1 μM). Group 9 served as the control for hearts taken from diabetic animals. In groups 10–12, hearts obtained from diabetic animals pretreated with 0.01, 0.1 and 1.0 μM CDU, respectively. In groups 13–15, hearts from diabetic rats were pretreated with 0.01, 0.1 and 1.0 μM HET0016, respectively. Group 16 consisted of hearts obtained from diabetic rats that were pretreated with both HET0016 (0.01 μM) and CDU (0.1 μM). In another group of experiments, the effects of glibenclamide (1 μM), an inhibitor of ATP-sensitive potassium channels, on the cardioprotective effects of CDU or HET0016 were investigated. Glibenclamide was used to determine if the ATP-sensitive potassium channels are involved in mediating the cardioprotective effect of CDU and HET0016. In this group of experiments, the hearts obtained from diabetic rats were pretreated with CDU (1 μM) + glibenclamide or HET0016 (0.1 μM) + glibenclamide for 30 min prior to I/R.
Statistics
Results are presented as mean values ± SEM. Analysis of variance followed by a post hoc test (Bonferroni) was used to determine the significance of differences in mean values between treatment groups. The difference was considered to be significant when P-value was <0.05. Results for the heart perfusion experiments are expressed as mean ± SEM. Reperfusion values were compared with their respective baseline controls using a two-tailed, paired t-test. The different experimental groups were compared using a general factorial analysis of variance. Computerized statistical analysis was performed with SPSS for Windows (V.6.0.1; SPSS Inc. Evanston, IL, USA). For further comparisons, univariate Scheffes’ confidence intervals were obtained for the parametric estimates.
Drugs and chemicals
1-Cyclohexyl-3-dodecyl urea was obtained from Calbiochem Company (La Jolla, CA, USA). N-hydroxy-N′-(4-butyl-2-methyl-phenyl)-formamidine (HET0016) was purchased from Cayman Chemicals Company (Ann Arbor, ME, USA). Glibenclamide and the other chemicals were purchased from Sigma Biochemicals (St Louis, MO, USA). Glibenclamide and HET0016 were dissolved in ethanol, while CDU was dissolved in dichloromethane.
Results
Hyperglycaemia and body weights
Fasting plasma glucose levels averaged 460 ± 15 mg dl−1 in STZ-treated rats as compared with 90 ± 6 mg dl−1 in the control animals. Body weight of the control animals averaged 327 ± 8 g in comparison to body weight of the diabetic rats which fell to 197 ± 6 g by the end of the study.
Metabolism of AA
A comparison of the metabolism of AA in microsomes prepared from the hearts of diabetic and control rats is presented in Fig. 1. Microsomes prepared from the hearts of control and diabetic animals produce EETs, DiHETEs and 20-HETE when incubated with AA. The production of 20-HETE was about two times higher in the hearts obtained from diabetic animals than controls rats. The production of EETs and DiHETEs, however, was not significantly different in the hearts obtained from control and diabetic animals.
Figure 1.
Cytochrome P-450 ω-hydroxylase and epoxygenase activity in microsomes prepared from the hearts of diabetic and non-diabetic rats: (a) 20-HETE formation was significantly elevated in microsomes prepared from the hearts of diabetic rats as compared to the non-diabetic animals; (b) DiHETEs and EETs formation were not different between diabetic and non-diabetic rats. Mean ± SEM (n = 5). *Significantly different compared to untreated control, P < 0.05.
Ischaemia–reperfusion studies
Cardiac function following 40 min of ischaemia and 30 min of reperfusion (I/R) was markedly impaired in hearts obtained from diabetic animals (Pmax = 4 ± 2 mmHg, LVEDP = 71.5 ± 5.3 mmHg, CF = 0.3 ± 0.1 ml min−1, CVR = 114.0 ± 25.6 mmHg ml−1 min−1) compared to the values seen in hearts obtained from non-diabetic rats (Pmax = 60 ± 4 mmHg, LVEDP = 20.0 ± 2.0 mmHg, CF = 7.0 ± 0.3 ml min−1, CVR = 18.0 ± 1.4 mmHg ml−1 min−1) (Tables 1 & 2 and Figs 2 & 3).
Table 1.
