Abstract
Obesity is considered a major cause of premature mortality and a potential threat to the longstanding secular decline in mortality in the United States. We measure relative and attributable risks associated with obesity among middle-aged adults using data from the Health and Retirement Study (1992–2004). Although class II/III obesity (BMI ≥ 35.0 kg/m2) increases mortality by 40% in females and 62% in males compared with normal BMI (BMI = 18.5–24.9), class I obesity (BMI = 30.0–34.9) and being overweight (BMI = 25.0–29.9) are not associated with excess mortality. With respect to attributable mortality, class II/III obesity (BMI ≥ 35.0) is responsible for approximately 4% of deaths among females and 3% of deaths among males. Obesity is often compared with cigarette smoking as a major source of avoidable mortality. Smoking-attributable mortality is much larger in this cohort: about 36% in females and 50% in males. Results are robust to confounding by preexisting diseases, multiple dimensions of socioeconomic status (SES), smoking, and other correlates. These findings challenge the viewpoint that obesity will stem the long-term secular decline in U.S. mortality.
Mortality among middle- and older-aged Americans continues to decline in the United States, as evidenced by recent figures on life expectancy (National Center for Health Statistics 2006). This achievement, part of a longstanding secular decline in U.S. mortality, is arguably threatened by rising obesity (Olshansky et al. 2005). Obesity is often compared with cigarette smoking as a major cause of avoidable mortality (Marshall 2004; Parloff 2003; U.S. Department of Health and Human Services 2001). Despite improved longevity among middle- and older-aged Americans, more than 30% of this group are obese (BMI ≥ 30.0 kg/m2), and an additional 40% are overweight (BMI = 25.0–29.9; Ogden et al. 2006). Furthermore, the association between obesity and the early onset of chronic illnesses and disability is increasingly evident (Al Snih et al. 2007; Alley and Chang 2007; Field et al. 2001; Reynolds, Saito, and Crimmins 2005). Recent investigations, however, have suggested a more limited role for obesity in shortening lifespan (Flegal et al. 2005; Kulminski et al. 2008), extending the ongoing debate on this topic. Moreover, claims that obesity is responsible for a substantial number of premature deaths must rest on an empirical framework that translates individual-level mortality, usually estimated by relative risk, into attributable mortality at a population level. Such a framework is rarely implemented in research on obesity and mortality.
DIVERGENT ESTIMATES OF EXCESS DEATHS ASSOCIATED WITH OBESITY
In 2005, Flegal et al. (2005) published an article in the Journal of the American Medical Association that prompted considerable controversy about the issue of excess deaths associated with obesity. Flegal et al. (2005) estimated that only about 25,800 adult U.S. deaths in 2000 were attributable to being overweight or obese. This figure reflected both 112,000 excess deaths from obesity and 86,000 fewer deaths from being overweight (i.e., being overweight was protective against mortality). The estimate by Flegal et al. (2005) was considerably smaller than previous findings by Mokdad et al. (2004, 2005), who estimated that 350,000 deaths in the United States for 2000 were attributable to being overweight or obese (the authors did not present separate figures for the overweight and obese). The work by Mokdad et al. (2004) has become very influential in staging the scientific and public debate on obesity as a major cause of death.1 Mokdad et al. (2004) concluded that excess body weight was second only to tobacco use (435,000 excess deaths) as a cause of avoidable mortality in 2000, while the more recent estimate by Flegal et al. (2005) suggested a considerably smaller role for excess body weight.
The divergent findings of Flegal et al. (2005) and Mokdad et al. (2004) are likely due to methodological differences between the studies. One notable difference is the treatment of age (Couzin 2005; Flegal, Graubard, and Williamson 2004). Flegal et al. (2005) stratified the analysis by age (25–59, 60–69, and 70+), which allowed relative mortality risks, from which attributable mortality is based, to vary by age. In contrast, Mokdad et al. (2004) followed the approach described by Allison et al. (1999) and pooled ages 18 and older, thereby treating age as a confounder and not as an effect modifier. Another difference is the period for which relative mortality risks were estimated. While both studies calculated attributable mortality for 2000, each relied on estimates of relative mortality risks derived from data sources covering distinct prior periods. Flegal et al. (2005) based their estimates of relative mortality risks using three National Health and Nutrition Examination Surveys (NHANES I, II, III), which had a combined mortality period of 1971–2000. In contrast, Mokdad et al. (2004) combined six epidemiological cohorts with a mortality follow-up encompassing 1948–1992. As suggested in Flegal et al.’s (2005) work, the relative risks associated with obesity may be declining over time. Hence, to the extent that the relative mortality risks are decreasing over time, estimates of attributable mortality based on earlier data will be higher.
THE PRESENT STUDY
We investigate obesity’s association with mortality using the Health and Retirement Study (HRS), and we address prior methodological concerns. The HRS offers numerous advantages relevant to current debates surrounding obesity and mortality. It collects data over a recent period, beginning in 1992, with mortality linkages available through 2004. Therefore, we do not rely on historical data to estimate risk ratios as in previous studies. Given that the treatment of age has contributed to prior controversies, the HRS is also advantageous because it is specifically designed for a birth cohort analysis across narrow age ranges (in contrast to NHANES, which is not designed for this purpose). Here, we examine Americans who were born between 1931 and 1941 and who were ages 50–61 when first interviewed in 1992. The HRS contains a sample of nearly 10,000 middle-aged adults (about four times that of NHANES III, which was conducted over a similar period), thus increasing the precision of estimates in this specific age group.
Because obesity’s effect on mortality among middle-aged adults is increasingly important to future life expectancy, we examine adults in their 50s and 60s. The proportion of Americans in their 50s and 60s is expected to grow from approximately one-fifth to one-quarter over the next decade (U.S. Census Bureau 2005). Our target population, the 1931–1941 birth cohort, is a predecessor of the numerically large baby boom cohort born between 1946 and 1964. Most baby boomers are moving through the fifth and sixth decades of life, and this study will provide insight into how obesity is affecting their mortality. We investigate middle-aged adults as opposed to the elderly because middle-aged adults are less burdened by age-associated comorbidities that obscure causal links (Manson et al. 2007). Additionally, changes in height and body mass composition common in older individuals complicate the measurement of weight status in the elderly (Gallagher et al. 1996; Janssen and Mark 2007). Finally, middle-aged adults are less influenced by selective forces that reduce the observed effect of excess body weight because of early deaths to at-risk subjects.
Most studies on obesity and mortality have relied on samples limited to specific segments of the population. These include the Nurses’ Health Study (Manson et al. 1995), the Physicians’ Health Study (Ajani et al. 2004), and the National Institutes of Health (NIH)-AARP cohort (Adams et al. 2006). Another contribution of this study is thus that we use nationally representative data.
ESTIMATING ATTRIBUTABLE MORTALITY
Previous work on excess deaths associated with obesity has centered on the concept of “attributable mortality,” which refers to the fraction of deaths that are avoided if a risk factor is eliminated from the population. Attributable mortality combines estimates of both relative risk and prevalence to summarize the harm of a risk factor. The appeal of providing a single summary figure that is easily interpretable has led to the use of attributable risk in setting public health priorities. For example, smoking-related attributable mortality is used to document the large mortality effect of smoking and to support public anti-tobacco spending (Centers for Disease Control and Prevention 2002). Although the interpretation of attributable mortality can be straightforward, important assumptions are implicit in its calculation, assumptions that have received little attention in the debate on obesity and mortality. Attributable mortality generally assumes a causal link between a risk factor and an outcome (Rockhill, Newman, and Weinberg 1998). It also sets the mortality of those with a risk factor to the mortality of the reference category (i.e., those without a risk factor; Levine 2007). In the case of obesity, this means that all the obese would instantaneously possess the same mortality as “normal” weight individuals, thus ignoring any previous effects of obesity earlier in life and any unobserved confounding.
Despite these limitations, attributable risk does have the advantage of combining an estimate of risk for an individual (such as the relative risk) with the prevalence of a risk factor at the population level. Therefore, examining attributable risk is useful in evaluating obesity’s role on macro-level trends in mortality and life expectancy. It also aids in the comparison of the effects of various risk factors. For example, the effect of obesity is often judged against the better-known effect of smoking on mortality, often without the calculation of attributable risk. In this study, we compare the attributable risk of obesity with that of smoking.
CONFOUNDING BY PREEXISTING ILLNESSES
Major health conditions—including cancer and respiratory disease—increase the risk of death but can also cause substantial weight loss. It is postulated that ignoring the role of disease-induced weight loss leads to underestimated effects of obesity (Manson et al. 2007). However, disease presence may operate both as a confounder and as a biological mediator between weight status and death. On the one hand, controlling for disease could increase the observed effect of obesity because it would adjust for recent weight loss experienced by leaner but sick individuals. On the other hand, controlling for disease could decrease the observed effect of obesity because it captures intermediary physiological links (e.g., obesity leads to cardiovascular disease, which in turn leads to death).
