Summary
This study examined the role of 20-hydroxyeicosatetraenoic (20-HETE) in altering vascular function in streptozotocin (STZ)-induced diabetic rats.
The expression of CYP4A protein and the formation of 20-HETE were elevated in the kidney, but not in the renal or mesenteric vasculature, of diabetic animals. The vasoconstrictor responses to norepinephrine (NE), endothelin-1 (ET-1), and angiotensin II (Ang II) were significantly enhanced in the isolated perfused mesenteric vascular bed and renal artery segments of diabetic rats. Chronic treatment of the diabetic rats with 1-aminobenzotriazole (ABT, 50 mg kg−1 alt−1 diem) or N-hydroxy-N′-(4-butyl-2-methylphenyl) formamidine (HET0016, 2.5 mg kg−1 day−1) attenuated the responses to these vasoconstrictors in both vascular beds.
The synthesis of 20-HETE in renal microsomes was reduced by >80% confirming that the doses of ABT and HET0016 were sufficient to achieve system blockade. Addition of HET0016 (1 μM) in vitro also normalized the enhanced vascular responsiveness of renal and mesenteric vessels obtained from diabetic animals to NE and inhibited the formation of 20-HETE by >90% while having no effect on the formation of epoxides. Vasodilator responses to carbachol and histamine were reduced in the mesenteric vasculature, but not in renal arteries, of diabetic rats. Treatment of the diabetic animals with HET0016 improved vasodilator responses in both vascular beds. Vascular sensitivity to exogenous 20-HETE was elevated in the mesenteric bed of diabetic animals compared to controls.
These results suggest that 20-HETE contributes to the elevation in vascular reactivity in diabetic animals. This effect is not due to increased vascular expression of CYP4A but may be related to either enhanced agonist-induced release of substrate (arachidonic acid) by the CaMKII/Ras-GTPase system and/or elevated vascular responsiveness to 20-HETE.
Keywords: arachidonic acid, cytochrome P-450, aminobenzotriazole, HET0016, noradernaline, endothelin, angiotensin II, eicosanoids
Introduction
Diabetes mellitus is associated with alterations in vascular reactivity that contributes to the development of cardiovascular complications including hypertension, arteriosclerosis, microangiopathy, end stage renal disease and congestive heart failure (Kamata et al., 1989; Feener & King, 2001; Kassab et al., 2001). Enhanced vascular response to vasoconstrictors and attenuated response to vasodilators have been reported in diabetic patients and animals (Kamata et al., 1989; Feener & King, 2001; Kassab et al., 2001). However, the factors contributing to these changes in vascular reactivity are not fully understood.
Recent studies have indicated that 20-hydroxyeicosatetraenoic (20-HETE) is an endogenously-formed vasoconstrictor that modulates the response of renal, cerebral and mesenteric arteries to angiotensin II (Ang II), vasopressin, endothelin (ET-1), and serotonin (5-HT), as well as the vasodilators nitric oxide (NO) and carbon monoxide (Muthalif et al., 1998; Croft et al., 2000; Roman et al., 2000; Kehl et al., 2002; Roman, 2002; Kaide et al., 2003). 20-HETE alters vascular tone by activating a number of signal transduction pathways including protein kinase C (PKC), rho-kinase, mitogen-activated protein kinase (MAPK) and tyrosine kinases (Roman et al., 2000; Roman, 2002). There is evidence that Ca2+/calmodulin-dependent protein kinase II (CaMKII) couples G protein receptors to activation of cytosolic phospholipase A2 (cPLA2) leading to increased release of arachidonic acid (AA) and formation of 20-HETE in vascular smooth muscle cells (VSMC) (Muthalif et al., 1998, 2002). It is also known that Ras-GTPase activates cPLA2 via a MAPK pathway again leading to 20-HETE formation in VSMC (Muthalif et al., 2000a). Moreover, activation of the 20-HETE/CaMKII and Ras-GTPase signal transduction pathway contributes to the elevated vascular tone in L-NAME treated rats and in Ang II- and deoxycorticosterone acetate (DOCA)-induced models of hypertension via a 20-HETE dependent mechanism (Su et al., 1998 ; Oyekan et al., 1999; Muthalif et al., 2000a,b, 2002; Benter et al., 2005a).
The expression of cytochrome P450 (CYP) 4A enzymes and the formation of 20-HETE has been reported to be elevated in the liver and kidney of diabetic animals but it remains to be determined whether the production of 20-HETE is altered in the vasculature and if this contributes to the elevated vascular reactivity and endothelial dysfunction seen in diabetes. (Shimojo et al., 1993). These findings suggest that changes in the vascular formation of CYP metabolites of AA may contribute to some of the vascular complications in diabetes. Indeed, inhibitors of CaMKII and Ras-GTPase, which promote the formation of 20-HETE in VSMC, oppose the development of abnormal vascular reactivity in diabetic animal models (Yousif et al., 2003, 2004; Benter et al., 2005a,b; Yousif, 2006). Thus, the present study examined the role of 20-HETE in altering vascular reactivity in the renal and mesenteric vascular beds in the streptozotocin (STZ) model of diabetes in rats.
Materials and methods
Experiments were performed in male Wistar rats weighing 200–250 g. The rats had free access to food and water throughout the study. All protocols were approved by the Institutional Committee for the Care and Use of Animals in Research and Education of Kuwait University and were in compliance with the principles for the care and use of animals approved by the American Physiological Society.
Diabetes was induced by administration of a single intraperitoneal (i.p.) injection of 55 mg kg−1 body weight of STZ dissolved in a 0.05 M citrate buffer (pH 4.5). Age-matched control rats were injected with the buffer alone. Body weight and plasma glucose levels were determined prior to and 48 h after administration of STZ, using a blood glucose analyzer (Glucometer Elite XL, Bayer Diagnostic, Berlin, Germany). Rats with a post-prandial blood glucose concentration above 300 mg dl−1 were included in the study. Body weight and plasma glucose levels were reassessed 4 weeks later, before sacrificing the animals for the vascular reactivity studies.
Experimental groups
Experiments were performed on six different groups of rats. In group 1, non-diabetic rats received daily i.p. injections of saline (n = 10) which served as the vehicle for all of the drug treatments. In group 2, non-diabetic rats received i.p. injections of 1-aminobenzotriazole (ABT, 50 mg kg−1 alt diem, n = 10) for 30 days to chronically inhibit the formation of 20-HETE and epoxyeicosatrienoic acids (EETs). Group 3 consisted of non-diabetic rats that received i.p. injections of N-hydroxy-N′-(4-butyl-2-methylphenyl) formamidine (HET0016) (2.5 mg kg−1, daily, n = 10) in an attempt to inhibit the formation of 20-HETE more selectively. Group 4 consisted of diabetic rats that received daily i.p. injections of saline. In groups 5 and 6, diabetic rats were chronically treated with i.p. injections of ABT (50 mg kg−1, alt diem, n = 10) or HET0016 (2.5 mg kg−1, daily, n = 10), respectively for 30 days. The doses of the inhibitors used are based on previous reports indicating that chronic treatment of rats with similar doses of ABT or HET0016 were sufficient to effectively block the renal formation of 20-HETE in vivo (Miyata et al., 2001; Hoagland et al., 2003).
