Abstract
Background
Incidence of asthma increases during the early adult years, but the relative influence of sex and early life factors in determining newly diagnosed asthma in young adults is unknown.
Methods
Healthy newborns (n=1246) were enrolled in the Tucson Children's Respiratory Study. Parental characteristics early life wheezing phenotypes, airway function and bronchial hyperresponsiveness to cold dry air and sensitization to Alternaria were determined by age 6 years. Physician diagnosed asthma, both chronic and newly diagnosed, and airway function were determined at age 22 years.
Findings
Average incidence of asthma between 16 and 22 years was 12.6 per thousand person-years. One fourth of all cases of active asthma at age 22 were newly diagnosed, of which 71% were females. Asthma remittance by age 22 was higher among males (p=0.008). Age at diagnosis was linearly associated with FEV1/FVC ratio at age 22. Late-onset (multinomial odds ratio [M-OR]= 7.4 [95% CI:3.9,14]) and persistent wheezing (M-OR=14.0 [6.8,28]) in early life, sensitization to Alternaria (M-OR=3.6 [2.1,6.4]), low airway function at age 6 (M-OR=2.1 [1.1,3.9]) and bronchial hyperresponsiveness at age 6 (M-OR=4.5 [1.9,10]) were independently associated with chronic asthma at age 22. Bronchial hyperresponsiveness (M-OR=6.9 [2.3,21]), low airway function at age 6 (M-OR=2.8 [1.1,6.9]), late-onset (M-OR=4.6 [1.7,12]) and persistent wheezing (M-OR=4.0 [1.2,14]) predicted newly diagnosed asthma at age 22.
Interpretation
Among young adults, females preferentially develop newly diagnosed asthma, but most had already wheezed in early life and had bronchial hyperresponsiveness by age 6. The roots of early adult onset asthma can be found in the preschool years.
Introduction
Several lines of evidence indicate that the majority of persons diagnosed with asthma during the first two decades of life had recurrent wheezing episodes during the preschool years (1), suggesting that the disease process may have started years before diagnosis. Prospective data from the 1958 British cohort indicated that an upsurge in incident cases of asthma and wheezing occurs during the early adult years (2). This “second wave” of newly diagnosed disease has not been extensively studied, in spite of the fact that it constitutes a high proportion of asthma in young adults and thus contributes significantly to respiratory morbidity in this age group, especially among women (3). It is currently unknown, for example, if factors present in early life contribute significantly to the risk of this “second asthma wave”, as they do for asthma occurring during the school years. Strachan et al (2) reported that preexisting allergic rhinitis was an important risk factor for new onset asthma in early adult life, and this was confirmed by Guerra et al (4), suggesting that allergy-related factors may play a role. To our knowledge, no studies have assessed if respiratory events and alterations in airway and immune reactivity directly ascertained during the preschool years may play a role in the incidence and prevalence of asthma in early adult life.
Children who have lower respiratory tract illnesses (LRI) in early life are at increased risk of subsequent wheezing and asthma (5, 6). We have previously shown in a longitudinal study of unselected children (1) that those who are wheezing at age 6 are at increased risk of subsequent asthma up to the age of 16 years, whereas “transient early wheezers” (i.e., those who wheeze with LRI but do not report wheezing at age 6) are not. What the relation is between these early wheezing phenotypes and new onset asthma in early adult life is unknown.
Bronchial hyperresponsiveness (BHR), a central characteristic of asthma regardless of age of onset (7), consists of an abnormal bronchoconstrictive response to a variety of stimuli. Previously we showed in this same longitudinal cohort that non-asthmatic children with BHR at age 6 were at increased risk of developing asthma by age 11, but the association was not independent of allergic sensitization and mild wheezing at age 6 (8). No studies have addressed the role of BHR during the preschool years in the development of new onset asthma in adult life.
The main objective of this paper was to determine if potential risk factors for asthma measured during the preschool years predicted prevalence, incidence, and remission of physician diagnosed asthma and asthma-like symptoms in early adult life.
Methods
Study Design
Healthy infants were enrolled at birth in the Tucson Children's Respiratory Study in Tucson, Arizona between 1980 and 1984 (n=1246) (9). Parents were contacted shortly after their child was born, and completed a questionnaire describing their ethnicity, history of physician-diagnosed asthma, years of education, and current smoking habits.
Early Life Assessments
Parents were instructed at enrollment to bring their child to the pediatrician at the first sign or symptoms of a lower respiratory illness (LRI) before age 3 (10), and wheezing was identified by the physician. Current physician diagnosed asthma and current wheeze during the previous year were assessed from questionnaires completed for the child at age 2 (mean±SD: 1.6±0.3yr, n=835), 3 (2.9±0.5yr, n=769), 6 (6.2±0.9yr, n=840), 8 (8.6±0.7yr, n=727), 11 (10.9±0.6yr, n=831), 13 (13.5±0.6yr, n=646) and 16 (16.6±0.6yr, n=712) years (sample sizes limited to those with information at age 22 years). If a physician diagnosis of asthma with active symptoms was ever reported on a questionnaire, the participant was classified as having asthma by age 16 years. Participants who completed one or more questionnaires and had no report of asthma on any questionnaire were considered as not having asthma by age 16 years.