Prior studies have used numerous methods to estimate the magnitude of confounding by illness, and findings are mixed. Some studies stratify the analysis by health status. The expectation is that those without a history of a major illness would exhibit higher effects of obesity on mortality compared with those with a preexisting condition. For example, when using the Cancer Prevention Study II, Calle et al. (1999) found a stronger effect of obesity (BMI ≥ 30.0) on mortality among those without a history of disease (including heart disease, cancer, stroke, and respiratory disease) compared with those with a history of disease, and among nonsmokers compared with former and current smokers. In contrast, Flegal et al. (2007b) reported that excluding those with preexisting illnesses (cancer and cardiovascular disease) and ever smokers had little effect on estimates of overweight- and obesity-attributable mortality using NHANES I–III. Another method is to stratify by time on study, which is guided by the notion that confounding by illness is stronger closer in time to study entry. In the NIH-AARP Cohort of adults aged 50–71 studied over 10 years, Adams et al. (2006) showed that the relative risks associated with obesity (BMI ≥ 30) were lower in the first five years of the study versus the final five years. However, other techniques related to time on study, such as excluding deaths occurring early in the study, have been reported to have little effect (Al Snih et al. 2007; Flegal et al. 2007b; Sempos et al. 1998). Given such discrepancies, we consider multiple methods in this study, offering a comparison of the results.
CONFOUNDING BY SOCIOECONOMIC STATUS (SES)
SES is also a potential confounder in the association between weight status and health, but its role has received less attention than the role of preexisting diseases. Even analyses restricted to specific occupational cohorts can be subject to residual confounding by SES (Lauderdale 2005). The most frequently used measure of SES in studies on obesity and mortality is education. Other dimensions of SES, such as income and wealth, have not been fully explored but are increasingly realized to have independent effects on health over and above education (Braveman et al. 2005). For example, income is known to be associated with a wide array of health endpoints (including mortality) net of education (Bond Huie et al. 2003; Braveman et al. 2005). Income differentials across BMI are well known and are particularly strong among women (Chang and Lauderdale 2005). Income can affect the ability to purchase healthy foods, which are more expensive than nutritionally poor and calorie-dense foods (Drewnowski and Specter 2004), and other resources (e.g., gym membership) associated with weight status.
Beyond education and income, wealth is independently associated with many health endpoints (Duncan et al. 2002; Pollack et al. 2007). Wealth is robust to short-term fluctuations in income and is an indicator of accumulated economic resources. Households with higher wealth are better able to afford high-quality medical care and to absorb the financial burden that arises from a new illness (Smith 1999). In middle-aged adults, accumulated wealth can differ markedly within any level of education or income (Krieger, Williams, and Moss 1997). Specific to weight status, households with higher wealth usually reside in advantaged communities that provide more favorable food and recreation environments (Chung and Myers 1999). These and other neighborhood-level advantages are shown to influence weight status (Chang 2006; Chang, Hillier, and Mehta 2009; Poortinga 2006). To our knowledge, no prior study has controlled for wealth in this context. The HRS contains detailed measurements of income and wealth, and we examine both these dimensions in addition to education.
SUMMARY
We examine relative and attributable mortality risks for higher weight status (BMI ≥ 25.0) in a nationally representative cohort of middle-aged Americans aged 50–61 in 1992 and followed through 2004. Given concerns that the effect of obesity on mortality has shifted, our results will provide a contemporary view on this relationship that is applicable to the aging baby boom cohorts. Obesity is thought to rival cigarette smoking as a major source of preventable mortality. We also estimate smoking-attributable mortality to compare with our obesity estimates. This comparison has not been done with a nationally representative data source. Contributions of our study also include evaluating the role of disease confounding using multiple methods and accounting for three major dimensions of SES, which could not be done with previous data.
METHODS
Data
The HRS is sponsored by the National Institute of Aging (Grant Number NIA U01AG009740) and is conducted by the University of Michigan. It is an ongoing longitudinal study of Americans aged 50 and older. This analysis is based on a nationally representative cohort born between 1931 and 1941. Respondents were first interviewed in 1992 at ages 50–61, and information on deaths is available through 2004. The HRS has two methods of determining deaths—through linkage with the National Death Index (NDI) and through the interview of a surviving household member. There is a high degree of concordance between death linkage and survivor reporting (approximately 99%, from authors’ tabulations). The results presented here are based on NDI linkages, but no difference resulted with models based on survivor reports. Observed mortality in the 1931–1941 HRS cohort is nearly identical to mortality reported in U.S. vital statistics (Health and Retirement Study 2007b).
The 1931–1941 HRS birth cohort has a total sample size of 9,749. The sample used in our analysis excludes data collected by proxy household members because of concerns of biased reporting of height and weight by proxies, and further excludes observations with imputed values for either height or weight. This resulted in a loss of 471 respondents, yielding an analytic sample of 9,278 (5,057 females and 4,221 males). Preliminary analysis indicated that the proxy sample and observations excluded because of height or weight imputations had similar baseline characteristics and mortality compared with the analytic sample even though the proxy sample was predominantly male (nearly 80%). Sensitivity regressions show nearly identical results in models that included the entire sample compared with the results presented here. Approximately 14% (n = 1,301) of the sample had died by the end of 2004. Analyses are conducted with the RAND HRS data, version F (RAND 2006), which we link to mortality data from the HRS tracker file, version 2.0 (Health and Retirement Study 2007a). Detailed data on cigarette smoking and imputation flags are added from the 1992 Core HRS file (Health and Retirement Study 2004).
Weight Status
We categorize BMI based on guidelines set forth by the NIH (National Heart, Lung, and Blood Institute 1998) and the World Health Organization (2000). The categories used here are underweight (BMI < 18.5), normal (BMI = 18.5–24.9), overweight (BMI = 25.0–29.9), class I obese (30.0–34.9), and class II/III obese (BMI ≥ 35.0).
The HRS relies on self-reports of height and weight. There is debate over the validity of self-reported data, but several studies have found self-reports to be a valid proxy for clinically measured values (Jeffery 1996; Spencer et al. 2002; Weaver et al. 1996). Nevertheless, in addition to our HRS analysis, we assess potential self-reporting bias with the aid of NHANES III (1988–1994), which contains data on both measured and self-reported height and weight. Here, we compare mortality estimates by using measured versus self-reported weight status on the same set of respondents. We restrict the NHANES III analysis to the same age range used in the HRS (ages 50–61). NHANES III collected baseline data between 1988 and 1994, and we model mortality through 2000, which is the latest year for which mortality information was released.
Other Predictors
We adjust for three sets of confounders: sociodemographic, SES, and behavioral. Sociodemographic confounders include race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other) and marital status (married, never married, divorced/separated, and widowed). We account for sex through stratification, and age is used as the x-axis variable in Cox proportional hazard models (analyses discussed below).
SES is measured by education, household income, and household wealth. Education is measured categorically: (1) no high school diploma, (2) high school diploma/GED, (3) some college, and (4) college degree. Alternative specifications based on years of schooling produced no substantive differences in results. The HRS contains detailed measurements of both income and wealth with relatively few imputed cases (Moon and Juster 1995; Smith 1995). Household income measures the income of both the respondent and spouse. Household wealth measures total household assets after accounting for debts, including the value of real estate, savings, retirement accounts, and investments. Based on published literature using the HRS, we measure both income and wealth continuously (Bond Huie et al. 2003). We logarithmically transform both income and wealth to produce more normal distributions. Given that wealth is negative in some respondents, we add a constant term to all values before taking the logarithm.
Behavioral confounders include cigarette smoking and physical activity. We construct a five-category smoking variable to capture incremental increases in the effect of smoking (Rogers et al. 2005): (1) never smoker, (2) former smoker, (3) current light smoker (<1 pack per day), (4) current moderate smoker (1 to <2 packs per day), and (5) current heavy smoker (≥2 packs per day). Although physical activity could be on the pathway between obesity and death, physical activity could also lead to a spurious association because low levels of physical activity could increase the risk of both obesity and death. Physical activity is measured as a dummy variable indicating vigorous physical activity (three or more times per week).
Previous studies in this area have invariably controlled for biological markers. We do not control for biological risk factors because they are likely on the causal pathway between weight status and mortality. For example, obesity may result in high blood pressure, which in turn increases the risk of cardiovascular mortality.
Analytical Approach
We first examine individual-level associations. We estimate the shape of the BMI and mortality relationship by using a lowess regression described by Gronniger (2006) with the specification , where i subscripts an individual, and βjXj is a vector of predictor variables and their estimated coefficients. The model treats BMI continuously and nonparametrically, thereby allowing the effect of BMI on mortality to differ across the BMI spectrum.
Next, relative mortality risks are estimated as hazard ratios obtained from multivariate Cox proportional hazard regressions modeling age at death (Korn, Graubard, and Midthune 1997). Hazard ratios are calculated with respect to both normal BMI and the BMI region associated with minimum mortality as identified in the lowess regression. The Cox model has the form , where i subscripts an individual, and a indexes age measured in days. The baseline hazard function is h(a). βjXj are estimated coefficients and predictor variables, including dummy variables for weight-status categories. The risk set begins at age 50.0. Respondents alive on December 31, 2004, are censored at their age on this date. Proportionality assumptions were confirmed by testing the slope of Schoenfeld residuals. We checked interactions of weight status with all other covariates but did not detect significant interactions. Robust standard errors are calculated to account for clustering at the household level because in some instances, multiple members of a single household were interviewed.
