Abstract
Background
Alcoholism, like other substance abuse disorders, is a chronically relapsing condition. Compared with other abused drugs, however, little is known about the neural mechanisms mediating ethanol (EtOH)-craving and -seeking behavior leading to relapse. This study, therefore, was conducted to identify candidate brain regions that are recruited by an EtOH-associated contextual stimulus (S+). A secondary objective was to determine whether EtOH S+-elicited neural recruitment patterns are modified by the opiate antagonist naltrexone (NTX), a compound that reduces cue-induced craving in alcoholics and attenuates ethanol seeking in animal models of relapse.
Methods
Rats were tested in a conditioned reinstatement model of relapse with subsequent examination of brain c-fos expression patterns elicited by an EtOH S+ versus a cue associated with nonreward (S−). In addition, modification of these expression patterns by NTX was examined.
Results
The EtOH S+ reinstated extinguished responding and increased c-fos expression within the prefrontal cortex, hippocampus, nucleus accumbens, and hypothalamic paraventricular nucleus (PVN). Naltrexone suppressed the S+-induced reinstatement and attenuated hippocampal CA3 c-fos expression, while increasing neural activity in the extended amygdala and PVN.
Conclusions
Ethanol-associated contextual stimuli recruit key brain regions that regulate associative learning, goal-directed behavior, and Pavlovian conditioning of emotional significance to previously neutral stimuli. In addition, the data implicate the hippocampus, amygdala, and PVN as potential substrates for the inhibitory effects of NTX on conditioned reinstatement.
Keywords: Amygdala, drug-seeking, hippocampus, HPA-axis, nucleus accumbens, relapse
A major factor contributing to the persistent nature of substance abuse disorders is the process of associative learning whereby environmental stimuli repeatedly paired with drug consumption acquire incentive-motivational value. Subjective responses to such stimuli have been implicated in maintaining ongoing drug use, eliciting drug craving, and precipitating relapse (O’Brien et al. 1998).
Substantial advances have been made in understanding the neurocircuitry mediating the conditioned effects of drug-related environmental stimuli in the case of cocaine and, to some extent, opiates and nicotine. Studies utilizing c-fos expression as a marker of neural activation have revealed that these stimuli recruit corticolimbic circuitry components including the medial prefrontal cortex (mPFC), basolateral amygdala (BLA), nucleus accumbens (NAC), and hippocampus (Ciccocioppo et al. 2001; Franklin and Druhan 2000; Neisewander et al. 2000; Schroeder et al 2000, 2001, 2003; Schroeder and Kelley 2002). Functional brain imaging data in humans (Childress et al. 1999; Daglish et al. 2003) and targeted lesion or pharmacological approaches in animals (Cardinal et al. 2002; Everitt et al. 2001; See et al. 2003a; Tzschentke and Schmidt 2000) have further confirmed a role of these brain regions in cue-induced drug seeking and reinstatement.
In contrast, little is known about the neural substrates controlling conditioned drug-seeking responses in the case of ethanol (EtOH) addiction, the most prevalent substance abuse disorder worldwide. To address this question, a c-fos mapping approach was employed to identify brain regions recruited by exposure to ethanol-associated contextual stimuli. A secondary objective, of relevance for understanding the neurocircuitry mediating conditioned ethanol-seeking, was to study the effects of the opiate antagonist naltrexone (NTX) on c-fos expression. Naltrexone reliably reduces ethanol cue reactivity and craving in alcoholics (Monti et al. 1999; O’Malley et al. 2002; Rohsenow et al. 2000), as well as cue-induced ethanol seeking in animal models of relapse (Ciccocioppo et al. 2002, 2003; Liu and Weiss 2002). To shed light on the neural substrates through which NTX inhibits conditioned ethanol seeking and to aid in identifying brain regions critical for the motivating effects of ethanol cues, the effects of NTX on c-fos expression patterns associated with ethanol cue exposure were examined.
Methods and Materials
Subjects
Male Wistar rats (Charles River, Raleigh, North Carolina) weighing 180 to 200 g on arrival were housed three per cage in a temperature and humidity controlled vivarium on a reversed 12-hour light/dark cycle (lights off 8:00 am). Training and testing were conducted daily from 12:00 pm to 3:00 pm. All procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by The Scripps Research Institute Institutional Animal Care and Use Committee.
Behavioral Testing Equipment
Behavioral procedures were conducted in standard operant conditioning chambers located inside sound-attenuating, ventilated cubicles (Coulbourn Instruments, Allentown, Pennsylvania). Drinking solutions were dispensed by syringe pumps into a drinking reservoir positioned 4 cm above the grid floor in the center of the chamber’s front panel (Weiss et al. 1993). A retractable lever was located 4.5 cm to the right of the drinking reservoir. Depression of the lever resulted in delivery of .1 mL liquid.
Ethanol Self-Administration
Rats were transiently (3 days) given restricted access to water (2 hours per day) to facilitate acquisition of operant responding for a liquid reinforcer. During 30-minute daily sessions, a single response at the lever resulted in delivery of a .2% (wt/vol) saccharin solution (.1 mL). During all subsequent training and testing, water was available ad libitum in the home cages. Following acquisition of saccharin-reinforced responding, rats were trained to self-administer ethanol using a sweet solution fading procedure. During the first 6 days of this phase, lever responses resulted in presentation of a .2% (wt/vol) saccharin solution containing 5.0% (wt/vol) ethanol. Starting on day 7, the concentration of ethanol was increased from 5.0% to 8.0% and finally to 10% (wt/vol), while the saccharin concentration was reduced to 0%.
Cue Conditioning Procedure
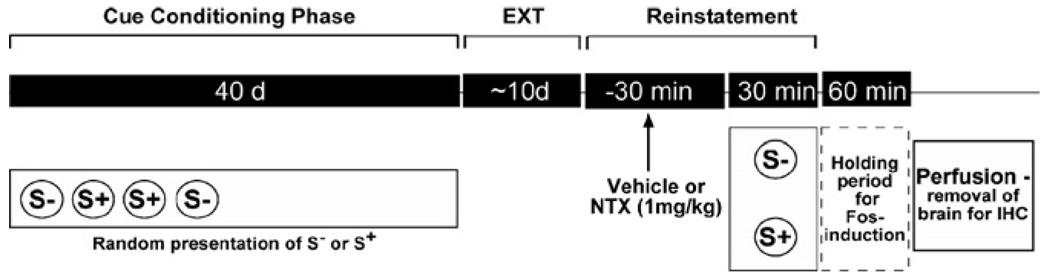
Beginning with self-administration training at the 10% ethanol concentration, rats were subjected to a reinforcement contingency (Figure 1) in which responses at the lever were differentially reinforced in the presence of olfactory discriminative stimuli (Sd) signaling availability of ethanol (S+) or nonreward (S−). Olfactory stimuli were generated by depositing six drops of distinct food flavor extracts into the bedding of the operant conditioning chamber 1 minute before extension of the lever. A banana flavor extract served to signal the availability of ethanol (S+), while an anise extract signaled nonavailability of ethanol (S−). Bedding was changed and trays thoroughly cleaned between sessions. Daily ethanol or nonreward sessions were scheduled in random sequence. Training continued until rats completed 20 ethanol and 20 nonreward sessions (i.e., a total of 40 conditioning sessions). After each session, liquid dispensers and waste trays were inspected for overflow to confirm that animals had been drinking.
Figure 1.
Schematic illustrating the ethanol training and test sequence. EXT, extinction; IHC, immunohistochemistry; NTX, naltrexone.
Extinction
After completion of the conditioning procedure, rats were subjected to daily 30-minute extinction sessions until a criterion of ≤6 responses/session over 3 consecutive days was reached. During these sessions, no Sd were presented and responses at the previously active lever activated the syringe pump motor but had no other scheduled consequences.
Conditioned Reinstatement Tests
Reinstatement tests began 1 day after the final extinction session. Rats were randomly divided into four groups: S−/vehicle (n = 9), S−/NTX (n = 7), S+/vehicle (n = 9), and S+/NTX (n = 7). Naltrexone hydrochloride dihydrate (Sigma, St. Louis, Missouri) (1.0 mg/kg) (SC) or its vehicle (VEH) saline was administered 30 minutes before test sessions. Reinstatement tests were conducted under extinction conditions, except that the S+ or S− stimuli were reintroduced before extension of the lever as during the conditioning phase.
Naltrexone is known to produce changes in brain activation, including disinhibition of the hypothalamic-pituitary-adrenal (HPA) axis and central nucleus of the amygdala (Oswald and Wand 2004; Park and Carr 1998). Therefore, to establish and control for the effects of NTX alone on Fos-immunoreactivity, a separate group of rats (n = 6) was treated with NTX 30 minutes before the final extinction session and in the absence of cue exposure (NTX control animals).