The effect of CDU or HET0016 on recovery in left ventricular contractility and vascular haemodynamics of perfused hearts isolated from control animals
| Pmax (mmHg) | LVEDP (mmHg) | CF (ml min−1) | CVR (mmHg ml−1 min−2) | |||||
|---|---|---|---|---|---|---|---|---|
| Groups studied | CON | REP | CON | REP | CON | REP | CON | REP |
| Wistar (C) | 97 ± 5 | 60 ± 4 | 5.5 ± 0.4 | 20.1 ± 2.0 | 12.1 ± 0.4 | 7.2 ± 0.3 | 5.1 ± 0.3 | 18.1 ± 1.4 |
| C + CDU (0.01 μM) | 87 ± 8 | 53 ± 4 | 6.1 ± 0.3 | 22.3 ± 2.0 | 12.2 ± 0.5 | 7.2 ± 1.0 | 5.2 ± 1.0 | 19.2 ± 1.4 |
| C + CDU (0.1 μM) | 99 ± 9 | 62 ± 4 | 6.1 ± 0.3 | 22.2 ± 1.5 | 13.1 ± 0.3 | 8.1 ± 0.4 | 5.2 ± 0.3 | 17.2 ± 1.5 |
| C + CDU (1 μM) | 108 ± 8 | 75 ± 6* | 5.2 ± 0.3 | 16.2 ± 2.0* | 14.1 ± 1.0 | 10.2 ± 0.4* | 4.1 ± 0.2 | 11.1 ± 0.5* |
| C + HET0016 (0.01 μM) | 90 ± 1 | 53 ± 3 | 5.2 ± 0.1 | 20.1 ± 1.0 | 9.2 ± 1.0 | 5.1 ± 0.5 | 5.2 ± 1.0 | 20.3 ± 1.5 |
| C + HET0016 (0.1 μM) | 88 ± 10 | 54 ± 2 | 5.2 ± 0.2 | 19.3 ± 0.5 | 9.2 ± 0.4 | 5.2 ± 0.1 | 5.3 ± 0.5 | 16.1 ± 1.0 |
| C + HET0016 (1 μM) | 95 ± 3 | 51 ± 1 | 6.2 ± 0.4 | 25.2 ± 1.5 | 10.1 ± 1.0 | 6.3 ± 1.0 | 5.1 ± 0.3 | 19.2 ± 1.0 |
| C + CDU (0.1 μM) + HET0016 (0.01 μM) | 97 ± 8 | 62 ± 7 | 5.2 ± 0.3 | 18.1 ± 0.5 | 11.5 ± 0.5 | 7.1 ± 0.5 | 4.3 ± 0.3 | 16.3 ± 1.5 |
The data were computed at 30-min reperfusion (37 °C) and expressed as mean ± SEM.
Pmax, left ventricular developed pressure; LVEDP, left ventricular end-diastolic pressure, CF, coronary flow; CVR, coronary vascular resistance, CON, baseline control; REP, reperfusion.
P < 0.05 compared to Wistar controls (C).
Table 2.
The effect of CDU or HET0016 on recovery in left ventricular contractility and vascular haemodynamics of perfused hearts isolated from diabetic animals
|
Pmax (mmHg) |
LVEDP (mmHg) |
CF (ml min−1) |
CVR (mmHg ml−1 min−2) |
|||||
|---|---|---|---|---|---|---|---|---|
| Groups studied | CON | REP | CON | REP | CON | REP | CON | REP |
| Diabetic Wistar (D) | 62 ± 10 | 4 ± 2 | 6.8 ± 0.5 | 71.5 ± 5.3 | 6.3 ± 0.6 | 0.3 ± 0.1 | 14.5 ± 3 | 114.0 ± 25.6 |
| D + CDU (0.01 μM) | 81 ± 5 | 12 ± 2* | 5.9 ± 0.4 | 43.8 ± 3.9* | 8.9 ± 0.5 | 1.3 ± 0.2* | 5.7 ± 0.3 | 45.3 ± 3.2* |
| D + CDU (0.1 μM) | 82 ± 8 | 20 ± 2* | 6.1 ± 0.2 | 36.3 ± 2.0* | 7.9 ± 0.2 | 2.7 ± 0.2* | 7.1 ± 0.5 | 43.0 ± 3.5* |
| D + CDU (1 μM) | 94 ± 5 | 47 ± 3* | 8.0 ± 0.3 | 37.4 ± 1.6* | 11.2 ± 0.2 | 6.9 ± 0.3* | 4.7 ± 0.2 | 20.8 ± 1.2* |
| D + HET0016 (0.01 μM) | 77 ± 4 | 14 ± 2* | 6.5 ± 0.3 | 45.0 ± 3.2* | 6.7 ± 0.5 | 1.2 ± 0.1* | 5.2 ± 0.4 | 34.0 ± 2.3* |
| D + HET0016 (0.1 μM) | 116 ± 5 | 49 ± 2* | 5.9 ± 0.3 | 28.5 ± 1.1* | 10.5 ± 0.4 | 3.4 ± 0.2* | 5.5 ± 0.3 | 28.5 ± 1.7* |
| D + HET0016 (1 μM) | 68 ± 3 | 9 ± 1* | 6.3 ± 0.4 | 45.7 ± 3.3* | 7.8 ± 0.7 | 1.5 ± 0.3* | 8.8 ± 0.4 | 65.7 ± 3.4* |
| D + CDU (0.1 μM) + HET0016 (0.01 μM) | 101 ± 4 | 43 ± 2*† | 6.0 ± 0.2 | 25.8 ± 2.0*† | 9.5 ± 0.6 | 3.5 ± 0.2*† | 5.7 ± 0.3 | 26.8 ± 2.3*† |
| D + CDU (1 μM) + HET0016 (0.1 μM) | 99 ± 3 | 48 ± 2* | 5.4 ± 0.2 | 23.0 ± 3.4* | 8.0 ± 0.4 | 4.1 ± 0.3* | 6.3 ± 0.3 | 26.0 ± 2.1* |
The data were computed at 30-min reperfusion (37 °C) and expressed as mean ± SEM.
D, diabetic; Pmax, left ventricular developed pressure; LVEDP, left ventricular end-diastolic pressure, CF, coronary flow; CVR, coronary vascular resistance; CON, baseline control; REP, reperfusion.
P < 0.05 compared to diabetic controls (D).
P < 0.05 compared to CDU (0.1 μM) alone or HET0016 (0.01 μM) alone.