We use the hazard ratios from the Cox proportional models to estimate macro-level effects. We calculate the population-attributable risk fraction (PAF), which indicates the fraction of deaths that would be avoided in a counterfactual situation where an exposure is eliminated from a population. The following formula for PAF is employed (for a discussion, see Rockhill et al. 1998):
PAFk is the PAF for the kth exposure category, and pdk is the fraction of total deaths that are exposed to the kth category. HRk is the hazard ratio of the kth category. Hazard ratios from Cox models can be used to calculate PAF (Allison et al. 1999; Benichou 2001; Flegal et al. 2005; Natarajan, Lipsitz, and Rimm 2007). The PAF is additive across discrete categories of a risk factor.2 We calculate the PAF for current and former smoking by adding the PAFs across individual smoking categories. We use the jackknife method (Lehnert-Batar, Pfahlberg, and Gefeller 2006) to derive 95% confidence intervals (CI). To calculate excess deaths, the PAF is multiplied with the total number of deaths in the United States occurring in the 1931–1941 birth cohort in 1999 (140,808 in females and 200,546 in males), which is the mean year of death in the sample.
RESULTS
Table 1 presents descriptive characteristics by sex. Approximately 58% of females and 70% of males fall into one of the three higher weight-status groups (overweight, obese I, or obese II/III). Females are more likely to be class II/III obese than males (8.2% vs. 4.5%), and both groups had a class I obesity prevalence of about 16%. Approximately 49% of males and 34% of females are overweight. Both sexes had a mean age of less than 56 years. Males have a slight education advantage and possess higher levels of household income and household wealth. Smoking status differed markedly by sex, with females more likely to have never smoked (45.2% versus 25.4%). Physical activity was comparable across the groups, with about 20% reporting vigorous physical activity at least three times per week. As expected, females (10.1%) experience lower mortality than males (16.8%).
Table 1.
Descriptive Characteristics by Sex, 1931–1941 HRS Cohort: 1992
| Characteristics | Females (N = 5,057) | Males (N = 4,221) |
|---|---|---|
| NIH/WHO BMI Categories | ||
| Underweight (<18.5) | 1.9 | 0.5 |
| Normal (18.5–24.9) | 40.0 | 29.4 |
| Overweight (25.0–29.9) | 33.8 | 49.3 |
| Class I obese (30–34.9) | 16.2 | 16.3 |
| Class II/III obese (≥35.0) | 8.2 | 4.5 |
| Mean Age, Years | 55.5 (2.9) | 55.6 (2.8) |
| Race/Ethnicity | ||
| White, non-Hispanic | 80.5 | 82.0 |
| Black, non-Hispanic | 10.8 | 9.4 |
| Hispanic | 6.5 | 6.3 |
| Other | 2.2 | 2.2 |
| Marital Status | ||
| Married | 71.4 | 81.1 |
| Never married | 3.2 | 4.3 |
| Divorced/separated | 15.8 | 12.6 |
| Widowed | 9.7 | 2.0 |
| Education | ||
| Less than a high school diploma | 23.9 | 21.6 |
| High school diploma/GED | 41.9 | 35.1 |
| Some college | 19.6 | 20.4 |
| College degree or higher | 14.6 | 23.0 |
| Mean Income, $1,000s | 44.3 (41.4) | 55.6 (54.1) |
| Mean Wealth, $1,000s | 224.6 (403.1) | 240.6 (439.7) |
| Smoking Status | ||
| Never smoker | 45.2 | 25.4 |
| Former smoker | 29.2 | 46.2 |
| Light smoker (<1 pack per day) | 10.1 | 7.7 |
| Moderate smoker (1 to <2 packs) | 13.1 | 15.0 |
| Heavy smoker (≥2 packs) | 2.4 | 5.6 |
| Vigorous Physical Activity (≥3 times per week) | 19.4 | 20.6 |
| % Deaths (number) | 10.1 (559) | 16.8 (742) |
Notes: Numbers are percentages unless otherwise noted. Standard deviations for continuous variables are in parentheses. Percentages, means, and standard deviations are weighted. Sample size (N) and number of deaths are not weighted.
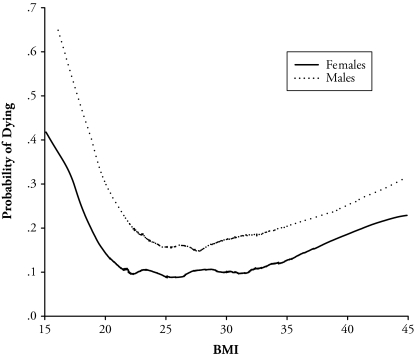
Shape of the BMI-Mortality Association
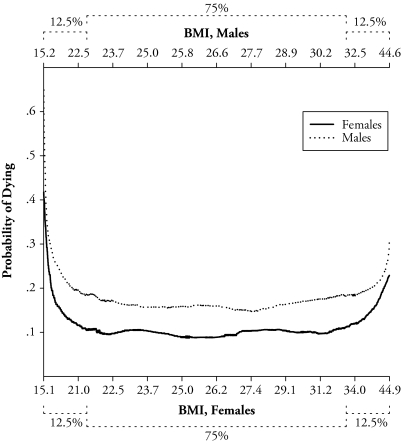
Figures 1 and 2 show the shape of the BMI and mortality association by sex based on the lowess regression. Figure 1 displays the probability of dying on the y-axis and BMI on the x-axis. Among females, the minimum probability of dying falls at a BMI of about 25.0 to 26.0. The minimum BMI value is slightly higher for males at about 27.0. Figure 2 shows the same model as Figure 1 but uses a nonstandard graph. Figure 2 graphs the probability of dying versus percentiles of the BMI distribution. The x-axis in Figure 2 is proportional to the BMI distribution rather than to actual BMI values. Formulating the graph in this way allows us to highlight the fact that the majority of females and males (roughly 75%) lies in a BMI region where the association is relatively flat (between a BMI of about 22.0 and 31.0). Accordingly, less than 13% of the samples are in a region where excess weight confers excess mortality (the right side of the figure). Given that minimum mortality for both females and males falls within the overweight range, we use this region as an alternative reference category to normal weight status in the Cox proportional hazard models.
Figure 1.
Probability of Dying Versus BMI by Sex, 1931–1941 HRS Cohort: 1992–2004
Notes: Semiparametric lowess model described by Gronniger (2006). Bandwidth = 0.30. Models adjust for sociodemographic characteristics (sex, race/ethnicity, and marital status), socioeconomic status (education, income, and wealth), and associated behaviors (smoking and physical activity).
Figure 2.
Probability of Dying Versus Percentiles of the BMI Distribution by Sex, 1931–1941 HRS Cohort: 1992–2004
Notes: Adapted from Figure 1, except with x-axis proportional to the BMI distribution of the samples. Dotted brackets indicate percentage of the sample.
Cox Proportional Hazard Models
Table 2 presents results for the Cox proportional hazard models by sex, with both normal (18.5–24.9) and overweight (25.0–29.9) as alternative reference categories. The overall pattern of results presented in Table 2 shows that being underweight or being class II/III obese is significantly associated with excess mortality. Risks are flat in the middle part of the distribution, with nonsignificant differences among the normal, overweight, and class I obese categories after accounting for all covariates. As expected, the hazard ratios in Table 2 are higher when overweight is used as the reference category because this BMI region better captures minimum mortality, but the increases are generally modest.
Table 2.
Hazard Ratios Predicting Mortality From Any Cause by Sex, 1931–1941 HRS Cohort: 1992–2004
| BMI Categories | Females (N = 5,057) |
Males (N = 4,221) |
||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 (+ SES) | Model 3 (+ behaviors) | Model 1 | Model 2 (+SES) | Model 3 (+ behaviors) | |
| Reference: Normal | ||||||
| Underweight | 3.57*** (2.34, 5.43) | 3.11*** (2.04, 4.74) | 2.64*** (1.70, 4.10) | 3.75*** (1.84, 7.64) | 3.50*** (1.76, 6.97) | 2.85** (1.44, 5.61) |
| Overweight | 0.94 (0.74, 1.18) | 0.85 (0.67, 1.08) | 0.84 (0.66, 1.07) | 0.82* (0.68, 0.98) | 0.83* (0.69, 1.00) | 0.90 (0.74, 1.08) |
| Class I obese | 0.90 (0.67, 1.20) | 0.80 (0.60, 1.06) | 0.83 (0.62, 1.10) | 0.92 (0.73, 1.16) | 0.89 (0.71, 1.13) | 1.01 (0.79, 1.28) |
| Class II/III obese | 1.68*** (1.26, 2.25) | 1.32 (0.97, 1.78) | 1.40* (1.02, 1.91) | 1.45* (1.04, 2.02) | 1.34 (0.95, 1.88) | 1.62** (1.15, 2.27) |
| Reference: Overweight | ||||||
| Underweight | 3.81*** (2.49, 5.83) | 3.67*** (2.40, 5.61) | 3.14*** (2.03, 4.86) | 4.60*** (2.26, 9.38) | 4.24*** (2.13, 8.45) | 3.18*** (1.61, 6.28) |
| Normal | 1.07 (0.85, 1.35) | 1.18 (0.93, 1.50) | 1.19 (0.93, 1.51) | 1.23* (1.02, 1.48) | 1.21* (1.00, 1.46) | 1.12 (0.93, 1.35) |
| Class I obese | 0.96 (0.72, 1.29) | 0.94 (0.71, 1.25) | 0.98 (0.73, 1.31) | 1.13 (0.91, 1.41) | 1.08 (0.87, 1.35) | 1.12 (0.90, 1.41) |
| Class II/III obese | 1.80*** (1.35, 2.41) | 1.55** (1.16, 2.08) | 1.66** (1.23, 2.24) | 1.78*** (1.28, 2.47) | 1.62** (1.16, 2.26) | 1.80*** (1.29, 2.51) |
Notes: Numbers in parentheses are 95% confidence intervals. Model 1 adjusts for sociodemographic characteristics (race/ethnicity and marital status). Model 2 adds SES (education, income, and wealth). Model 3 adds associated behaviors (smoking status and physical activity). The data are weighted.
