Abstract
Robust data from a voter registry show that Costa Rican nonagenarians have an exceptionally high live expectancy. Mortality at age 90 in Costa Rica is at least 14% lower than an average of 13 high-income countries. This advantage increases with age by 1% per year. Males have an additional 12% advantage. Age-90 life expectancy for males is 4.4 years, one-half year more than any other country in the world. These estimates do not use problematic data on reported ages, but ages are computed from birth dates in the Costa Rican birth-registration ledgers. Census data confirm the exceptionally high survival of elderly Costa Ricans, especially males. Comparisons with the United States and Sweden show that the Costa Rican advantage comes mostly from reduced incidence of cardiovascular diseases, coupled with a low prevalence of obesity, as the only available explanatory risk factor. Costa Rican nonagenarians are survivors of cohorts that underwent extremely harsh health conditions when young, and their advantage might be just a heterogeneity in frailty effect that might disappear in more recent cohorts. The availability of reliable estimates for the oldest-old in low-income populations is extremely rare. These results may enlighten the debate over how harsh early-life health conditions affect older-age mortality.
Two key findings have emerged from recent studies of old-age mortality in humans (Vaupel et al. 1998): (1) mortality rates are declining substantially, and (2) the increase of death rates with age decelerates among the oldest-old. In the words of Vaupel et al. (1998), these findings are perplexing and hard to reconcile: according to evolutionary biology, there is no possible selection against mutations occurring after reproduction and nurturing have ceased. A possible explanation of the old-age deceleration is heterogeneity in frailty; that is, as the frail die at early ages, the old tend to be a select subpopulation of the fit-test (Barbi, Caselli, and Vallin 2003; Horiuchi and Wilmoth 1998; Vaupel et al. 1998). In turn, a possible explanation of the mortality decline at old ages is a cohort effect of past improvements in health conditions at early ages; that is, recent improvements in health status among the elderly would echo events that happened decades ago when cohorts were young. These two explanations are somehow contradictory: does high, early-life mortality make a cohort stronger by eliminating the frail, or does the cohort become weaker because of accumulated injuries? An important scientific debate is taking place in this regard (Barbi and Vaupel 2005; Finch and Crimmins 2004, 2005).
The heterogeneity in frailty argument has been mostly supported by mathematical and simulation models (Vaupel, Manton, and Stallard 1979); by indirect evidence from genetic homogeneous populations such as twins (Yashin and Iachine 1997); and by observations in other species, such as the Mediterranean fruit fly (Vaupel and Carey 1993). Indirect methods have been developed to determine the existence of heterogeneity from cohort mortality patterns (Manton, Stallard, and Vaupel 1981). Data showing low death rates at old ages in low-income populations that saw harsh health conditions at young ages might support the heterogeneity in frailty argument, given the prejudice that the poor cannot be healthy nor live longer. Coale and Kisker (1986) offered two possible explanations of the observed mortality crossover at old ages among some socioeconomically disadvantaged populations: selection in heterogeneity or bad data. They concluded that bad data was the probable cause of these crossovers given the positive association between mortality in childhood and at young adult ages, and mortality in old age that was observed in cohorts with good data. A social selection argument, which has been used to explain good health in low-income populations (especially immigrants), parallels the heterogeneity in frailty argument. A well-known example of this is the “Hispanic paradox” (i.e., that Hispanics have lower mortality than whites) in the United States, which some researchers explain by several types of selection biases (Khlat and Darmon 2003; Palloni and Morenoff 2001).
Genetic makeup as well as nutrition, well-being, access to health care, lifestyles, and environmental conditions (contemporary and past) are, of course, determinants of old-age mortality above and beyond selection effects. The relative importance of these factors is, however, unknown. In addition, determinants of mortality might act differently at old ages than at young ages, challenging conventional wisdom that extrapolates to old ages what has been observed for younger ages. For example, Okinawa displays exceptional longevity even though it is one of the least developed regions in Japan (Cockerham and Yamori 2001). Another challenging example is that of Hispanics in the United States, who have lower adult mortality than whites in spite of Hispanics’ lower socioeconomic status and limited access to health care (Elo et al. 2004). And there is also the case of exceptional longevity in Sardinia, Italy, where old-age life expectancy is higher than in the much richer northern region of the country (Caselli and Lipsi 2006). Are elderly Okinawans, U.S. Hispanics, and Sardinians really exceptions to the rule of a socioeconomic gradient in mortality? Might it be that at old ages, the rules of survival are different than at young ages? To what extent do poor health conditions early in life strengthen or weaken a cohort at old ages? If dietary caloric restriction slows aging in other species (Roth et al. 2002), could certain human populations that were undernourished when young have an advantage for survival at old age?
An obstacle to answering these questions is the absence of sufficiently accurate data about old-age mortality in low-income populations. Costa Rica may be an exception. Since 1961, the United Nations has graded the Costa Rican vital statistics system—which was established in 1883––as “complete” (United Nations 1961). The country also has a carefully kept population registry used for voting purposes. Costa Rica is one of 11 developing countries whose vital registration statistics in 1995 are characterized by Hill et al. as both complete (recording at least 90% of births and deaths) and accurate (producing mortality estimates similar to those based on census and survey data) (Hill et al. 1999).
With its 4.5 million inhabitants, Costa Rica is the second most-densely populated country in the Continental Americas. (El Salvador ranks first.) Located in the Central American Isthmus, Costa Rica somehow escaped the wars and turbulences of the region in the 1980s and has enjoyed political stability for many decades. In economic terms, Costa Rica does not differ from the Latin American average. According to the World Bank (2006), its per capita income is about $4,600 per year, compared with the $3,600 annual average for Latin America. In terms of equity in income distribution, social security coverage, access to public health services and sanitation, labor laws, and protection of the environment, Costa Rica ranks among the highest in the Americas. Costa Rica has both a mixed economy with open markets and government control of key areas, such as health, education, banking, energy, communications, and insurance (Mesa-Lago 2000). The Human Development Index of the United Nations ranks Costa Rica as 48th in the world and 4th in Latin America (after Chile, Argentina, and Uruguay).
The country has essentially completed its demographic transition (World Bank 2006). Its life expectancy is the second highest in the Americas (Canada is first), which is higher than in the United States. The total fertility rate of 2.00 in 2005 is lower than in the United States (2.04 births), and it is the second lowest in Latin America after Cuba. Costa Rica is also one of the few Latin American countries with a substantial stock of international immigrants. Ten percent of the total population is foreign-born, a figure not that different from the 12% foreign-born population of the United States (United Nations Population Division 2006). Because the demographic transition was so quick and recent, a population aging process has not yet occurred: only 5.6% of Costa Ricans are aged 65 and older, although this will change very quickly in the next few decades, surpassing 20% by the year 2050 (INEC and CCP 2002).
