Abstract
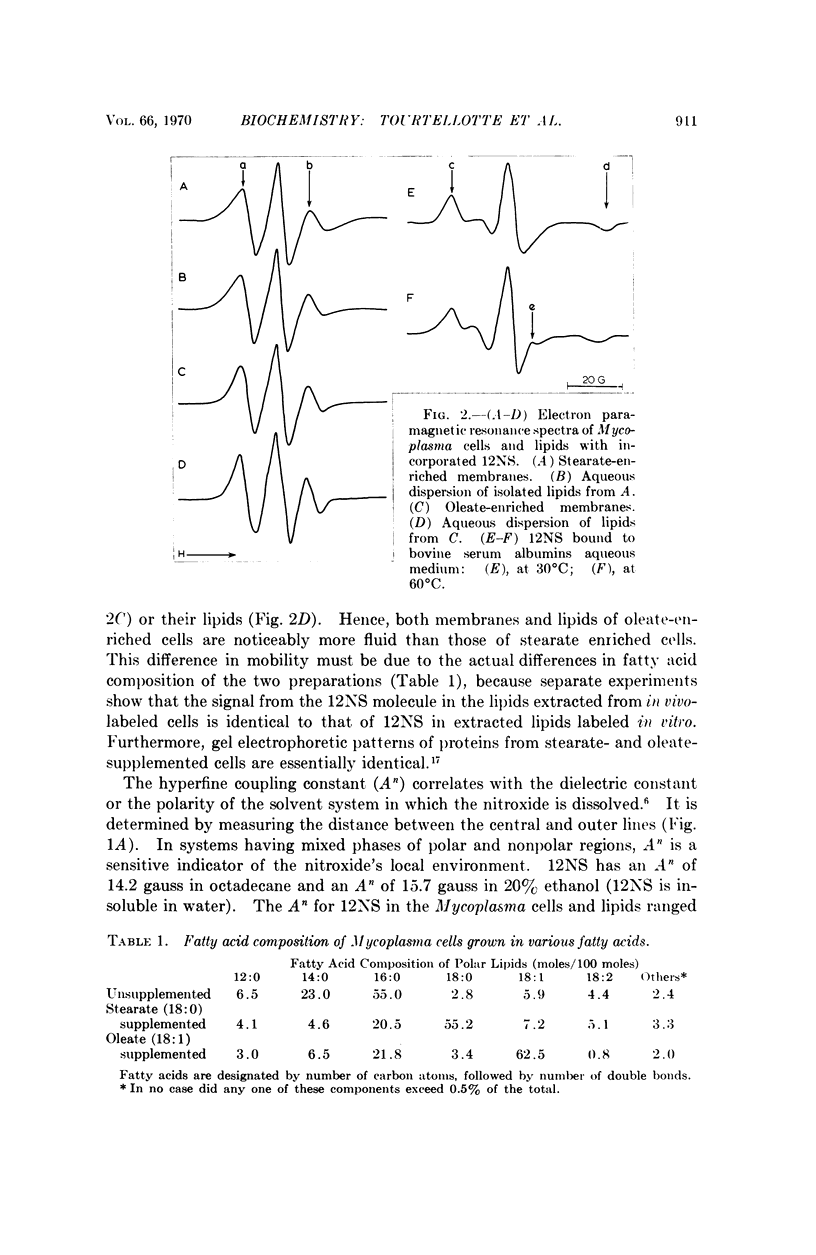
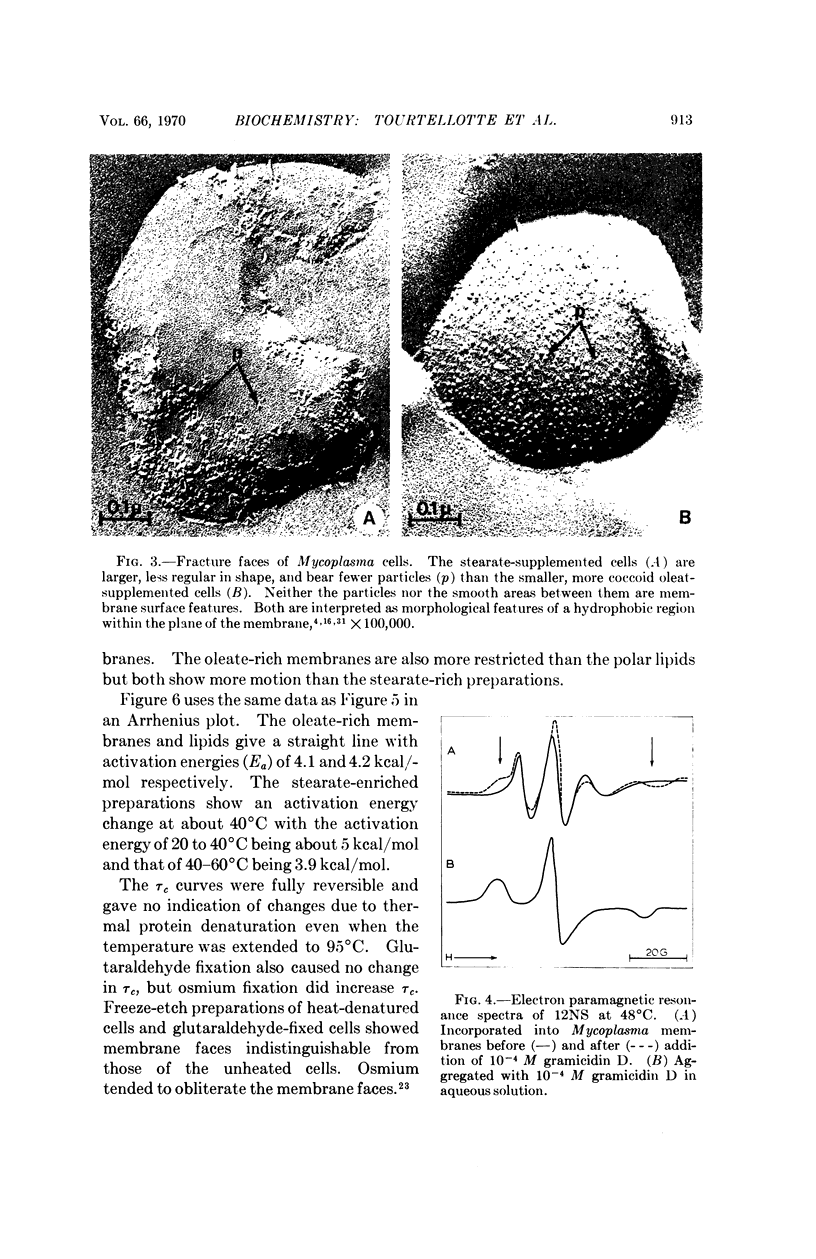
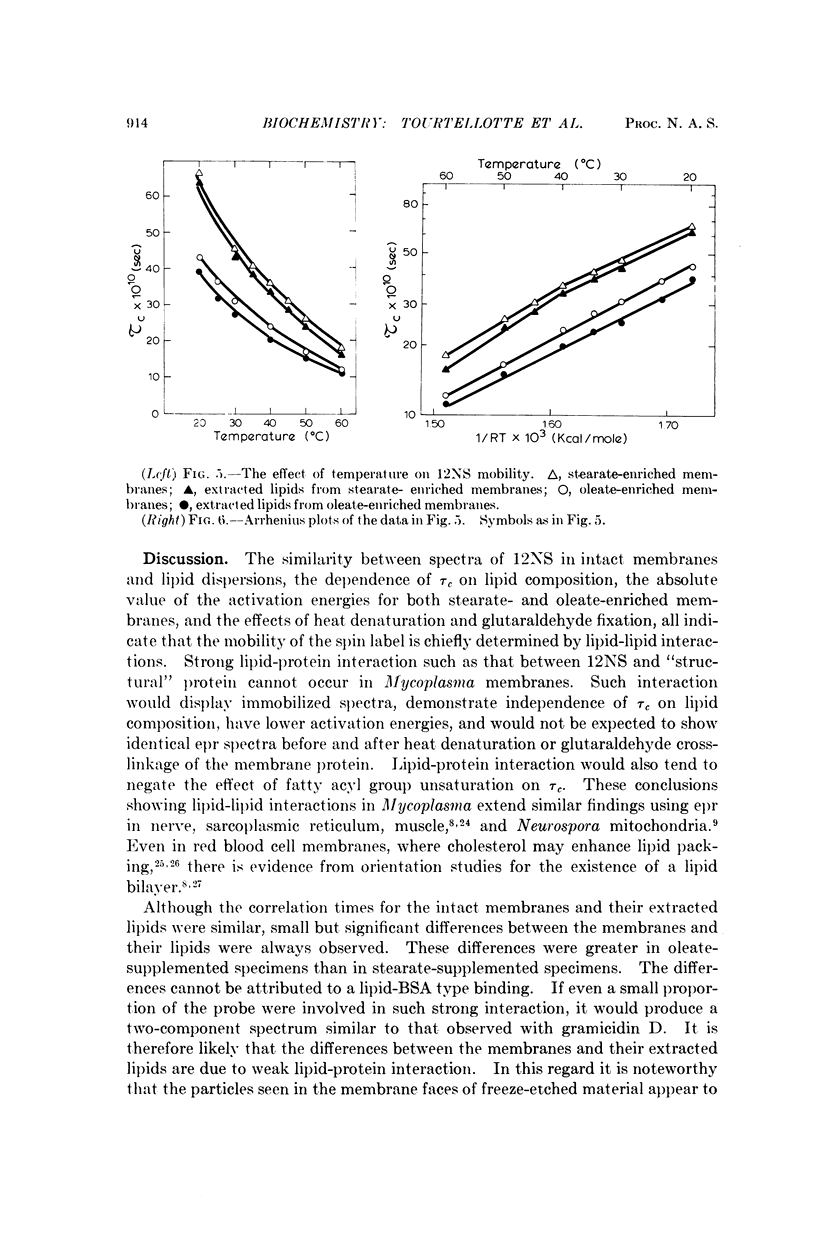
A spin-labeled fatty acid was incorporated in vivo into the polar lipids of Mycoplasma laidlawii membranes. The electron paramagnetic resonance signal from either intact cells or their extracted lipids reflected the fatty acid composition of the Mycoplasma membranes. Comparison of signals from intact cells, gramicidin-treated cells, heat-treated cells, and extracted lipids indicates that a major portion of the membrane lipids is in a semiviscous hydrocarbon environment. The results also show that the spin label in the intact membrane is slightly but significantly less mobile than it is in protein-free lipid extracts made from these membranes. Correlated electron microscope examinations using the freeze-etch technique reveal particulate components in the hydrophobic region of the membrane. The mobility of the lipids in the intact cell membrane may be influenced by their association with these particles.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branton D. Fracture faces of frozen membranes. Proc Natl Acad Sci U S A. 1966 May;55(5):1048–1056. doi: 10.1073/pnas.55.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deamer D. W., Branton D. Fracture planes in an ice-bilayer model membrane system. Science. 1967 Nov 3;158(3801):655–657. doi: 10.1126/science.158.3801.655. [DOI] [PubMed] [Google Scholar]