Fos-Protein Immunohistochemistry
Ninety minutes after initiation of reinstatement test (or final extinction sessions in NTX control animals), a time point within the window of peak Fos-protein levels (Kovacs 1998; Dragunow and Faull 1989), animals were deeply anesthetized and transcardially perfused with 2% sodium nitrite solution followed by 4% formaldehyde in .1 mol/L phosphate buffer (pH 7.4). Brains were removed, postfixed (2 hours, at 4°C) in the same fixative solution and cryoprotected (12 hours, 10% sucrose in .1 mol/L phosphate buffer, pH 7.4 at 4°C). Serial 40-µm coronal sections of the forebrain were cut on a freezing microtome, and a 1-in-4 series of brain sections was processed for immunohistochemical detection of Fos-protein. Sections were incubated in the following series of antibodies: primary Fos (18 hours, 1:5000, rabbit polyclonal) (Santa Cruz Biotechnology, Santa Cruz, California), biotinylated anti-rabbit 1gG (2 hours, 1:300) (Jackson ImmunoResearch, West Grove, Pennsylvania). Sections were then incubated in an avidin-biotin-horseradish peroxidase complex solution (2 hours) (Vector Elite Kit, Vector Labs, Burlingame, California). Horseradish peroxidase activity was visualized with nickel-diaminobenzidine. Sections from each experimental group were processed simultaneously. Omission of the primary antisera on a subset of sections resulted in a loss of immunoreactivity. Sections were mounted onto chrome-alum slides, dehydrated, and coverslipped.
Analysis of Fos-Protein Immunohistochemistry
Cresyl violet-labeled sections were used in conjunction with the Paxinos and Watson (1997) rat brain atlas to identify brain regions for Fos-positive cell analysis. Fos-positive nuclei were counted blind to treatment and within the entire nucleus under question, using a Nikon light microscope equipped with a dark-field diaphragm to aid in the identification of anatomical landmarks. Given that counts were performed on every fourth 40-µm section, each section being spaced 160 µm from the next, no single nucleus would have been counted in multiple sections from any animal. Therefore, any systematic error in the counting procedure was consistently applied to all groups. Considering these factors were identical for all sections, animals, and treatment groups, a stereological analysis was not considered necessary for this material.
Within the medial prefrontal cortex, bilateral Fos-positive cell counts were made in the prelimbic (Pre-L) and infralimbic (Infra-L) divisions (2.54 mm to 2.7 mm rostral to bregma); anterior cingulate (AC) cortex area 2 (Cg2) (1.6 mm to 1.44 mm rostral to bregma); the nucleus accumbens core (NACc) and shell (NACsh) (1.76 mm to 1.6 mm rostral to bregma); the anterior-medial (AM), latero-ventral (LV), and latero-dorsal (LD) divisions of the bed nucleus of the stria terminalis (BST) (.08 mm to −.4 mm relative to bregma); the central nucleus of the amygdala (CeA) and basolateral amygdala (BLA) (from −2.12 mm to −2.92 mm relative to bregma); the dorsal hippocampus (HIPPO), including the CA1, CA3, and dentate gyrus (DG) divisions (−2.80 mm to −3.12 mm relative to bregma); as well as the medial parvocellular paraventricular nucleus (mpPVN) and magnocellular paraventricular nucleus (mgPVN) of the hypothalamus (−1.48 mm to −2.12 mm relative to bregma). Counts of Fos-positive cells were also made within brain regions associated with the processing of olfactory information, including the anterior piriform cortex (PXa) (2.54 mm to 2.7 mm rostral to bregma) and posterior piriform cortex (PXp) (−2.3 mm to −2.46 mm caudal to bregma), the entorhinal cortex (EC) (−3.14 mm to −3.30 mm caudal to bregma), and the ventrolateral orbitofrontal cortex (VLO) (2.54 mm to 2.7 mm rostral to bregma). Digital images were taken on a Zeiss microscope and imported into Adobe Photoshop.
Statistical Analysis
Differences in lever responses during reinstatement tests were analyzed by mixed factorial analysis of variance (ANOVA) with treatment (S−/VEH, S+/VEH, S−/NTX, S+/NTX) as the between-subjects factor and session (extinction and reinstatement) as the within-subjects factor. Fisher’s protected least square difference (PLSD) post hoc tests were employed to subsequently verify differences between extinction, VEH, and NTX treatment conditions.
For analysis of Fos-immunoreactivity data, raw counts of Fos-positive cells from each neuronal population of interest first were normalized by log transformation due to violation of homogeneity of variance as determined by Bartlett’s test. The data then were analyzed by three-way ANOVA with brain division, cue condition (S+ or S−), and drug treatment (VEH or NTX) as independent factors. Following significant main effects or interactions, differences in Fos-positive cell counts were analyzed separately using multifactor ANOVA within the following brain divisions: 1) the mPFC, divided into its Pre-L, Infra-L, and AC divisions; 2) the NAC shell/core; 3) BLA; 4) the HIPPO, including the CA1, CA3, and dentate gyrus subregions; 5) the CeA; 6) BST; 7) paraventricular nucleus (PVN); and 8) brain regions associated with olfactory information processing, the PXa, PXp, EC, and VLO.
Fos-positive cell counts in the mPFC, NAC, and HIPPO were each analyzed by three-way ANOVA with brain division, cue condition, and drug treatment as independent factors. Differences among individual means were subsequently confirmed by PLSD post hoc tests. In the case of the BLA, CeA, PVN, and BST, data were analyzed by separate two-way ANOVAs with drug treatment and cue condition as independent factors, followed by PLSD post hoc tests. Given that NTX by itself is known to increase c-fos expression within the extended amygdala (including the NACsh) (Carr et al. 1998, 1999; Park and Carr 1998), the effects of the opiate antagonist on cue S− and S+ elicited Fos-immunoreactivity were also compared with NTX control animals (i.e., rats with the same reinforcement and ethanol history but not presented with the S− and S+). These data were analyzed by separate two-way ANOVAs with brain division and cue condition as independent factors, followed by PLSD post hoc tests. For brain regions associated with olfactory information processing, data were analyzed by separate two-way ANOVAs with brain region and cue condition as independent factors, followed by PLSD post hoc tests.
Results
Ethanol Self-Administration, Conditioning, and Extinction
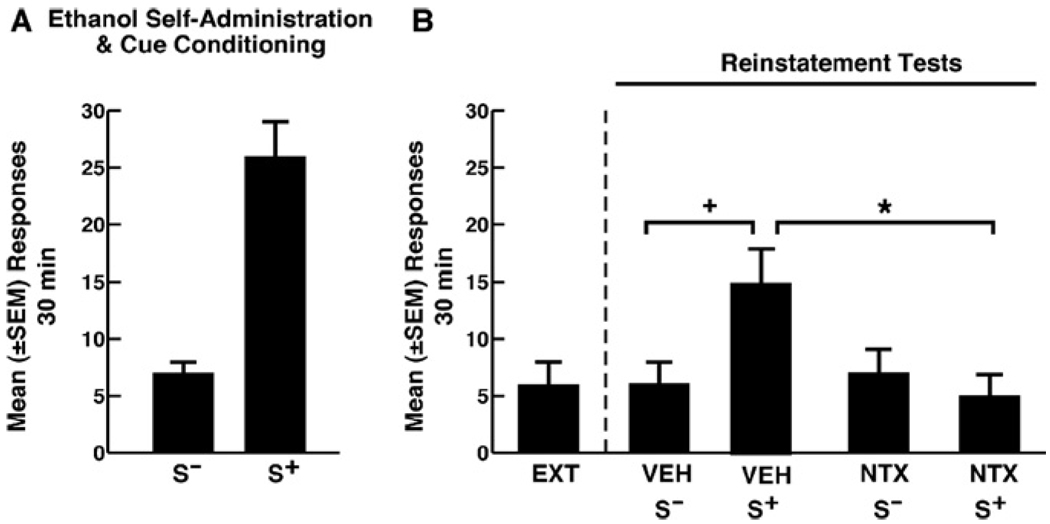
All rats acquired responding reinforced by 10% ethanol and developed stable ethanol self-administration during the conditioning phase. The mean (± SEM) number of responses averaged across the three final sessions was 26 ± 3 during ethanol and 7 ± 1 during nonreward sessions (Figure 2A). Rats emitted 16 ± 2 responses during the first extinction session. All rats reached the extinction criterion within 10 days.
Figure 2.
(A) Mean number (±SEM) of lever-press responses over 30 minutes during the last 3 days of self-administration training in the presence of the ethanol-predictive S+ or the nonreward S−. (B) In VEH-treated animals, the ethanol S+ elicited significant recovery of responding compared with the S−/VEH-treated animals (+p < .05). This effect was fully reversed by naltrexone (NTX) (*p < .05). Responding in the presence of the S− remained at extinction levels. S+, ethanol; S−, nonreward; VEH, vehicle; NTX, naltrexone.