p < .05;
p < .01;
p < .001
Model 1 adjusts only for sociodemographic characteristics (race/ethnicity and marital status). With respect to normal weight status (top of Table 2), class II/III obesity is associated with an excess risk of 68% (p < .001) in females and 45% (p < .05) in males. Model 2 further adjusts for SES (education, income, and wealth). Given the negative association between SES and weight status, the hazard ratios of the three higher weight-status categories show the expected decrease between Model 1 and Model 2. After we control for SES, class II/III obesity loses statistical significance relative to normal weight in both females and males, although the hazard ratios still indicate an excess risk of about 30% in both sexes. With respect to being overweight (bottom of Table 2), class II/III obesity is associated with a 55% (p < .01) increase in risk among females and a 62% (p < .01) increase among males in Model 2. Income and wealth had a modest effect on the hazard ratio of class II/III obesity (BMI ≥ 35.0) after education and sociodemographic characteristics are accounted for (an approximate 5% and 7% reduction in females and males, respectively; not shown in table). In contrast to the effect of SES, inclusion of behaviors (smoking and physical activity) in Model 3 results in an increase in the hazard ratios of each of the higher weight-status categories. Most of this change can be attributed to the inclusion of smoking status (i.e., smokers die at a high rate and tend to be leaner). In fact, physical activity alone had a negligible effect on the hazard ratios of the three higher weight-status categories (not shown in table).
In sum, the Cox hazard models presented in Table 2 suggest that excess mortality risk is limited to the tails of the BMI distribution. In the fully adjusted Model 3, females in the highest obesity category (class II/III) have a 40% (p < .05) higher risk of dying relative to those in the normal category; for males, the risk is about 62% (p < .01). If we compare class II/III obesity with overweight, excess risks are 66% (p < .001) for females and 80% (p < .01) for males, after accounting for all confounders.
Preexisting Illnesses
A critique of the approach used in Table 2 is that we ignore confounding by illness, perhaps resulting in underestimated effects of obesity (Manson et al. 1987; Willett et al. 2005). Another possibility is that illness positively associates with weight status through, for example, limiting physical activity. In Table 3, we implement a series of models attempting to account for illness confounding. We combine both sexes for clarity because sex-stratified models did not lead to different conclusions. The first model in Table 3 (Model 4) adjusts for all sociodemographic, SES, and behavioral characteristics as in our previous fully adjusted models. A straightforward method to account for confounding by illness is to control for health status. Model 5 controls for disease presence by including dummy variables for history of a major illness (heart disease, stroke, respiratory illness, and cancer) and favorable self-rated health (excellent/very good/good versus fair/poor). Self-rated health is included because it is a global measure of health status that may accurately measure underlying or undiagnosed disease processes (Idler and Benyamini 1997). As expected, adjustment for health status decreases the hazard ratio for underweight because it accounts for the disproportionate number of ill respondents in this category, yet the effect remains statistically significant (2.07; p < .001). Furthermore, the effect of class II/III obesity also decreased (from 1.53 to 1.29 in Model 5). Smaller decreases are observed in the overweight and class I obese categories as well. Hence, adjustment for health status does not produce an increased effect of obesity.
Table 3.
Hazard Ratios Predicting Mortality From Any Cause Accounting for the Role of Pre existing Illnesses, 1931–1941 HRS Cohort: 1992–2004
| Characteristics | Model 4 | Model 5 | Model 6 Limited to No History of Major Illness | Model 7 Limited to Excellent/Very Good/Good Self-Rated Health |
|---|---|---|---|---|
| BMI Categories (ref. = normal) | ||||
| Underweight | 2.74*** (1.92, 3.93) | 2.07*** (1.42, 3.02) | 2.36** (1.36, 4.09) | 1.60 (0.83, 3.10) |
| Overweight | 0.87 (0.75, 1.01) | 0.86* (0.74, 1.00) | 0.88 (0.73, 1.07) | 0.84 (0.69, 1.03) |
| Class I obese | 0.93 (0.77, 1.11) | 0.84 (0.70, 1.01) | 1.03 (0.82, 1.31) | 1.10 (0.86, 1.40) |
| Class II/III obese | 1.53*** (1.22, 1.91) | 1.29* (1.03, 1.61) | 1.67*** (1.24, 2.26) | 1.52* (1.08, 2.12) |
| History of Major Illnessa | — | 2.19*** (1.92, 2.49) | — | — |
| Self-Rated Healthb (excellent/very good/good) | — | 0.48*** (0.41, 0.56) | — | — |
| Sample Size | 9,278 | 9,278 | 7,432 | 7,192 |
| Number of Deaths | 1,301 | 1,301 | 739 | 690 |
Notes: Numbers in parentheses are 95% confidence intervals. Data are for both sexes combined. All models adjust for sociodemographic characteristics (sex, race/ethnicity, and marital status), socioeconomic status (education, income, and wealth), and associated behaviors (smoking and physical activity). The data are weighted except for sample size and number of deaths.
Self-reported history of ever having heart disease, stroke, respiratory illness, or cancer.
Coefficient is with reference to fair or poor self-rated health.
p < .05;
p < .01;
p < .001
Another strategy is to condition the sample on healthy respondents (Models 6 and 7). Model 6 limits the sample to respondents who report no history of major illness, and Model 7 is limited to those with favorable self-rated health. Although some evidence suggests that the effect of obesity is higher in those reporting no major illness (Model 6), as in our earlier estimates, only class II/III obesity is significant (1.67; p < .001), and there is only a modest increase compared with Model 4 (1.53; p < .001) in this category. Limiting the sample to favorable self-rated health (Model 7) also results in a similar pattern compared with Model 4, which uses the entire sample. In addition, restricting the sample to never smokers also resulted in a pattern whereby class II/III obesity is the only higher weight-status category significantly associated with excess mortality relative to normal weight status (results not shown)
As a final sensitivity analysis, we interact weight status with time in the study (Table 4). Previous studies have stratified the follow-up period by two or more periods, often using cut points between two and five years. There has been no theoretical justification as to the appropriateness of using these specific cut points. Here, we measure time continuously and are thus able to assess an overall trend of any interaction. In Model 8 (Table 4), we use time as the x-axis variable as opposed to age, as we did in prior models (because we are interested in capturing interactions with time in the study). Age is included as a covariate. We also collapse the overweight and class I obese groups into one aggregate category to reduce the number of interaction terms increasing the power of the model. Model 8 indicates no significant interactions between higher weight status and time, suggesting that effects do not differ in the early (vs. later) parts of the study.
Table 4.
Interaction of Weight Status With Time in the Study, 1931–1941 HRS Cohort: 1992–2004
| Characteristics | Model 8 |
|---|---|
| Main Effects (ref. = normal) | |
| Underweight | 3.56*** (2.22, 5.72) |
| Overweight/class I obese | 0.93 (0.69, 1.25) |
| Class II/III obese | 1.68* (1.10, 2.56) |
| Interaction Effects | |
| Underweight × Time | 0.85 (0.65, 1.12) |
| Overweight/class I obese × Time | 0.96 (0.82, 1.13) |
| Class II/III obese × Time | 0.93 (0.74, 1.16) |
| Sample Size | 9,278 |
| Number of Deaths | 1,301 |
Notes: Numbers in parentheses are 95% confidence intervals. The data are for both sexes combined. The model adjusts for sociodemographic characteristics (age, sex, race/ethnicity, and marital status), socioeconomic status (education, income, and wealth), and associated behaviors (smoking and physical activity). Time is used as x-axis variable. The data are weighted except for sample size and number of deaths.
p < .05;
p < .01;
p < .001
In total, we do not find a systematic underestimation of the effect of obesity due to either observed or occult diseases. The effect of class II/III obesity (BMI ≥ 35.0) is larger in those without a history of a major illness compared with the entire sample, but the difference is modest. Moreover, there is no evidence that higher weight status (BMI ≥ 25.0) interacts with time, suggesting that any unmeasured acute illnesses affecting mortality in the short term do not confound longer-term associations.
Attributable Mortality
Table 5 presents estimates of attributable mortality and excess deaths for higher weight status and smoking. Attributable mortality is given as a percentage and reflects the percentage of deaths avoidable if the excess mortality of the risk factor were eliminated. Negative percentages indicate relative risks that are less than 1.0 and imply that mortality would increase if the risk category were eliminated. Excess deaths are calculated for 1999, which is the mean year of death. Values in Table 5 are based on hazard ratios from the fully adjusted Model 3 of Table 2. We use both normal and overweight as alternative reference categories for weight status. Class II/III obesity is the only weight-status category associated with significant and positive attributable mortality. With reference to normal weight status, class II/III obesity is responsible for approximately 3.8% (p < .05) and 2.5% (p < .05) of deaths among females and males, respectively. This translates into 5,416 (of 140,808) and 5,034 (of 200,546) excess deaths among females and males, respectively. With reference to the overweight category, the attributable mortality percentages are slightly higher and are 5.4% (p < .01) for females and 2.9% (p < .01) for males. In contrast, smoking-attributable mortality is substantial: 35% (p < .001) for females and 50% (p < .001) for males. This translates into approximately 50,000 and 100,000 excess deaths for females and males, respectively.