Official life tables for 1995–2000 (Rosero-Bixby, Brenes-Camacho, and Collado-Chaves 2004) suggest exceptionally high old-age longevity in Costa Rica. Comparing the age-80 life expectancy in those tables with 13 high-income countries in exactly the same period in a database kept at the Max Plank Institute (Kannisto et al. 1994),1 Costa Rican males are the leaders with 8.2 years, followed by Japan with 7.6 years, and Iceland with 7.4 years. Costa Rican females, with 9.0 years of age-80 life expectancy, are in the middle of this elite pack: for example, Japan has 10.0 years, and Iceland has 8.7 years.
Costa Rica is well known as a country with outstanding health indicators. For example, it was included as one of the four study cases in the Rockefeller Foundation report on “Good Health at Low Cost” (Halstead, Walsh, and Warren 1985). However, there is a huge difference between having good health indicators and being the world-champion in longevity, leaving clear grounds for skepticism. Past claims of exceptional longevity in communities in the Andes and in the Caucasus have not resisted scientific scrutiny (Garson 1991). Demographers know well that age exaggeration among the elderly in censuses leads to substantial inflation of old-age populations and, consequently, underestimated mortality rates (Coale and Kisker 1986; Preston, Elo, and Stewart 1999). With these antecedents, academic circles may disregard this Costa Rican claim as just another case of “bad data.” This article relies on new data of very high quality to validate the patterns observed in the life tables and to obtain a more refined estimate of late-life longevity.
DATA AND METHODS
In an attempt to avoid data errors that have hampered studies of mortality of the oldest-old in other populations (Garson 1991; Kannisto 1988), the estimates in this article do not use conventional data sources. In particular, this article avoids using information on reported age from censuses or vital statistics but instead uses the Costa Rican national population voter registry, from which a database was created to study 24,400 Costa Rican nonagenarians in 1983–2004. This database includes, essentially, extinct birth cohorts born in 1878–1903 and quasi-extinct cohorts born in 1904–1913.
The Supreme Electoral Tribunal (Tribunal Supremo de Elecciones) provided the voter registry, which includes databases of births, naturalizations, and deaths as well as the voting lists (the padrón) for the 1990, 1994, 1998, and 2002 elections. The computerized birth registry, which is supposed to include all ever-living Costa Ricans, includes individuals who contacted the civil registration system since its computerization in 1970. Individuals contacted the registry because of registration (or certification requests) of vital events such as births, deaths, or marriages as well as to obtain (or renew every 10 years) an identification card, or cédula. The databases of the registry are linked by the unique identification (ID) number that each Costa Rican is given as of birth registration or naturalization. Given that this ID number also appears on the cédula, it is known in Costa Rica as the cédula number. A “survival time” data set was created using STATA (Statacorp 2005) with information on sex as well as dates of birth, death, and likely place of residence in each election year for all Costa Rican nonagenarians ever living in 1983–2004; the entry date is January 1, 1983 or the 90th birthday date, and the exit date is the date of death or October 30, 2004. Note that ages (at death or at any time of observation) in this data set do not come from reports but rather from computations based on dates documented in the registry.
Data Quality
The quality of the database of nonagenarians is crucial for this article. Three potential biases may occur and need to be validated: (1) selection bias, if the registry does not include all individuals and if those excluded have differential mortality; (2) underregistration of deaths, which would result in an underestimation of death rates, as well as an overcount of individuals still alive, especially toward the end of the observation period; and (3) age-misreporting biases, which, in other studies, underestimate mortality as result of age exaggerations.
How complete is the Costa Rican registry? It is almost impossible that a Costa Rican adult lived in the country since 1970 without ever having his/her cédula and, thus, never appearing in the registry. The cédula is required everywhere for all kinds of transactions, public or private. Besides, no deceased can be buried (keep in mind that the great majority of the studied nonagenarians have died) without a death certificate issued by either the Civil Register offices or, in remote locations, by the Rural Guard. Thus, all the dead are in the database, and a selection bias by exclusion of individuals from the registry is unlikely. A cross section from the nonagenarian database revealed 5,900 people alive and aged 90 or older at the time of the 2000 census. The census count was 7,000, or about 20% more; the percentage is similar by sex. This discrepancy does not come from deficiency in the registry but from overcounting in the census that is likely due to age exaggeration, as it was reported in the evaluation of the 2000 census (INEC and CCP 2002). Moreover, a study of the 1984 census estimated about a 50% overcount of population aged 80 and older, which is also due to age exaggeration (MIDEPLAN, CELADE, and DGEC 1988).
The second potential bias, the inflation in the count of people alive if the registry failed to exclude some of the dead, is addressed by looking at cohorts that should be extinct. Cohorts born in 1880–1895 were indeed extinct by 2004 in the registry. The maximum age reached by any of the 24,400 nonagenarians was 109; three died at this age. If under-registration of deaths occurred, one would see individuals still alive at age 120 or so, which is not the case. Therefore, estimates for extinct cohorts are, by definition, free of error from death underregistration. In addition, if the analysis showed that mortality in nonextinct cohorts is not significantly different from mortality in extinct cohorts, it would suggest that there are not missing deaths in the two groups.
Regarding the third potential bias, as mentioned earlier, this article’s information about age (at death or at any time during survival) does not come from reports but is instead computed from the dates in birth and death certificates, avoiding the most problematic data error in studies of mortality of the oldest old: age exaggeration that translates to underestimated mortality. In addition, information within the ID number (cédula) that each Costa Rican receives at birth allows for a second check of possible birth-date errors. This number is given to each individual at birth registration (or naturalization) and includes the number of the ledger and page where the person is registered. Because the ledgers are uniquely and sequentially numbered since the beginning of the civil registration system in 1880, looking at the ID number can establish the year when each individual was actually registered. Those with timely registration—say, within a year of their stated birth date—cannot have their age exaggerated. A person who appears timely registered could make consistent the two years (birth and registration) only by moving ahead the birth year, never by moving it back (which would cause the problematic age-exaggeration error). In contrast, those who registered late (some of them as adults, including foreigners who are citizens by naturalization) may have reporting errors in their birth date that produce age exaggeration. For example, somebody born in 1920 and registered in 1960 can have a wrong birth year of, say, 1900, which will result in 20-year age exaggeration. The analysis in this article tests for significant mortality differences by registration timing.
About 1,100 individuals (or 5%) in the database are centenarians: that is, they were alive at their 100th birthday. Although this is a small figure, it is worth exploring its validity by checking the reliability criteria used by Kannisto (1988) in his article on centenarians. Two indicators of data reliability in centenarians are applicable to the data in this article (Kannisto 1988: table 1). First, deaths of those aged 105 and older as a percentage of deaths at ages 100 or older are expected to be less than 5% and to be lower for men than for women. In the Costa Rican database, this indicator is 5.2% for men and 5.9% for women after excluding late registration births. These figures are borderline acceptable and much better than in (for example) the United States (7% and 8% among whites, and 30% among nonwhites), Spain (10% and 11%), and Portugal (16%) (Kannisto 1988: table 1). Second, the probability of dying is expected to be higher at age 101 than at age 100, and the ratio between them (q100 / q101) should be below unity. In the Costa Rican database, this ratio was 0.94 for men and 0.79 for women, far lower than most populations in the Kannisto article. For example, the ratio is 1.19 and 0.99 in Japan, 1.16 and 1.31 in Spain, 1.02 and 0.97 for whites in the United States, and 1.25 and 1.38 for nonwhites in the United States (Kannisto 1988: table 1).