Vascular reactivity in isolated perfused mesenteric vascular bed
After 30 days of treatment with vehicle or the various inhibitors, the diabetic and non-diabetic rats were killed with an i.p. injection of pentobarbital (100 mg kg−1) and the mesenteric vascular bed was isolated and transferred into a Petri dish containing Krebs Henseleit solution containing (in mM): NaCl (118.3), KCl (4.7), CaCl2 (2.5), MgSO4 (1.2), NaHCO3 (25), KH2PO4 (1.2) and glucose (11.2) that was equilibrated with 95% O2 and 5% CO2. The mesenteric artery was cannulated and the bed was placed in a water-jacketed chamber maintained at 37 °C. The preparation was continuously perfused at a rate of 6 ml min−1. Changes in perfusion pressure following close intra-arterial injections of various agonists were recorded with a pressure transducer and a chart recorder. Following a 45 min equilibration period, the vasoconstrictor responses to NA (10, 100 and 1000 nM), ET-1 (0.01, 0.1 and 1.0 nM) and Ang II (0.1, and 1.0 nM) were studied. The order of administration of the vasoconstrictors was randomized between animals. In a separate group of experiments using control and diabetic animals, vasodilator responses to carbachol, histamine and sodium nitroprusside (SNP) were investigated in the perfused mesenteric vascular bed. The perfused mesenteric bed was constricted by perfusion with Krebs Henseleit solution containing NA (10−6 M). After establishing a steady level of contraction, successive doses of carbachol (1 and 10 nmol), histamine (10 and 100 nmol) or SNP (0.1, 1.0 and 10 nmol) were given at regular intervals. The vasodilator response is expressed as % of the pre-contraction induced by NA (10−6 M). 5–10 min were allowed for full recovery between doses and the order of vasodilator administration was randomized between animals.
Vascular reactivity in isolated renal artery segments
After 30 days of treatment with vehicle or the various inhibitors, rats were killed and the renal artery isolated and transferred into a Petri dish containing oxygenated Krebs Henseleit buffer solution. The vessels were cut into 5 mm rings that were mounted in organ baths containing 50 ml Krebs Henseleit solution, pH 7.4. The baths were maintained at 37 °C and equilibrated with 95% O2 and 5% CO2. Isometric contractions were recorded using a force transducer and a chart recorder. The preparations were preloaded with 0.75 g of tension and 45 min were allowed for equilibration of the preparations.
Following the equilibration period, cumulative concentration response curves to the vasoconstrictor responses to NA (10, 100 and 1000 nM), ET-1 (0.01, 0.1 and 1.0 nM) and Ang II (0.1, and 1.0 nM) were constructed. The agonists were added sequentially and the maximal increase in tension was recorded. Eight to ten independent experiments were performed per constrictor agonist and only one constrictor substance was studied per ring. The recovery time between doses of the agonists was 5–10 min for NA and Ang II and at least 20 min for ET-1. The concentrations of the vasoactive agonists were chosen based on our previous results indicating that these concentrations typically span the range of responses from the EC30 to the maximal response in these vascular beds and are useful to demonstrate the changes in the vascular reactivity. In a separate series of experiments, the vasodilator responses to carbachol, histamine and SNP were determined using other vascular rings. In these experiments, segments of renal artery were contracted with a sub-maximal concentration of NA (10−6 M). After a steady state level of tension was obtained, cumulative concentration response curves to carbachol (10−7, 10−6 and 10−5 M), histamine (10−6, 10−5 and 10−4 M) and SNP (10−8, 3 × 10−8 and 10−7 M) were constructed.
Effect of acute in vitro blockade of the synthesis of 20-HETE with HET0016 (1 μM) in vitro on the vasoconstrictor response of mesenteric and renal vasculature to NA
Further experiments were performed to determine if the elevated vascular tone of diabetic animals is dependent on the synthesis of 20-HETE and if this could be reversed by acute administration of HET0016, reported to be a highly selective inhibitor of 20-HETE synthesis in vitro (Miyata et al., 2001). The mesenteric vascular bed from diabetic animals treated with vehicle was isolated and perfused as described above. Following a 30 min equilibration period, vasoconstrictor responses to NA (3, 10 and 30 nM) were determined. After a 30 min perfusion with HET0016 (1 μM), vasoconstrictor responses to NA was re-assessed. Similar experiments were performed on renal artery rings prepared from diabetic animals. In these experiments the vasoconstrictor responses to NA (10−8, 3 × 10−8 and 10−7 M) were determined before and 30 min after addition of HET0016 (1 μM) to the bath.
Vascular responsiveness to exogenous 20-HETE
Another series of experiments examined whether vascular reactivity to 20-HETE itself was altered in diabetes. A concentration response curve for 20-HETE (0.001, 0.01, 0.03 and 0.1 nmol) was constructed using perfused mesenteric beds from control and diabetic animals. The vasoconstrictor response to 20-HETE was examined on basal perfusion pressure and also against a perfusion pressure elevated by perfusion with phenylephrine (10−6 M). After establishing a steady level of vasoconstriction, successive doses of 20-HETE were administered. The vasoconstrictor response was expressed as the change in perfusion pressure in mmHg, and was calculated as the area under the curve.
Expression of CYP4A protein in the kidney and renal vasculature
Renal cortical microsomes and renal artery homogenates (20 μg) were separated by electrophoresis on 7.5% SDS-PAGE gels. The gels were transferred to nitrocellulose membranes blocked overnight with 10% non-fat dry milk in a 10 mM Tris buffered saline containing Tween-20 and then incubated with a 1:4000 dilution of CYP4A primary antibody for 2 h. The membranes were washed and incubated for 1 h with a 1:2000 dilution of a horseradish peroxidase coupled second antibody. The membranes were coated with an enhanced chemoluminese reagent (West Pico, Pierce Chem. Corp.) and exposed to x-ray film. The relative intensity of the CYP4A bands (50 Kd) were determined using UnScanit software.
Renal metabolism of arachidonic acid
Microsomes were prepared from renal cortical tissue obtained from diabetic and non-diabetic by differential centrifugation as previously described (Imig et al., 1996; Kehl et al., 2002). The metabolism of arachidonic acid (AA) was determined by incubating the microsomes (0.5 mg of protein) with 14C-AA (0.1 μCi, 42 μM) for 30 min at 37 °C in 1 ml of a 100 mM KPO4 buffer (pH 7.4) containing 10 mM MgCl2,1 mM EDTA, 1 mM NADPH and an NADPH-regenerating system (10 mM isocitrate and isocitrate dehydrogenase, 0.4 U ml−1). The reactions were terminated by acidification to pH 3.5 using 1 M formic acid and the samples were extracted with ethyl acetate. The metabolites formed were separated using a 2 mm × 25 cm C18-reverse phase HPLC column and a linear elution gradient ranging from acetonitrile: water: acetic acid (50/50/0.1) to acetonitrile: acetic acid (100/0.1) over 40 min. The radioactive products were monitored using a radioactive flow detector (Model 120, Radiomatic Instrument Co., Tampa, FL, USA). Values are expressed as picomoles formed per minute per milligram of protein.
LC/MS evaluation of the metabolism of AA in isolated renal and mesenteric arteries
Intact renal and mesenteric arteries were microdissected from diabetic and non-diabetic rats and incubated in 2 ml physiological salt solution containing 40 μM AA, 1 mM NADPH, and 2 μM indomethacin. The reactions were gently swirled for 90 min at 37 °C under an atmosphere of 100% O2 maintaining a PO2 in the assay buffer of 150 torr. The reactions were terminated by acidification to pH 3.5 using 1 M formic acid. The arteries were homogenized using a ground glass tissue grinder and 2 ng of an internal standard 5, 14, 20-hydroxydienoic acid added. An aliquot of the homogenate was taken for measurement of sample protein content and the remaining sample was extracted with ethyl acetate. The organic layer was dried under nitrogen, reconstituted in methanol and then resolved by HPLC on a Betabasic C18 column (150 × 2.1 mm, 3 μm; Thermo Hypersil-Keystone) using a step gradient ranging from acetonitrile:methanol:water:acetic acid (51:9:40: 0.01) for 15 min. The eluent from the HPLC was ionized using negative ion electrospray. Peaks eluting with a mass/charge ratio (m/z) of 319 > 301 (HETEs and EETs), 337 > 319 (DiHETEs) or 323 > 270 (internal standard) were isolated and measured using a triple quadruple mass spectrometer (API 3000, Applied Biosystems, Inc., Foster City, CA, USA) Standard curves for 20-, 12-, 15- and 5-HETE, 11, 12-, 14, 15- and 8,9 DiHETEs and EETs over a range from 2 pg to 2 ng were generated with each batch of samples. Values are expressed as picomoles formed per minute per milligram of protein.
Statistics
Mean values ± SE are presented. The significance of differences in mean values between treatment groups was determined using an analysis of variance followed by a post hoc test (Bonferroni). P-value <0.05 was considered to be significant.