Table 5.
Attributable Mortality (%) and 1999 Excess Deaths in U.S. Target Population for BMI Categories and Smoking, 1931–1941 HRS Cohort: 1992–2004
| Females (total deaths, 1999 = 140,808) |
Males (total deaths, 1999 = 200,546) |
|||
|---|---|---|---|---|
| Attributable Mortality (%) | Number of Excess Deaths, 1999 | Attributable Mortality (%) | Number of Excess Deaths, 1999 | |
| Reference: Normal | ||||
| Overweight | −5.6 (−13.8, 2.5) | −7,933 (−19,392, 3,527) | −5.0 (−13.7, 3.7) | −10,023 (−27,486, 7,441) |
| Class I obese | −3.1 (−7.6, 1.5) | −4,308 (−10,739, 2,123) | 0.1 (−3.7, 4.0) | 224 (−7,475, 7,923) |
| Class II/III obese | 3.8* (0.1, 7.6) | 5,416* (180, 10,652) | 2.5* (0.5, 4.6) | 5,034* (939, 9,128) |
| Reference: Overweight | ||||
| Class I obese | −0.3 (−4.6, 4.0) | −431 (−6,430, 5,568) | 1.8 (−1.7, 5.3) | 3,584 (−3,430, 18,634) |
| Class II/III obese | 5.4** (1.9, 8.8) | 7,559** (2,669, 12,440) | 2.9** (0.9, 4.9) | 5,889** (1,898, 9,880) |
| Reference: Never Smoker | ||||
| Former smoker | 13.2*** (7.1, 19.3) | 18,583*** (9,990, 27,175) | 18.1*** (11.2, 25.0) | 36,286*** (22,518, 50,053) |
| Current light smoker | 6.7*** (3.2, 10.3) | 9,495** (4,459, 14,531) | 7.8*** (5.1, 10.5) | 15,582*** (10,179, 20,984) |
| Current moderate smoker | 11.5*** (7.2, 15.8) | 16,195*** (10,144, 22,246) | 18.2*** (14.1, 22.3) | 36,586*** (28,369, 44,802) |
| Current higher smoker | 3.9*** (1.6, 6.1) | 5,467*** (2,301, 8,634) | 5.8*** (3.4, 8.1) | 11,571*** (6,824, 16,317) |
| Cigarette smoking (total) | 35.3*** (26.7, 43.9) | 49,740*** (37,664, 61,817) | 49.9*** (39.2, 60.6) | 100,024*** (78,576, 121,471) |
Notes: Numbers in parentheses are 95% confidence intervals. The number of excess deaths is calculated in 1999, the mean year of death. The 1931–1941 birth cohort was aged 57–68 in 1999. The data reflect sampling weights.
p < .05;
p < .01;
p < .001
Self-Reporting Bias in NHANES III
Table 6 considers the potential bias of self-reported BMI in a similarly aged (50–61 years) sample from NHANES III (N = 1,943). We compare two models from an identical set of respondents: NHANES-M based on clinically measured BMI, and NHANES-SR based on self-reported BMI. Sex-stratified models do not result in different conclusions; hence, both sexes are combined for clarity.
Table 6.
Distribution of Weight Status and Hazard Ratios From Clinically Measured and Self-Reported Height/Weight in NHANES III, Ages 50–61: 1988–2000
| Sample | BMI Category |
||||
|---|---|---|---|---|---|
| Underweight | Normal | Overweight | Class I Obese | Class II/III Obese | |
| Percentage Distribution | |||||
| NHANES-M (N = 1,943) | 1.4 (0.7, 2.1) | 31.2 (28.4, 34.1) | 36.7 (33.7, 39.7) | 20.3 (17.7, 22.8) | 10.4 (8.6, 12.1) |
| NHANES-SR (N = 1,943) | 1.5 (0.7, 2.2) | 33.2 (30.4, 36.1) | 40.1 (37.1, 43.2) | 16.7 (14.4, 18.9) | 8.5 (6.9, 10.1) |
| Hazard Ratiosa | |||||
| NHANES-M | 1.52 (0.47, 4.94) | 1.00 | 1.47 (0.94, 2.30) | 1.27 (0.72, 2.24) | 2.31* (1.18, 4.52) |
| NHANES-SR | 1.55 (0.47, 5.14) | 1.00 | 1.11 (0.71, 1.73) | 1.33 (0.76, 2.32) | 2.31* (1.16, 4.61) |
Notes: Numbers in parentheses are 95% confidence intervals. NHANES-M uses clinically measured height and weight. NHANES-SR uses self-reported height and weight from an identical set of respondents. The data are weighted.
Multivariate Cox regression with age as x-axis adjusting for sex, race/ethnicity, education, marital status, and smoking status. Baseline measurements are taken from 1988–1994, with mortality follow-up through 2000.
p < .05;
p < .01;
p < .001
The first section of Table 6 shows the distribution of weight status. The prevalence of weight-status categories across the two models are highly consistent, with the 95% confidence intervals substantially overlapping for each weight-status category. As expected, NHANES-SR underestimates the prevalence of class I and class II/III obesity compared with NHANES-M, although the bias is not large. For example, the prevalence of class II/III obesity is 8.5% (95% CI = 6.9, 10.1) in NHANES-SR and 10.4% (95% CI = 8.6, 12.1) in NHANES-M. The last section of Table 6 presents adjusted hazard ratios from Cox regressions. Mortality is modeled through 2000. We adjust for sex, race/ethnicity, education, marital status, and smoking status—variables comparable between the HRS and NHANES. Similar to the HRS results presented above, the only higher weight-status category in NHANES-M that has a significant and positive association with mortality is class II/III obesity (hazard ratio = 2.31; p < .05). Furthermore, the hazard ratios of the obese categories are virtually the same in NHANES-M and NHANES-SR. Hence, reliance on self-reported BMI does not necessarily underestimate obesity’s effect on mortality when using an identical set of respondents, which is the most appropriate comparison group.
In supplementary analyses, we created a misclassification variable for respondents who were classified into the wrong weight category because of biased self-reporting. We then interacted this misclassification indicator with the “true” weight-status category in a series of Cox proportional hazard models. We found no significant interactions between misclassification and weight status, thus indicating that those who misclassified did not have significantly different mortality than respondents who reported accurately in each respective weight-status category. We also tested interactions with a multicategory variable by distinguishing underreporters from overreporters. Again, no significant interactions were found.
DISCUSSION
Mortality attributable to obesity continues to be a highly researched topic engendering much controversy. Concerns focus on the treatment of age and associated confounders, the period of data, and applicability to a representative U.S. population. We addressed each concern in a sample of middle-aged adults, a group that is experiencing improving mortality but also rising obesity. We found that about 75% of middle-aged Americans possess a BMI whose association with mortality is flat (roughly between a BMI of 22 and 31). In Cox proportional hazard models, we found that being overweight or being class I obese is not associated with a higher risk of dying compared with normal BMI. On the other hand, class II/III obesity does confer significantly higher mortality, ranging from about a 40% (females) to 62% (males) excess risk relative to normal BMI. With respect to macro-level effects, we found that class II/III obesity is associated with a small amount of attributable mortality: less than 4% in females and 3% in males with reference to normal BMI. Although obesity-attributable mortality is modest, smoking-attributable mortality is large: about 35% in females and 50% in males. Our findings are robust to confounding by detailed sociodemographic and behavioral characteristics as well as three dimensions of SES. Other contributions of this research include multiple checks for confounding by preexisting illness and an assessment of potential bias attributable to self-reporting.
Our results indicate a considerably smaller mortality effect of obesity compared with the findings of Allison et al. (1999) and Mokdad et al. (2004), whose estimates indicate that about 13%–15% of all deaths in 1991 and 2000 can be attributed to obesity among adults aged 18 and older. The age-dependent nature of the BMI and mortality association makes direct comparison with these findings difficult because neither study stratified by age. Our findings are consistent with those of Flegal et al. (2005), whose estimates suggest that approximately 5% of deaths among adults aged 25 and older in 2000 were attributable to obesity (BMI ≥ 30.0). Using clinically measured height and weight data from NHANES I, II, and III, Flegal et al. (2005) found significant excess mortality only in the obese II/III (BMI ≥ 35.0) relative to normal (BMI = 18.5–24.9), which is consistent with our estimates. Their estimates covered 1971–2000, and our estimates cover 1992–2004. We also use more finely detailed measurements of confounding variables. For example, we adjusted for five categories of incremental exposure to cigarette smoking, but Flegal et al. (2005) used only three (never, former, and current). We also included three dimensions of SES—education, income, and wealth—but Flegal et al. (2005) adjusted only for education (in sensitivity models).