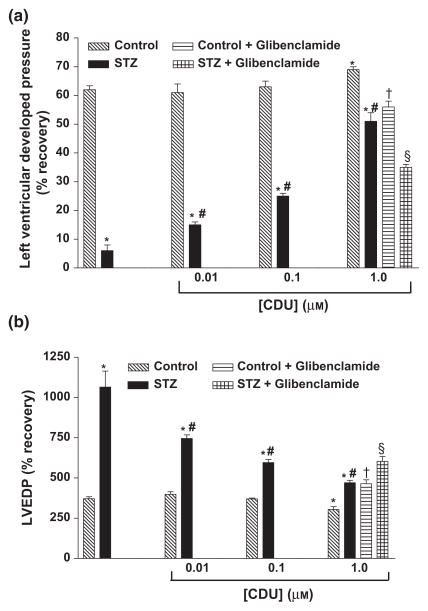
Figure 2.
Effect of CDU (0.01, 0.1 and 1.0 μM) and combination treatment with CDU (1.0 μM) and glibenclamide (1 μM) on (a) % recovery in Pmax and (b) LVEDP in perfused hearts of control and diabetic rats. Mean ± SEM (n = 5). *Significantly different compared to untreated control, #Significantly different compared to untreated-STZ, †Significantly different compared to control-CDU, §Significantly different compared to STZ-CDU, P < 0.05.
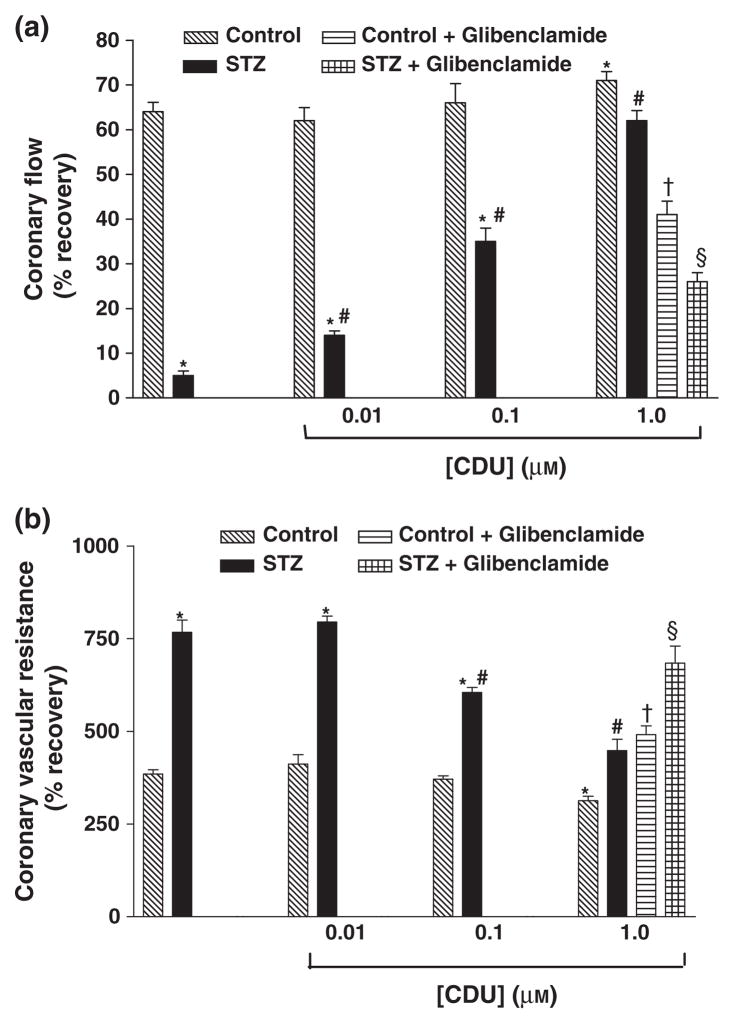
Figure 3.
Effect of CDU (0.01, 0.1 and 1.0 μM) and combination treatment with CDU (1.0 μM) and glibenclamide (1 μM) on (a) % recovery in CF and (b) CVR in perfused hearts of control and diabetic rats. Mean ± SEM (n = 5). *Significantly different compared to untreated control, #Significantly different compared to untreated-STZ, †Significantly different compared to control-CDU, §Significantly different compared to STZ-CDU, P < 0.05.
Effect of perfusion with an inhibitor of soluble epoxide hydrolase (CDU) on recovery of cardiac function
Pretreatment of hearts obtained from diabetic rats with CDU produced a dose-dependent improvement of all parameters of cardiac function following I/R (Tables 1 & 2 and Figs 2 & 3). Perfusion with CDU also had a beneficial effect of the recovery of the performance of the hearts obtained from non-diabetic animals but only at the highest dose (1.0 μM) (Tables 1 & 2 and Figs 2 & 3). The effect of glibenclamide, an inhibitor of ATP-sensitive potassium channels, on CDU-mediated cardioprotection was also investigated. Combination treatment with CDU and glibenclamide before I/R resulted in significant attenuation of the beneficial effects of CDU in both control and diabetic animals (Tables 1 & 2 and Figs 2 & 3).