Results
Hyperglycemia and body weights
Blood glucose levels following an overnight fast averaged 576 + 20 mg dl−1 in STZ-induced diabetic rats as compared with 94 + 2 mg dl−1 in control animals. The body weight of diabetic rats fell to 162 ± 6 g over the course of the study. In comparison, body weight of the non-diabetic control animals averaged 275 ± 4 g. Chronic treatment with ABT or HET0016 had no significant effect on body weight or fasting blood glucose concentration in either the diabetic or non-diabetic rats.
Effects on the vasoconstrictor response of renal and mesenteric arteries
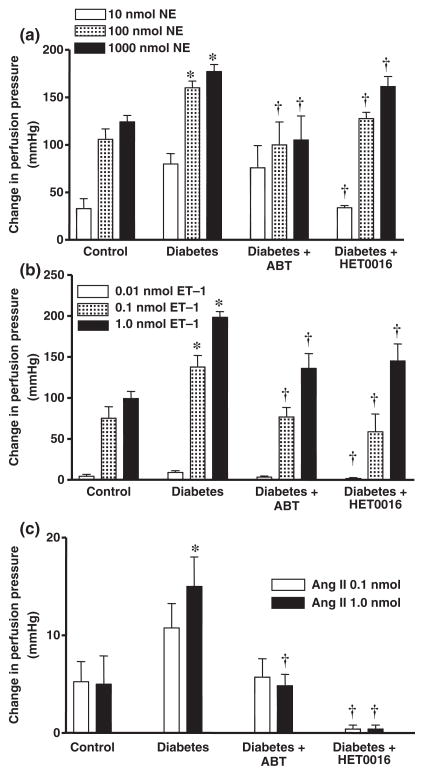
Vasoconstrictor responses to NA, ET-1 or Ang II were all significantly elevated in the perfused mesenteric vascular bed of STZ-induced diabetic rats as compared to the responses observed in rats treated with vehicle (Fig. 1). The enhanced vasoconstrictor responses to all of these agonists were significantly reduced in diabetic rats chronically treated with ABT or HET0016. ABT had no significant effect on the vasoconstrictor responses to NA, ET-1 and Ang II in the perfused mesenteric vascular bed of non-diabetic control rats. However, vasoconstrictor responses to NA (100 nmol) and ET-1 (1.0 nmol) were significantly reduced by 40% and 27%; respectively, in the mesenteric vascular bed of control rats treated with HET0016. In contrast, the vasoconstrictor response to AngII was not affected in these animals.
Figure 1.
Comparison of vasoconstrictor responses to (a) NA, (b) ET-1 and (c) Ang II in the perfused mesenteric vascular bed of control, diabetic and diabetic rats chronically treated with ABT or HET0016. Mean values ± SE from 8 rats per group are presented. *Significant difference from control. †Significantly different from the corresponding value in diabetic rats.
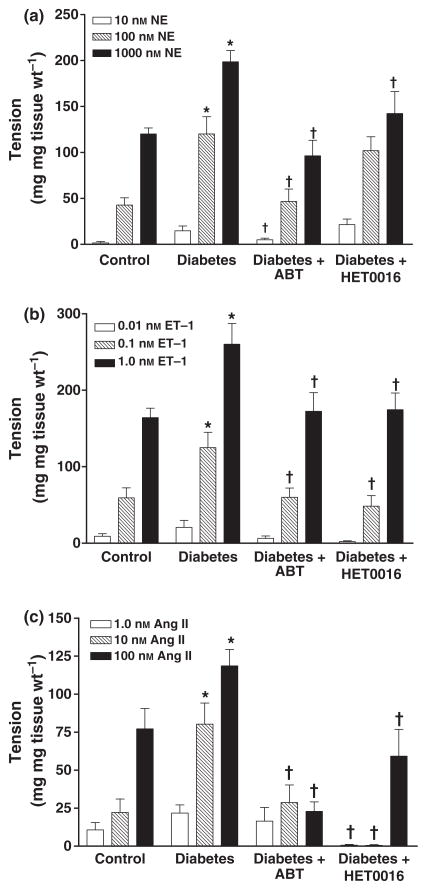
The vasoconstrictor responses to NA, ET-1 or Ang II were all significantly greater in renal artery rings prepared from diabetic rats than in the control rats treated with vehicle (Fig. 2). The enhanced vascular responses to these agents was attenuated in diabetic rats chronically treated with ABT or HET0016. The vasoconstrictor response to ET-1 was also reduced in the renal arteries obtained from non-diabetic control rats that were chronically treated with ABT or HET0016 (data not shown) while the responses to NA and Ang II were unaltered.
Figure 2.
Comparison of vasoconstrictor responses to (a) NA, (b) ET-1 and (c) Ang II in renal artery ring segments prepared from control, diabetic, and diabetic rats chronically treated with ABT or HET0016. Mean values ± SE from 10 rats per group are presented. *Significant difference from control, †Significant difference from the corresponding value in diabetic rats.
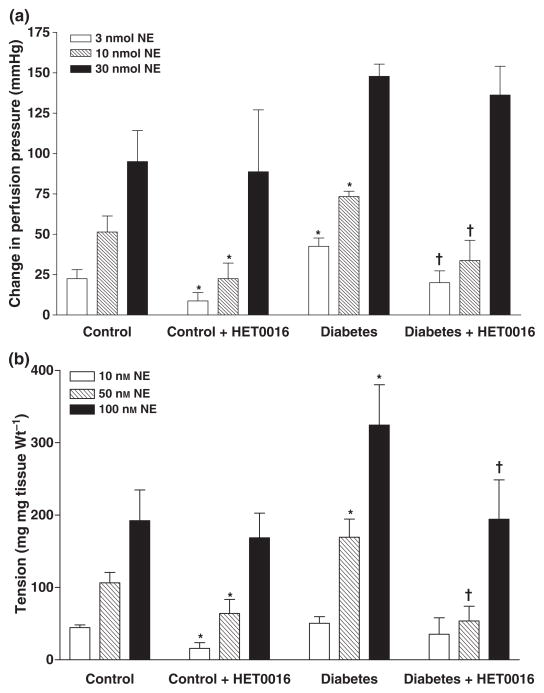
The effect of acute blockade of the synthesis of 20-HETE in vitro with HET0016 (1 μM) on the vasoconstrictor response in the renal and mesenteric vaculature to NA are presented in Fig. 3. The vasoconstrictor response to NA was greater in the perfused mesenteric vascular bed and in renal arteries of diabetic animals as compared with the responses seen in non-diabetic animals. Addition of HET0016 (1 μM) had no effect on the basal perfusion pressure of the mesenteric bed or the basal tone of renal artery rings in either diabetic or non-diabetic animals. HET0016 however, attenuated the vasconstrictor response to NA in both the mesenteric vascular bed and in renal artery rings of both diabetic and control rats (Fig. 3).
Figure 3.
Effect of acute blockade of the synthesis of 20-HETE with HET0016 (1 μM) in vitro on the vasoconstrictor response to noradrenaline in the (a) perfused mesenteric vascular bed and (b) renal artery rings of control and diabetic rats. Mean values ± SE from 4–7 rats per group are presented. *Significant difference from control, †Significant difference from the corresponding value in diabetic rats.