Skin prick tests to 7 local aeroallergens (Bermuda grass, Alternaria alternata, careless weed, house dust mix, and mesquite, mulberry and olive tree pollens) were performed at age six as previously described (11). Tests were read at 20 minutes and the sum of the largest wheal diameter plus the perpendicular diameter recorded. Wheal sizes greater than or equal to 3mm, after subtracting the negative control, were considered positive.
Study subjects participated in a cold air challenge at a mean age of 6.1±0.5yr (8). Children who were actively wheezing, had used 'breathing meds' during the past 48 hours, had a lower respiratory illness during the previous 6 weeks, or upper respiratory tract infection during the previous 3 weeks were rescheduled for testing. Those who required continuous treatment or could not be rescheduled were not tested. Baseline maximal expiratory flow at functional residual capacity (V'maxFRC, ml/sec) was recorded from the best of three voluntary partial expiratory maneuvers as previously described (8). The children then breathed CO2-enriched cold (-20°C) dry air for 6 minutes and the mean of the first two values measured within 5 minutes was taken as the post-challenge value. Bronchial responsiveness was calculated as percent fall = (pre-post)/pre x 100. Cold air bronchial hyper-responsiveness (CA-BHR) was defined as a drop in V'maxFRC greater than 41.1%, the 90th percentile of decline for reference children (skin test negative, never wheeze, never diagnosed with asthma by age 6) (8).
We included as a risk factor in these analyses the early wheezing phenotypes as previously described (5): “persistent wheezers” (children who wheeze during LRI before age 3 and were still wheezing at age 6), “late onset wheezers” (those who did not have wheezing LRI in early life but started wheezing by age 6), “transient early wheezers” (those who wheezed with LRI but did not report wheezing at age 6) and “never wheezers” (no wheezing LRIs and no wheeze at age 6).
Year 22 Assessments
Data regarding the occurrence of respiratory symptoms during the previous year were obtained from questionnaires completed at the in-depth evaluation at age 22 (n=735), and, if no data was available at that age, data from questionnaires at ages 24 (n=46) or 18 (n=77) years were used. Of the 1246 participants, 858 had questionnaire information (“age 22”, mean age 21.7yr, SD=1.2, range: 17.8 to 26.4yr). Current wheeze was defined as having had at least one self-reported episode during the previous year. Shortness of breath with wheeze (SOB) was defined as any, infrequent (1-3 times) or frequent (4 or more times) episodes during the previous year. Current asthma at age 22 years was defined as having ever had a physician diagnosis with active symptoms (attacks, episodes or wheeze) during the previous year. Current asthma at age 22 was subdivided into four categories (Figure 1): “newly diagnosed asthma” (n=49, subjects with no report of physician diagnosed asthma between ages 2 and 16 but with a diagnosis and active symptoms at age 22); “inactive asthma” (n=74, subjects with diagnosis and active asthma between ages 2 and 16 but no active symptoms at age 22); “chronic asthma” (n=132, subjects with a diagnosis and active asthma between ages 2 and 16 and active symptoms also at age 22); and “no asthma” (n=594, subjects who had at least one questionnaire between age 2 and 16 with no report of physician diagnosed active asthma and no reported active asthma at age 22). Those with current asthma were further subdivided into those who had taken any prescription medications for asthma or wheeze in the past year and those who had not. Current cigarette smoking was determined from the questionnaires.
Figure 1.
Definitions of asthma at age 22: Inactive asthma is a physician diagnosis of asthma between 2 and 16 years but no current symptoms at age 22, newly diagnosed asthma is first diagnosis and current symptoms at age 22, chronic asthma is a diagnosis between 2 and 16 years and current symptoms at age 22 and no asthma is no diagnosis of asthma through age 22 years.
Allergy skin prick tests were performed at the age 22 in-depth evaluation (n=462) for 17 local aero-allergens including: house dust mix, cat hair, cat pelt, dog, cockroach, Dermatophagoides farinae, Penicillium, Aspergillus fumigatus, Hormodendrum Cladosporioides, Alternaria alternata, and the pollens of Bermuda grass, olive tree, careless weed, mesquite tree, mulberry tree, and ragweed. Methods were the same as the testing at age 6 years.
Spirometry was performed in 456 individuals at the age 22 in-depth evaluation using a portable Schiller Spirovit SP-1 (Schiller AG, Baar, Switzerland)(1). Systems were calibrated with a Jones flow-volume calibrator (Model FVC-3000; Jones Medical Instrumentation Company, Oakbrook, IL). None of the participants had used a bronchodilator within 6 hours of testing. Study nurses recorded height, weight, and age at the time of testing. Subsequent to baseline measurements, a fixed dose of 2 puffs of albuterol (180 μg) was administered from a metered-dose inhaler and aerochamber holding device (Monaghan Medical Corp, Plattsburgh, NY) and the post bronchodilator spirometry obtained after 15 minutes. Spirometry indices included the forced vital capacity (FVC, milliliters) and forced expiratory volume in one second (FEV1, milliliters). Response to bronchodilator was calculated as 200*((post-pre)/(post+pre)).
Statistical methods
Proportions were compared with chi-square analysis or Fisher's Exact Test as appropriate; odds ratio were computed using logistic regression. Multinomial logistic regression was used to estimate multinomial odds ratios (M-OR, also known as relative risk ratios) for categorical outcomes. To allow for all participating subjects to be included in the regression models, dummy “missing” categories were used when predictor variables had missing information. Full regression models included all variables, best-fitting models included those variables with p<0.1 in the full model. Attributable risk was calculated as ((OR-1)/OR) × P (where P is the proportion of the cases who had the risk factor). Two-tail p-values less than 0.05 were considered significant. Statistical analyses were carried out using SPSS for Windows 15.0 and STATA 10.0.