Effect of perfusion with an inhibitor of 20-HETE synthesis (HET0016) on recovery of cardiac function
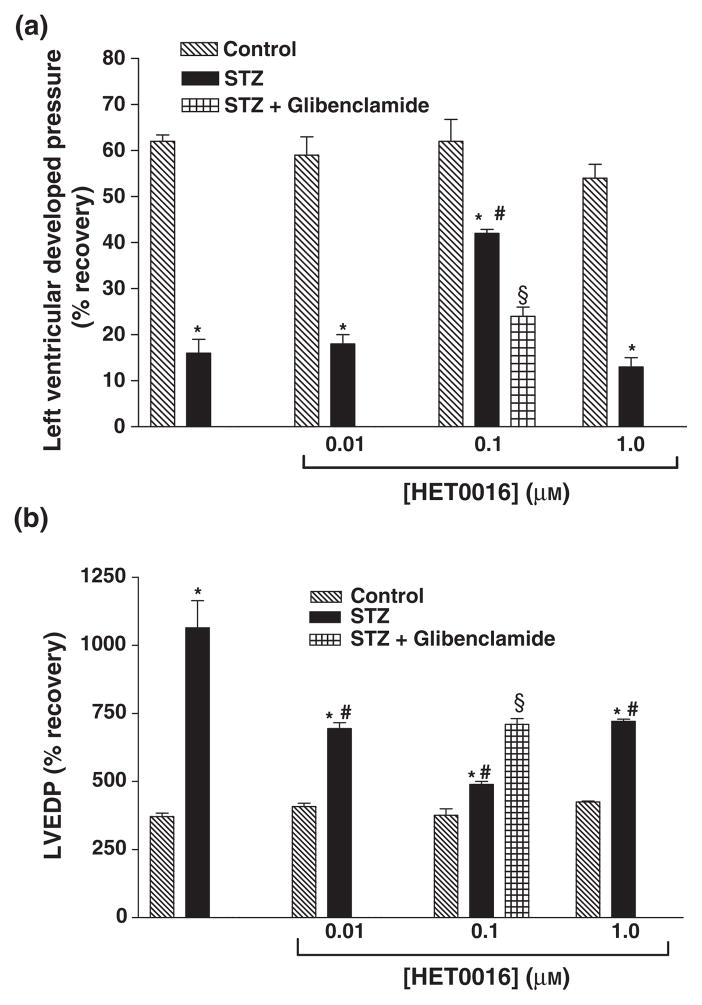
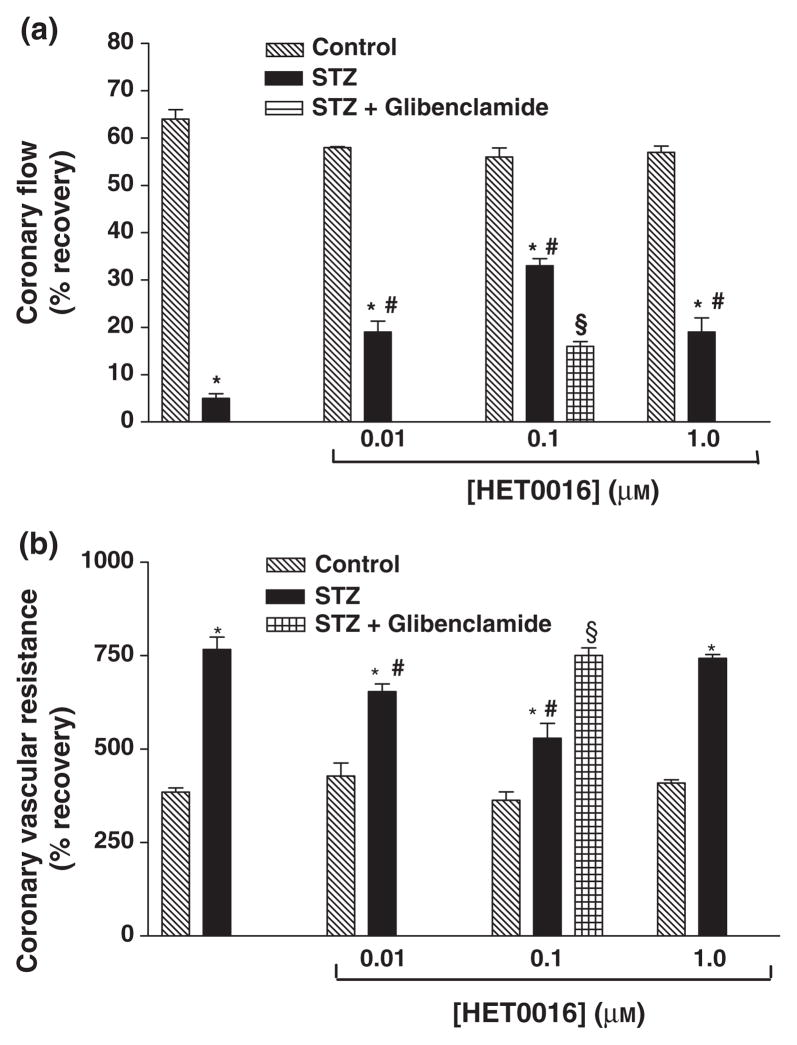
Perfusion with HET0016 before ischaemia had no significant effect on the recovery of function following I/R in hearts obtained from non-diabetic animals. However, pretreatment of the hearts of diabetic animals with HET0016 improved the functional recovery following IR injury (Tables 1 & 2 and Figs 4 & 5). The best recovery was seen using a concentration of 0.1 μM HET0016. The effect of glibenclamide on HET0016-mediated cardioprotection was also investigated. Combination treatment with HET0016 and glibenclamide before I/R resulted in significant attenuation of the beneficial effects of HET0016 in diabetic animals (Tables 1 & 2 and Figs 4 & 5).
Figure 4.
Effect of HET0016 (0.01, 0.1 and 1.0 μM) on (a) % recovery in Pmax and (b) LVEDP in perfused hearts of control and diabetic rats. Effect of combination treatment with HET0016 (0.1 μM) and glibenclamide (1 μM) was investigated in hearts from diabetic rats only. Mean ± SEM (n = 5). *Significantly different compared to untreated control, #Significantly different compared to untreated-STZ, §Significantly different compared to STZ-HET0016, P < 0.05.