Table 1.
Selected Data on Costa Rican Nonagenarians, 1983–2004
| Total | Birth Cohort |
|||
|---|---|---|---|---|
| 1878–1893 | 1894–1903 | 1904–1913 | ||
| Number of Individuals | 24,438 | 2,150 | 7,692 | 14,596 |
| Number of Observed Years | ||||
| Total | 101,439 | 8,778 | 38,981 | 53,680 |
| In 1983–1993 | 33,409 | 8,611 | 24,798 | 0 |
| In 1994–2004 | 68,030 | 167 | 14,183 | 53,680 |
| Mean Number of Observed Years | 4.15 | 4.08 | 5.07 | 3.68 |
| Deceased (%) | 73 | 100 | 97 | 57 |
| Mean Age at Death | 93.8 | 96.1 | 94.4 | 92.9 |
| Mean Observed Age | 92.7 | 94.9 | 93.2 | 92.0 |
| Female Ratio | 1.28 | 1.20 | 1.24 | 1.33 |
| Late Birth Registry (%) | 17 | 36 | 17 | 13 |
| Central Region (%) | 71 | 76 | 71 | 69 |
Source: National Registry of the Tribunal Supremo de Elecciones.
Characteristics of the Nonagenarians in the Database
The database of nonagenarians rendered about 101,000 person-years for 24,400 individuals born from 1878 to 1913 (Table 1). More than two-thirds of the observation segments correspond to the 1994–2004 period. Almost all individuals born before 1904 are deceased; these individuals were dubbed “extinct cohorts.” Mean age at death is 93.8 years, ranging from 96.1 in the oldest cohorts to 92.9 in the youngest one. These figures and trends are, however, severely biased by censoring effects: left-censoring for oldest cohorts because observation starts in 1983, and right-censoring for the youngest because observation stops in 2004. They are not good indicators of life expectancy. From those born in 1904–1913, 43% were still alive at closing date in 2004. Each individual was observed a little more than four years on average. The mean observed age is about 93 years. The female to male ratio is 1.28, with an increasing trend in more recent cohorts, which indicates that the sex gap in mortality is widening. The proportion of late-registered births is 17% overall and substantially higher (36%) in the cohorts born before 1893. Almost three-fourths of the observations correspond to the Central region.
Estimates of Mortality and Life Expectancy
This article estimates the mortality rates of Costa Rican nonagenarians using the “extinct cohort” method, which was developed for European countries by Vincent (1951) and was used in the United States by (among others) Rosenwaike (1981). The denominators for the rates in this article based on microdata are exact counts of person-years lived in each age.2 Survival-time routines in the STATA software facilitated these computations (Statacorp 2005).
The observed age-sex rates are summarized and smoothed out using a three-parameter relational model of mortality adapted from the Coale’s model for marital fertility (Coale 1977). The death rate m at age x and sex d (dummy variable equal to 1 for males) is modeled as a function of an old-age standard mortality schedule V of high-income countries; I refer to this as the Kannisto-Thatcher standard.3 The modeled death rate is a product of the standard V and the parameters M, denoting the relative level of mortality of females at age 90; A, representing the effect of aging above and beyond the standard schedule; and S, representing the effect of sex above and beyond the standard schedule. Values of 1 for the parameters indicate a behavior identical to the standard schedule. In symbols,
This model is preferable to a parametric hazard regression model for two reasons: (1) parameters are meaningful in substantive terms and not just in mathematical terms; and (2) death rates are not forced to follow a mathematical function, such as Gompertz or Weibull, but are allowed to adjust to patterns observed in other populations; this is an analytic strategy with a long tradition in demographic modeling that includes the Louis Henry model of natural fertility, the Ansley Coale models for fertility and nuptiality, and the William Brass models for survival (Brass 1971; Coale 1977; Henry 1972).
This article estimates model parameters using Poisson regression, following to Rodríguez and Cleland (1988), who estimated the analogous Coale/Page model for fertility using this log-linear regression model. Given that the mortality rate m is the ratio of the count of deaths Y and the number of person-years of exposure N, the Poisson regression models expected number of deaths E[Y] as the dependent variable:
where the product NV is an offset term (McCullagh and Nelder 1989), and bi are the estimated regression coefficients, which when exponentiated, render the M, A, and S parameters, respectively. The model’s parameters bi were estimated using STATA (Statacorp 2005).4
To investigate mortality covariates, the three parameters of the model are also estimated for subgroups defined by variables of interest. Those variables are included as additional terms in the Poisson regression model as well as their interactions with x and d. The only additional variables available in the database of nonagenarians are the calendar year of observation (1983–2004); whether the individual was registered in a timely manner at birth (an indication that age is error-free); the place of residence, which is a time-varying variable (for each age-segment, the most recent voting place listed on the padrón), and the month of birth (a proxy to assess the effect of early life-health conditions). Preliminary analyses showed that most geographic variations are captured by the dummy variable “residence in the Central region,” which includes the capital city.
Causes of Death
Six broad groups of causes of death, plus a residual category, are defined as follows, with the codes from the 9th International Classification of Diseases (ICD-9) listed in parentheses after each cause: (1) communicable diseases (1–139, 460–490); (2) cancer (140–239); (3) cardiovascular diseases (390–459); (4) Chronic respiratory diseases (491–519); (5) diabetes (250); and (6) accidents and violence (800–999). The information on causes of death comes from the vital statistics system because the voter registry does not have these data. Age-specific mortality rates were computed for the six groups of causes of death for the period 1990–1999. For comparative purposes, standardized death rates were also computed for the United States (white population only) and Sweden for the period 1994–1996. Data disaggregated by age and causes of death were not readily available for ages 85 or older in these or other countries. The comparison thus refers to the group aged 85 or older and uses the “indirect” procedure of standardization (Shryock and Siegel 1976), with the Costa Rican rates as the standard.5
RESULTS
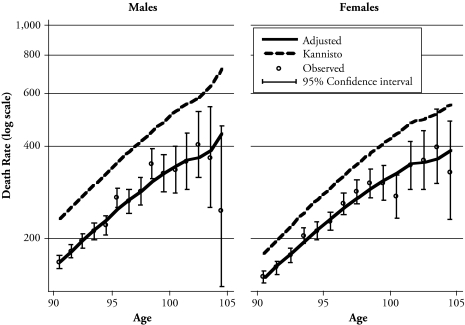
The age-specific death rates from the Costa Rican database are substantially lower than the average of 13 high-income countries (listed in footnote 1)—the Kannisto-Thatcher standard (see Figure 1). The Costa Rican advantage is larger for males, which means a narrower sex gap in Costa Rica, analogous to one observed in Sardinia, Italy (Robine et al. 2006). The rates increase with age, with a slope resembling that of the standard. In populations with bad data at these ages, one usually observes flat curves. There is some deceleration in the increases at advanced ages—a phenomenon observed in other populations and species as well, which is the subject of intense scrutiny (Horiuchi and Wilmoth 1998).