Informed consent was obtained from the parents for their children, or by the enrollees themselves if appropriate, and the Institutional Review Board of the University of Arizona approved the study.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Participants
Children who had information for asthma and respiratory symptoms at age 22 (n=858) were more likely to have non-smoking, non-Hispanic White parents with more years of education compared to children who did not have information (n=388, Table 1). There was no difference in the proportion of males, early wheezing phenotypes, atopy or Alternaria sensitization at age 6, or parental history of asthma between the two groups.
Table 1.
Characteristics of participants with data at age 22 (n=858) compared to those without data at age 22 (n=388)
| Characteristics* | Adult Data % (n+/n total) |
No Adult Data % (n+/n total) |
p-value |
|
|---|---|---|---|---|
| Male | 47.8 (410/858) | 52.3 (203/388) | 0.14 | |
| Ethnicity | 64.6 (554/858) | 46.4 (180/388) | <0.0001 | |
| Early Wheezing Phenotypes | ||||
| Never | 51.7 (355/687) | 50.4 (70/139) | ||
| Transient | 20.1 (138/687) | 18.7 (26/139) | ||
| Late | 15.7 (108/687) | 11.5 (16/139) | ||
| Persistent | 12.5 (86/687) | 19.4 (27/139) | 0.1, 3df | |
| Any skin test, age 6yr | 39.0 (260/667) | 35.8 (34/95) | 0.6 | |
| Alternaria skin test, age 6yr | 17.1 (114/666) | 20.0 (19/95) | 0.5 | |
| Parental Characteristics: | ||||
| Asthma | Maternal | 10.7 (90/845) | 11.9 (37/310) | 0.5 |
| Paternal | 12.1 (98/812) | 12.1 (34/282) | 0.9 | |
| Smoking | Maternal | 14.9 (128/858) | 23.9 (92/385) | <0.001 |
| Paternal | 27.8 (235/846) | 39.5 (150/380) | <0.001 | |
| Ed>12yrs | Maternal | 74.7 (640/857) | 53.9 (207/384) | <0.001 |
| Paternal | 76.5 (644/842) | 56.5 (212/375) | <0.001 | |
Two non-Hispanic white parents; Alternria=allergy skin test positive to Alternaria alternata at age 6 years
At age 22, 30.0% (255/849) of the participants reported ever receiving a physician diagnosis of asthma. Active asthma was reported by 21.3% (184/849) and wheeze without a diagnosis of asthma was reported by 19.2% (163/849). Shortness of breath with wheeze during the previous year was reported by 19.2% (163/850) and 7.3% (61/850) reported experiencing such symptoms frequently. One quarter of the participants reported currently smoking cigarettes at age 22 (26.3%, 224/851).
Early Life Risk Factors for Asthma, Wheeze and SOB at age 22
Parental asthma and age 6 CA-BHR, Alternaria skin test reactivity and low V'maxFRC were all strongly associated with an increased risk for asthma and SOB at age 22 (Supplement Table 1). Ethnicity (data not shown) and eczema at age 2 years were unrelated to asthma symptoms at age 22. Children with persistent and late onset wheeze during the preschool years (see definitions in Methods) were more likely to have current asthma and SOB with wheeze at age 22 than those who had no reports of wheezing by age 6 years (Supplement Table 1). Smoking at age 22 was associated with increased reports of asthma and wheeze at that age. When multinomial logistic regression was performed with “current asthma” and “wheeze only” as the outcomes compared to the “no asthma and no wheeze” group, late and persistent wheezing, parental asthma and age 6 CA-BHR, sensitization to Alternaria and low V'maxFRC were all positively and independently associated with current asthma and SOB with wheeze at age 22 years (Supplement Table 2).
Characterization of Asthma at age 22
Of the 849 individuals with information about asthma at age 22, 58.3% completed all 7 questionnaires administered between ages 2 and 16, 39.5% completed 4-6 questionnaires, 2.1% completed 1-3 questionnaires. On average, subjects had 6.3 completed questionnaires. By age 16, 24.3% (206/849) had reported a diagnosis of asthma at least once. Among the 643 individuals who never reported physician diagnosed asthma by age 16 years, 64.1% (412/643) had at least one report of wheeze between ages 2 and 16 years.
Current asthma at age 22 was grouped into inactive, newly diagnosed and chronic categories based on the history of physician diagnosis through age 16 years and current symptoms at age 22 (Figure 1; see Methods for definitions).
Incidence of newly diagnosed asthma at age 22 was 7.6% (49/643), with an average yearly incidence of 12.6 per thousand person-years. When compared to no asthma, newly diagnosed and chronic asthma were strongly associated with current SOB and cough (Supplement Table 3). All three asthma categories were associated with concurrent skin test positivity.
In order to assess asthma severity, newly diagnosed and chronic asthma were further divided according to whether any prescription medications were used for asthma or wheeze during the previous year (Supplement Table 4). For newly diagnosed asthma, there was no difference in SOB with wheeze, cough or skin test positivity between those using medication and those not using medication. In contrast, subjects with chronic asthma who were using medications were more likely to have SOB with wheeze than those not using such medications (75.7% vs. 40.3% respectively, p<0.0001). Prevalence of cough and skin test positivity was similar in both groups.