Effects on the vasodilator responses of renal and mesenteric arteries
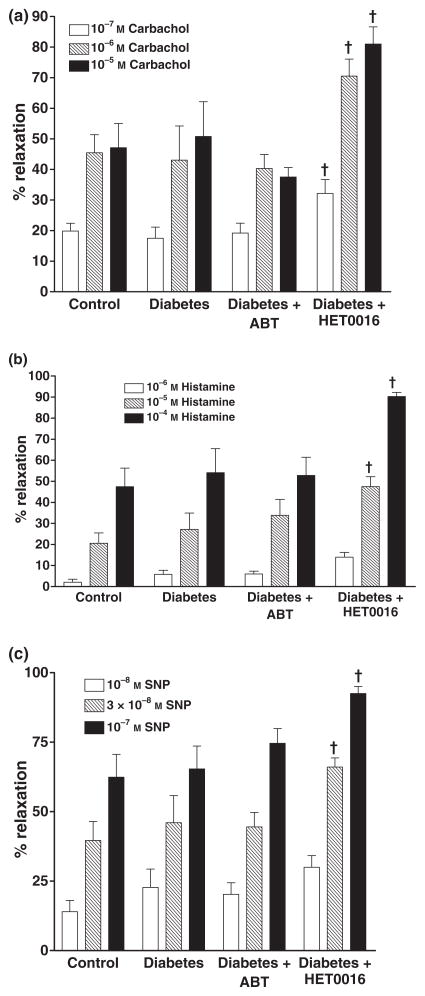
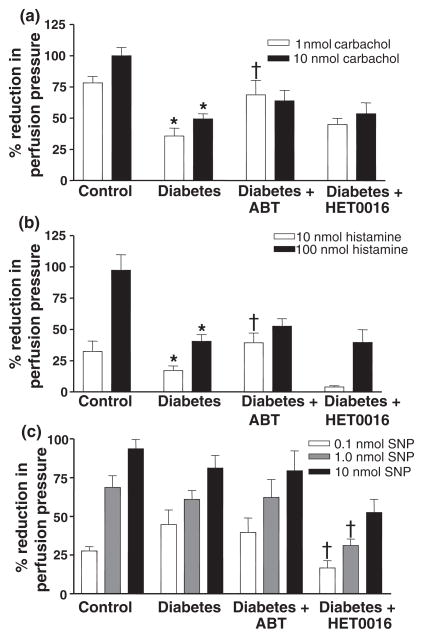
The effects of ABT and HET0016 on the vasodilator response of renal arteries to carbachol, histamine and SNP are presented in Fig. 4. The vasodilator responses to these agents in renal artery rings prepared from diabetic rats were not significantly different from those obtained using renal artery rings from control rats. Chonic blockade of the formation of EETs and 20-HETE with ABT had no significant effect on the vasodilator response of renal arteries to carbachol, histamine or SNP in the renal arteries of the diabetic rats. In contrast, HET0016 potentiated the vasodilator response of renal arteries to carbachol, histamine and SNP obtained from diabetic rats. Chronic treatment of control rats with ABT no signficant effect on the vasodilator responses of renal arteries to these vasodilators. However, the vasodilator response to histamine was significantly increased (60% increase at 10−4 M and 18% at 5 × 10−3 M) in renal arteries obtained from control animals chronically treated with HET0016, while the vasodilator responses to carbachol or SNP were unaffected.
Figure 4.
Comparison of the vasodilator responses to (a) carbachol, (b) histamine or (c) sodium nitroprusside (SNP) in isolated renal artery ring segments prepared from control, diabetic and diabetic rats chronically treated with ABT or HET0016. Mean values ± SE from 10 rats per group are presented. † Significant difference from the corresponding value in diabetic rats.
Vasodilator responses to carbachol, histamine, and SNP in mesenteric arteries are shown in Fig. 5. The vasodilator responses to carbachol and histamine, but not SNP, were reduced by about 50% in the isolated perfused mesenteric vascular bed of diabetic animals as compared to the responses seen in the non-diabetic control animals. Chronic blockade of the formation of 20-HETE and EETs in diabetic rats with ABT enhanced the vasodilator response of mesenteric arteries to low concentrations of carbachol and histamine, but not to SNP. Chronic treatment of the diabetic animals with HET0016 had no effect on the vasodilator response to either carbachol or histamine, but it did reduce the reponse to SNP. In perfused mesenteric beds obtained from control animals chronically-treated with ABT or HET0016, the vasodilator responses to carbachol, histamine or SNP were reduced.
Figure 5.
Comparison of the vasodilator responses to (a) carbachol, (b) histamine and (c) sodium nitroprusside in isolated perfused mesenteric vascular bed of control, diabetic and diabetic rats chronically treated with ABT or HET0016. Mean values ± SE from 10 rats per group are presented. *Significant difference from control. † Significant difference from the corresponding value in diabetic rats.
Vascular responsiveness to exogenous 20-HETE
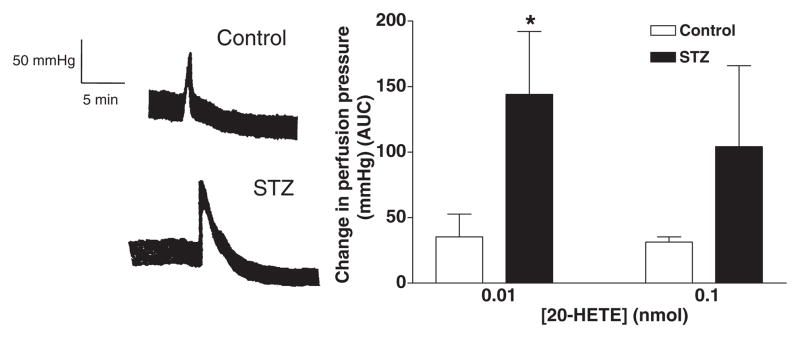
20-HETE elicited a small vasoconstrictor response in the mesenteric bed of both control and diabetic animals perfused under baseline conditions. The magnitude of the response was similar in control and diabetic animals. However, after elevating vascular tone with phenylephrine (10−6M), the vasconstrictor response to 20-HETE was markedly potentiated in the mesenteric vasculature of both diabetic and control animals. In addition, the magnitude of the response to 20-HETE was significantly greater in the mesenteric vascular bed of diabetic rats than that seen in control animals. (Fig. 6a and b).
Figure 6.
(a) Representative traces showing the vasoconstrictor response (mmHg) to 20-HETE (0.01 nmol) in the perfused mesenteric vascular bed of control (upper panel) and diabetic rats (lower panel) pre-constricted with phenylephrine (PE, 10−6 M). (b) Comparison of 20-HETE-induced vasoconstrictor responses in the perfused mesenteric vascular bed of control and diabetic rats. *Significant difference from control.
Expression of CYP4A enzymes and formation of 20-HETE in the kidney and vasculature
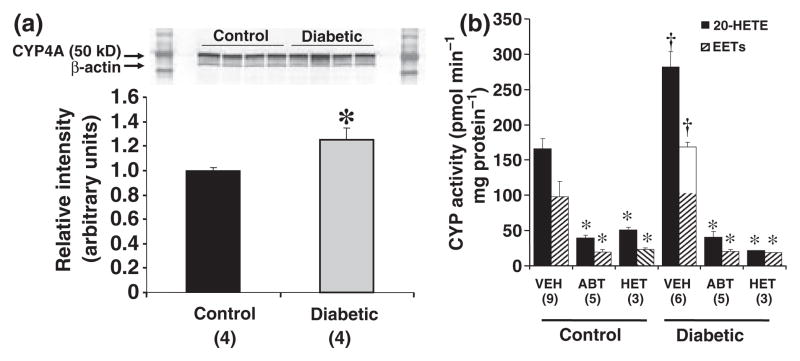
The expression of CYP4A protein and the formation of 20-HETE were 20% and 60% greater in microsomes prepared from the kidney of diabetic rats as compared to the levels seen in the kidney of control rats (Fig. 7a and b). Chronic treatment of the rats with ABT or HET0016 inhibited the renal formation of 20-HETE by more than 80% in both non-diabetic and diabetic rats (Fig. 7b) confirming that the doses used were sufficient to achieve systemic blockade. Chronic treatemed of the rats with the non-selective inhibitor ABT, reduced the renal formation of EETs by 80%. The renal production of EETs was also reduced in the rats chronically treated with HET0016 (Fig. 7b) which has been previously reported to be a highly selective inhibitor of the synthesis of 20-HETE in vitro (Miyata et al., 2001) and after acute administration at a dose of 1 mg Kg−1 to rats in vivo (Hoagland et al., 2003).
Figure 7.