Behavioral Effects of Ethanol Cue Exposure and Modification by Naltrexone
Rats exposed to the ethanol S+ and pretreated with vehicle or NTX (S+/VEH) showed significant reinstatement (Figure 2B). Responding in the presence of the S− (S−/VEH) remained at extinction levels. Naltrexone attenuated S+-induced responding compared with the S+/VEH condition but did not modify responding in the S− condition [Figure 2B; main effect of treatment: F(3,29) = 3.16; p < .05; and session x treatment interaction: F(3,29) = 4.27; p < .05].
Effects of Ethanol Cue Exposure on Fos-Protein Immunoreactivity
Given the primary objective of these studies (i.e., to identify brain sites that are responsive to ethanol cues), EtOH cue effects on c-fos expression in VEH-treated rats will be presented first and separately from those in NTX-treated animals.
Overall, three-way ANOVA of Fos-positive cell counts from all brain regions and treatment groups revealed a significant brain region x cue condition x drug treatment interaction [F(11,334) = 4.97; p < .001]. Subsequent two-way ANOVA confirmed a significant effect of the EtOH S+ on Fos-positive cell numbers in vehicle-treated rats compared with those tested in the absence of the ethanol cue—the S− condition [brain region x cue condition interaction: F(11,334) = 2.20; p < .05].
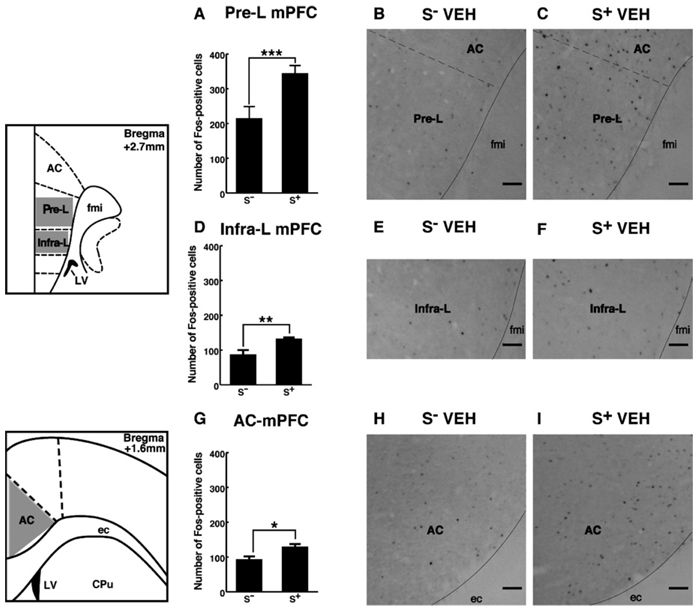
In the mPFC, increased Fos-positive cells were found following S+ exposure with significant effects in the Pre-L (Figure 3A–C), Infra-L (Figure 3D–F), and AC (Figure 3G–I) compared with the nonethanol cue (S−) condition [main effect of cue condition: F(1,84) = 34.42; p < .001]. No differences were found between the superficial and deep layers of the prefrontal cortex.
Figure 3.
Schematic drawings and photomicrographs depicting typical regions of analysis for the prelimbic (Pre-L), infralimbic (Infra-L), and anterior cingulate (AC) divisions of the medial prefrontal cortex (mPFC). Presentation of the ethanol S+ significantly increased numbers of Fos-positive cells within the Pre-L (A–C), Infra-L (D–F), and AC (G–I) compared with the nonreward S−. Scale bar = 50 µm. Schematics adapted from Paxinos and Watson (1997). LSD post hoc tests: *p < .05, **p < .01, ***p < .001. Pre-L, prelimbic; Infra-L, infralimbic; AC, anterior cingulate; mPFC, medial prefrontal cortex; S+, ethanol; S−, nonreward; ec, external capsule; CPu, caudate putamen; fmi, forceps minor of corpus callosum; LV, lateral ventricle; LSD, least significant difference.
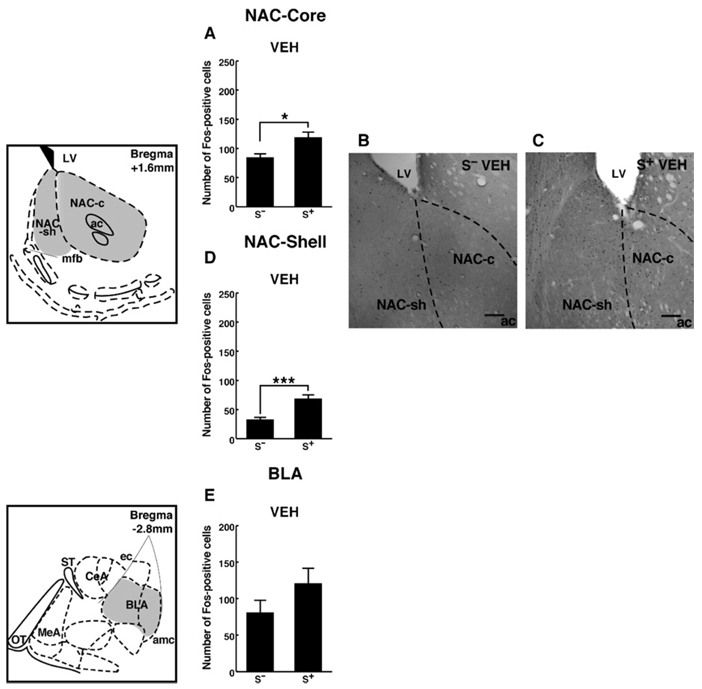
In both the NAC core and shell, Fos-positive cell counts were increased in EtOH S+-exposed rats compared with S−-exposed controls [cue x drug interaction: F(1,56) = 52.37; p < .001] (Figure 4A–D).
Figure 4.
Schematic drawings and photomicrographs depicting typical regions of analysis for the NAC core, shell, and basolateral amygdala. Presentation of the ethanol-associated S+ significantly increased Fos-positive cells within the NAC core (A–C) and shell (B–D). In the BLA (E), a trend toward increased numbers of Fos-positive cells was observed in vehicle-treated animals exposed to the ethanol-associated S+ compared with the S−, but this effect failed to reach statistical significance. Scale bar = 100 µm. Schematics adapted from Paxinos and Watson (1997). LSD post hoc tests: *p < .05, ***p < .001. NAC, nucleus accumbens; S+, ethanol; BLA, basolateral amygdala; S−, nonreward; amc, amygdala capsule; ac, anterior commissure; ec, external capsule; LV, lateral ventricle; MeA, medial amygdala; mfb, medial forebrain bundle; ST, stria terminalis; OT, optic tract; LSD, least significant difference.
A trend toward increased Fos-positive cells was seen within the BLA, but this effect failed to reach statistical significance (Figure 4E).
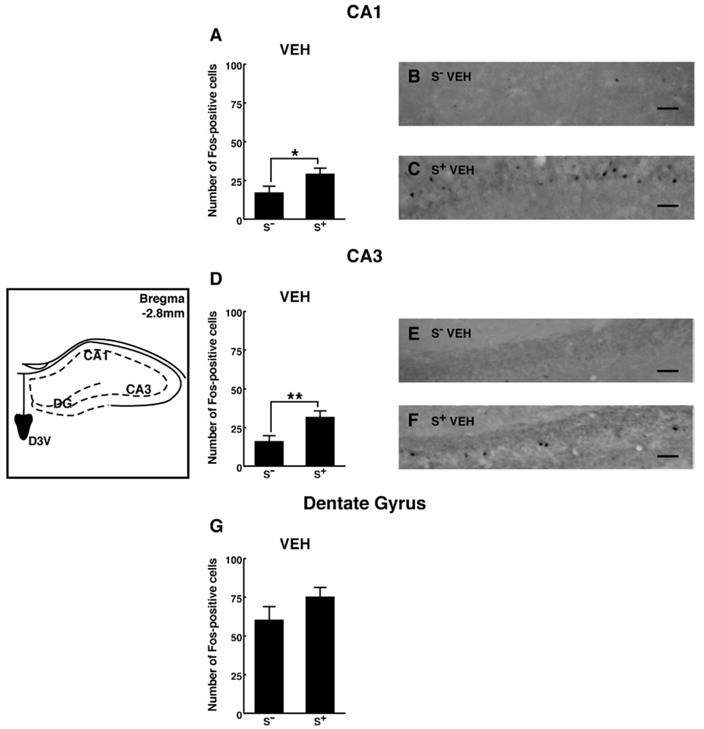
In the HIPPO, S+ exposure significantly increased Fos-positive cells within the CA1 (Figure 5A–C) and CA3 (Figure 5D–F) regions [main effect of cue condition: F(1,84) = 40; p < .001]. Fos-immunoreactivity in the dentate gyrus was not increased over that in rats tested in the absence (S−) of the ethanol cue (Figure 5G).