Lung Function and Asthma at age 22 Years
Similar values for pre- and post-bronchodilator FEV1/FVC ratio and response to bronchodilator were observed for the inactive asthma and no asthma groups at age 22 (Table 2). In contrast, the pre- and post-bronchodilator FEV1/FVC ratio was significantly lower in both newly diagnosed and chronic asthma compared to no asthma. The response to bronchodilator was significantly higher in chronic asthma, but not in newly diagnosed asthma, as compared to no asthma. When current asthma was further subdivided by prescription medications use for asthma during the previous year, different patterns were observed for the newly diagnosed and chronic groups (Supplement Table 5). Whereas newly diagnosed asthmatics have a lower FEV1/FVC ratio regardless of medication use, among children with chronic asthma, only those currently using medication had a lower FEV1/FVC ratio (compared to those not using medication).
Table 2.
Pre- and post-bronchodilator FEV1/FVC ratio and response to bronchodilator for asthma at age 22 years
| Asthma Groups | Pre-BD FEV1/FVC ratio* (z-scores) |
Age 22 Years Post-BD FEV1/FVC ratio* (z-scores) |
Bronchodilator Response*† (z-scores) |
||||
|---|---|---|---|---|---|---|---|
| n | mean (sem) | p‡ | mean (sem) | p‡ | mean (sem) | p‡ | |
| No Asthma | 317 | 0.10 (0.05) | ref | 0.09 (0.05) | ref | -0.08 (0.05) | ref |
| Inactive | 44 | 0.09 (0.15) | 0.9 | 0.16 (0.14) | 0.6 | -0.07 (0.15) | 0.9 |
| Newly Diagnosed | 24 | -0.49 (0.20) | 0.005 | -0.47 (0.19) | 0.009 | 0.21 (0.30) | 0.4§ |
| Chronic | 69 | -0.37 (0.15) | 0.004§ | -0.33 (0.15) | 0.009§ | 0.32 (0.15) | 0.016§ |
Each lung function outcome was adjusted for sex in a linear regression and the standardized residuals from the regression (z-scores) were saved and used as the outcome measures for this table. A z-score of 1 represents one standard deviation from the group mean of zero.
Bronchodilator response calculated using FEV1 (ml) as 2*((post-pre)/(post+pre))*100
p-values computed using linear regression for each outcome with the no asthma group as the reference group,
Levene's test for equality of variances was significant for this comparison and so we used an unequal variances t-test to compute the p-value with reference to the no asthma group
Age at Diagnosis and Asthma at age 22
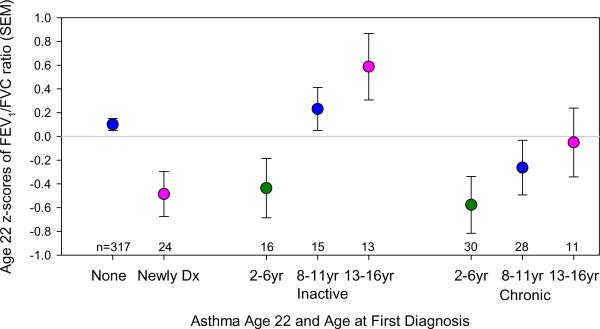
Subjects with asthma at age 22 years were subdivided into three categories a priori, depending on their prospectively assessed age at diagnosis: 2-6yrs, 8-11yrs and 13-16yrs. There was no significant difference at age of first diagnosis between inactive and chronic asthma (32.4% vs. 44.7% diagnosed 2-6yr, 40.5% vs. 34.8% diagnosed 8-11yr, and 27.0 vs. 20.5% diagnosed 13-16yr, respectively, p=0.2). However, there was a significant impact of age at diagnosis on the FEV1/FVC ratio at age 22 years (Figure 2). For both chronic and inactive asthma, age at diagnosis was significantly and linearly related to the FEV1/FVC ratio, p=0.009, after adjusting for asthma status and sex.
Figure 2.
FEV1/FVC ratio and asthma at age 22 years by age at first asthma diagnosis. FEV1/FVC ratio was adjusted for sex in a linear regression and the standardized residuals from the regression (z-scores) were saved and used as the outcome measure for this figure (a z-score of 1 represents one standard deviation from the group mean of zero). Age at first diagnosis was divided into three groups based on the questionnaire when the diagnosis was first reported: 2-6yrs, 8-11yrs and 13-16yrs. Newly Dx is first diagnosis of asthma and current symptoms at age 22 years, inactive is first diagnosis of asthma between 2 and 16 years with no symptoms at age 22 years, chronic is first diagnosis between 2 and 16 years with active symptoms at age 22 years, the none group never received a diagnosis of asthma during the study period. Age at diagnosis was significantly and linearly related to the FEV1/FVC ratio, p=0.009, among inactive and chronic asthmatics at age 22 years, after adjusting for asthma status and sex.
Early Life Risk Factors for Asthma at age 22 years
Univariate and multinomial analyses for the association between early life risk factors and asthma at age 22 years are shown in Tables 3 and 4, respectively. Newly diagnosed asthma was twice as likely to occur in females as in males. Parental asthma and both late onset and persistent wheezing during the first 6 years of life were associated with inactive, newly diagnosed and chronic asthma (Tables 3 and 4 and Supplement Figure 1). In contrast, eczema by age 2 years and Alternaria sensitization at age 6 years were associated with inactive and chronic asthma but not with newly diagnosed asthma. Low V'maxFRC at age 6 years was associated with newly diagnosed and chronic asthma but not inactive asthma at age 22 years. There was a strong positive association between CA-BHR and both newly diagnosed asthma (M-OR=6.9 [2.3, 21]) and chronic asthma (M-OR=4.5 [1.9, 10]). The population attributable risk of CA-BHR for newly diagnosed and chronic asthma was 33% and 26%, respectively. No significant association was found between inactive asthma at age 22 years and CA-BHR at age 6 years.