Panel a. Expression of CYP4A protein in microsomes prepared from kidneys of control and diabetic rats. Each lane was loaded with 20 μg of protein. *Significant difference from the expression level in the control rats. Panel b. Cytochrome P-450 ω-hydroxylase and epoxygenase activity in microsomes prepared from the kidney of control and STZ-treated diabetic rats chronically treated with vehicle, ABT, or HET0016 for 30 days. The microsomes (0.5 mg protein) were incubated with 14C-AA (0.1 μCi, 42 μM) for 30 min in the presence of NADPH (1 mM). The reactions were stopped with formic acid, extracted with ethyl acetate. The products formed were separated by HPLC and detected using a radioactive flow detector. *Significant difference from the corresponding value in vehicle treated rats, †Significant difference from the corresponding value in non-diabetic control animals.
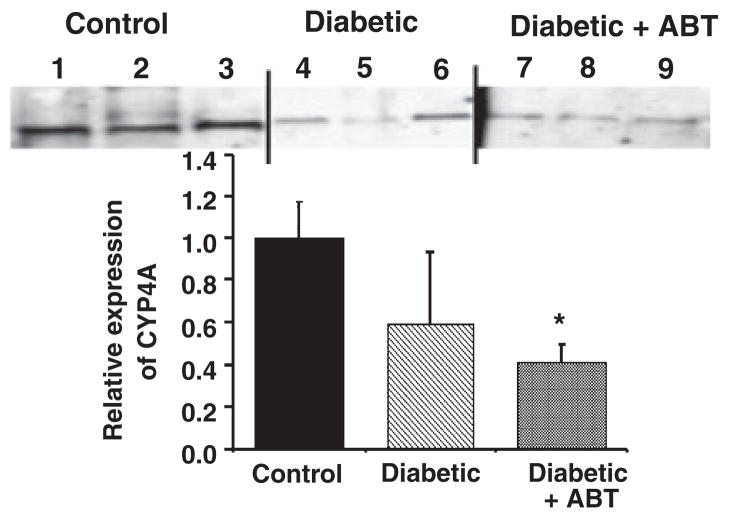
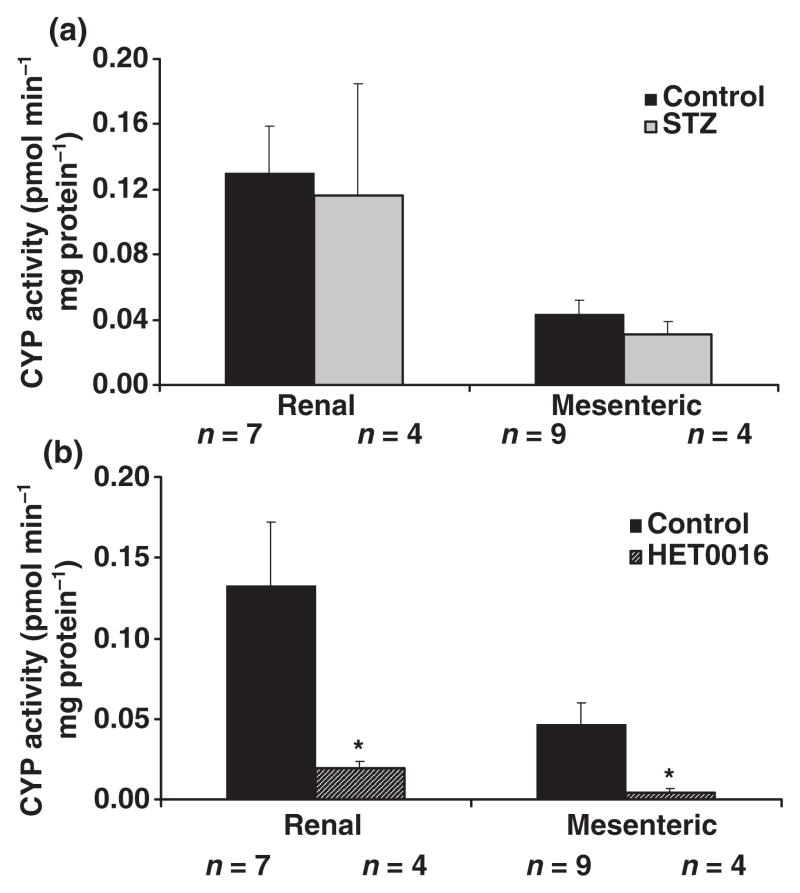
In contrast to the kidney, the expression of CYP4A protein was not elevated in renal vasculature microdissected from the kidneys of diabetic rats (Fig. 8). Rather, the expression of CYP4A protein, if anything, tended to be reduced rather than elevated. Similarly, Fig. 9 shows that the production of 20-HETE was not significantly different in either renal or mesenteric arteries obtained from diabetic and non-diabetic control animals. We demonstrated that the dose of HET0016 (1 μM), which we found to inhibit vasoconstrictor responses in vitro, completely blocked the formation of 20-HETE in isolated renal and mesenteric arteries obtained from non-diabetic animals. In vitro addition of 1 μM HET0016 significantly inhibited the formation of 20-HETE in renal and mesenteric arteries of non-diabetic rats by more than 80% (Fig. 9b). HET0016 had no significant effect on vascular epoxygenase activity (sum of EETs + DiHETEs) in these vessels which averaged 45 ± 18 and 64 ± 10 pmol min−1 mg−1 protein, respectively in control (n = 7) and HET0016-treated (n = 4) renal vessels and 6.5 + 4.9 and 9.7 + 2.6 pmol min−1 mg−1 protein in control (n = 9) and HET0016-treated (n = 4) mesenteric arteries.
Figure 8.
Comparison of the expression of Cytochrome P4504A protein in homogenates prepared from the renal artery of control, diabetic, and diabetic rats chronically treated with ABT. The data are expressed as relative to mean intensity recorded in renal arteries obtained from the control animals. Each lane was loaded with 20 μg protein prepared from the renal of individual rats. *Significant difference compared to the level of expression in the control group.
Figure 9.
Panel a. Comparison of the production of 20-HETE in intact renal and mesenteric arteries obtained from diabetic and non-diabetic rats. Vessels were microdissected from the kidney and mesenteric beds of control and diabetic animals and incubated for 1 h in a physiologic salt solution containing 42 μM arachidonic acid and 1 mM NADPH. The vessels were homogenized in the buffer and extracted with ethyl acetate. The products formed were analysed by LC/MS as described in the methods section. Panel b presents the effects of in vitro addition of HET0016 (1 μM) on the production of 20-HETE by intact renal and mesenteric arteries. *Significant difference from the corresponding control value.
Discussion
Arachidonic acid is primarily metabolized by enzymes of the CYP4A and perhaps the 4F families in small arteries in the brain, lung, kidney and peripheral vasculature to produce 20-HETE, and this compound has been shown to play a critical role in the regulation of vascular tone (Alonso-Galicia et al., 1997; Oyekan & Mcgiff, 1998; Oyekan et al., 1999; Croft et al., 2000; Roman et al., 2000;, Kehl et al., 2002; Roman, 2002; Kaide et al., 2003). Considerable evidence has accumulated to indicate that the production of 20-HETE is altered in the kidney of several models of hypertension and that this contributes to the development of hypertension and associated end-organ damage (Muthalif et al., 2002, 2000a,b; Benter et al., 2005a,b; Oyekan et al., 1999; Su et al., 1998).
In this study, we found that the vasoconstrictor responses to NA, ET-1 and Ang II were potentiated in the isolated perfused mesenteric vascular bed and in renal arteries obtained from STZ-induced diabetic rats. Chronic treatment of both diabetic and non-diabetic rats with ABT or HET0016 reduced the formation of 20-HETE in renal microsomes confirming that the doses employed were sufficient to produce a systemic blockade of CYP4A activity. Unfortunately, neither drug selectively inhibited the synthesis of 20-HETE in the kidney, as both compounds reduced epoxygenase activity. While this outcome was anticipated for the ABT, HET0016 is a more specific inhibitor of the synthesis of 20-HETE which exhibits a 1000-fold greater selectivity for inhibition of the synthesis of 20-HETE vs. EETs, DiHETEs and prostaglandins, at least in vitro (Miyata et al., 2001). It has also been shown to selectively block the renal formation of 20-HETE when given acutely at a dose of 1 mg Kg−1 to rats in vivo (Hoagland et al., 2003). Thus, the lack of a selective blockade of the synthesis of 20-HETE in the kidney of these rats was surprising. HET0016 is highly lipid soluble and accumulates in the brain and kidney at concentrations 4–10-fold higher than that seen in plasma. So it is possible to reach levels sufficient to inhibit renal but not vasculature epoxygenase activity. Alternatively, the reduction in renal epoxygenase activity in rats chronically treated with HET0016 may not be a direct inhibitory effect of HET0016 but simply a compensatory response since the expression of renal epoxygenase enzymes are regulated by a number of factors such as salt balance and the renin angiotensin system, that can be influenced by long-term blockade of the synthesis of 20-HETE.