Figure 5.
Schematic drawings and photomicrographs depicting typical regions of analysis for the CA1, CA3, and DG of the dorsal hippocampus. Presentation of the ethanol S+ significantly increased Fos-positive cell counts in VEH-treated animals within the CA1 (A–C) and CA3 (D–F) regions of the hippocampus. A trend toward increased c-fos expression was observed within the dentate gyrus (DG) (G), but this effect only approached statistical significance. Scale bar = 10 µm. Schematics adapted from Paxinos and Watson (1997). LSD post hoc tests: *p < .05, **p < .01. DG, dentate gyrus; S+, ethanol; VEH, vehicle; D3V, dorsal third ventricle; LSD, least significant difference.
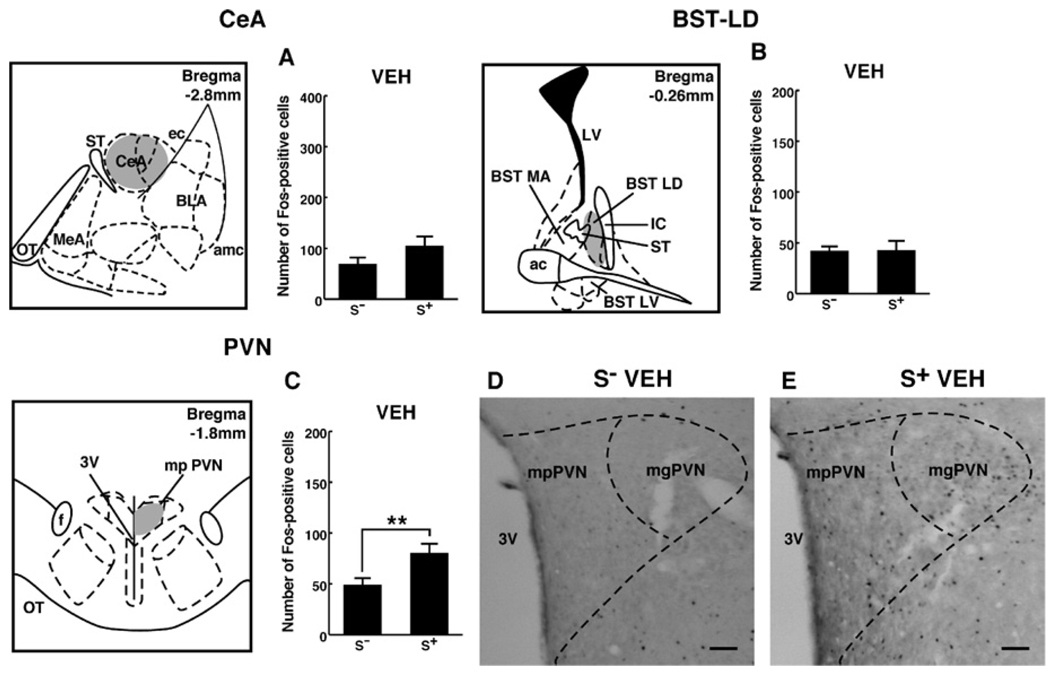
Within stress-regulatory sites, Fos-positive cells in the S+ and S− groups were statistically indistinguishable between the CeA and BST-LD (Figure 6A and B). However, Fos-positive cells in S+-exposed rats were increased both in the mpPVN [main effect of cue condition: F(1,28) = 8; p < .01] and mgPVN [S−, 8 ± 2; S+, 24 ± 4 (mean ± SEM); main effect of cue condition: F(1,9.6) = 9.6; p < .001; Figure 6C and D]. No differences in Fos-immunoreactivity due to cue condition were observed in the BST-AM or BST-LV.
Figure 6.
Schematic drawings and photomicrographs depicting typical regions of analysis for the central nucleus of the amygdala (CeA), latero-dorsal bed nucleus of the stria terminalis (BST-LD), and the medial parvocellular paraventricular nucleus (mpPVN). Counts of Fos-positive cells in vehicle-treated animals from the S+ and S− groups in the CeA or BST-LD were statistically indistinguishable (A, C). However, an increase in Fos-positive cells was observed in the mpPVN of S+-exposed rats (C–E). A small but significant increase in Fos-positive cell numbers was also seen in the mgPVN in S+ compared with S− exposed rats. Scale bar = 100µm. Schematics adapted from Paxinos and Watson (1997). LSD post hoc tests: **p < .01. CeA, central amygdala; BST-LD, latero-dorsal bed nucleus of the stria terminalis; mpPVN, medial parvocellular paraventricular nucleus; S+, ethanol; S−, nonreward; mgPVN, magnocellular paraventricular nucleus; amc, amygdala capsule; ac, anterior commissure; BST-MA, antero-medial bed nucleus of the stria terminalis; BLA, basolateral amygdala; ec, external capsule; f, fornix; IC, internal capsule; LV, lateral ventricle; BST-LV, latero-ventral bed nucleus of the stria terminalis; MeA, medial amygdala; mp, medial parvocellular; mg, magnocellular; PVN, paraventricular nucleus; ST, stria terminalis; OT, optic tract; 3V, third ventricle; LSD, least significant difference.
No statistical differences in Fos-positive cell counts were found between ethanol S+ and S− conditions in brain regions linked to olfactory information processing, including the PXa, PXp, EC, and VLO.
Effects of Naltrexone on Cue-Elicited Fos-Immunoreactivity
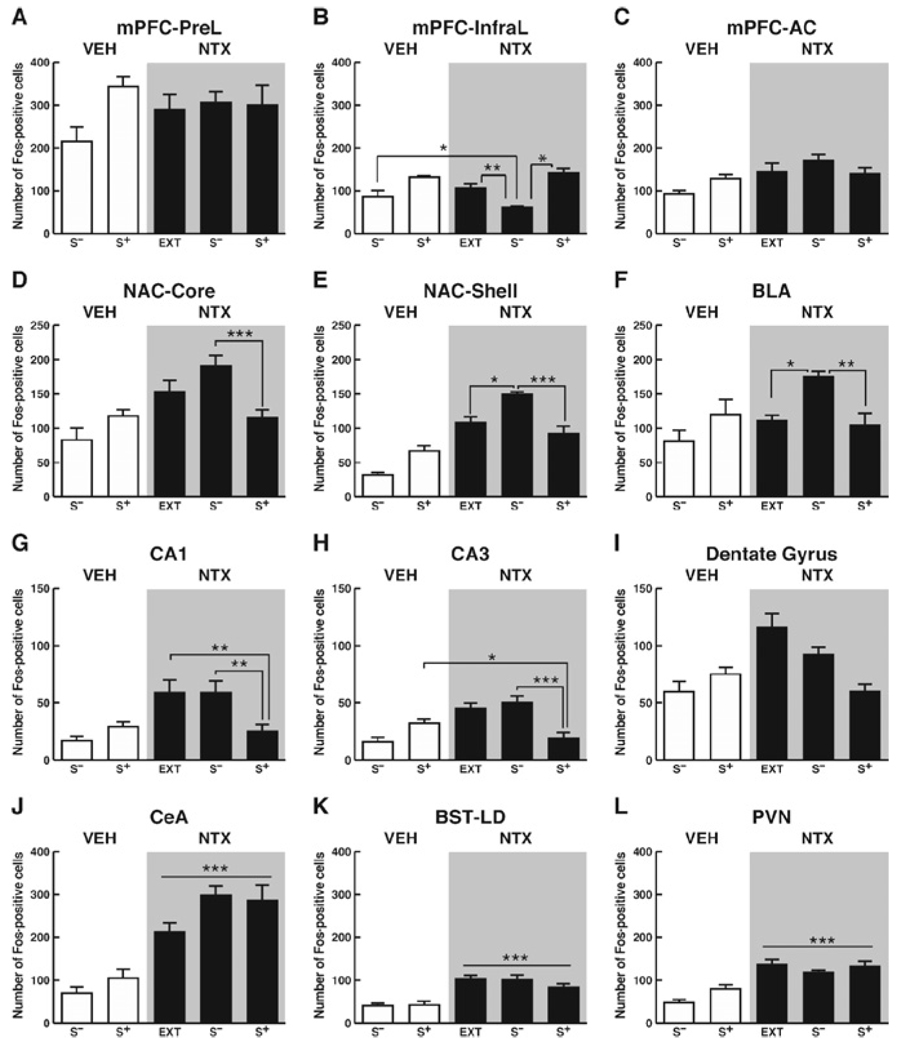
In contrast to its clear-cut behavioral actions (i.e., reversal of EtOH S+-induced reinstatement), NTX produced a complex pattern of changes in Fos-immunoreactivity. However, consistent with previous reports (Oswald and Wand 2004; Park and Carr 1998), naltrexone’s prevailing effect, regardless of cue condition, was disinhibitory and resulted in increased Fos-positive cells within the majority of brain regions. Nonetheless, distinct brain region-dependent effects of NTX were observed in four brain regions, including 1) the mPFC (Figure 7A–C); 2) the NAC and BLA (Figure 7D–F); 3) the HIPPO (Figure 7G–I); and 4) brain sites implicated in stress-regulatory functions (CeA, BST-LD, mpPVN; Figure 7J–L).