Table 3.
Proportion (%) of subjects in different categories for early life risk factors and current smoking who had inactive, newly diagnosed and chronic asthma at age 22 years
| No Asthma |
Inactive |
Asthma at Age 22 Years Newly Diagnosed |
Chronic |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk Factors* | Categories | Total n | (n) | %† (n+) | p‡ | %† (n+) | p‡ | %† (n+) | p‡ |
| Sex | Male | 404 | (271) | 14.5 (46) | 4.9 (14) | 21.2 (73) | |||
| Female | 445 | (323) | 8.0 (28) | 0.008 | 9.8 (35) | 0.023 | 15.4 (59) | 0.045 | |
| Parental asthma | Neither | 622 | (468) | 9.8 (51) | 6.0 (30) | 13.5 (73) | |||
| Either | 179 | (93) | 17.7 (20) | 0.018 | 15.5 (17) | 0.001 | 34.5 (49) | <0.0001 | |
| Parental smoking | No | 565 | (395) | 12.0 (54) | 7.3 (31) | 17.7 (85) | |||
| Yes | 273 | (194) | 8.5 (18) | 0.18 | 8.1 (17) | 0.7 | 18.5 (44) | 0.8 | |
| Eczema 2yr | No | 696 | (502) | 9.2 (51) | 7.9 (43) | 16.6 (100) | |||
| Yes | 79 | (40) | 28.6 (16) | <0.0001 | 9.1 (4) | 0.8 | 32.2 (19) | 0.004 | |
| Early Wheezing Phenotypes | Never | 354 | (297) | 6.0 (19) | ref | 4.2 (13) | ref | 7.8 (25) | ref |
| Transient | 135 | (99) | 10.0 (11) | 0.16 | 9.2 (10) | 0.055 | 13.2 (15) | 0.090 | |
| Late Onset | 107 | (47) | 27.7 (18) | <0.0001 | 14.6 (8) | 0.004 | 42.0 (34) | <0.0001 | |
| Persistent | 86 | (27) | 38.6 (17) | <0.0001 | 12.9 (4) | 0.044 | 58.5 (38) | <0.0001 | |
| Alternaria positive 6yr | No | 546 | (399) | 10.5 (47) | 7.2 (31) | 14.7 (69) | |||
| Yes | 113 | (51) | 22.7 (15) | 0.006 | 5.6 (3) | 0.7 | 46.3 (44) | <0.0001 | |
| CA-BHR 6yr | No | 330 | (262) | 8.7 (25) | 4.0 (11) | 10.9 (32) | |||
| Yes | 58 | (29) | 19.4 (7) | 0.048 | 19.4 (7) | 0.001 | 34.1 (15) | <0.0001 | |
| V'maxFRC Quartiles 6yr | High | 132 | (106) | 8.6 (10) | ref | 6.2 (7) | ref | 7.8 (9) | ref |
| Med-high | 132 | (91) | 12.5 (13) | 0.4 | 3.2 (3) | 0.3 | 21.6 (25) | 0.005 | |
| Med-low | 132 | (91) | 15.0 (16) | 0.15 | 6.2 (6) | 0.9 | 17.3 (19) | 0.036 | |
| Low | 132 | (75) | 14.8 (13) | 0.17 | 11.8 (10) | 0.17 | 31.2 (34) | <0.0001 | |
| Smoking 22yr | No | 625 | (439) | 12.4 (62) | 6.4 (30) | 17.6 (94) | |||
| Yes | 224 | (155) | 7.2 (12) | 0.068 | 10.9 (19) | 0.058 | 19.7 (38) | 0.5 | |
Eczema=physician diagnosis of eczema by age 2 years; CA-BHR=bronchial hyperresponsiveness to cold air challenge at age 6 years; Alternaria=allergy skin test positive to Alternaria alternata at age 6 years
Percentages for each asthma group were calculated with respect to the no asthma group after excluding the other two asthma groups.
p-values for the association between each individual risk factor and the asthma groups were estimated using multinomial logistic regression with respect to the no asthma group.
Table 4.