Figure 1.
Observed and Adjusted Age-Specific Death Rates: Costa Rica (1983–2004) and Kannisto-Thatcher Average (1990–1999)
The three-parameter model provides a reasonable adjustment of the Costa Rican rates in Figure 1. Smoothing the rates with the model seems necessary to eliminate large, random fluctuations. The 95% confidence intervals (CI) illustrate that the observed rates become highly unreliable by age 98 and beyond because of random errors originated in small numbers of observations. The relational model in this article corrects these probably random fluctuations and purposely imposes a monotonic pattern of increasing rates with age, as observed in 13 developed populations.
Table 2 shows the three parameters of the mortality model estimated for Costa Rican nonagenarians and used to smooth the rates in Figure 1. The M parameter is estimated at 0.829; that is, Costa Rica has 17% lower mortality at age 90 than the Kannisto-Thatcher standard for high-income countries. The A parameter is estimated at 0.989; aging occurs more slowly in Costa Rica than in the standard, at a rate 1.1% slower for each extra year of age. The S parameter came out as 0.878; Costa Rican males have an additional advantage of 12% lower-than-expected death rates.
Table 2.
Three Models Describing Mortality of Costa Rican Nonagenarians Estimated With Poisson Regression, Robust Estimates: Models Differ by the Explanatory Variables Included
| Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| Parameter | 95% Confidence Interval | Parameter | 95% Confidence Interval | Parameter | 95% Confidence Interval | |
| M Level | 0.829 | 0.808–0.851 | 0.847 | 0.814–0.882 | 0.859 | 0.833–0.886 |
| S Male = 1 | 0.878 | 0.853–0.905 | 0.888 | 0.862–0.915 | 0.874 | 0.848–0.904 |
| A Age (90 = 0) | 0.989 | 0.984–0.994 | 0.991 | 0.985–0.997 | 0.990 | 0.984–0.997 |
| Effects on M | ||||||
| Late registry | 0.985 | 0.947–1.026 | 0.937 | 0.887–0.990 | ||
| Year (1995 = 0) | 0.991 | 0.987–0.996 | 0.996 | 0.992–1.000 | ||
| Non-Central region | 0.892 | 0.863–0.922 | 0.851 | 0.809–0.894 | ||
| Non-extinct cohort | 1.005 | 0.955–1.058 | ||||
| Born in March or April | 1.036 | 0.996–1.078 | 1.070 | 1.009–1.133 | ||
| Effects on S | ||||||
| Late registry | 1.088 | 1.006–1.177 | ||||
| Effects on A | ||||||
| Year (1995 = 0) | 0.998 | 0.997–0.999 | ||||
| Non-Central region | 1.013 | 1.001–1.024 | ||||
| Born in March or April | 0.990 | 0.977–1.004 | ||||
| Effects on Year | ||||||
| Non-Central region | 1.007 | 1.001–1.013 | ||||
Notes: The effects on sex, age and year were estimated by including the respective interaction variable in the model. See the text for an explanation of the parameters.
Table 2 shows estimates for two additional models. The second model allows for variation in mortality levels (the M parameter) with five additional variables. It is useful just to show that there is no significant difference in mortality of extinct and non-extinct cohorts, which is an assurance that there are no death underregistration errors. By including statistical interactions,6 the third model also allows variation in the sex and age effects. Being a late-birth registry (a proxy for potential age errors) reduces mortality by 6%, but this effect occurs only among women, as shown by the interaction effect with sex. There is also a significant trend of mortality reduction over time of 0.4% per year, but this trend occurs only in the Central region (as shown by the region-year interaction) and dissipates at older ages (age-year interaction). The non-Central regions have 15% lower mortality by 1995. Given that those regions are the most remote and least developed, one wonders whether this apparent advantage may come from data errors. The advantage of non-Central regions, however, disappears at older ages and more recent times, as shown by the corresponding interactions. Finally, those born in March or April have a 7% higher mortality at age 90, a disadvantage that diminishes with age. Analogous effects of month of birth observed in other populations have been taken as indication of the direct correlation between early-life conditions (in utero and neonatal) and old-age health, linked to the shortage of food during winter.7 Costa Rica does not see that kind of food shortage, but seasonality does occur for other factors, particularly those linked to the dry season that goes from January to April. In particular, conceptions and diarrhea used to peak in January and February, months in which people also used to be very busy harvesting coffee and celebrating the extra income from this and other harvests and the dry season. It may be that infections in the final months of pregnancy that hampered in utero development affect the health of these newborn babies even when they reach very old ages.
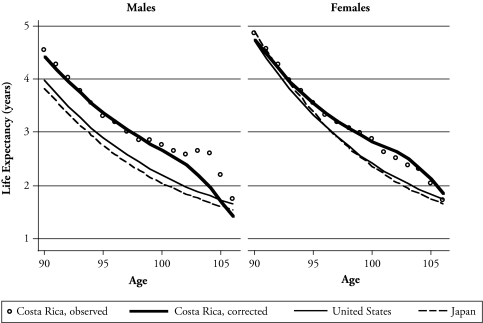
Restricting the analysis only to the Central region and timely registered births assures high-quality estimates, although these may be conservative. With these two restrictions, the parameters for aging (A) and sex (S) are about the same as in the simple model presented earlier. The parameter for mortality level (M) is a bit higher, and the advantage for Costa Rica declines from 17% to 14%. The exceptional longevity of Costa Ricans does not seem sensitive to this refinement. Figure 2 shows life expectancy by age originally estimated with the observed death rates, as well as that estimated with the rates from the model and restricted to timely registered births and the Central region. No important differences are seen between the two series up to age 102. The figure also shows that while Costa Rican females differ little from those in Japan and the United States, Costa Rican nonagenarian males have a one-half year advantage in life expectancy at all ages. By age 100, Costa Rican males have 2.7 years of life expectancy, and females have 2.8 years; in the United States (whites only), males have 2.2 years, and females have 2.4 years. However, after about age 100, comparisons must be taken cautiously because of uncertainties originated in the small number of observations in Costa Rica and the sensitivity of the estimates to small variations in computation methods or in data errors.
Figure 2.
Life Expectancy, by Age and Sex: Costa Rica (1983–2004), the United States (whites only, 1990–1995), and Japan (1990–1995)
Note: “Costa Rica, corrected” refers to Costa Rican estimates based on death rates smoothed with the regression model and restricted to timely registered births and residence in Central region.