Chronic administration of ABT and HET0016 normalized the enhanced vascular responses to NA, ET-1 and Ang II in the mesenteric circulation and renal arteries of diabetic rats. These results are most likely due to blockade of the vasoconstrictor actions of 20-HETE in diabetic rats rather than an effect of ABT and HET0016 to block the synthesis of the vasodilator EETS in the mesenteric and renal circulation. In further studies, we demonstrated that acute treatment of mesenteric and renal arteries with HET0016 (1 μM) in vitro selectively reduced the formation of 20-HETE by 90% and had no effect on the production of EETs or DiHETEs. In these in vitro experiments, HET0016 still reduced NA-induced vasoconstrictor responses in the perfused mesenteric bed and isolated renal artery obtained from control and diabetic animals. However, the percent attenuation of the vasoconstrictor response to NA was significantly greater in the vessels of the diabetic animals than in the non-diabetic controls. These findings further support our view that 20-HETE rather than EETs play at least a permissive role in elevating the vascular responsiveness of renal and mesenteric arteries to G-protein coupled receptor mediated vasoconstrictors in diabetic rats.
In this study, we demonstrated that the inhibitors of the synthesis of 20-HETE cause a much larger attenuation of the vasoconstrictor responses to Ang II and ET-1 than NA. The reason for this differential effect remains to be determined but could be related to the fact that 20-HETE augments vascular tone by blocking Ca2+activated K+ channels leading to membrane depolarization and greater calcium influx through voltage sensitive channels (Sun et al., 1998; Roman, 2002). In this regard, previous studies have demonstrated that the vasoconstrictor response to Ang II is highly dependent on Ca2+influx through the voltage sensitive channels, whereas the vasoconstrictor response to NA is much more dependent on the release of calcium from intracellular stores in the sarcoplasmic reticulum (Bhugra & Gulati, 1996;, Baan et al., 1999; Garcha et al., 2001; Lee et al., 2001; Roman, 2002; Arun et al., 2005; Shin et al., 2005; Villalba et al., 2007).
We also found that HET0016 or ABT did not have much effect on the baseline vasoconstrictor responses to Ang II, ET-1 or NA in the renal artery of control animals. However, HET0016 did attenuate the vasoconstrictor responses to NA and ET-1 in the mesenteric vascular bed in control animals. The finding that HET0016 attenuates vascular reactivity in the perfused mesenteric bed, but not in a larger vessels such as the renal artery, fits with previous observations that the expression of CYP4A enzymes and production of 20-HETE is much greater in microvessels <100 μM in diameter than in larger conduit vessels (Roman, 2002).
Additional experiments were performed to determine whether upregulation of the vascular expression of CYP4A contributes to the enhanced 20-HETE-dependent vascular reactivity to constrictor stimuli seen in diabetic animals. We observed that the expression of CYP4A protein and synthesis of 20-HETE is elevated in the kidney of the diabetic rats. These results confirm previous reports indicating that the expression of CYP4A protein is elevated in the kidney and liver of diabetic animals (Shimojo et al., 1993; Engels et al., 1999; Enriquez et al., 1999; Takatori et al., 2002; Yoshinari et al., 2006). However, the expression of CYP4A protein tended to be reduced rather than elevated in renal arteries and the synthesis of 20-HETE was not significantly different in either the renal or mesenteric arteries obtained from control and diabetic rats. These studies indicate that upregulation of CYP4A expression does not mediate the increase in vascular responsiveness to vasoconstrictors seen in diabetic rats and that some other mechanism must be involved.
Previous studies have indicated that NA, Ang II and ET-1 stimulate production of 20-HETE in VSMC by enhancing the availability of substrate through the release of AA from membrane phospholipid stores (Muthalif et al., 1998). Recent studies in VSMC have shown that CaMKII participates in coupling G-protein receptors to activation of PLA2 and the release of AA, which can be subsequently metabolized by CYP4A enzymes to 20-HETE (Muthalif et al., 1998). Ras-GTPase also activates cPLA2 via a MAPK pathway again leading to 20-HETE formation in VSMC (Muthalif et al., 1998, 2000a,b). Recent studies have shown that chronic treatment of diabetic rats with KN-93 or FTPIII to block CaMKII and Ras-GTPase pathways is just as effective as inhibitors of the synthesis of 20-HETE in preventing the enhanced vasoconstrictor responses to NA, ET-1 and Ang II in the renal and mesenteric circulation of diabetic rats (Yousif et al., 2003, 2004; Benter et al., 2005a,b; Yousif, 2006). These findings suggest that increased expression or activity of CaMKII or Ras-GTPase pathways might be responsible for the enhanced 20-HETE dependent vascular reactivity to vasoconstrictors in diabetic rats by enhancing agonist-induced release of substrate (AA) and subsequent production of 20-HETE in the vasculature of diabetic animals in the absence of upregulation of the expression of CYP4A protein. Another possibility is that the CaMKII or Ras-GTPase system might alter signal transduction pathways by which 20-HETE alters vascular reactivity. In this regard the present finding of enhanced vascular responsiveness to 20-HETE in the mesenteric vasculature of diabetic rats supports the latter hypothesis.
Endothelial dysfunction is also associated with diabetes (Kamata et al., 1989; Feener & King, 2001). Reports about the effect of experimentally induced diabetes on endothelium-dependent vascular relaxation have been inconsistent. Endothelium-dependent relaxation has been shown to be unaffected (Beenen et al., 1996; Garcia et al., 1999), attenuated (Kamata & Hosokawa, 1997), or augmented (Gebremedhin et al., 1989) in animal models of diabetes. In this study, we confirmed previous reports that the vasodilator responses to carbachol and histamine are significantly reduced in the perfused mesenteric bed of the diabetic rats. Hyperglycemia results in diminished release and/or bioavailability of nitric oxide (Oyama et al., 1986; Feener & King, 2001; Ramana et al., 2003). Thus, impaired NO synthesis may be partially responsible for the impaired vascular reactivity observed in diabetic animals. In addition, reduced availability of NO may increase the formation of 20-HETE in VSM since NO directly inhibits the formation of 20-HETE (Sun et al., 2000; Roman, 2002). In this study, the vasodilator responses of vascular rings prepared from the main renal artery to carbachol, histamine or SNP were not significantly altered in diabetic rats. However, we clearly confirmed that the responses to all of these vasodilators were markedly diminished in the isolated perfused mesenteric vascular bed of diabetic animals. Selective blockade of the formation of 20-HETE with HET0016 enhanced the vasodilator responses in the mesenteric vascular bed to carbachol, histamine and SNP in diabetic rats. These findings suggest that 20-HETE reduces the responsiveness to these NO dependent vasodilators in STZ rats. The mechanism involved remains to be determined but two recent studies have indicated that 20-HETE elevates oxidative stress in endothelial cells (Wang et al., 2006; Guo et al., 2007). Elevated production of superoxide via a 20-HETE dependent pathway could impair vascular responses to NO dependent dilators by scavenging NO. There is also recent evidence that overexpression of CYP4A protein and the production of 20-HETE leads to NO synthase uncoupling thereby reducing the NO by endothelial cells (Wang et al., 2006).