- Green D. E., Perdue J. F. Membranes as expressions of repeating units. Proc Natl Acad Sci U S A. 1966 May;55(5):1295–1302. doi: 10.1073/pnas.55.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Orientation and motion of amphiphilic spin labels in membranes. Proc Natl Acad Sci U S A. 1969 Sep;64(1):20–27. doi: 10.1073/pnas.64.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Spin-label studies of the excitable membranes of nerve and muscle. Proc Natl Acad Sci U S A. 1968 Sep;61(1):12–16. doi: 10.1073/pnas.61.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith A. D., Waggoner A. S., Griffith O. H. Spin-labeled mitochondrial lipids in Neurospora crassa. Proc Natl Acad Sci U S A. 1968 Nov;61(3):819–826. doi: 10.1073/pnas.61.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libertini L. J., Waggoner A. S., Jost P. C., Griffith O. H. Orientation of lipid spin labels in lecithin multilayers. Proc Natl Acad Sci U S A. 1969 Sep;64(1):13–19. doi: 10.1073/pnas.64.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney R. N., Tourtellotte M. E. Mycoplasma membrane lipids: variations in fatty acid composition. Science. 1969 Apr 25;164(3878):433–434. doi: 10.1126/science.164.3878.433. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Branton D. Membrane splitting in freeze-ethching. Covalently bound ferritin as a membrane marker. J Cell Biol. 1970 Jun;45(3):598–605. doi: 10.1083/jcb.45.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Cosenza B. J., Tourtellotte M. E. Variations in Mycoplasma morphology induced by long-chain fatty acids. J Gen Microbiol. 1966 Jan;42(1):139–145. doi: 10.1099/00221287-42-1-139. [DOI] [PubMed] [Google Scholar]