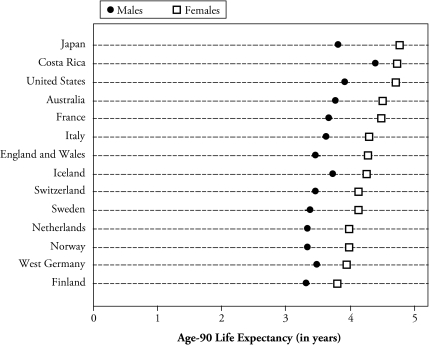
Age-90 life expectancy—a summary of the mortality of nonagenarians—in this new data set resulted in 4.7 and 4.4 years for women and men, respectively—figures almost identical to those in the official life tables for 1995–2000. Figure 3 compares my estimate for Costa Rica for the period 1983–2004 (central year 1994) with high-income countries in the aforementioned Kannisto-Thatcher database for the period 1992–1998 (with 1995 as the central year). The estimates in the figure are thus contemporary to the same period. Costa Rican males have the highest life expectancy, which is one-half year more than the United States, Japan, Australia, and Iceland. Costa Rican females are essentially tied in first place with Japan and the United States. The sex gap in life expectancy is notoriously smaller in Costa Rica: 0.3 years at age 90 compared with 1.1 year for France or the United States (see Figure 3).
Figure 3.
Age-90 Life Expectancy, by Sex, for Selected Countries Ordered by Female Life Expectancy: Costa Rica (1983–2004) and Other Countries (1990–1995)
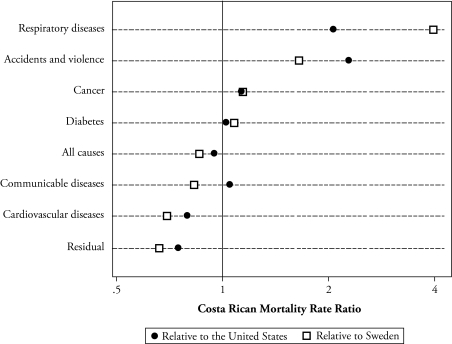
Data on causes of death may help to understand the Costa Rican advantage. Cardiovascular diseases (CVDs) are, by far, the leading cause of death, accounting for nearly 50% of all deaths of nonagenarians. Chronic respiratory diseases (mostly “other chronic airway obstructive diseases”), communicable diseases (mostly bronchopneumonia and pneumonia), and cancer have similar importance, each accounting about 12% of old-age deaths. A comparison with the United States and Sweden in Figure 4 points out that the Costa Rican advantage is mostly due to its lower CVD mortality. The age-adjusted rate of mortality from CVD, at ages 85 and older, is 20% lower in Costa Rica than in the United States and 30% lower than in Sweden. In turn, mortality by communicable diseases is similar to that in the United States and lower than in Sweden. By contrast, Costa Rican elders have substantially higher mortality from chronic respiratory diseases and accidents (huge rate ratios on the order of 200%–400%). Cancer is another pathology from which Costa Ricans have slightly higher mortality rates than do U.S. and Swedish citizens (about 15% higher), mostly attributable to stomach cancer.
Figure 4.
Mortality Rate Ratio by Cause of Death in Costa Rica Relative to the United States and Sweden for Those Aged 85 and Older in the 1990s: Indirect Standardization by Age and Sex
DISCUSSION
Fresh data from a population registry kept in Costa Rica for voting purposes confirms early estimates of exceptional longevity of its elders. Life expectancy for nonagenarian males is one-half year more in Costa Rica than in any other country, with reliable statistics. Although this life expectancy is still less than that for females, the difference is only 0.3 years, which is the smallest recorded by national populations at these mortality levels.
Are these figures valid or just a product of “bad data”? How could they be valid, considering that well-being and health services in Costa Rica are far behind those in high-income countries?
This article is mostly concerned with the validity of the estimates. By not basing the estimates on self-reported age data, it avoids age exaggeration, which is the most problematic and pervasive data error at old ages. By double-checking birth dates and excluding individuals whose birth dates are fully documented but whose registration did not occur close to birth, almost no possibility of age errors exists. The only possibility of error would come from massive identity suplantation of older and deceased individuals by younger ones. Such a massive fraud does not seem plausible.
Estimates in this article for extinct cohorts are free from death underregistration errors: cohorts were not extinct if there was a failure to register some deaths. Independent census data, which are also free of missing-death errors, confirm that survival of Costa Rican nonagenarians may be exceptional. Table 3 shows that although the percentage of nonagenarians in the total population is not impressive (0.2%) because of very rapid population growth in the past, the nonagenarian rate is exceptionally high for Costa Rican males: more than twofold those of France, Italy, Sweden, or the United States, and four times higher than in Russia. Following common practice in demographic analyses of centenarians (see, e.g., Robine and Paccaud 2005), the nonagenarian rate was defined as the ratio of the population aged 90 and older in 2000 to the population 60 and older in 1970: that is, a 30-year cohort survival proportion among the elderly and assuming null migration. A problem with these census data is age exaggeration that might inflate the nonagenarian rate. An independent evaluation of the 2000 census found that age exaggeration did indeed exist, especially among the oldest-old.8 Correction of the age-reporting errors reduces the Costa Rican nonagenarian rate from 6.9% to 5.6% for males (Table 3, second row), but this figure continues to be more than twice as large as in France, Italy, and Sweden. The nonagenarian sex ratio in Table 3 is also exceptional in Costa Rica: there are only 30% or 40% more women than men, compared with the 200% or 300% excess of women in the other countries in the table. This result corroborates this article’s finding that the mortality sex gap among Costa Rican nonagenarians is substantially smaller than in other countries. It is reassuring to reach the same result with two independent data sources. This Costa Rican peculiarity has also been observed in the island of Sardinia of Italy (Robine et al. 2006).
Table 3.
Nonagenarians in the Population, Cohort Nonagenarian Rate,a and Female to Male Ratio in Nonagenarians: Costa Rica and Selected Countries, Circa 2000
| Country | In Population (%) |
Cohort Rate (%) |
Female Ratio | ||
|---|---|---|---|---|---|
| Female | Male | Female | Male | ||
| Costa Rica, Observed | 0.22 | 0.17 | 9.2 | 6.9 | 1.4 |
| Costa Rica, Corrected | 0.19 | 0.14 | 7.4 | 5.6 | 1.3 |
| France | 1.09 | 0.33 | 6.1 | 2.5 | 3.5 |
| Italy | 0.90 | 0.31 | 5.4 | 2.3 | 3.0 |
| Japan | 0.77 | 0.26 | 8.3 | 3.3 | 3.0 |
| Russia | 0.39 | 0.10 | 2.8 | 1.6 | 4.2 |
| Sweden | 1.05 | 0.37 | 5.6 | 2.2 | 2.9 |
| United States | 0.78 | 0.26 | 6.9 | 2.9 | 3.1 |
Sources: Data for Costa Rica are from the country’s 2000 census, observed and corrected figures, correction in (INEC and CCP 2002). Data for other countries are from the Human Mortality Database (http://www.mortality.org).
Nonagenarian rate = the population aged 90 and older in 2000 / Population aged 60 and older in 1970.