Unlike most major studies, we use data from the 1990s and 2000s, providing a contemporary study of obesity and mortality. In another recent study conducted between 1994 and 2003, Kulminski et al. (2008) reported similar findings for a sample of adults aged 65 and older. Their study, based on the National Long Term Care Survey, found that being overweight (BMI = 25.0–29.9) or being obese I (BMI = 30.0–34.9) were not associated with excess mortality relative to a reference BMI of 22.0–24.9 in both sexes. We extended these findings to a middle-aged population in their 50s and 60s and generally found no statistically significant differences in mortality across the normal, overweight, and mildly obese levels. In fact, the minimum mortality region appears to be in the overweight range although the difference between the overweight and normal categories was not always statistically significant. Another recent study using adults aged 50–70 from the NIHAARP cohort (1995–2005) found no significant excess mortality in overweight groups (BMI = 25.0–29.9) relative to a BMI 23.5–24.9 among men, and a small excess relative risk among women (approximately 6% to 7% for a BMI of 28.0–29.9) after adjusting for sociodemographic and behavioral correlates and using self-reported BMI (Adams et al. 2006). The authors reported that being obese I (BMI = 30.0–34.9) was significantly associated with excess mortality of about 18% in women and 10% in men, which is in slight contrast to our finding of no significant effect for either sex, though the reference ranges used differed across the studies. Another difference is that the NIH-AARP cohort is a nonrepresentative sample based on a mailed questionnaire. In contrast, the data used here are from a probability-based, nationally representative survey with high response rates and weighting adjustments for nonresponse.
Flegal et al. (2005) suggested that the relative risk of obesity may have declined since the early 1970s, based on data from successive NHANES surveys. They found a smaller association of obesity and mortality in NHANES II (1976–1980) and NHANES III (1988–1994) compared with NHANES I (1971–1975). Gregg et al. (2005) provided indirect evidence supporting a declining risk of mortality among those with a BMI ≥ 25.0. They found that overweight and obese individuals in 1999–2000 had a lower prevalence of high total cholesterol, high blood pressure, and smoking compared with overweight and obese individuals in the 1960s and 1970s. These favorable trends occurred in all weight-status groups, but the reductions in high cholesterol and high blood pressure were generally higher among the overweight and obese (significant only for high cholesterol), suggesting a relative improvement in these risk factors over time. Reasons behind these changes are not fully understood. Heavier individuals could have disproportionately benefited from better medical management of risk factors for cardiovascular disease and other illnesses associated with excess weight. For example, the rapid dissemination of lipid-lowering drugs (e.g., statins) during the 1990s could play an important role (Carroll et al. 2005). Changes in social values and patterns of discrimination could also be informative. Increasing obesity levels over time may lead to improvements in the relative status of the overweight and mildly obese, who may face less social isolation and less employment and health care discrimination as their body type becomes more commonplace.
Although health-related risk factors among the overweight and mildly obese may be declining and the mortality effect may be weak, there is emerging evidence of a parallel increase in disability among the obese (Alley and Chang 2007). Moreover, there has been a disproportionate rise in diabetes in this group relative to leaner individuals (Gregg et al. 2005). Taken in tandem with our findings of a weak effect of obesity on mortality, these unfavorable trends may be a product of obese individuals living longer than in the past but also acquiring a number of comorbid and disabling conditions as they age (Alley and Chang 2007). Two recent studies examining mortality and disability simultaneously in older populations showed the stronger effect of obesity on disability compared with mortality (Al Snih et al. 2007; Reynolds et al. 2005), further highlighting these contrasting trends.
It is suggested that preexisting diseases confound the association between weight status and mortality (Manson et al. 2007; Willett et al. 2005). However, statistical techniques used to account for confounding raise other methodological problems. We restricted our analysis to respondents aged 50–61 at baseline to limit the problem of preexisting diseases (as well as related problems associated with compositional changes in body mass), but in a middle-aged population, we would still expect the prevalence of chronic illness to be relatively high. Therefore, we implement multiple methods to account for confounding by illness. Our results do not find supporting evidence that preexisting illnesses substantially confound the association between weight status and mortality. Models that alternatively controlled for health status, excluded those with unfavorable health status, or allowed risks to vary with time did not indicate that our estimates were substantially biased. Other recent papers have reached similar conclusions with respect to confounding by illness (Al Snih et al. 2007; Flegal et al. 2007b). Nonetheless, given the complex pathways among body weight, disease, and death, we cannot entirely rule out residual illness confounding. Furthermore, confounding by SES appears modest and most pronounced in the highest BMI category.
Selective survival should minimally bias our results because we excluded older adults. Selective survival may help explain the weak effects of obesity on mortality in elderly populations (Janssen and Mark 2007; Manson et al. 2007). In a cohort of middle-aged adults, however, a similar argument is unlikely to hold: in a low-mortality population, relatively few deaths occur before the fifth and sixth decades of life. Approximately 17% of the 1931–1941 U.S. birth cohort examined in this study died by age 50. For selective survival to be important, most early deaths would have to be to individuals who possess excess weight. Yet in the United States, deaths at younger ages are more likely attributable to accidents or cancer (Jemal et al. 2005). Accidents are a cause of death not likely to be associated with weight status, and mortality from many types of cancers (particularly at younger ages) are shown to be weakly related to excess weight (Flegal et al. 2007a; Krueger et al. 2004).
This study has limitations. First, we used BMI as a proxy for adiposity when other anthropometric measures are perhaps more suitable (e.g., waist circumferences, waist-to-hip ratio; Kalmijn et al. 1999; Price et al. 2006; Seidell and Visscher 2000; Visscher et al. 2001). Most nationally representative data use BMI as a measure of weight status because of its ease of collection. By using BMI, we are able to compare our results with prior major studies. The usefulness of BMI versus other measures of weight status—such as waist circumference and the waist-to-hip ratio—is still not fully explored among middle- and older-aged adults (Price et al. 2006; Simpson et al. 2007). For example, Woo et al. (2002) found that in a sample of adults aged 70 and older, both BMI and waist circumference similarly predicted disease outcomes and mortality, but waist-to-hip ratio was not predictive of these events. In a sample of women aged 30 to 55, Manson et al. (1995) found that the waist-to-hip ratio was more weakly related to overall mortality compared with BMI over a 16-year period (though the waist-to-hip ratio was a strong predictor of heart disease mortality). We also measured BMI in middle adulthood, though it is likely that the deleterious health effects of excess body weight accumulate over a lifetime (Jeffreys et al. 2003). Given that requisite large-scale data containing both weight-status measurements early in life and mortality measurements later in life are not readily available, most research examining obesity and mortality have taken an approach similar to ours. Studies have found that childhood and adulthood BMI are moderately and positively correlated (Casey et al. 1992; Guo et al. 1994; Serdula et al. 1993), suggesting that many respondents in our study who were obese in 1992 were obese when they were younger (i.e., middle adulthood BMI proxies for BMI at younger ages). Moreover, a previous study reported similar patterns for weight status and mortality between recall measurements of early life BMI (age 21) and measurements taken at older ages (Corrada et al. 2006).
Finally, HRS data are nationally representative of the noninstitutionalized population, but deaths will come from both the community and institutions. Based on the 2000 census, only 1.1% of Americans aged 50–61 resided in institutional settings. It is likely that some respondents entered institutions after they were interviewed. Deaths of these individuals are still observed by mortality linkages used here. Therefore, we still capture a portion of deaths in institutions.
Despite concerns that increasing obesity levels will threaten future life expectancy improvements in the United States, our findings lend support for the notion of a weak effect of obesity on current mortality, as indicated by both its relative and attributable risks. An important contribution is that we focus on middle-aged adults, a group at high risk for cardiovascular and other chronic conditions that are thought to link obesity to death. A number of studies showed that excess body weight is not strongly associated with an increased risk of death in the elderly (Al Snih et al. 2007; Flegal et al. 2005; Grabowski and Ellis 2001; Reynolds et al. 2005). For obesity to have a large effect on life expectancy, it would have to be responsible for a substantial burden of premature deaths at middle and older ages, when the majority of deaths in the United States occur. Yet, we found that only a BMI ≥ 35.0 confers excess mortality, which comprises a minority of individuals who possess higher weight status (BMI ≥ 25.0). Nevertheless, the excess mortality associated with having a BMI ≥ 35.0 is not insubstantial; it is approximately 40% to 60% higher than that associated with having normal BMI (18.5–24.9), roughly similar to the mortality difference between non-Hispanic blacks and non-Hispanic whites at middle age.
There are considerable differences in past estimates of obesity-related attributable mortality. Reasons for these differences are numerous and complex, partly reflecting sample composition, techniques accounting for age effects, and the period of data collection. This investigation examined a nationally representative sample of middle-aged adults based on data that were designed for birth-cohort analysis and that possessed high-quality information on deaths and sociodemographic indicators. In sum, we found that obesity is not a large source of attributable mortality among middle-aged adults and that some prior estimates of obesity-related mortality were overestimated. These findings challenge the viewpoint that obesity will stem the long-term secular decline in U.S. mortality.
Acknowledgments
The authors thank Samuel Preston and Irma Elo for their guidance and suggestions. We are also grateful to three anonymous reviewers for their helpful comments.
Footnotes
Dr. Mehta is currently a Robert Wood Johnson Foundation Health & Society Scholar at the University of Michigan. This research was completed when Dr. Mehta was supported by a T32 Predoctoral Institutional Training Grant from the National Institutes of Health (AG000177; PI: Dr. Beth Soldo, University of Pennsylvania). Dr. Chang was supported in part by Grant K12HD043459 from the National Institutes of Health.