Figure 5.
Effect of HET0016 (0.01, 0.1 and 1.0 μM) on (a) % recovery in CF and (b) CVR in perfused hearts of of control and diabetic rats. Effect of combination treatment with HET0016 (0.1 μM) and glibenclamide (1 μM) was investigated in hearts from diabetic rats only. Mean ± SEM (n = 5). *Significantly different compared to untreated control, #Significantly different compared to untreated-STZ, §Significantly different compared to STZ-HET0016, P < 0.05.
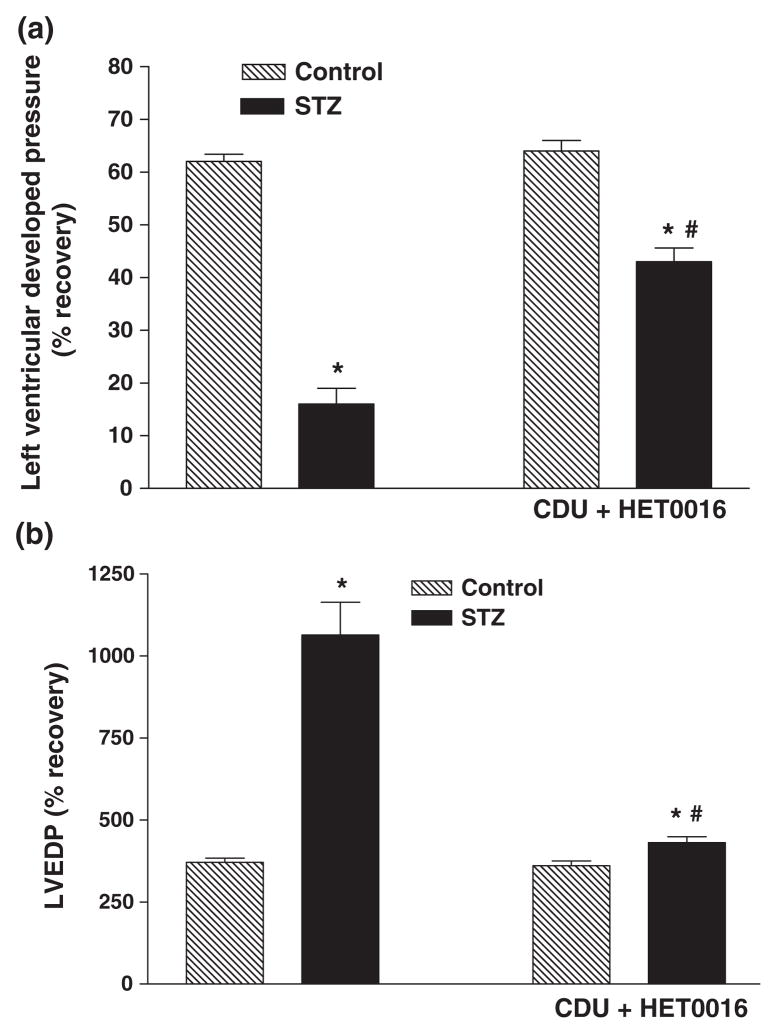
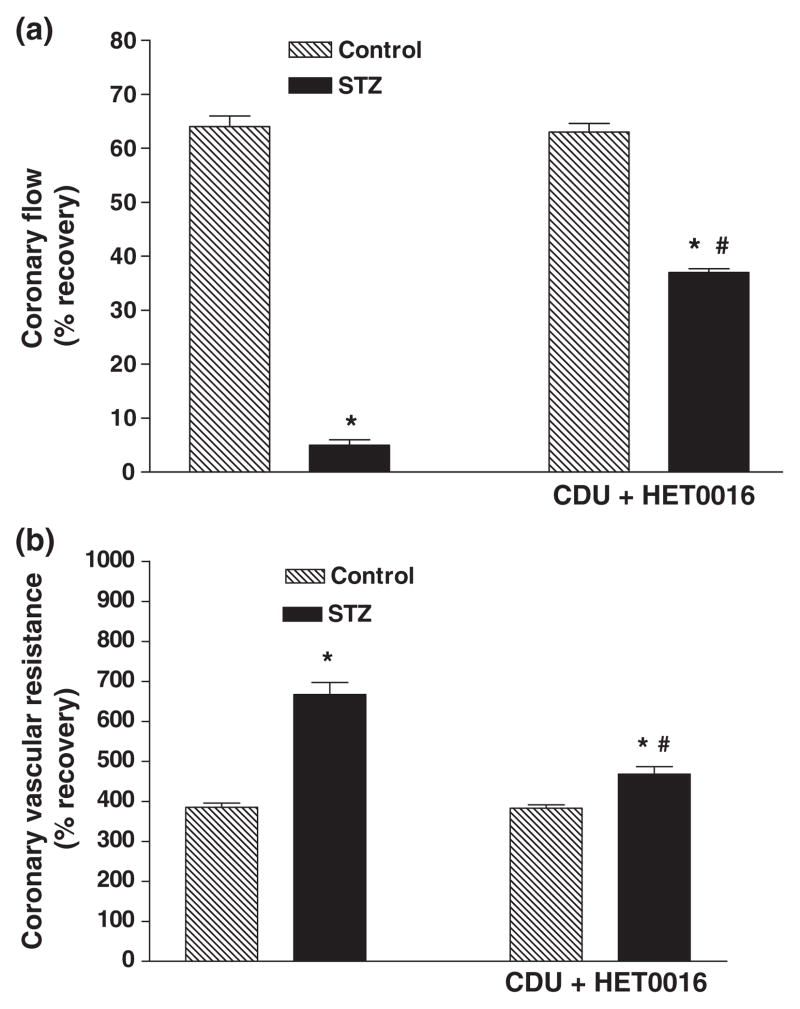
Effect of perfusion with a combination of CDU ± HET0016 on recovery of cardiac function
Pretreatment of the hearts of diabetic animals with a combination of CDU (0.1 μM) and HET0016 (0.01 μM) resulted in a much better recovery of function following I/R injury than that seen when either drug was given alone (Tables 1 & 2 and Figs 6 & 7). We also performed preliminary experiments investigating the benefit of combining 1 μM CDU with 0.1 μM HET0016. However, this combination did not produce an additive effect.