According to the World Bank (2006), by 2004, Costa Rica had a per capita gross national income of about US$4,700 and a health expenditure of $310. These figures are about one-tenth those in high-income countries. In the United States, these amounts were $41,400 and $5,700, respectively. Indicators of health services, such as per capita physicians and hospital beds, are also substantially lower in Costa Rica: they equate to only one-third the number of U.S. physicians and one-tenth the number of Japanese beds. It is perplexing that a country with these modest levels of well-being, health investments, and infrastructure may be the one with the highest life expectancy among the elderly.
Broad explanations of Costa Rica’s health achievements in the literature include the orientation of the government toward equity and social development, with large social investments being possible, in part, because of the absence of military expenditures (Rosero-Bixby 1991). The 1949 constitution abolished the armed forces. Investments in education and the very high coverage of health insurance are often mentioned as key factors (Caldwell 1986). Health insurance covers 82% of the population, including the 9% population deemed destitute, whose insurance is paid by the government (Rosero-Bixby 2004). Provision of primary health care, particularly to remote or poor populations, has a quantifiable impact on death rates, especially among children (Rosero-Bixby 1986). A 17-year follow-up of a group of Costa Rican elderly has shown no meaningful differences in survival by socio economic condition (education or wealth) nor by being covered by the national health insurance9 (Rosero-Bixby, Dow, and Lacle 2005); this suggests that the Costa Rican advantage at old ages may be present across the entire society, with no clear-cut health interventions or classic socioeconomic gradients as explanation.
Data on causes of death suggested that the Costa Rican advantage comes mostly from CVDs. However, the comparison with Sweden and the United States must be taken cautiously because differences may be an artifact from variations in how causes of death are registered in each country, as well as from age misreporting errors or possibly under-registration of deaths. The data for this comparison are regular data from vital statistics, which are good albeit not perfect in Costa Rica. However, it is worth noting that an early study (Rosero-Bixby 1996) among young adults found similar patterns. For example, it found that mortality by heart disease among males aged 25–74 is 42% lower in Costa Rica than in the United States. The CVD advantage of Costa Rican males does not seem to occur only among the oldest old, and this advantage is so large that its being the result of bad data is hard to believe.
In Sardinia, another place with exceptional old-age longevity among males and a small sex gap, Caselli and Lipsi (2006) also found that low CVD mortality explains the survival advantage of elderly Sardinians compared with other Italians.
Another suggestive result regarding causes of death is that old-age mortality by communicable diseases is similar to that in the United States and is lower than in Sweden. This result somehow confirms the high level of development of the current Costa Rican primary health care system, which other studies have identified as an important factor for the low mortality at earlier ages in Costa Rica (Rosero-Bixby 1986, 2004).
One can safely assume that the lower CVD mortality of elderly Costa Rican males does not come from access to superb health care. Costa Rica has a good health care system, especially at the primary level, with almost universal coverage. However, the Costa Rican health care system is not comparable with the health infrastructure of Sweden and the United States, especially considering the access to health care that Medicare provides to the elderly in the United States. So what are the preventive, genetic, or behavioral factors that protect Costa Ricans from high CVD rates? Table 4 provides some hints by comparing selected markers from National Health and Nutrition Examination Survey (NHANES) 2001–2002 in the United States and results from an ongoing study, Costa Rica: Estudio de Longevidad y Envejecimiento Saludable (Costa Rican Study of Longevity and Healthy Aging; CRELES). Smoking, past and present, is not a factor among males, nor is high blood pressure or elevated cholesterol or triglycerides levels. It does not seem that Costa Ricans have the genes or a diet that reduce these risk factors. The only lowered risk factor for which Costa Rican males have a clear advantage is a lesser prevalence of obesity. Prevalence of obesity in Costa Rican males is two-thirds that found in the United States. This probably results in the significantly lower prevalence of uncontrolled diabetes in males as measured by the glycohemoglobin level, the only other factor in Table 4 that shows a Costa Rican advantage. Other factors that may be worth investigating are levels of stress, support networks, and the like.
Table 4.
Proportion of Those Aged 60–90 Who Suffer From Selected Risk Factors, by Sex: Costa Rica (2005) and United States (2001–2002)
| Risk Factor | Males |
Females |
||
|---|---|---|---|---|
| Costa Rica | United States | Costa Rica | United States | |
| Obese: BMI ≥ 30 | .16 | .22 * | .31 | .27 |
| Waist ≥ 94/80cm, Male/Female | .48 | .80 * | .86 | .87 |
| Ever Smoked | .68 | .66 * | .22 | .42 * |
| Currently Smokes | .14 | .07 * | .04 | .06 * |
| High Blood Pressure, Diastolic > 90 | .37 | .04 * | .41 | .03 * |
| High Blood Pressure, Systolic > 140 | .67 | .38 * | .69 | .51 * |
| HDL Cholesterol ≤ 40/50 mg/dl, Male/Female | .46 | .31 * | .59 | .28 * |
| Total Cholesterol > 250 | .14 | .07 * | .26 | .16 * |
| Triglycerides ≥ 150 mg/dl | .43 | .44 | .48 | .50 |
| Glycohemoglobin ≥ 6.5% | .12 | .14 * | .20 | .11 * |
| Average N | 1,176 | 557 | 1,410 | 607 |
| Mean Age | 75 | 75 | 75 | 75 |
Notes: Figures are age-adjusted proportions with logistic regression to age 75. Figures in gray boxes indicate risk factors for which Costa Ricans have a significant advantage.
Sources: For the United States, data come from the Centers for Disease Control (CDC), National Health and Nutrition Examination Survey, 2001–2002 (http://www.cdc.gov/nchs/nhanes.htm). For Costa Rica, the data come from the study “Costa Rican Study of Longevity and Healthy Aging” (CRELES), Centro Americano de Población, Universidad de Costa Rica (http://ccp.ucr.ac.cr/creles).
Difference is significant at p < .05.
This article does not have an answer to the question of why elderly Costa Ricans do so well. It could be a genetic factor, lifestyle, social factors, or the environment. It could also be just a heterogeneity in frailty effect. Costa Rican nonagenarians are true survivors of cohorts that underwent extremely harsh health conditions when young. For example, they survived infant mortality rates in the range of 250 per thousand prevalent in Costa Rica in the early twentieth century. Malaria, tuberculosis, and diarrheic diseases decimated these cohorts when they were young. It looks like the selection-of-the-fittest effect prevailed over the weakening effect. In addition, modern health evils, such as obesity and a sedentary lifestyle, are less common among them. Finally, a reasonably good health care system is currently protecting them from dying of communicable diseases.
These explanations, however, say nothing regarding why the Costa Rican advantage occurs mostly among males, or why the sex gap in mortality is so small. The only thing known so far is that this population exhibits low cardiovascular mortality and that Costa Rican males of these ages are thin. Comparatively, Costa Rican women tend to be obese, which perhaps is due to their high fertility in the past; each extra pregnancy usually increases mother’s weight, as shown, for example, by Arroyo et al. (1995) for Mexican women.