Multinomial odds ratio for inactive, newly diagnosed, and chronic asthma at age 22 years by different risk factors in early life
| Risk Factors* | Categories | Inactive | Asthma at Age 22 Years Newly Diagnosed | Chronic | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M-OR† | (95%CI) | p | M-OR | (95%CI) | p | M-OR | (95%CI) | p | ||
| Parental asthma | Yes | 2.0 | 1.1, 3.6 | 0.030 | 2.7 | 1.4, 5.2 | 0.004 | 3.2 | 1.9, 5.4 | <0.0001 |
| Eczema 2yr | Yes | 3.8 | 1.9, 7.8 | 0.0002 | 1.1 | 0.4, 3.3 | 0.9 | 2.0 | 1.0, 4.1 | 0.047 |
| Early | Never | ref | ref | ref | ||||||
| wheezing | Transient Early | 1.6 | 0.7, 3.5 | 0.3 | 2.0 | 0.8, 4.8 | 0.14 | 1.4 | 0.7, 2.9 | 0.3 |
| phenotypes | Late Onset | 5.4 | 2.5, 11 | <0.0001 | 4.6 | 1.7, 12 | 0.003 | 7.4 | 3.9, 14 | <0.0001 |
| Persistent | 8.9 | 4.0, 20 | <0.0001 | 4.0 | 1.2, 14 | 0.027 | 14 | 6.8, 28 | <0.0001 | |
| Alternaria positive 6yr | Yes | 2.0 | 1.0, 4.0 | 0.067 | 0.6 | 0.2, 2.2 | 0.4 | 3.6 | 2.1, 6.4 | <0.0001 |
| CA-BHR 6yr | Yes | 2.4 | 0.9, 6.5 | 0.083 | 6.9 | 2.3, 21 | 0.0006 | 4.5 | 1.9, 10 | 0.0006 |
| V'maxFRC quartiles 6yr | Low | 1.1 | 0.5, 2.4 | 0.8 | 2.8 | 1.1, 6.9 | 0.029 | 2.1 | 1.1, 3.9 | 0.021 |
Eczema=physician diagnosis of eczema by age 2 years; Alternaria=allergy skin test positive to Alternaria alternata at age 6 years; CA-BHR=bronchial hyperresponsiveness to cold air challenge at age 6 years; V'maxFRC=lowest quartile compared to upper three quartiles combined
M-OR multinomial odds ratio (estimated using multinomial logistic regression) with all risk factors listed in the table included in the model with the no asthma group as the reference group. Models were additionally adjusted for ethnicity, sex and current smoking at age 22
Discussion
This is the first study of the relation between potential risk factors directly ascertained during the first years of life (i.e., not obtained by retrospective questionnaire later in life) and both chronic and incident physician diagnosed asthma in early adult life. We found that, in over 70% cases of cases of current asthma and in 63% of those with newly diagnosed asthma at age 22 years, episodes of wheezing had already occurred during the first 3 years of life and/or were reported by parents at age 6 (Table 3). CA-BHR (but not sensitization to Alternaria) at age 6, late-onset and persistent wheezing by age 6, and female sex, were independent predictors of incident physician diagnosed asthma at age 22. Moreover, CA-BHR and sensitization to Alternaria at age 6, the main asthma-associated allergen in the Tucson area (11), together with persistent and late onset wheezing by that age, were significant, independent predictors of chronic asthma at age 22. It is possible that early sensitization to other allergens prevalent in other locales may show similar strong associations with adult asthma as seen with Alternaria in our locale. Male sex was a significant predictor of asthma remission at age 22. Our findings support our previous proposition that the roots of most forms of asthma can be found in early life (12), but we now extend that proposition to asthma that is reported as being newly diagnosed in early adult life.
Few studies have assessed prospectively the early life risk factors for prevalent, incident and remitted asthma in early adult life (3). In the most comprehensive study, Strachan et al (13) assessed the 1958 British cohort and reported a yearly incidence of asthma (11.1/1000 person-years) between the ages of 17 and 33 years similar to our 12.6/1000 person-years between 16 and 22 years. In Sweden, Larsson et al (14) reported an incidence of 11.1/1000 person-years between ages 16 and 19, strikingly similar to that reported by Strachan et al (2) and in this paper. In neither of the previous two studies, however, was prospectively obtained information from the first years of life available. Our study thus confirms that newly diagnosed asthma in early adult life contributes significantly to asthma morbidity in this age group.
We found that both chronic and newly diagnosed asthma at age 22 were much more common (4.5 and 6.9 times more likely, respectively) in children with CA-BHR than in children without CA-BHR at age 6 (Table 4), and that this association was independent of current asthma symptoms by that age. These results suggest that asymptomatic alterations in the regulation of airway tone are already present during the preschool years and strongly predict the likelihood of having asthma in early adult life. Although we had previously shown that CA-BHR at age 6 is associated with allergic sensitization at that age (8), the association between CA-BHR and incident physician diagnosed asthma at age 22 was independent of sensitization to Alternaria (11). However, newly diagnosed asthmatics were more likely to be sensitized to aeroallergens at age 22 than subjects with no asthma. These results strongly suggest that, much like chronic asthma, newly diagnosed asthma at age 22 is associated with the clinical expression of CA-BHR already present during the preschool years. However, and contrary to chronic asthma, newly diagnosed asthma is associated with late onset sensitization and is unrelated to early sensitization to local aeroallergens.
Persistent wheezing during the preschool years was a strong predictor of both chronic and incident asthma at age 22. We had previously shown that transient early wheezing was unrelated to the risk of having asthma symptoms between ages 8 and 16 years (1), whereas persistent and late onset wheezing were consistently associated with these symptoms during that same age interval (1). We interpret these findings as indicating that children classified as persistent or late onset wheezers in early life are predisposed to chronic symptoms that will either persist throughout childhood or manifest themselves more intensely in early adult life, especially in females.
Compared to males, females were twice as likely to have obtained a new asthma diagnosis between ages 16 and 22 years (Table 3). Moreover, over 70% of subjects with newly diagnosed asthma at age 22 were females. Conversely, males were significantly more likely to have inactive asthma at age 22 (Table 3) which suggests higher rates of asthma remission in males between 16 and 22 years. These findings confirm and extend those reported by several other longitudinal studies in this age group, which have suggested a gradual change in the relative prevalence of asthma between males and females between the pubertal years and early adult life (15-18).