Figure 7.
Graphs (A–L) demonstrating the effects ofNTXon c-fos expression in animals exposed to either extinction, the ethanol S+, or the nonreward S−. Effects of the ethanol S+ or the nonreward S− in VEH-treated animals have been re-illustrated for ease of comparison. LSD post hoc tests: *p < .05, **p < .01, ***p < .001. (A–C): Within the Pre-L (A) and AC (C) divisions of the mPFC, NTX pretreatment of extinction control animals produced an identical pattern of Fos-immunoreactivity to that observed in S−/ and S+/NTX-treated groups. Within the Infra-L (B), Fos-immunoreactive neurons in S−/NTX-treated animals were significantly less than the extinction/NTX, S+/NTX group, and the VEH/S− group. (D–F): In theNACcore (D), no differences in Fos-immunoreactivity were observed between extinction NTX and S−/NTX-treated animals. However, S−/NTX-treated animals displayed significantly greater Fos-positive cells than S−/NTX rats. In the NAC shell (E) as well as in the BLA (J), naltrexone increased numbers of Fos-positive cells within the S− nonreward condition to a greater extent than in all other groups. (G–I): Within the CA1 (G) and CA3 (H) regions of the hippocampus, Fos-positive cell counts within the NTX-treated extinction group were identical to those in the S−/NTX group. However, counts from extinction and S−/NTX-treated conditions in both the CA1 and CA3 were significantly greater than the S−/NTX group. Moreover, Fos-positive CA3 cell counts in the S+/NTX condition were significantly reduced compared with the S+/VEH group. In the DG (I), despite similar trends to those in other parts of the hippocampus observed, all changes in Fos-immunoreactivity within the DG failed to reach statistical significance. (J–L): In the CeA, the BST-LD, and the mpPVN, NTX significantly increased Fos-positive cell counts over those in vehicle-treated rats, regardless of cue exposure condition (extinction, S+, or S−). NTX, naltrexone; S+, ethanol; S−, nonreward; VEH, vehicle; LSD, least significant difference; Pre-L, prelimbic; AC, anterior cingulate; mPFC, medial prefrontal cortex; Infra-L, infralimbic; NAC, nucleus accumbens; BLA, basolateral amygdala; DG, dentate gyrus; CeA, central amygdala; BST-LD, latero-dorsal bed nucleus of the stria terminalis; mpPVN, medial parvocellular paraventricular nucleus.
Effects of NTX on mPFC Neurons
Within the Pre-L and AC, the pattern of Fos-immunoreactivity in NTX (extinction) control animals was identical to that in the S−/NTX and S+/NTX groups. In these brain regions, the opiate antagonist produced neural activation in NTX control animals identical to that in VEH-treated rats exposed to the ethanol S+ (Figure 7A and C).
Within the Infra-L, numbers of Fos-positive neurons in NTX control animals were equivalent to those in NTX-treated rats exposed to the ethanol S+. Fos-immunoreactivity in S−/NTX-treated animals, however, was significantly lower than in NTX control animals or the S+/NTX group [brain region x cue interaction: F (4,51) = 7.15; p < .001; Figure 7A–C]. Moreover, compared with the VEH/S− group, significantly fewer Fos-positive cells were observed in S−/NTX-treated animals [brain region set x cue condition x drug interaction: F(2,84) = 7.28; p < .01; Figure 7B).
Effects of NTX on NAC and BLA Neurons
In the NAC core, no differences in Fos-immunoreactivity were observed between NTX control animals and S−/NTX-treated animals. However, Fos-positive cells were significantly increased in the S−/NTX compared with the S+/NTX condition. Fos-positive cell numbers in the NAC shell and BLA of NTX control animals were indistinguishable from those in NTX-treated animals exposed to the S+. However, Fos-positive cell counts in both the NTX control animals and NTX/S+ group were significantly lower than in the S−/NTX condition [NAC shell main effect of cue condition: F(2,34) = 13.09; p < .001; BLA main effect of cue condition: F(2,17) = 6.2; p < .01]. In fact, Fos-positive cell counts in NAC and BLA neurons of the S−/NTX group were overall substantially greater than in any other treatment condition, including those in the S+/VEH and S−/VEH conditions [NAC, cue condition x drug interaction: F(1,56) = 52.37; BLA, significant cue condition x drug interaction: F (1,28) = 8.18; p < .01; Figure 7E and F].
Effects of NTX on Dorsal Hippocampal Neurons
Within the hippocampal CA1 (Figure 7G) and CA3 (Figure 7H) regions, Fos-positive cell counts in NTX control animals were identical to those in the S−/NTX group. In both the CA1 and CA3, Fos-immunoreactivity was significantly greater in the NTX control and S−/NTX conditions compared with the S+/NTX group [main effect of cue condition: F(2,51) = 14.38; p < .001]. Moreover, Fos-positive CA3 cells in the S+/NTX condition were significantly reduced compared with the S+/VEH group [significant cue condition x drug interaction: F (1,84) = 39.9; p < .001; Figure 7H]. Thus, unlike in other brain sites, NTX reversed the S+-induced elevations in Fos-immunoreactivity observed in VEH-treated rats within the CA3 region. In the DG (Figure 7I), all changes in Fos-immunoreactivity failed to reach statistical significance, despite trends in the same direction as in the CA1 and CA3 (Figure 7I).
Effects of NTX on Stress-Regulatory Sites
Within the CeA, BST-LD, and mpPVN of NTX control animals, numbers of Fos-immunoreactive cells did not differ from those in NTX-treated animals exposed to the S+ or S−. In the CeA [main effect of drug: F (1,28) = 41.0; p < .001; Figure 7J], the BST-LD [main effect of drug: F (1,36) = 41.0; p < .001; Figure 7K), and the mpPVN [main effect of drug: F (1,28) = 58.0; p < .001; Figure 7L], NTX significantly increased Fos-positive cell counts over those in vehicle-treated rats, regardless of cue exposure condition (S+ or S−). The opiate antagonist did not alter Fos-positive cell numbers in the BST-MA and BST-LV.
Discussion
Confirming earlier findings (Ciccocioppo et al. 2002, 2003; Katner and Weiss 1999; Liu and Weiss 2002), an olfactory stimulus (S+) conditioned to ethanol reward, but not a cue associated with nonreward (S−), elicited recovery of responding at a previously ethanol-paired lever. Exposure to the ethanol S+ and ensuing ethanol-seeking behavior was associated with activation of the Pre-L, Infra-L, and AC mPFC divisions; the NAC; and dorsal HIPPO, indicating that major components of the incentive motive circuit (Everitt and Wolf 2002; Kalivas and McFarland 2003; Shalev et al. 2002; Weiss 2005) are also activated by ethanol-related stimuli. Additionally, the ethanol S+ increased Fos-positive neurons within hypothalamic sites regulating neuroendocrine and autonomic responses to stress.
In the medial prefrontal cortex, neural activation induced by the ethanol S+ was most prominent within the Pre-L and Infra-L divisions but with clearly significant effects also in AC. The increase in Fos-positive neurons within the Pre-L and AC is consistent with reports of increased c-fos expression in these regions in rats showing conditioned place preference (CPP) for environments previously paired with ethanol, cocaine, nicotine, or morphine (Franklin and Druhan 2000; Neisewander et al. 2000; Schroeder et al. 2000, 2001, 2003; Schroeder and Kelley 2002; Topple et al. 1998) and reinstatement induced by cocaine-related contextual stimuli (Ciccocioppo et al. 1999). Moreover, Fos-positive nuclei were increased in the Pre-L by discrete ethanol-paired conditioned stimuli (Wedzony et al. 2003). In the case of cocaine, functional studies (Fuchs et al. 2005; McLaughlin and See 2003) have confirmed that increased c-fos expression within the Pre-L and AC translates into a direct role in reinstatement by conditioned discrete and contextual stimuli (McLaughlin and See 2003; Weissenborn et al. 1997). The consistency of neural activation in the Pre-L and AC by stimuli conditioned to different classes of drugs of abuse and under widely different contingencies strongly implicates a direct functional role for these brain regions in ethanol-seeking, although this remains to be confirmed directly.