Eric Oliver (2006) argued that political interests have taken precedent over scientific interests in debates surrounding obesity as a public health peril. Oliver contended that the excess death figures produced by Mokdad et al. (2004) “had all the trappings of official truth” because of the prestige of the Journal of the American Medical Association, where the article was published, as well as author affiliations with the Centers for Disease Control and Prevention (Oliver 2006:3).
The PAF is generally not additive across separate risk factors except where there are no individuals in cross-classified cells (e.g., no smokers who are also obese), or there are no interactions of the joint risk factors (Benichou 2001). Summing the individual PAFs across multiple risk factors can lead to a value greater than 1.0 (more than 100% of deaths are attributable to the multiple causes) because deaths in cross-classified cells would be double counted (or, a single death can be attributed to more than one cause).
REFERENCES
- Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. “Overweight, Obesity, and Mortality in a Large Prospective Cohort of Persons 50 to 71 Years Old”. New England Journal of Medicine. 2006;355:763–78. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- Ajani UA, Lotufo PA, Gaziano JM, Lee IM, Spelsberg A, Buring JE, Willett WC, Manson JE. “Body Mass Index and Mortality Among US Male Physicians”. Annals of Epidemiology. 2004;14:731–39. doi: 10.1016/j.annepidem.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Al Snih S, Ottenbacher KJ, Markides KS, Kuo YF, Eschbach K, Goodwin JS. “The Effect of Obesity on Disability vs. Mortality in Older Americans”. Archives of Internal Medicine. 2007;167:774–80. doi: 10.1001/archinte.167.8.774. [DOI] [PubMed] [Google Scholar]
- Alley DE, Chang VW. “The Changing Relationship of Obesity and Disability, 1988–2004”. Journal of the American Medical Association. 2007;298:2020–27. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. “Annual Deaths Attributable to Obesity in the United States”. Journal of the American Medical Association. 1999;282:1530–38. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- Benichou J. “A Review of Adjusted Estimators of Attributable Risk”. Statistical Methods in Medical Research. 2001;10:195–216. doi: 10.1177/096228020101000303. [DOI] [PubMed] [Google Scholar]
- Bond Huie SA, Krueger PM, Rogers RG, Hummer RA. “Wealth, Race, and Mortality”. Social Science Quarterly. 2003;84:667–84. [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. “Socioeconomic Status in Health Research: One Size Does Not Fit All”. Journal of the American Medical Association. 2005;294:2879–88. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr “Body-Mass Index and Mortality in a Prospective Cohort of U.S. Adults”. New England Journal of Medicine. 1999;341:1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- Carroll MD, Lacher DA, Sorlie PD, Cleeman JI, Gordon DJ, Wolz M, Grundy SM, Johnson CL. “Trends in Serum Lipids and Lipoproteins of Adults, 1960–2002”. Journal of the American Medical Association. 2005;294:1773–81. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]
- Casey V, Dwyer J, Coleman K, Valadian I. “Body Mass Index From Childhood to Middle Age: A 50-Year Follow-Up”. American Journal of Clinical Nutrition. 1992;56:14–18. doi: 10.1093/ajcn/56.1.14. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention “Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Economic Costs—United States, 1995–1999”. Morbidity and Mortality Weekly Report. 2002;51:300–303. [PubMed] [Google Scholar]
- Chang VW. “Racial Residential Segregation and Weight Status Among US Adults”. Social Science & Medicine. 2006;63:1289–303. doi: 10.1016/j.socscimed.2006.03.049. [DOI] [PubMed] [Google Scholar]
- Chang VW, Hillier AE, Mehta NK. “Neighborhood Racial Isolation, Disorder, and Obesity”. Social Forces. 2009;87:2063–92. doi: 10.1353/sof.0.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang VW, Lauderdale DS. “Income Disparities in Body Mass Index and Obesity in the United States, 1971–2002”. Archives of Internal Medicine. 2005;165:2122–28. doi: 10.1001/archinte.165.18.2122. [DOI] [PubMed] [Google Scholar]
- Chung C, Myers SL. “Do the Poor Pay More for Food? An Analysis of Grocery Store Availability and Food Price Disparities”. Journal of Consumer Affairs. 1999;33:276. [Google Scholar]
- Corrada MM, Kawas CH, Mozaffar F, Paganini-Hill A. “Association of Body Mass Index and Weight Change With All-Cause Mortality in the Elderly”. American Journal of Epidemiology. 2006;163:938–49. doi: 10.1093/aje/kwj114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin J. “A Heavyweight Battle Over CDC’s Obesity Forecasts”. Science. 2005;308:770–71. doi: 10.1126/science.308.5723.770. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Specter S. “Poverty and Obesity: the Role of Energy Density and Energy Costs”. American Journal of Clinical Nutrition. 2004;79:6–16. doi: 10.1093/ajcn/79.1.6. [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Daly MC, McDonough P, Williams DR. “Optimal Indicators of Socioeconomic Status for Health Research”. American Journal of Public Health. 2002;92:1151–57. doi: 10.2105/ajph.92.7.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA. “Impact of Overweight on the Risk of Developing Common Chronic Diseases During a 10-Year Period”. Archives of Internal Medicine. 2001;161:1581–86. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, Williamson DF. “Methods of Calculating Deaths Attributable to Obesity”. American Journal of Epidemiology. 2004;160:331–38. doi: 10.1093/aje/kwh222. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, Williamson DF, Gail MH. “Excess Deaths Associated With Underweight, Overweight, and Obesity”. Journal of the American Medical Association. 2005;293:1861–67. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, Williamson DF, Gail MH. “Cause-Specific Excess Deaths Associated With Underweight, Overweight, and Obesity”. Journal of the American Medical Association. 2007a;298:2028–37. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, Williamson DF, Gail MH. “Impact of Smoking and Preexisting Illness on Estimates of the Fractions of Deaths Associated With Underweight, Overweight, and Obesity in the US Population”. American Journal of Epidemiology. 2007b;166:975–82. doi: 10.1093/aje/kwm152. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. “How Useful Is Body Mass Index for Comparison of Body Fatness Across Age, Sex, and Ethnic Groups?”. American Journal of Epidemiology. 1996;143:228–39. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- Grabowski DC, Ellis JE. “High Body Mass Index Does Not Predict Mortality in Older People: Analysis of the Longitudinal Study of Aging”. Journal of the American Geriatric Society. 2001;49:968–79. doi: 10.1046/j.1532-5415.2001.49189.x. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, Narayan KMV, Williamson DF. “Secular Trends in Cardiovascular Disease Risk Factors According to Body Mass Index in US Adults”. Journal of the American Medical Association. 2005;293:1868–74. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- Gronniger JT. “A Semiparametric Analysis of the Relationship of Body Mass Index to Mortality”. American Journal of Public Health. 2006;96:173–78. doi: 10.2105/AJPH.2004.045823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Roche A, Chumlea W, Gardner J, Siervogel R. “The Predictive Value of Childhood Body Mass Index Values for Overweight at Age 35 y”. American Journal of Clinical Nutrition. 1994;59:810–19. doi: 10.1093/ajcn/59.4.810. [DOI] [PubMed] [Google Scholar]
- Health and Retirement Study 20041992 Core File, Version 20 Public Use Dataset Ann Arbor, MI: University of Michigan; [producer] with funding from the National Institute on Aging (grant number NIA U01AG009740). [Google Scholar]
- Health and Retirement Study 2007aSample Sizes and Response Rates (2002 and beyond)Available online at http://hrsonline.isr.umich.edu/intro/sho_uinfo.php?hfyle=sample_new_v3&xtyp=2
- Health and Retirement Study 2007b2004 Tracker File, Version 20 Public Use Dataset Ann Arbor, MI: University of Michigan; [producer] with funding from the National Institute on Aging (Grant Number NIA U01AG009740). [Google Scholar]
- Idler EL, Benyamini Y. “Self-Rated Health and Mortality: A Review of Twenty-Seven Community Studies”. Journal of Health & Social Behavior. 1997;38:21–37. [PubMed] [Google Scholar]
- Janssen I, Mark AE. “Elevated Body Mass Index and Mortality Risk in the Elderly”. Obesity Reviews. 2007;8:41–59. doi: 10.1111/j.1467-789X.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- Jeffery RW. “Bias in Reported Body Weight as a Function of Education, Occupation, Health and Weight Concern”. Addictive Behaviors. 1996;21:217–22. doi: 10.1016/0306-4603(95)00050-x. [DOI] [PubMed] [Google Scholar]
- Jeffreys M, McCarron P, Gunnell D, McEwen J, Smith GD. “Body Mass Index in Early and Mid-Adulthood, and Subsequent Mortality: A Historical Cohort Study”. International Journal of Obesity and Related Metabolic Disorders. 2003;27:1391–97. doi: 10.1038/sj.ijo.0802414. [DOI] [PubMed] [Google Scholar]