Figure 6.
Effect of combined treatment with CDU (0.1 μM) and HET0016 (0.01 μM) on (a) % recovery in Pmax and (b) LVEDP in perfused hearts of control and diabetic rats. Mean ± SEM (n = 5). *Significantly different compared to untreated control, #Significantly different compared to untreated-STZ, P < 0.05.
Figure 7.
Effect of combined treatment with CDU (0.1 μM) and HET0016 (0.01 μM) on (a) % recovery in CF and (b) CVR in perfused hearts of of control and diabetic rats. Mean ± SEM (n = 5). *Significantly different compared to untreated control, #Significantly different compared to untreated-STZ, P < 0.05.
Discussion
This study examined the possible role of CYP450 eicosanoids in contribution to cardiac dysfunction following I/R in hearts from diabetic and control animals. The results indicate that the production of 20-HETE but not EETs or DiHETEs is elevated in cardiac tissue obtained from diabetic animals and the hearts from diabetic animals showed poor recovery of function following I/R injury. We also found that the performance of the hearts of diabetic animals can be significantly improved by administration of HET0016, a selective inhibitor of the formation of 20-HETE, or by administration of the sEH inhibitor, CDU. HET0016 had no effect on the functional recovery of hearts obtained from non-diabetic animals following I/R injury. These results suggest that upregulation of the formation of 20-HETE in the hearts of diabetic animals may contribute to increased injury following ischaemia. Overall, the present findings suggest that inhibitors of the formation of 20-HETE and sEH may provide a novel and effective therapeutic strategy to minimize cardiac dysfunction following I/R injury especially in diabetes.
The present findings implicating a possible role for 20-HETE in cardiac ischaemia reperfusion injury are consistent with the results of previous studies indicating that the levels of 20-HETE in the coronary venous plasma are elevated in the late stages of ischaemia and early stages of reperfusion and that inhibitors of the synthesis of 20-HETE reduce infarct size following transient occlusion and reperfusion of the left descending coronary artery in dogs. (Nithipatikom et al., 2004, 2006a). Our finding that the formation of 20-HETE is elevated in the hearts of diabetic animals is also consistent with previous reports that the expression and activity of enzymes of the CYP4A family are elevated in the liver and kidney of diabetic animals (Shimojo et al., 1993). Finally, the present results indicating that administration of HET0016 improved cardiac recovery in the hearts obtained from diabetic but not control animals suggests that enhanced formation of 20-HETE in the hearts of diabetic rats may contribute to the elevated I/R cardiac injury that occurs in diabetic rats. Overall, the present findings are consistent with the results of a recent study in which inhibitors of the synthesis of 20-HETE attenuated N-nitro-L-arginine methyl ester-induced cardiac dysfunction in SHR (Benter et al., 2005a). The present data suggest that the protective effects of HET0016 in the diabetic hearts are at least partially due to opening of K(ATP) channels as co-administration of HET0016 with glibenclamide attenuated the beneficial effects of HET0016 on the recovery of cardiac function. This observation suggests that diabetes-induced increase in 20-HETE levels may have an inhibitory effect on cardiac mitoK (ATP) channels during I/R similar to the well-known effect of 20-HETE to block Ca2+-activated K+ channels that share the same Kir subunit (Gebremedhin et al., 2008). Another possible explanation for the mechanism of action of 20-HETE inhibitors came from a recent study where it was shown that CYP ω-hydroxylase inhibition by HET0016 reduced the infarct size of heart, decreased DNA fragmentation, reduced TUNEL-positive cells, attenuated caspase-3 activation, and modulated genes associated with apoptosis in rats rendered ischaemia followed by reperfusion (Lv et al., 2008). The authors of this study concluded that CYP ω-hydroxylase inhibition protects I/R-induced heart injury by reducing apoptosis associated with ERK1/2 activation (Lv et al., 2008).
Recent studies have also suggested that EETs may oppose ischaemic damage to the heart following I/R (Seubert et al., 2004, 2007; Nithipatikom et al., 2006b; Gauthier et al., 2007). Indeed the present results indicating that administration of an inhibitor of sEH improved cardiac function following I/R injury in hearts obtained from both control and diabetic animals are consistent with this conclusion. Interestingly, the beneficial effects of CDU were greater in hearts from diabetic than those obtained from non-diabetic rats. This difference in diabetic hearts compared to controls could be due to diabetes-induced changes in metabolism of EETs. This hypothesis is supported by a previous observation which demonstrated that rates of degradation of EETs are higher in diabetic mice because of elevated sEH mRNA expression (Rodriguez & Clare-Salzler, 2006).