In summary, this study indicates that chronic blockade of the production of 20-HETE normalizes the enhanced vasoconstrictor responses to Ang II, NA and ET-1 in the renal and mesenteric circulation of diabetic rats. Acute treatment of isolated renal and mesenteric vessels with HET0016 (1 μM) in vitro also normalized the enhanced vascular responsiveness of these vessels to NA in diabetic animals and selectively inhibited the formation of 20-HETE. Treatment of the diabetic animals with HET0016 improved vasodilator responses in the mesenteric vascular bed. Vascular sensitivity to exogenous 20-HETE was markedly elevated in the mesenteric bed of diabetic animals compared with controls. These results suggest that 20-HETE plays a role in elevating vascular reactivity in diabetic animals. This effect is not due to increased vascular expression of CYP4A but may be related to elevated vascular responsiveness to 20-HETE.
Acknowledgments
This work was supported in part by a grant from Kuwait University Research Administration given to Ibrahim F. Benter (RM02/03) and NIH grants HL29587 and 36279 awarded to Richard Roman.
References
- ALONSO-GALICIA M, DRUMMOND HA, REDDY KK, FALCK JR, ROMAN RJ. Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension. 1997;29:320–325. doi: 10.1161/01.hyp.29.1.320. [DOI] [PubMed] [Google Scholar]
- ARUN KH, KAUL CL, RAMARAO P. AT1 receptors and L-type calcium channels: functional coupling in supersensitivity to angiotensin II in diabetic rats. Cardiovasc Res. 2005;65:374–386. doi: 10.1016/j.cardiores.2004.10.010. [DOI] [PubMed] [Google Scholar]
- BAAN J, JR, PFAFFENDORF M, VAN DER WAL AC, CHANG PC, VAN ZWIETEN PA. Influence of losartan and nicardipine on the contractile responses of human subcutaneous arteries and veins to angiotensin II. Fundam Clin Pharmacol. 1999;13:43–49. doi: 10.1111/j.1472-8206.1999.tb00319.x. [DOI] [PubMed] [Google Scholar]
- BEENEN OH, MATHY MJ, PFAFFENDORF M, VAN ZWIETEN PA. Vascular responsiveness in isolated perfused kidneys of diabetic hypertensive rats. J Hypertension. 1996;14:1125–1130. doi: 10.1097/00004872-199609000-00013. [DOI] [PubMed] [Google Scholar]
- BENTER IF, FRANCIS I, COJOCEL C, JUGGI JS, YOUSIF MHM, CANATAN H. Contribution of cytochrome P450 metabolites of Arachidonic acid to hypertension and end-organ damage in spontaneously hypertensive rats with L-NAME. Pharmacol Res. 2005a;25:143–154. doi: 10.1111/j.1474-8673.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- BENTER IF, YOUSIF MH, CANATAN H, AKHTAR S. Inhibition of Ca2+/calmodulin-dependent protein kinase II, RAS-GTPase and 20-hydroxyeicosatetraenoic acid attenuates the development of diabetes-induced vascular dysfunction in the rat carotid artery. Pharmacol Res. 2005b;52:252–257. doi: 10.1016/j.phrs.2005.04.001. [DOI] [PubMed] [Google Scholar]
- BHUGRA P, GULATI OD. Interaction of calcium channel blockers with different agonists in aorta from normal and diseased rats. Indian J Physiol Pharmacol. 1996;40:109–119. [PubMed] [Google Scholar]
- CROFT KD, MCGIFF JC, SANCHEZ-MENDOZA A, CARROLL MA. Angiotensin II releases 20-HETE from rat renal microvessels. Am J Physiol Renal Physiol. 2000;279:F544–F551. doi: 10.1152/ajprenal.2000.279.3.F544. [DOI] [PubMed] [Google Scholar]
- ENGELS W, VAN BILSEN M, WOLFFENBUTTEL BH, VAN DER VUSSE GJ, GLATZ JF. Cytochrome P450, peroxisome proliferation, and cytoplasmic fatty acid-binding protein content in liver, heart and kidney of the diabetic rat. Mol Cell Biochem. 1999;192:53–61. [PubMed] [Google Scholar]
- ENRIQUEZ A, LECLERCQ I, FARRELL GC, ROBERTSON G. Altered expression of hepatic CYP2E1 and CYP4A in obese, diabetic ob/ob mice, and fa/fa Zucker rats. Biochem Biophys Res Commun. 1999;255:300–306. doi: 10.1006/bbrc.1999.0202. [DOI] [PubMed] [Google Scholar]
- FEENER EP, KING GL. Endothelial dysfunction in diabetes mellitus: role in cardiovascular disease. Heart Fail Monit. 2001;1:74–82. [PubMed] [Google Scholar]
- GARCHA RS, SEVER PS, HUGHES AD. Mechanism of action of angiotensin II in human isolated subcutaneous resistance arteries. Br J Pharmacol. 2001;134:188–196. doi: 10.1038/sj.bjp.0704222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA VM, OCHOA JE, ELIAS MM. Effect of early stage of experimental diabetes on vascular functions in isolated perfused kidneys. J Auton Pharmacol. 1999;19:97–103. doi: 10.1046/j.1365-2680.1999.00122.x. [DOI] [PubMed] [Google Scholar]
- GEBREMEDHIN D, KOLTAI MZ, POGATSA G, MAGYAR K, HADHAZY P. Altered responsiveness of diabetic dog renal arteries to acetylcholine and phenylephrine: role of endothelium. Pharmacology. 1989;38:177–184. doi: 10.1159/000138535. [DOI] [PubMed] [Google Scholar]
- GUO AM, ARBAB AS, FALCK JR, CHEN P, EDWARDS PA, ROMAN RJ, SCICLI AG. Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferation. J Pharmacol Exp Ther. 2007;321:18–27. doi: 10.1124/jpet.106.115360. [DOI] [PubMed] [Google Scholar]
- HOAGLAND KM, FLASCH AK, ROMAN RJ. Inhibitors of 20-HETE formation promote salt-sensitive hypertension in rats. Hypertension. 2003;42:669–673. doi: 10.1161/01.HYP.0000084634.97353.1A. [DOI] [PubMed] [Google Scholar]
- IMIG JD, ZOU AP, STEC DE, HARDER DR, FALCK JR, ROMAN RJ. Formation and actions of 20-hydroxyeicosatetraenoic acid in rat renal arterioles. Am J Physiol. 1996;270:R217–R227. doi: 10.1152/ajpregu.1996.270.1.R217. [DOI] [PubMed] [Google Scholar]
- KAIDE J, WANG MH, WANG JS, ZHANG F, GOPAL VR, FALCK JR, NASJLETTI A, LANIADO-SCHWARTZMAN M. Transfection of CYP4A1 cDNA increases vascular reactivity in renal interlobar arteries. Am J Physiol Renal Physiol. 2003;284:F51–F56. doi: 10.1152/ajprenal.00249.2002. [DOI] [PubMed] [Google Scholar]
- KAMATA K, HOSOKAWA M. Endothelial dysfunction in the perfused kidney from the streptozotocin-induce diabetic rat. Res Commun Mol Pathol Pharmacol. 1997;96:57–70. [PubMed] [Google Scholar]
- KAMATA K, MIYATA N, KASUYA Y. Impairment of endothelium-dependent relaxation and changes in levels of cyclic GMP in aorta from streptozotocin-induced diabetic rats. Br J Pharmacol. 1989;97:614–618. doi: 10.1111/j.1476-5381.1989.tb11993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASSAB E, MCFARLANE SI, SOWERS JR. Vascular complications in diabetes and their prevention. Vasc Med. 2001;6:249–255. doi: 10.1177/1358836x0100600409. [DOI] [PubMed] [Google Scholar]
- KEHL F, CAMBJ-SAPUNAR L, MAIER KG, et al. 20-HETE contributes to the acute fall in cerebral blood flow after subarachnoid hemorrhage in the rat. Am J Physiol Heart Circ Physiol. 2002;282:H1556–H1565. doi: 10.1152/ajpheart.00924.2001. [DOI] [PubMed] [Google Scholar]