Neural activation by the ethanol S+ was also observed in the Infra-L, similar to previous reports on activation of this structure by discrete ethanol-paired cues (Wedzony et al. 2003). Functional integrity of the Infra-L is apparently not required for conditioned reinstatement by cocaine-paired stimuli (McLaughlin and See 2003); although, like the effects of the ethanol S+, a contextual cocaine cue increased c-fos expression in this brain region (Franklin and Druhan 2000). It is possible that increased c-fos expression within the Infra-L by ethanol- or cocaine-associated stimuli is related to conditioned autonomic responses that are typically associated with drug cue exposure in humans (Sinha et al. 2000; Stormark et al. 1995). Additionally, the Infra-L is implicated in regulating impulsivity (Chudasama et al. 2003), and manipulations interfering with Infra-L cortex function retard extinction of conditioned fear and increases the recovery of appetitive Pavlovian responses (Frysztak and Neafsey 1994; Pratt and Mizumori 2001; Rhodes and Killcross 2004). Thus, the Infra-L cortex warrants further attention with respect to a role in conditioned drug-seeking and relapse.
Ethanol S+ exposure significantly increased c-fos expression within the hippocampal CA1 and CA3 regions, similar to the effects of exposure to environments previously associated with cocaine (Brown et al. 1992; Neisewander et al. 2000) or ethanol (Topple et al. 1998) in CPP studies. The HIPPO has an established role in contextual memory retrieval and the occasion-setting actions of contextual stimuli (Holland and Bouton 1999; Honey and Good 2000). These functions extend to control of drug-seeking behavior since functional inactivation of the dorsal HIPPO suppresses reinstatement of cocaine seeking by contextual cues (Fuchs et al. 2005). The activation of the dorsal HIPPO by the ethanol S+ is consistent with this finding and strongly suggests a role for the HIPPO in controlling conditioned ethanol-seeking as well.
In the NAC, the ethanol S+ increased Fos-positive nuclei within both the core and shell. Based largely on studies of conditioned cocaine-seeking behavior, the NAC core is thought to preferentially mediate the effects of discrete drug-conditioned stimuli, whereas the NAC shell has been implicated in drug seeking controlled by contextual stimuli (Ghitza et al. 2003; Ito et al. 2004; Cardinal and Everitt 2004; Weiss 2005). It is possible, under the present contingencies, the S+ (a contextual cue), via its occasion-setting properties, may have conveyed conditioned reinforcing properties to discrete ethanol-paired cues in the environment such as the sound of the lever mechanism, resulting in activation not only of NAC shell but also core neurons — a hypothesis that awaits further testing. Moreover, regionally specific activation of the NAC by contextual stimuli conditioned to different classes of abused drugs remains to be more systematically examined since cocaine CPP (Franklin and Druhan 2000; Neisewander et al. 2000) but not CPP for ethanol, nicotine, or morphine have been reported to elicit activation of both NACsh and core neurons (Schroeder et al. 2000, 2001, 2003; Schroeder and Kelley 2002; Topple et al. 1998).
In the BLA, an important neural substrate for appetitive conditioning (Cardinal et al. 2002), only a tendency toward increased Fos-immunorectivity was observed, in contrast to an earlier finding in which CPP for a beer-paired environment was accompanied by activation of the BLA (Topple et al. 1998). This discrepancy may be related to differences in learning and motivational processes that control behavior in CPP versus reinstatement procedures. However, similar to the present findings, re-exposure to morphine or nicotine-paired environments in CPP studies failed to increase c-fos expression in the BLA (Schroeder et al. 2000, 2003; Schroeder and Kelley 2002). Conversely, the BLA consistently shows elevated c-fos expression in rats exposed to cocaine-related contextual cues (Ciccocioppo et al 2001; Franklin and Druhan 2000; Neisewander et al. 2000), and a direct role for this site in conditioned reinstatement has been demonstrated repeatedly in the case of cocaine (Di Ciano and Everitt 2004; Fuchs et al. 2002, 2005; Fuchs and See 2002; See et al 2003b). One may speculate that the modest activation of the BLA in the present study is related to differences in the sensory modality required for processing of the stimuli (i.e., the reliance on olfactory cues in the present versus nonolfactory cues in other studies). However, the lack of a selective recruitment of the olfactory cortex and related circuitry by the S+ argues against this possibility. Further elucidation of the precise role of the BLA, specifically in conditioned reinstatement by contextual ethanol cues, will remain for future investigation.
The ethanol S+ significantly increased c-fos expression in brain sites not traditionally linked to conditioned reinstatement: the medial parvocellular (mp) and magnocellular (mg) paraventricular nucleus of the hypothalamus (PVN). Activation of mp-PVN neurons is positively correlated with HPA axis activation (Buller et al. 1998; Dayas et al. 1999), suggesting that the S+ elicited a “stress-like” neuroendocrine response. In humans, drug cue manipulations induce a similar pattern of cue reactivity and craving as that induced by stress, and these effects are accompanied by increased HPA axis activity and anxiety (Fox et al. 2005; Sinha et al. 2000, 2003). Thus, subjective responses to drug cues include stress-like reactions, which is consistent with the S+-induced increase in mpPVN cell activity. Elevated Fos-immunoreactivity was also observed in the magnocellular PVN (mg-PVN), an effect consistent with psychological stressors (Dayas et al. 1999), lending further support to the hypothesis that the ethanol S+ produced stress-like effects. In addition to influencing the HPA axis, activated PVN neurons, through descending brainstem projections (Dayas et al. 2004), may influence autonomic responses associated with the anticipation of ethanol reward predicted by the S+ as observed in alcoholic subjects (Sinha et al. 2000; Stormark et al. 1995).
Since the ethanol S+ was an odor cue, it was also important to evaluate c-fos expression within brain regions related to olfactory processing and memory (Illig 2005; Schoenbaum and Eichenbaum 1995), including the piriform cortex, its hippocampal relay station the entorhinal cortex (Haberly and Price 1978), and the orbitofrontal cortex. These reciprocally connected brain regions are significant efferent targets of the olfactory bulb (Illig 2005; Schoenbaum and Eichenbaum 1995). Of importance, the orbito-frontal cortex has been shown to play a pivotal role in goal-directed behavior, including cue-induced cocaine seeking (Fuchs et al. 2004; Gallagher et al. 1996; Lipton et al. 1999; Schoenbaum et al. 1999). However, the effects of the S+ and S− on Fos-immunoreactivity in these parts of the olfactory cortex were statistically indistinguishable and, therefore, did not reflect the differential behavioral effect of these cues. In support of this conclusion, behavioral data show that different types of olfactory stimuli used as an S+ during conditioning produce identical levels of reinstatement (Katner and Weiss 1999). A tentative interpretation for this finding is that these regions are involved in the processing of the sensory aspect of olfactory cues, whereas sites such as the Pre-L and Infra-L prefrontal cortex and HIPPO are involved in the integration and interpretation of the significance of these stimuli, such as their association with reward.
The observed brain activation pattern points toward a role in cue-induced ethanol-seeking for brain regions regulating associative learning and goal-directed behavior — as in the case of other drugs of abuse — as well as of sites regulating neuroen-docrine responses to stress. While increased c-fos expression does not provide direct support for a role of these sites in ethanol-seeking behavior, such a role appears likely in view of ample evidence that transient lesion and local pharmacological manipulation of these brain regions modify cocaine-seeking responses controlled by stimuli conditioned to cocaine (Everitt and Wolf 2002; Kalivas and McFarland 2003; Shalev et al. 2002; Weiss 2005). To further pinpoint brain regions that may be critical specifically for the motivating effects of ethanol-related stimuli, the effects of nonselective opiate receptor antagonist NTX on brain activation patterns induced by the ethanol S+ were examined. As NTX reduces both ethanol craving elicited by ethanol cues in abstinent alcoholics (Monti et al. 1999; O’Malley et al. 2002; Rohsenow et al. 2000) and reinstatement induced by ethanol cues in rats (Ciccocioppo et al. 2002, 2003; Katner and Weiss 1999; Liu and Weiss 2002), modification of cue-induced c-fos expression by NTX would be expected to reveal brain sites with a possibly critical role in mediating the motivating effects of ethanol cues.
In contrast to earlier observations with a dopamine D1 antagonist that was found to suppress cue-induced c-fos expression while concomitantly reversing conditioned reinstatement in the case of cocaine (Ciccocioppo et al. 2001), the prevailing effects of NTX on c-fos expression was disinhibitory — an effect consistent with previous reports (Carr et al. 1998, 1999; Oswald and Wand 2004; Park and Carr 1998). Despite the complexity of naltrexone’s effects, careful examination of the c-fos expression data and integration with existing clinical and preclinical literature provides at least three “leads” potentially relevant for naltrexone’s antireinstatement actions: 1) the disinhibitory effects of NTX in the CeA, BST-LD, and PVN; 2) the effects of NTX on the dorsal HIPPO where S+-induced c-fos expression was suppressed (particularly within the CA3); and 3) the specific increase in c-fos expression under S− conditions within the BLA and NAC.