In this study, we also found that glibenclamide attenuated CDU-mediated cardioprotection indicating that opening of mitoK(ATP) channels perhaps by increased levels of EETs may oppose cardiac injury following I/R and contribute to in the improved function recovery seen in hearts treated with CDU. This finding is in agreement with other reports which indicated that KATP channel activation in the myocardium may play an important role in the cardioprotective actions of EETs (Seubert et al., 2004; Gauthier et al., 2007). Previous results obtained in dogs demonstrated that the cardioprotective effect of the EETs was completely blocked by glibenclamide (Lu et al., 2001). It has also been shown that activation of sarcKATP channels by EETs can protect against ischaemic cardiac reperfusion injury by hyperpolarizing the cell, thereby limiting calcium entry, preserving ATP utilization, and maintaining cardiac membrane potential and contractility (Lu et al., 2001). Batchu et al. have recently shown that EETs improve functional recovery and reduce injury through enhanced postischaemic ventricular repolarization and attenuated electrocardiogram abnormalities via activation of sarcKATP channels and a PKA-dependent signalling (Batchu et al., 2009). EETs have also been shown to produce cardioprotective effects through activation of the reperfusion injury salvage kinase pathway which involves activation of the phosphatidylinositol 3-kinase/Akt pathway (Seubert et al., 2004; Gross et al., 2007; Yellon & Hausenloy, 2007). In this study, treatment with a combination of 1 μM CDU and 0.1 μM HET0016 did not produce an additive effect suggesting that these drugs might act on the same or convergent signalling pathways. However, our observation that pretreatment of the hearts with a combination of CDU (0.1 μM) and HET0016 (0.01 μM) resulted in a much better recovery of function following I/R injury than that seen when either drug was given alone suggests that further studies will be needed to characterize the mechanism of action of these drugs.
In summary, the results of this study suggest that inhibition of the formation of 20-HETE and prevention of hydrolysis and inactivation of EETs have a beneficial effect on the recovery of cardiac function following I/R injury especially in diabetic animals. These findings suggest that inhibitors of the synthesis of 20-HETE and/or sEH inhibitors may have therapeutic potential to reduce cardiac injury especially in diabetes in which oxidative stress is elevated and endothelial function is compromised.
Acknowledgments
This work was supported by Kuwait University Research Administration Grant RM02/03 (I.F.B and M.H.Y.) and grants HL29587 and HL36279 (R.J.R.).
References
- BATCHU SN, LAW E, BROCKS DR, FALCK JR, SEUBERT JM. Epoxyeicosatrienoic acid prevents postischemic electrocardiogram abnormalities in an isolated heart model. J Mol Cell Cardiol. 2009;46:67–74. doi: 10.1016/j.yjmcc.2008.09.711. [DOI] [PubMed] [Google Scholar]
- BENTER IF, FRANCIS I, COJOCEL C, JUGGI JS, YOUSIF MH, CANATAN H. Contribution of cytochrome P450 metabolites of arachidonic acid to hypertension and end-organ damage in spontaneously hypertensive rats treated with L-NAME. Auton Autacoid Pharmacol. 2005a;25:143–154. doi: 10.1111/j.1474-8673.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- BENTER IF, YOUSIF MH, CANATAN H, AKHTAR S. Inhibition of Ca2+/calmodulin-dependent protein kinase II, Ras-GTPase and 20-HETE attenuates the development of diabetes-induced vascular dysfunction in the rat carotid artery. Pharmacol Res. 2005b;52:252–257. doi: 10.1016/j.phrs.2005.04.001. [DOI] [PubMed] [Google Scholar]
- CAMPBELL WB, FALCK JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49:590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- CAMPBELL WB, GEBREMEDHIN D, PRATT PF, HARDER DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- DAVIS BB, THOMPSON DA, HOWARD LL, MORISSEAU C, HAMMOCK BD, WEISS RH. Inhibitors of soluble epoxide hydrolase attenuate vascular smooth muscle cell proliferation. Proc Natl Acad Sci USA. 2002;99:2222–2227. doi: 10.1073/pnas.261710799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLOZIER TC, KISSLING GE, COULTER SJ, DAI D, FOLEY JF, BRADBURY JA, MURPHY E, STEENBERGEN C, ZELDIN DC, GOLDSTEIN JA. Detection of human CYP2C8, CYP2C9 and CYP2J2 in cardiovascular tissues. Drug Metab Dispos. 2007;35:682–688. doi: 10.1124/dmd.106.012823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANG X, WEINTRAUB NL, MCCAW RB, HU S, HARMON SD, RICE JB, HAMMOCK BD, SPECTOR AA. Effect of soluble epoxide hydrolase inhibition on epoxyeicosatrienoic acid metabolism in human blood vessels. Am J Physiol Heart Circ Physiol. 2004;287:H2412–H2420. doi: 10.1152/ajpheart.00527.2004. [DOI] [PubMed] [Google Scholar]
- GAUTHIER KM, YANG W, GROSS GJ, CAMPBELL WB. Roles of epoxyeicosatrienoic acids in vascular regulation and cardiac preconditioning. J Cardiovasc Pharmacol. 2007;50:601–608. doi: 10.1097/FJC.0b013e318159cbe3. [DOI] [PubMed] [Google Scholar]