If the high longevity of elderly Costa Ricans is mostly a result of a selection process of the less frail, this may be an ephemeral advantage that may disappear as more frail individuals reach old ages, thanks to the rapid progress that took place in the past.10 Analyses in other low-income countries with adequately robust data are needed to see whether the early harsh conditions generally faced 80–100 years ago in these countries lead to similar patterns of low mortality at old ages.
Appendix Table A1.
Age and Sex Mortality Rates per 1,000 Population
| Age | Kannisto-Thatcher |
Costa Rica (1983–2004) |
||||
|---|---|---|---|---|---|---|
| Males | Females | Males | Na | Females | Na | |
| 90 | 231 | 178 | 166 | 9,391 | 149 | 11,780 |
| 91 | 253 | 198 | 181 | 7,704 | 160 | 9,790 |
| 92 | 277 | 220 | 195 | 5,925 | 175 | 7,652 |
| 93 | 302 | 243 | 211 | 4,529 | 203 | 5,891 |
| 94 | 328 | 268 | 220 | 3,430 | 211 | 4,419 |
| 95 | 357 | 295 | 271 | 2,505 | 227 | 3,286 |
| 96 | 387 | 323 | 264 | 1,781 | 259 | 2,359 |
| 97 | 419 | 352 | 285 | 1,236 | 284 | 1,656 |
| 98 | 453 | 382 | 351 | 844 | 303 | 1,123 |
| 99 | 489 | 412 | 326 | 561 | 303 | 756 |
| 100 | 526 | 444 | 335 | 362 | 274 | 515 |
| 101 | 556 | 482 | 355 | 236 | 346 | 344 |
| 102 | 582 | 495 | 405 | 146 | 358 | 218 |
| 103 | 635 | 518 | 365 | 77 | 397 | 128 |
| 104 | 721 | 553 | 246 | 49 | 330 | 79 |
| 105 | 853 | 604 | 274 | 29 | 369 | 49 |
| 106 | 1,054 | 672 | 344 | 17 | 441 | 25 |
N = person-years observed.
Footnotes
The Wellcome Trust Foundation (Grant No. 072406/Z/03/Z) and the Florida Ice and Farm Co. of Costa Rica provided support for this and other studies on aging in Costa Rica. The Costa Rican Tribunal Supremo de Elecciones provided the databases. Daniel Antich from the Universidad de Costa Rica provided assistance in processing the databases. Albert I. Hermalin from the University of Michigan provided suggestions and encouragement to improve this manuscript. German Rodríguez from Princeton University provided statistical advice to estimate the model.
The 13 countries are Australia, England and Wales, Finland, France, West Germany, Iceland, Italy, Japan, the Netherlands, Norway, Sweden, Switzerland, and the United States. Kannisto et al. (1994) judged that these countries, with the exceptions of the United States and Australia, have “highly reliable data.” The data were taken from the following Web site: http://www.demogr.mpg.de/databases/ktdb/.
For example, a man born on February 1, 1900 and deceased on May 1, 1995 will enter into observation in 1990. He will contribute 11 person-months to the denominator of the rate in that year and age 90; one personmonth to age 90, year 1991; 11 months to age 91, year 1991; and so on until his final segment of three months at age 95 and year 1995, which ends in a death and contributes 1 to the rate’s numerator. If this person were still alive at the end of the observation period on October 30, 2004, his last segment would be 9 months at age 104, ending in censoring.
I averaged the 1992–1998 data for the 13 countries listed in footnote 1 to define an old-age standard mortality schedule for high-income countries. Appendix Table A1 shows rates in the standard schedule along with observed rates in the Costa Rican database of 20,000 nonagenarians, which were estimated using an exact account of person-years in the denominator.
The data set for estimating the model is of the survival-time type, with censoring at the end of 2004 and entry to observation at the 90th birthday (or January 1983 for individuals older than 90 and alive at that time). Each observation was split into single age units to properly model the effect of age as time-varying covariate. The use of Poisson regression for grouped data generated with the STATA command “strate” is a logical choice because the dependent variable is a count of deaths in each age and the exposure is the number of person-years observed. A problem with these grouped data is that the sample size is inflated: each individual is counted several times, one in each age until death. Standard errors were estimated with STATA regression models using individual-level data and “robust” estimates, which take into account that information has been replicated for each person. Statisticians have been using Poisson regression to fit survival models for decades, and even Cox’s partial likelihood approach has been shown to be a form of Poisson regression (Clayton and Cuzick 1985; Whitehead 1980). In the present case, it is not necessary to prove the count is Poisson (the 0–1 death outcome at the individual level might not be) but just that the likelihoods of the survival and Poisson models are equivalent, which has been demonstrated by Holford (1980) and by Laird and Olivier (1981). Some statisticians refer to this approach to fit survival models as the “Poisson trick.” Regarding the naive issue of equality of mean and variance as requirement to use Poisson, it is true that the variance equals the mean in a Poisson distribution, but estimates obtained by maximizing the Poisson likelihood are optimal under the weaker condition that the variance is proportional to the mean, which is another standard result in generalized linear models (Wedderburn 1974). The standard errors are typically underestimated with overdispersed data, but one can estimate the proportionality factor via Pearson’s chi-square or by using robust standard errors, as done here.
The specific data sources on causes of death were as follows: for Costa Rica, the death data base provided by the National Statistical and Census Institute (INEC) and available online at http://censos.ccp.ucr.ac.cr/; for the United States, the WONDER system of the Centers for Disease Control at http://wonder.cdc.gov/; and for Sweden, information provided by Professor Charli Eriksson from data in the Swedish National Institute of Public Health.
Only significant effects are included in the third regression. Significance was tested by looking at the variation in the log-likelihood ratio when the variable and its relevant interactions are included in the model.
Adult mortality is higher for those born in spring: April to June in the northern hemisphere, and October to December in the southern hemisphere (Doblhammer 2004).
I compared the census-reported age with the age in the national identification card—the cédula—in a sample of 7,400 seniors. Among those aged 90 and older, about 30% of the individuals exaggerated their age by more than six years on average, compared with 10% by those in their 60s (Rosero-Bixby et al. 2004).
There seems to be a selection bias in this lack of insurance effect because the frail may tend to seek out insurance coverage more frequently.
Life expectancy at birth in Costa Rica rose from 46 to 63 years from 1940–1960, which means a gain of 19 hours of life every single day in a 20-year period. In the 1970s, there were again gains at the same staggering speed, which raised life expectancy to 73 years in 1980. In 2000, life expectancy was 78 years (Rosero-Bixby 2004).