The NTX-induced increase in Fos-immunoreactivity within the CeA, BST-LD, and PVN is consistent with naltrexone’s known disinhibitory actions on mpPVN, CeA, and BST-LD neurons (Carr et al. 1998, 1999; Oswald and Wand 2004; Park and Carr 1998) and resulting HPA axis activation. Interestingly, HPA axis activation has been implicated in the anticraving effects of NTX and acamprosate (Kiefer et al. 2006; O’Malley et al. 2002). Alcoholics show an impaired HPA axis function, and there is growing evidence of a link between HPA axis dysregulation and ethanol relapse (Adinoff et al. 2005a, 2005b, 2005c; Kiefer et al. 2006; O’Malley et al. 2002). It has been suggested that NTX-induced HPA axis activation suppresses ethanol craving and reduces relapse risk by substituting for the HPA axis-activating effects of ethanol (Kiefer et al. 2006; Kreek et al. 2002; McCaul et al. 2001; O’Malley et al. 2002). Consistent with this hypothesis, NTX increased Fos-positive mpPVN cells and nearly doubled the number of activated Fos-positive neurons in the BST-LD and CeA, activation of which is directly linked to recruitment of the mpPVN and HPA axis activation (Buller et al. 1998; Dayas et al. 1999).
The disinhibitory effects of NTX on “extended amygdala” neurons (i.e., the CeA, BST-LD, and including the NACsh) are also worth considering in the context of findings by Carr et al. (1998, 1999) and Park and Carr (1998) showing that NTX-induced c-fos expression within the CeA, BST-LD, and NACsh is linked to the suppression of positive hedonic responses to food. These authors propose that “the Fos-response induced by NTX may reflect activation of neurons that antagonize reward-related processes in this circuitry.” Such effects, although not yet directly confirmed, may also contribute to suppressed ethanol craving — consistent with reports that CeA-directed injections of opiate antagonists modulate ethanol-reinforced behaviors (Foster et al. 2004; Heyser et al. 1999) and suppress the desire to consume a preferred diet (Glass et al. 2000).
A second finding with likely implications for the “anti-reinstatement” actions of NTX is the suppression of hippocampal CA3 c-fos expression. The HIPPO contains a high density of opioid receptors (Mansour et al. 1987) and opioids modulate learning and memory-related events in this brain region (Bramham and Sarvey 1996; Jamot et al. 2003; Jang et al. 2003; Simmons and Chavkin 1996). Moreover, μ-opioid receptor antagonist injections into the dorsal HIPPO impair spatial memory retrieval (Meilandt et al. 2004). Thus, the suppression of S+-elicited neural activation in the HIPPO may reflect NTX-mediated dampening of learned contextual associations between environmental stimuli and drug reward, leading to reduced drug-seeking normally evoked by such cues. Further support for this interpretation comes from recent findings that attenuation of EtOH cue effects on reinstatement by LY379268, a Group II metabotropic glutamate receptor agonist (mGlu2/3) that reduces hippocampal neural excitability (Kew et al. 2002; Kilbride et al. 1998), is associated with reversal of EtOH cue-induced hippocampal c-fos expression (Weiss et al. 2005).
Thirdly, the effect of NTX to increase c-fos expression under S− conditions, specifically within the NAC shell and BLA, to levels significantly greater than in all other NTX-treated groups (i.e., NTX control and NTX/S+) may reflect a strengthening of response inhibition. Contrary to this interpretation, the changes in c-fos expression in the NTX/S− group were not accompanied by reduced behavioral responding compared with either extinction levels or responding during S− exposure in VEH-treated animals. However, it cannot be ruled out that “floor effects” in responding under the extinction and S− conditions prevented detection of an active inhibitory process that is augmented by NTX. Thus, this question will require further investigation.
An interesting observation potentially related to the enhanced c-fos expression in the BLA and NAC of S−-exposed rats was the reduction in Infra-L c-fos expression after NTX in the same S− cue condition and to the VEH/S− condition — an effect of NTX that was different than in other mPFC regions. Given that Infra-L mPFC projections target the NAC shell and BLA (Gabbott et al. 2005; Vertes 2004), one possible explanation for the increased c-fos expression under S− conditions within these brain regions is an NTX-induced alteration in Infra-L excitatory output onto intervening gamma-aminobutyric acid (GABA)ergic interneurons within the NACsh or BLA. These hypotheses and their relevance to the anti-reinstatement effects of NTX remain speculative until further scrutiny.
Conclusions
Ethanol-associated contextual stimuli elicit specific recruitment patterns within the mPFC, NAC, and HIPPO in rats similar to those produced by other abused drugs, as well as brain activation patterns evoked by ethanol cues in alcoholics (Grusser et al. 2004; Maas et al. 1998; Myrick et al. 2004). The findings also reveal that the effects of the “anticraving” agent NTX on this recruitment profile are complex. While it must be taken into account that c-fos expression is not a comprehensive marker of neural activity and that pathways contributing to NTX’s “anti-relapse” actions may not be revealed by Fos-mapping, the results identify the extended amygdala, PVN, and HIPPO as regions for further scrutiny as neural substrates for the therapeutic actions of opiate antagonists.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants AA10531 and AA014351 from the National Institute on Alcohol Abuse and Alcoholism (FW) and a CJ Martin Fellowship from the National Health and Medical Research Council of Australia (CVD). This is publication number 17324-NP from The Scripps Research Institute.
We thank Kris Trulock and Charles Peto (The Salk Institute, Laboratory of Neuronal Structure and Function) for assistance with production of the photomicrographs; Paul Kenny, Harinder Aujla, and Rémi Martin-Fardon for helpful discussion; and Mike Arends for assistance with the preparation of the manuscript.
References
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: Implications for relapse. Alcohol Clin Exp Res. 2005a;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 1: Adrenocortical and pituitary glucocorticoid responsiveness. Alcohol Clin Exp Res. 2005b;29:517–527. doi: 10.1097/01.ALC.0000158940.05529.0A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 2: Response to ovine corticotropin-releasing factor and naloxone. Alcohol Clin Exp Res. 2005c;29:528–537. doi: 10.1097/01.ALC.0000158939.25531.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Sarvey JM. Endogenous activation of mu and delta-1 opioid receptors is required for long-term potentiation induction in the lateral perforant path: Dependence on GABAergic inhibition. J Neurosci. 1996;16:8123–8131. doi: 10.1523/JNEUROSCI.16-24-08123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: Role of forebrain limbic structures. J Neurosci. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller KM, Xu Y, Day TA. Indomethacin attenuates oxytocin and hypothalamic-pituitary-adrenal axis responses to systemic interleukin-1 beta. J Neuroendocrinol. 1998;10:519–528. doi: 10.1046/j.1365-2826.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: Links to drug addiction. Curr Opin Neurobiol. 2004;14:156–162. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carr KD, Kutchukhidze N, Park TH. Differential effects of mu and kappa opioid antagonists on Fos-like immunoreactivity in extended amygdala. Brain Res. 1999;822:34–42. doi: 10.1016/s0006-8993(99)01088-4. [DOI] [PubMed] [Google Scholar]
- Carr KD, Park TH, Zhang Y, Stone EA. Neuroanatomical patterns of Fos-like immunoreactivity induced by naltrexone in food-restricted and ad libitum fed rats. Brain Res. 1998;779:26–32. doi: 10.1016/s0006-8993(97)01074-3. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: Differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Chhada M, Perfumi M, Froldi R, Massi M. Conditioned taste aversion induced by ethanol in alcohol-preferring rats: Influence of the method of ethanol administration. Pharmacol Biochem Behav. 1999;64:563–566. doi: 10.1016/s0091-3057(99)00104-5. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F. Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: Effects of naltrexone. Psychopharmacology (Berl) 2003;168:208–215. doi: 10.1007/s00213-002-1380-z. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F. Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology. 2002;27:391–399. doi: 10.1016/S0893-133X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: Reversal by D(1) antagonists. Proc Natl Acad Sci U S A. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Lingford-Hughes A, et al. Functional connectivity analysis of the neural circuits of opiate craving: “More” rather than “different”? Neuroimage. 2003;20:1964–1970. doi: 10.1016/j.neuroimage.2003.07.025. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: Evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Martin-Fardon R, Thorsell A, Weiss F. Chronic footshock, but not a physiological stressor, suppresses the alcohol deprivation effect in dependent rats. Alcohol Alcohol. 2004;39:190–196. doi: 10.1093/alcalc/agh046. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: A neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KL, McKay PF, Seyoum R, Milbourne D, Yin W, Sarma PV, et al. GABA(A) and opioid receptors of the central nucleus of the amygdala selectively regulate ethanol-maintained behaviors. Neuropsychopharmacology. 