- LEE CH, POBURKO D, SAHOTA P, SANDHU J, RUEHLMANN DO, VAN BREEMEN C. The mechanism of phenylephrine-mediated [Ca(2+)](i) oscillations underlying tonic contraction in the rabbit inferior vena cava. J Physiol. 2001;534:641–650. doi: 10.1111/j.1469-7793.2001.t01-1-00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYATA N, TANIGUCHI K, SEKI T, et al. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol. 2001;133:325–329. doi: 10.1038/sj.bjp.0704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUTHALIF MM, BENTER IF, KARZOUN N, FATIMA S, HARPER J, UDDIN MR, MALIK KU. 20-HETE mediates calcium/calmodulin-dependent protein kinase activation in VSMC. Proc Natl Acad Sci USA. 1998;95:12701–12706. doi: 10.1073/pnas.95.21.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUTHALIF MM, BENTER IF, KHANDEKAR Z, GABER L, ESTES A, MALIK S, PARMENTIER JH, MANNE V, MALIK KU. Contribution of Ras-GTPase GTPase/MAP kinase and cytochrome P450 metabolites to deoxycorticosterone-salt-induced hypertension. Hypertension. 2000a;35:457–463. doi: 10.1161/01.hyp.35.1.457. [DOI] [PubMed] [Google Scholar]
- MUTHALIF MM, KARZOUN NA, GABER L, KHANDEKAR Z, BENTER IF, SAEED AE, PARMENTIER JH, ESTES A, MALIK KU. Ang II–induced hypertension: contribution of Ras-GTPase/Mitogen-activated protein kinase and cytochrome P450 metabolites. Hypertension. 2000b;36:604–609. doi: 10.1161/01.hyp.36.4.604. [DOI] [PubMed] [Google Scholar]
- MUTHALIF M, KARZOUN NA, BENTER IF, GABER L, LJUCA F, UDDIN MR, KHANDEKAR Z, ESTES A, MALIK KU. Functional significance of activation of calcium/calmodulin-dependent protein kinase II in angiotensin II-induced vascular hyperplasia and hypertension. Hypertension. 2002;39:704–709. doi: 10.1161/hy0202.103823. [DOI] [PubMed] [Google Scholar]
- OYAMA Y, KAWASAKI H, HATTORI Y, KANNO M. Attenuation of endothelium-dependent relaxation in aorta from diabetic rats. Eur J Pharmacol. 1986;132:75–78. doi: 10.1016/0014-2999(86)90013-0. [DOI] [PubMed] [Google Scholar]
- OYEKAN AO, MCGIFF JC. Functional response of the rat kidney to inhibition of nitric oxide synthesis: role of cytochrome p450-derived arachidonate metabolites. Br J Pharmacol. 1998;125:1065–1073. doi: 10.1038/sj.bjp.0702171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OYEKAN AO, MCAWARD K, CONETTA J, ROSENFELD L, MCGIFF JC. Endothelin-1 and CYP450 arachidonate metabolites interact to promote tissue injury in DOCA-salt hypertension. Am J Physiol. 1999;276:R766–R775. doi: 10.1152/ajpregu.1999.276.3.R766. [DOI] [PubMed] [Google Scholar]
- RAMANA KV, CHANDRA D, SRIVASTAVA S, BHATNAGAR A, SRIVASTAVA SK. Nitric oxide regulates the polyol pathway of glucose metabolism in vascular smooth muscle cells. FASEB J. 2003;17:417–425. doi: 10.1096/fj.02-0722com. [DOI] [PubMed] [Google Scholar]
- ROMAN RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- ROMAN RJ, MAIER KG, SUN CW, HARDER DR, ALONSO-GALICIA M. Renal and cardiovascular actions of 20-hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids. Clin Exp Pharmacol Physiol. 2000;27:855–865. doi: 10.1046/j.1440-1681.2000.03349.x. [DOI] [PubMed] [Google Scholar]
- SHIMOJO N, ISHIZAKI T, IMAOKA S, FUNAE Y, FUJII S, OKUDA K. Changes in amounts of cytochrome P450 isoenzymes and levels of catalytic activities in hepatic and renal microsomes of rats with streptozocin-induced diabetes. Biochem Pharmacol. 1993;46:621–627. doi: 10.1016/0006-2952(93)90547-a. [DOI] [PubMed] [Google Scholar]
- SHIN IW, SOHN JT, KIM HJ, KIM C, LEE HK, CHANG KC, CHUNG YK. Etomidate attenuates phenylephrine-induced contraction in isolated rat aorta. Can J Anaesth. 2005;52:927–934. doi: 10.1007/BF03022053. [DOI] [PubMed] [Google Scholar]
- SU P, KAUSHAL KM, KROETZ DL. Inhibition of renal arachidonic acid omega-hydroxylase activity with ABT reduces blood pressure in the SHR. Am J Physiol Regulatory Integrative Comp Physiol. 1998;275:R426–R438. doi: 10.1152/ajpregu.1998.275.2.R426. [DOI] [PubMed] [Google Scholar]
- SUN CW, ALONSO-GALICIA M, TAHERI MR, FALCK JR, HARDER DR, ROMAN RJ. Nitric oxide-20-hydroxyeicosatetraenoic acid interaction in the regulation of K+ channel activity and vascular tone in renal arterioles. Circ Res. 1998;83:1069–1079. doi: 10.1161/01.res.83.11.1069. [DOI] [PubMed] [Google Scholar]
- SUN CW, FALCK JR, OKAMOTO H, HARDER DR, ROMAN RJ. Role of cGMP versus 20-HETE in the vasodilator response to nitric oxide in rat cerebral arteries. Am J Physiol Heart Circ Physiol. 2000;279:339–350. doi: 10.1152/ajpheart.2000.279.1.H339. [DOI] [PubMed] [Google Scholar]
- TAKATORI A, AKAHORI M, KAWAMURA S, ITAGAKI S, YOSHIKAWA Y. The effects of diabetes with hyperlipidemia on P450 expression in APA hamster livers. J Biochem Mol Toxicol. 2002;16:174–181. doi: 10.1002/jbt.10036. [DOI] [PubMed] [Google Scholar]
- VILLALBA N, STANKEVICIUS E, GARCIA-SACRISTAN A, SIMONSEN U, PRIETO D. Contribution of both Ca2+ entry and Ca2+ sensitization to the alpha1-adrenergic vasoconstriction of rat penile small arteries. Am J Physiol Heart Circ Physiol. 2007;292:H1157–H1169. doi: 10.1152/ajpheart.01034.2006. [DOI] [PubMed] [Google Scholar]
- WANG JS, SINGH H, ZHANG F, et al. Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ Res. 2006;98:962–969. doi: 10.1161/01.RES.0000217283.98806.a6. [DOI] [PubMed] [Google Scholar]
- YOSHINARI K, TAKAGI S, SUGATANI J, MIWA M. Changes in the expression of cytochromes P450 and nuclear receptors in the liver of genetically diabetic db/db mice. Biol Pharm Bull. 2006;29:1634–1638. doi: 10.1248/bpb.29.1634. [DOI] [PubMed] [Google Scholar]
- YOUSIF MH. Signal transduction through Ras-GTPase and Ca2+/calmodulin-dependent protein kinase II contributes to development of diabetes-induced renal vascular dysfunction. Cell Biochem Funct. 2006;244:299–305. doi: 10.1002/cbf.1301. [DOI] [PubMed] [Google Scholar]
- YOUSIF MHM, BENTER IF, AKHTAR S. Inhibition of calcium/calmodulin-dependent protein kinase II normalizes diabetes-induced abnormal vascular reactivity in the rat perfused mesenteric vascular bed. Auton Autacoid Pharmacol. 2003;23:27–33. doi: 10.1046/j.1474-8673.2003.00282.x. [DOI] [PubMed] [Google Scholar]
- YOUSIF MHM, BENTER IF, ABRAHAM S, AKHTAR S. Inhibition of Ras-GTPase improves diabetes-induced abnormal vascular reactivity in the rat perfused mesenteric vascular bed. Med Princ Pract. 2004;13:57–62. doi: 10.1159/000075629. [DOI] [PubMed] [Google Scholar]