- GEBREMEDHIN D, YAMAURA K, HARDER DR. Role of 20-HETE in the hypoxia-induced activation of Ca2+-activated K+ channel currents in rat cerebral arterial muscle cells. Am J Physiol Heart Circ Physiol. 2008;294:H107–H120. doi: 10.1152/ajpheart.01416.2006. [DOI] [PubMed] [Google Scholar]
- GROSS GJ, HSU A, FALCK JR, NITHIPATIKOM K. Mechanisms by which epoxyeicosatrienoic acids (EETs) elicit cardioprotection in rat hearts. J Mol Cell Cardiol. 2007;42:687–691. doi: 10.1016/j.yjmcc.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN SH, QUON MJ, KOH KK. Reciprocal relationships between abnormal metabolic parameters and endothelial dysfunction. Curr Opin Lipidol. 2007;18:58–65. doi: 10.1097/MOL.0b013e328012b627. [DOI] [PubMed] [Google Scholar]
- IMIG JD, ZHAO X, CAPDEVILA JH, MORISSEAU C, HAMMOCK BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- KROETZ DL, XU F. Regulation and inhibition of arachidonic acid hydrolases and 20-HETE formation. 2005 doi: 10.1146/annurev.pharmtox.45.120403.100045. [DOI] [PubMed] [Google Scholar]
- LARSEN BT, MIURA H, HATOUM OA, CAMPBELL WB, HAMMOCK BD, ZELDIN DC, FALCK JR, GUTTERMAN DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H491–H499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LU T, HOSHI T, WEINTRAUB NL, SPECTOR A, LEE HC. Activation of ATP-sensitive K(+) channels by epoxyeicosatrienoic acids in rat cardiac ventricular myocytes. J Physiol. 2001;537:811–827. doi: 10.1111/j.1469-7793.2001.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LV X, WAN J, YANG J, CHENG H, LI Y, AO Y, PENG R. Cytochrome P450 omega-hydroxylase inhibition reduces cardiomyocyte apoptosis via activation of ERK1/2 signaling in rat myocardial ischemia-reperfusion. Eur J Pharmacol. 2008;596:118–126. doi: 10.1016/j.ejphar.2008.08.008. [DOI] [PubMed] [Google Scholar]
- MUNIYAPPA R, MONTAGNANI M, KOH KK, QUON MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28:463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- NITHIPATIKOM K, GROSS ER, ENDSLEY MP, MOORE JM, ISBELL MA, FALCK JR, CAMPBELL WB, GROSS GJ. Inhibition of cytochrome P450ω-hydroxylase: a novel endogenous cardioprotective pathway. Circ Res. 2004;95:e65–e71. doi: 10.1161/01.RES.0000146277.62128.6f. [DOI] [PubMed] [Google Scholar]
- NITHIPATIKOM K, ENDSLEY MP, MOORE JM, ISBELL MA, FALCK JR, CAMPBELL WB, GROSS GJ. Effects of selective inhibition of cytochrome p450 omega-hydroxylase and ischemic preconditioning in myocardial protection. Am J Physiol Heart Circ Physiol. 2006a;290:H500–H505. doi: 10.1152/ajpheart.00918.2005. [DOI] [PubMed] [Google Scholar]
- NITHIPATIKOM K, MOORE JM, ISBELL MA, FALCK JR, GROSS GJ. Epoxyeicosatrienoic acids in cardioprotection: ischemic versus reperfusion injury. Am J Physiol Heart Circ Physiol. 2006b;291:H537–H542. doi: 10.1152/ajpheart.00071.2006. [DOI] [PubMed] [Google Scholar]
- OMURA T, TANAKA Y, MIYATA N, et al. Effect of a new inhibitor of the synthesis of 20-HETE on cerebral ischaemia reperfusion injury. Stroke. 2006;37:1307–1313. doi: 10.1161/01.STR.0000217398.37075.07. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ M, CLARE-SALZLER M. Eicosanoid imbalance in the NOD mouse is related to a dysregulation in soluble epoxide hydrolase and 15-PGDH expression. Ann N Y Acad Sci. 2006;1079:130–134. doi: 10.1196/annals.1375.019. [DOI] [PubMed] [Google Scholar]
- ROMAN RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- SCHMELZER KR, KUBALA L, NEWMAN JW, KIM IH, EISERICH JP, HAMMOCK BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEUBERT J, YANG B, BRADBURY J, et al. Enhanced postischemic functional recovery in cyp2j2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 pathway. Circ Res. 2004;95:506–514. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- SEUBERT JM, ZELDIN DC, NITHIPATIKOM K, GROSS GJ. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischaemia/reperfusion injury. Prostaglandins Other Lipid Mediat. 2007;82:50–59. doi: 10.1016/j.prostaglandins.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMOJO N, ISHIZAKI T, IMAOKA S, FUNAE Y, FUJII S, OKUDA K. Changes in amounts of cytochrome P450 isozymes and levels of catalytic activities in hepatic and renal microsomes of rats with streptozotocin-induced diabetes. Biochem Pharmacol. 1993;46:621–627. doi: 10.1016/0006-2952(93)90547-a. [DOI] [PubMed] [Google Scholar]
- SPECTOR AA, FANG X, SNYDER GD, WEINTRAUB NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- WANG JS, SINGH H, ZHANG F, et al. Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ Res. 2006;98:962–969. doi: 10.1161/01.RES.0000217283.98806.a6. [DOI] [PubMed] [Google Scholar]
- YELLON DM, HAUSENLOY DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- YU Z, XU F, HUSE LM, et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- ZHAO X, YAMAMOTO T, NEWMAN JW, KIM IH, WATANABE T, HAMMOCK BD, STEWART J, POLLOCK JS, POLLOCK DM, IMIG JD. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol. 2004;15:12442–12445. [PubMed] [Google Scholar]