We found that, as expected, mean FEV1/FVC ratio was significantly lower at age 22 years in subjects with both newly diagnosed and chronic asthma when compared with subjects with inactive asthma and no asthma (Table 2). However, a positive response to bronchodilators was only present among chronic asthmatics, suggesting irreversible deficits in lung function in newly diagnosed asthma. Of particular interest was the fact that, in both inactive and chronic asthma, FEV1/FVC ratio at age 22 yrs was strongly and linearly correlated with age at asthma diagnosis assessed prospectively (Figure 2). Taken together, these findings support the contention that alterations in airway structure and function are more likely to occur when initiation of the airway inflammatory processes associated with childhood asthma occurs during the preschool years. On the other hand, chronic airway hyperresponsiveness during the school years, even in the absence of symptoms, is associated with deficits in lung function growth (19) that may predispose to the development of asthma in early adult life.
We found that active smoking was a strong predictor of asthma, current wheezing and current shortness of breath with wheeze in early adult life. These findings are also in agreement with those of other studies in this age group (20) and support the contention that active smoking has deleterious, clinically manifested effects on lung health that can be detected relatively soon after smoking initiation.
Our study has limitations that need to be taken into account when interpreting our findings. As with most long-term cohort studies, by age 22 we had lost track of over one-third of our enrollees and the remaining participants were better educated, less likely to be minorities and less exposed to parental smoking than those who withdrew from the study (Table 1). In addition, almost half of subjects still participating in the study at age 22 years had moved out of Tucson and could not be tested for lung function or allergy. Due to concerns about the ethics of performing airway challenges in very young children, we did not test for CA-BHR at age 6 years children with current wheezing or those requiring active asthma treatment at that age; it is thus possible that our results may underestimate the association between CA-BHR and chronic and incident asthma in early adult life. Finally, we relied on physician diagnosis reported by parental questionnaire to assess the presence of asthma at all ages. This epidemiologic approach has been widely used to assess asthma incidence and prevalence both in longitudinal studies (21) and in national asthma surveys (22, 23). Although subject to diagnostic drift and bias, physician-diagnosed asthma is a strong indicator of need for health care utilization in subjects with asthma-like symptoms and thus allows differentiation of subjects with mild or misinterpreted wheezing episodes from those with symptoms worrisome enough to require physician attention. Indeed, almost two-thirds of all children without a diagnosis of asthma during our follow-up had at least one report of wheezing between ages 2 and 16. Our study should thus be interpreted as assessing risk factors for asthma symptoms significant enough to induce a diagnosis of asthma by a physician. Others have proposed to use objective markers such as concomitant increased responses to albuterol or methacholine to assess the presence of asthma in symptomatic subjects (24). We have previously shown, however, that each of these objective markers identifies different asthma phenotypes (25), and thus their isolated use is likely to introduce analytical biases in favor of different forms of asthma that coexist during the growing years.
In summary, we found that, in the majority of cases where asthma was first diagnosed in early adult life, wheezing episodes had already occurred or had been reported during the preschool years. We also found that bronchial hyperresponsiveness measured as early as age 6 and female gender were strong predictors of newly diagnosed asthma in young adults. These findings confirm and extend to the first years of life the observations made in the Dunedin (26) and in the Melbourne (27) cohorts, which showed strong correlations between asthma symptoms, lung function and bronchial responsiveness assessed during the school years and chronic asthma up to the fifth decade of life. We conclude that asthma which apparently develops in early adult life is a condition that affects mainly females and that commonly has its roots in the clinical expression of latent alterations of airway responses, which are already present in the preschool years. From the point of view of public health, primary prevention of this form of asthma will only become possible when the genetic and environmental factors that determine these alterations have been identified and their effects blocked or reversed.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the contributions of Lynn Taussig, M.D., who started the Tucson Children's Respiratory Study in 1980. We thank Bruce Saul for data management and our study nurses, Marilyn Lindell and Lydia de la Ossa, for data collection and participant follow-up. This work was supported by National Heart Lung and Blood Institute grants HL-56177.
Supported by a grant (HL-56177) from the National Heart Lung and Blood Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement We declare that we have no conflict of interest.