2004;29:269–284. doi: 10.1038/sj.npp.1300306. [DOI] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology. 2005;30:880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur J Neurosci. 2000;12:2097–2106. doi: 10.1046/j.1460-9568.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Res. 1994;643:181–193. doi: 10.1016/0006-8993(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus-and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 2002;160:425–433. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Weber SM, Rice HJ, Neisewander JL. Effects of excitotoxic lesions of the basolateral amygdala on cocaine-seeking behavior and cocaine conditioned place preference in rats. Brain Res. 2002;929:15–25. doi: 10.1016/s0006-8993(01)03366-2. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Landfield PW, McEwen B, Meaney MJ, Rapp PR, Sapolsky R, et al. Hippocampal neurodegeneration in aging. Science. 1996;274:484–485. doi: 10.1126/science.274.5287.484. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci. 2003;23:7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass MJ, Billington CJ, Levine AS. Naltrexone administered to central nucleus of amygdala or PVN: Neural dissociation of diet and energy. Am J Physiol Regul Integr Comp Physiol. 2000;279:R86–R92. doi: 10.1152/ajpregu.2000.279.1.R86. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. J Comp Neurol. 1978;178:711–740. doi: 10.1002/cne.901780408. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Roberts AJ, Schulteis G, Koob GF. Central administration of an opiate antagonist decreases oral ethanol self-administration in rats. Alcohol Clin Exp Res. 1999;23:1468–1476. [PubMed] [Google Scholar]
- Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Curr Opin Neurobiol. 1999;9:195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Honey RC, Good M. Associative components of recognition memory. Curr Opin Neurobiol. 2000;10:200–204. doi: 10.1016/s0959-4388(00)00069-6. [DOI] [PubMed] [Google Scholar]
- Illig KR. Projections from orbitofrontal cortex to anterior piriform cortex in the rat suggest a role in olfactory information processing. J Comp Neurol. 2005;488:224–231. doi: 10.1002/cne.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Jamot L, Matthes HW, Simonin F, Kieffer BL, Roder JC. Differential involvement of the mu and kappa opioid receptors in spatial learning. Genes Brain Behav. 2003;2:80–92. doi: 10.1034/j.1601-183x.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- Jang CG, Lee SY, Yoo JH, Yan JJ, Song DK, Loh HH, et al. Impaired water maze learning performance in mu-opioid receptor knockout mice. Brain Res Mol Brain Res. 2003;117:68–72. doi: 10.1016/s0169-328x(03)00291-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Katner SN, Weiss F. Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin Exp Res. 1999;23:1751–1760. [PubMed] [Google Scholar]
- Kew JN, Pflimlin MC, Kemp JA, Mutel V. Differential regulation of synaptic transmission by mGlu2 and mGlu3 at the perforant path inputs to the dentate gyrus and CA1 revealed in mGlu2 −/− mice. Neuropharmacology. 2002;43:215–221. doi: 10.1016/s0028-3908(02)00084-9. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Otte C, Naber D, Wiedemann K. Hypothalamic-pituitary-adrenocortical axis activity: A target of pharmacological anti-craving treatment? Biol Psychiatry. 2006;60:74–76. doi: 10.1016/j.biopsych.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Kilbride J, Huang LQ, Rowan MJ, Anwyl R. Presynaptic inhibitory action of the group II metabotropic glutamate receptor agonists, LY354740 and DCG-IV. Eur J Pharmacol. 1998;356:149–157. doi: 10.1016/s0014-2999(98)00526-3. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ. c-Fos as a transcription factor: A stressful (re)view from a functional map. Neurochem Int. 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002;1:710–726. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- Lipton PA, Alvarez P, Eichenbaum H. Crossmodal associative memory representations in rodent orbitofrontal cortex. Neuron. 1999;22:349–359. doi: 10.1016/s0896-6273(00)81095-8. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: Exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, et al. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradio-graphic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Stauffer R, Lee SM, Rohde CA. Naltrexone dampens ethanol-induced cardiovascular and hypothalamic-pituitary-adrenal axis activation. Neuropsychopharmacology. 2001;25:537–547. doi: 10.1016/S0893-133X(01)00241-X. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Meilandt WJ, Barea-Rodriguez E, Harvey SA, Martinez JL., Jr Role of hippocampal CA3 mu-opioid receptors in spatial learning and memory. J Neurosci. 2004;24:2953–2962. doi: 10.1523/JNEUROSCI.5569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, et al. Naltrexone’s effect on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res. 1999;23:1386–1394. [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: Relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: Can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Park TH, Carr KD. Neuroanatomical patterns of fos-like immunoreactivity induced by a palatable meal and meal-paired environment in saline- and naltrexone-treated rats. Brain Res. 1998;805:169–180. doi: 10.1016/s0006-8993(98)00719-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson W. The Rat Brain in Stereotaxic Coordinates. 3rd ed. Sydney, Australia: Academic Press; 1997. [Google Scholar]
- Pratt WE, Mizumori SJ. Neurons in rat medial prefrontal cortex show anticipatory rate changes to predictable differential rewards in a spatial memory task. Behav Brain Res. 2001;123:165–183. doi: 10.1016/s0166-4328(01)00204-2. [DOI] [PubMed] [Google Scholar]
- Rhodes SE, Killcross S. Lesions of rat infralimbic cortex enhance recovery and reinstatement of an appetitive Pavlovian response. Learn Mem. 2004;11:611–616. doi: 10.1101/lm.79704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, Colby SM, Kaplan GB. Naltrexone’s effects on reactivity to alcohol cues among alcoholic men. J Abnorm Psychol. 2000;109:738–742. [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbito-frontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. II. Ensemble activity in orbitofrontal cortex. J Neurophysiol. 1995;74:751–762. doi: 10.1152/jn.1995.74.2.751. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105:535–545. doi: 10.1016/s0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Holahan MR, Landry CF, Kelley AE. Morphine-associated environmental cues elicit conditioned gene expression. Synapse. 2000;37:146–158. doi: 10.1002/1098-2396(200008)37:2<146::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Kelley AE. Conditioned Fos expression following morphine-paired contextual cue exposure is environment specific. Behav Neurosci. 2002;116:727–732. doi: 10.1037//0735-7044.116.4.727. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Schiltz CA, Kelley AE. Neural activation profile elicited by cues associated with the anxiogenic drug yohimbine differs from that observed for reward-paired cues. Neuropsychopharmacology. 2003;28:14–21. doi: 10.1038/sj.npp.1300007. [DOI] [PubMed] [Google Scholar]
- See RE, Fuchs RA, Ledford CC, McLaughlin J. Drug addiction, relapse, and the amygdala. Ann N Y Acad Sci. 2003a;985:294–307. doi: 10.1111/j.1749-6632.2003.tb07089.x. [DOI] [PubMed] [Google Scholar]
- See RE, McLaughlin J, Fuchs RA. Muscarinic receptor antagonism in the basolateral amygdala blocks acquisition of cocaine-stimulus association in a model of relapse to cocaine-seeking behavior in rats. Neuroscience. 2003b;117:477–483. doi: 10.1016/s0306-4522(02)00665-6. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: A review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Simmons ML, Chavkin C. Endogenous opioid regulation of hippocampal function. Int Rev Neurobiol. 1996;39:145–196. doi: 10.1016/s0074-7742(08)60666-2. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Stormark KM, Laberg JC, Bjerland T, Nordby H, Hugdahl K. Autonomic cued reactivity in alcoholics: The effect of olfactory stimuli. Addict Behav. 1995;20:571–584. doi: 10.1016/0306-4603(95)00017-7. [DOI] [PubMed] [Google Scholar]
- Topple AN, Hunt GE, McGregor IS. Possible neural substrates of beer-craving in rats. Neurosci Lett. 1998;252:99–102. doi: 10.1016/s0304-3940(98)00574-6. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward. Crit Rev Neurobiol. 2000;14:131–142. [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Koros E, Czyrak A, Chocyk A, Czepiel K, Fijal K, et al. Different pattern of brain c-Fos expression following re-exposure to ethanol or sucrose self-administration environment. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:331–341. doi: 10.1007/s00210-003-0811-7. [DOI] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: Genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Weissenborn R, Robbins TW, Everitt BJ. Effects of medial prefrontal or anterior cingulate cortex lesions on responding for cocaine under fixed-ratio and second-order schedules of reinforcement in rats. Psychopharmacology (Berl) 1997;134:242–257. doi: 10.1007/s002130050447. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Dayas C, Aujla H, Baptista MAS, Martin-Fardon R, Weiss F. Activation of Groip II mGluRs attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. doi: 10.1523/JNEUROSCI.2384-06.2006. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]