- Jemal A, Ward E, Hao Y, Thun M. “Trends in the Leading Causes of Death in the United States, 1970–2002”. Journal of the American Medical Association. 2005;294:1255–59. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- Kalmijn S, Curb JD, Rodriguez BL, Yano K, Abbott RD. “The Association of Body Weight and Anthropometry with Mortality in Elderly Men: The Honolulu Heart Program”. International Journal of Obesity and Related Metabolic Disorders. 1999;23:395–402. doi: 10.1038/sj.ijo.0800832. [DOI] [PubMed] [Google Scholar]
- Korn EL, Graubard BI, Midthune D. “Time-to-Event Analysis of Longitudinal Follow-up of a Survey: Choice of the Time-Scale”. American Journal of Epidemiology. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- Krieger N, Williams DR, Moss NE. “Measuring Social Class in US Public Health Research: Concepts, Methodologies, and Guidelines”. Annual Review of Public Health. 1997;18:341–78. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- Krueger PM, Rogers RG, Hummer RA, Boardman JD. “Body Mass, Smoking, and Overall and Cause-Specific Mortality Among Older U.S. Adults”. Research on Aging. 2004;26:82–107. [Google Scholar]
- Kulminski AM, Arbeev KG, Kulminskaya IV, Ukraintseva SV, Land K, Akushevich I, Yashin AI. “Body Mass Index and Nine-Year Mortality in Disabled and Nondisabled Older U.S. Individuals”. Journal of the American Geriatrics Society. 2008;56:105–10. doi: 10.1111/j.1532-5415.2007.01494.x. [DOI] [PubMed] [Google Scholar]
- Lauderdale DS. “Adiposity and Physical Activity as Predictors of Mortality”. New England Journal of Medicine. 2005;352:1381–84. [PubMed] [Google Scholar]
- Lehnert-Batar A, Pfahlberg A, Gefeller O. “Comparison of Confidence Intervals for Adjusted Attributable Risk Estimates Under Multinomial Sampling”. Biometrics. 2006;48:805–19. doi: 10.1002/bimj.200510215. [DOI] [PubMed] [Google Scholar]
- Levine B. “What Does the Population Attributable Fraction Mean?”. Preventing Chronic Disease. 2007;4(1):A14. [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Bassuk SS, Hu FB, Stampfer MJ, Colditz GA, Willett WC. “Estimating the Number of Deaths Due to Obesity: Can the Divergent Findings Be Reconciled?”. Journal of Women’s Health. 2007;16:168–76. doi: 10.1089/jwh.2006.0080. [DOI] [PubMed] [Google Scholar]
- Manson JE, Stampfer MJ, Hennekens CH, Willett WC. “Body Weight and Longevity. A Reassessment”. Journal of the American Medical Association. 1987;257:353–58. [PubMed] [Google Scholar]
- Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE. “Body Weight and Mortality Among Women”. New England Journal of Medicine. 1995;333:677–85. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- Marshall E. “Public Enemy Number One: Tobacco or Obesity?”. Science. 2004;304:804. doi: 10.1126/science.304.5672.804. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. “Actual Causes of Death in the United States, 2000”. Journal of the American Medical Association. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. “Correction: Actual Causes of Death in the United States, 2000”. Journal of the American Medical Association. 2005;293:293–94. doi: 10.1001/jama.293.3.293. [DOI] [PubMed] [Google Scholar]
- Moon M, Juster FT. “Economic Status Measures in the Health and Retirement Study”. Journal of Human Resources. 1995;30:S138–S157. [Google Scholar]
- Natarajan S, Lipsitz SR, Rimm E. “A Simple Method of Determining Confidence Intervals for Population Attributable Risk From Complex Surveys”. Statistics in Medicine. 2007;26:3229–39. doi: 10.1002/sim.2779. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics . Health, United States, 2006 With Chartbook on Trends in the Health of Americans. Hyattsville, MD: 2006. [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute . Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Washington, DC: U.S. Public Health Service; 1998. [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. “ Prevalence of Overweight and Obesity in the United States, 1999–2004”. Journal of the American Medical Association. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Oliver JE. Fat Politics: the Real Story Behind America’s Obesity Epidemic. New York: Oxford University Press; 2006. [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. “A Potential Decline in Life Expectancy in the United States in the 21st Century”. New England Journal of Medicine. 2005;352:1138–45. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- Parloff R. “Is Fat the Next Tobacco?”. Fortune. 2003;147:50–54. [PubMed] [Google Scholar]
- Pollack CE, Chideya S, Cubbin C, Williams B, Dekker M, Braveman P. “Should Health Studies Measure Wealth? A Systematic Review”. American Journal of Preventive Medicine. 2007;33:250–64. doi: 10.1016/j.amepre.2007.04.033. [DOI] [PubMed] [Google Scholar]
- Poortinga W. “Perceptions of the Environment, Physical Activity, and Obesity”. Social Science and Medicine. 2006;63:2835–46. doi: 10.1016/j.socscimed.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Price GM, Uauy R, Breeze E, Bulpitt CJ, Fletcher AE. “Weight, Shape, and Mortality Risk in Older Persons: Elevated Waist-Hip Ratio, Not High Body Mass Index, Is Associated With a Greater Risk of Death”. American Journal of Clinical Nutrition. 2006;84:449–60. doi: 10.1093/ajcn/84.1.449. [DOI] [PubMed] [Google Scholar]
- RAND . RAND HRS Data, Version F. Santa Monica, CA: RAND Center for the Study of Aging [producer] with funding from the National Institute on Aging and the Social Security Administration; 2006. [Google Scholar]
- Reynolds SL, Saito Y, Crimmins EM. “The Impact of Obesity on Active Life Expectancy in Older American Men and Women”. Gerontologist. 2005;45:438–44. doi: 10.1093/geront/45.4.438. [DOI] [PubMed] [Google Scholar]
- Rockhill B, Newman B, Weinberg C. “Use and Misuse of Population Attributable Fractions”. American Journal of Public Health. 1998;88:15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RG, Hummer RA, Krueger PM, Pampel FC. “Mortality Attributable to Cigarette Smoking in the United States”. Population & Development Review. 2005;31:259–92. doi: 10.1111/j.1728-4457.2005.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidell JC, Visscher TL. “Body Weight and Weight Change and Their Health Implications for the Elderly”. European Journal of Clinical Nutrition. 2000;54:S33–S39. doi: 10.1038/sj.ejcn.1601023. [DOI] [PubMed] [Google Scholar]
- Sempos CT, Durazo-Arvizu R, McGee DL, Cooper RS, Prewitt TE. “The Influence of Cigarette Smoking on the Association Between Body Weight and Mortality. The Framingham Heart Study Revisited”. Annals of Epidemiology. 1998;8:289–300. doi: 10.1016/s1047-2797(97)00233-0. [DOI] [PubMed] [Google Scholar]
- Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. “Do Obese Children Become Obese Adults? A Review of the Literature”. Preventive Medicine. 1993;22:167–77. doi: 10.1006/pmed.1993.1014. [DOI] [PubMed] [Google Scholar]
- Simpson JA, MacInnis RJ, Peeters A, Hopper JL, Giles GG, English DR. “A Comparison of Adiposity Measures As Predictors of All-Cause Mortality: The Melbourne Collaborative Cohort Study”. Obesity. 2007;15:994–1003. doi: 10.1038/oby.2007.622. [DOI] [PubMed] [Google Scholar]
- Smith JP. “Racial and Ethnic Differences in Wealth in the Health and Retirement Study”. Journal of Human Resources. 1995;30:S158–S183. [Google Scholar]
- Smith JP. “Healthy Bodies and Thick Wallets: The Dual Relation Between Health and Economic Status”. Journal of Economic Perspectives. 1999;13:145–66. [PMC free article] [PubMed] [Google Scholar]
- Spencer EA, Appleby PN, Davey GK, Key TJ. “Validity of Self-Reported Height and Weight in 4808 EPIC-Oxford Participants”. Public Health Nutrition. 2002;5:561–65. doi: 10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau 2005Interim State Projections of Population for Five-Year Age Groups and Selected Age Groups by Sex: July 1, 2004 to 2030, File 2Available online at http://www.census.gov/population/www/projections/projectionsagesex.html
- U.S. Department of Health and Human Services . The Surgeon General’s Call to Action to Prevent and Decrease Overweight and Obesity. Rockville, MD: U.S. Public Health Service, Office of the Surgeon General; 2001. [PubMed] [Google Scholar]
- Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC. “A Comparison of Body Mass Index, Waist-Hip Ratio and Waist Circumference as Predictors of All-Cause Mortality Among the Elderly: The Rotterdam Study”. International Journal of Obesity. 2001;25:1730–35. doi: 10.1038/sj.ijo.0801787. [DOI] [PubMed] [Google Scholar]
- Weaver TW, Kushi LH, McGovern PG, Potter JD, Rich SS, King RA, Whitbeck J, Greenstein J, Sellers TA. “Validation Study of Self-Reported Measures of Fat Distribution”. International Journal of Obesity and Related Metabolic Disorders. 1996;20:644–50. [PubMed] [Google Scholar]
- Willett WC, Hu FB, Colditz GA, Manson JE. “Underweight, Overweight, Obesity, and Excess Deaths”. Journal of the American Medical Association. 2005;294:551. doi: 10.1001/jama.294.5.551-a. [DOI] [PubMed] [Google Scholar]
- Woo J, Ho SC, Yu AL, Sham A. “Is Waist Circumference a Useful Measure in Predicting Health Outcomes in the Elderly?”. International Journal of Obesity and Related Metabolic Disorders. 2002;26:1349–55. doi: 10.1038/sj.ijo.0802080. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. Geneva: World Health Organization Technical Report Series; 2000. [PubMed] [Google Scholar]