- Razin S. The cell membrane of mycoplasma. Ann N Y Acad Sci. 1967 Jul 28;143(1):115–129. doi: 10.1111/j.1749-6632.1967.tb27651.x. [DOI] [PubMed] [Google Scholar]
- Razin S., Tourtellotte M. E., McElhaney R. N., Pollack J. D. Influence of lipid components of Mycoplasma laidlawii membranes on osmotic fragility of cells. J Bacteriol. 1966 Feb;91(2):609–616. doi: 10.1128/jb.91.2.609-616.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steim J. M., Tourtellotte M. E., Reinert J. C., McElhaney R. N., Rader R. L. Calorimetric evidence for the liquid-crystalline state of lipids in a biomembrane. Proc Natl Acad Sci U S A. 1969 May;63(1):104–109. doi: 10.1073/pnas.63.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Engelman D. M. Current models for the structure of biological membranes. J Cell Biol. 1969 Sep;42(3):613–646. doi: 10.1083/jcb.42.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLMER E. N. Steroids and cell surfaces. Biol Rev Camb Philos Soc. 1961 Aug;36:368–398. doi: 10.1111/j.1469-185x.1961.tb01295.x. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S., Kingzett T. J., Rottschaefer S., Griffith O. H., Keith A. D. A spin-labeled lipid for probing biological membranes. Chem Phys Lipids. 1969 Sep;3(3):245–253. doi: 10.1016/0009-3084(69)90016-4. [DOI] [PubMed] [Google Scholar]