References
- 1.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, Taussig LM, Wright AL, Martinez FD. Outcome of asthma and wheezing in the first 6 years of life: Follow-up through adolescence. Am J Respir Crit Care Med. 2005;172:1253–1258. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national british cohort. British Medical Journal. 1996;312:1195–1199. doi: 10.1136/bmj.312.7040.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King ME, Mannino DM, Holguin F. Risk factors for asthma incidence. A review of recent prospective evidence. Panminerva Med. 2004;46:97–110. [PubMed] [Google Scholar]
- 4.Guerra S, Sherrill DL, Martinez FD, Barbee RA. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol. 2002;109:419–425. doi: 10.1067/mai.2002.121701. [DOI] [PubMed] [Google Scholar]
- 5.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. The Group Health Medical Associates. Asthma and wheezing in the first six years of life. New England Journal of Medicine. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 6.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: A birth cohort study. Lancet. 2006;368:763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 7.Pattemore PK, Asher MI, Harrison AC, Mitchell EA, Rea HH, Stewart AW. The interrelationship among bronchial hyperresponsiveness, the diagnosis of asthma, and asthma symptoms. Am Rev Respir Dis. 1990;142:549–554. doi: 10.1164/ajrccm/142.3.549. [DOI] [PubMed] [Google Scholar]
- 8.Lombardi E, Morgan WJ, Wright AL, Stein RT, Holberg CJ, Martinez FD. Cold air challenge at age 6 and subsequent incidence of asthma. A longitudinal study. American Journal of Respiratory & Critical Care Medicine. 1997;156:1863–1869. doi: 10.1164/ajrccm.156.6.9612066. [DOI] [PubMed] [Google Scholar]
- 9.Taussig LM, Wright AL, Morgan WJ, Harrison HR, Ray CG. The tucson children's respiratory study. I. Design and implementation of a prospective study of acute and chronic respiratory illness in children. American Journal of Epidemiology. 1989;129:1219–1231. doi: 10.1093/oxfordjournals.aje.a115242. [DOI] [PubMed] [Google Scholar]
- 10.Wright AL, Taussig LM, Ray CG, Harrison HR, Holberg CJ. The tucson children's respiratory study. Ii. Lower respiratory tract illness in the first year of life. American Journal of Epidemiology. 1989;129:1232–1246. doi: 10.1093/oxfordjournals.aje.a115243. [DOI] [PubMed] [Google Scholar]
- 11.Halonen M, Stern DA, Wright AL, Taussig LM, Martinez FD. Alternaria as a major allergen for asthma in children raised in a desert environment. American Journal of Respiratory and Critical Care Medicine. 1997;155:1356–1361. doi: 10.1164/ajrccm.155.4.9105079. [DOI] [PubMed] [Google Scholar]
- 12.Martinez FD. Toward asthma prevention--does all that really matters happen before we learn to read? N Engl J Med. 2003;349:1473–1475. doi: 10.1056/NEJMe030041. [DOI] [PubMed] [Google Scholar]
- 13.Anderson HR, Pottier AC, Strachan DP. Asthma from birth to age 23: Incidence and relation to prior and concurrent atopic disease. Thorax. 1992;47:537–542. doi: 10.1136/thx.47.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsson L. Incidence of asthma in swedish teenagers: Relation to sex and smoking habits. Thorax. 1995;50:260–264. doi: 10.1136/thx.50.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolai T, Pereszlenyiova-Bliznakova L, Illi S, Reinhardt D, von Mutius E. Longitudinal follow-up of the changing gender ratio in asthma from childhood to adulthood: Role of delayed manifestation in girls. Pediatr Allergy Immunol. 2003;14:280–283. doi: 10.1034/j.1399-3038.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 16.Ownby DR, Johnson CC, Peterson EL. Incidence and prevalence of physician-diagnosed asthma in a suburban population of young adults. Ann Allergy Asthma Immunol. 1996;77:304–308. doi: 10.1016/S1081-1206(10)63325-X. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Dales R, Tang M, Krewski D. Obesity may increase the incidence of asthma in women but not in men: Longitudinal observations from the canadian national population health surveys. Am J Epidemiol. 2002;155:191–197. doi: 10.1093/aje/155.3.191. [DOI] [PubMed] [Google Scholar]
- 18.Wright AL, Stern DA, Kauffmann F, Martinez FD. Factors influencing gender differences in the diagnosis and treatment of asthma in childhood: The tucson children's respiratory study. Pediatr Pulmonol. 2006;41:318–325. doi: 10.1002/ppul.20373. [DOI] [PubMed] [Google Scholar]
- 19.Xuan W, Peat JK, Toelle BG, Marks GB, Berry G, Woolcock AJ. Lung function growth and its relation to airway hyperresponsiveness and recent wheeze. Results from a longitudinal population study. Am J Respir Crit Care Med. 2000;161:1820–1824. doi: 10.1164/ajrccm.161.6.9809118. [DOI] [PubMed] [Google Scholar]
- 20.Avila L, Soto-Martinez ME, Soto-Quiros ME, Celedon JC. Asthma, current wheezing, and tobacco use among adolescents and young adults in costa rica. J Asthma. 2005;42:543–547. doi: 10.1080/02770900500214791. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, Sears MR. Risk factors for airway remodeling in asthma manifested by a low postbronchodilator fev1/vital capacity ratio: A longitudinal population study from childhood to adulthood. Am J Respir Crit Care Med. 2002;165:1480–1488. doi: 10.1164/rccm.2108009. [DOI] [PubMed] [Google Scholar]
- 22.Anderson HR, Gupta R, Strachan DP, Limb ES. 50 years of asthma: Uk trends from 1955 to 2004. Thorax. 2007;62:85–90. doi: 10.1136/thx.2006.066407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, Scalia MR, Akinbami LJ. National surveillance for asthma--united states, 1980-2004. MMWR Surveill Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 24.Toelle BG, Peat JK, Salome CM, Mellis CM, Woolcock AJ. Toward a definition of asthma for epidemiology. American Review of Respiratory Disease. 1992;146:633–637. doi: 10.1164/ajrccm/146.3.633. [DOI] [PubMed] [Google Scholar]
- 25.Stein RT, Holberg CJ, Morgan WJ, Wright AL, Lombardi E, Taussig L, Martinez FD. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax. 1997;52:946–952. doi: 10.1136/thx.52.11.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 27.Phelan PD, Robertson CF, Olinsky A. The melbourne asthma study: 1964-1999. J Allergy Clin Immunol. 2002;109:189–194. doi: 10.1067/mai.2002.120951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.