REFERENCES
- Arroyo P, Avila-Rosas H, Fernandez V, Casanueva E, Galvan D. “Parity and the Prevalence of Overweight”. International Journal of Gynaecology and Obstetrics. 1995;48:269–72. doi: 10.1016/0020-7292(94)02284-6. [DOI] [PubMed] [Google Scholar]
- Barbi E, Caselli G, Vallin J.2003“Trajectories of Extreme Survival in Heterogeneous Populations” Population(English ed)5843–65. [Google Scholar]
- Barbi E, Vaupel JW. “Comment on ‘Inflammatory Exposure and Historical Changes in Human Life-Spans.’”. Science. 2005;308:1743. doi: 10.1126/science.1108707. [DOI] [PubMed] [Google Scholar]
- Brass W. Biological Aspects of Mortality. London: Taylor and Francis Ltd; 1971. “On the Scale of Mortality.”; pp. 69–110. [Google Scholar]
- Caldwell JC. “Routes to Low Mortality in Poor Countries”. Population and Development Review. 1986;12:171–220. doi: 10.1111/j.1728-4457.2010.00353.x. [DOI] [PubMed] [Google Scholar]
- Caselli G, Lipsi RM.2006“Survival Differences Among the Oldest Old in Sardinia: Who, What, Where, and Why?” Demographic Research 14article 13267–94.Available online at http://www.demographic-research.org/volumes/vol14/13 [Google Scholar]
- Clayton DG, Cuzick J. “The EM Algorithm for Cox’s Regression Model Using GLIM”. Applied Statistics. 1985;34:148–56. [Google Scholar]
- Coale AJ.1977“The Development of New Models of Nuptiality and Fertility.” Populationnuméro spécial131–54. [Google Scholar]
- Coale AJ, Kisker EE. “Mortality Crossovers: Reality or Bad Data”. Population Studies. 1986;40:389–401. [Google Scholar]
- Cockerham WC, Yamori Y. “Okinawa: An Exception to the Social Gradient of Life Expectancy in Japan”. Asia and Pacific Journal of Clinical Nutrition. 2001;10:154–58. doi: 10.1111/j.1440-6047.2001.00232.x. [DOI] [PubMed] [Google Scholar]
- Doblhammer G. The Late Life Legacy of Very Early Life. Berlin: Springer-Verlag; 2004. [Google Scholar]
- Elo IT, Turra CM, Kestenbaum B, Ferguson BR. “Mortality Among Elderly Hispanics in the United States”. Demography. 2004;41:109–20. doi: 10.1353/dem.2004.0001. [DOI] [PubMed] [Google Scholar]
- Finch CE, Crimmins EM. “Inflammatory Exposure and Historical Changes in Human Life”. Science. 2004;305:1736–39. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- Finch CE, Crimmins EM. “Response to Comment on ‘Inflammatory Exposure and Historical Changes in Human Life-Spans.’”. Science. 2005;308:1743. doi: 10.1126/science.1108707. [DOI] [PubMed] [Google Scholar]
- Garson LK. “The Centenarian Question: Old-Age Mortality in the Soviet Union, 1897 to 1970”. Population Studies. 1991;45:265–78. doi: 10.1080/0032472031000145436. [DOI] [PubMed] [Google Scholar]
- Halstead SB, Walsh JA, Warren KS. Proceedings of a Conference Held at the Bellagio Conference Center. New York: The Rockefeller Foundation; 1985. “Good Health at Low Cost”. [Google Scholar]
- Henry L. On the Measurement of Human Fertility: Selected Writings. Amsterdam: Elsevier; 1972. [Google Scholar]
- Hill K, Pande R, Mahy M, Jones G. Trends in Child Mortality in the Developing World: 1960 to 1996. New York: UNICEF; 1999. [Google Scholar]
- Holford TR. “The Analysis of Rates and of Survivorship Using Log-Linear Models”. Biometrics. 1980;36:299–305. [PubMed] [Google Scholar]
- Horiuchi S, Wilmoth JR. “Deceleration in the Age Pattern of Mortality at Older Ages”. Demography. 1998;35:391–412. [PubMed] [Google Scholar]
- Instituto Nacional de Estadística y Censos (INEC) and Centro Centroamericano de Población (CCP) 2002Costa Rica: Estimaciones y proyecciones de población 1970–2050 actualizadas al año 2000 y evaluación del censo del 2000 y otras fuentes de información[Costa Rica: Population estimates and projections 1970–2050, updated by the year 2000, and evaluation of the 2000 census and other data sources]San José, Costa Rica: INEC [Google Scholar]
- Kannisto V. “On the Survival of Centenarians and the Span of Life”. Population Studies. 1988;42:389–406. [Google Scholar]
- Kannisto V, Lauritsen J, Thatcher AR, Vaupel JW. “Reductions in Mortality at Advanced Ages: Several Decades of Evidence From 27 Countries”. Population and Development Review. 1994;20:793–810. [Google Scholar]
- Khlat M, Darmon N. “Is There a Mediterranean Migrants Mortality Paradox in Europe?”. International Journal of Epidemiology. 2003;32:1115–18. doi: 10.1093/ije/dyg308. [DOI] [PubMed] [Google Scholar]
- Laird N, Olivier D. “Covariance Analysis of Censored Survival Data Using Log-linear Analysis Techniques”. Journal of the American Statistical Association. 1981;76:231–40. [Google Scholar]
- Manton KG, Stallard E, Vaupel JW. “Methods for Comparing the Mortality Experience of Heterogeneous Populations”. Demography. 1981;18:389–410. [PubMed] [Google Scholar]
- McCullagh P, Nelder JA. Generalized Linear Models. London: Chapman and Hall; 1989. [Google Scholar]
- Mesa-Lago C. Market, Socialist, and Mixed Economies: Comparative Policy and Performance— Chile, Cuba, and Costa Rica. Baltimore, MD: Johns Hopkins University Press; 2000. [Google Scholar]
- Ministerio de Planificacion Nacional y Politica Economica (MIDEPLAN), U.N. Centro Latinoamericano de Demografia (CELADE), and Direccion General de Estadistica y Censos (DGEC) 1988Costa Rica. Estimaciones y Proyecciones de Población 1950–2025[Costa Rica. Population Estimates and Projections 1950–2025]San José, Costa Rica: Imprenta Nacional [Google Scholar]
- Palloni A, Morenoff J. “Interpreting the Paradoxical in the Hispanic Paradox: Demographic and Epidemiological Approaches”. Annals of the New York Academy of Sciences. 2001;954:140–74. doi: 10.1111/j.1749-6632.2001.tb02751.x. [DOI] [PubMed] [Google Scholar]
- Preston SH, Elo IT, Stewart Q. “Effects of Age Misreporting on Mortality Estimates at Older Ages”. Population Studies. 1999;53:165–77. [Google Scholar]
- Robine JM, Caselli G, Rasulo D, Cournil A. “Differentials in the Femininity Ratio Among Centenarians: Variations Between Northern and Southern Italy From 1870”. Population Studies. 2006;60:99–113. doi: 10.1080/00324720500466000. [DOI] [PubMed] [Google Scholar]
- Robine JM, Paccaud F. “Nonagenarians and Centenarians in Switzerland, 1860–2001: A Demographic Analysis”. Journal of Epidemiology and Community Health. 2005;59:31–37. doi: 10.1136/jech.2003.018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez G, Cleland J. “Modelling Marital Fertility by Age and Duration: An Empirical Appraisal of the Page Model”. Population Studies. 1988;42:241–57. [Google Scholar]
- Rosenwaike I. “A Note on New Estimates of the Mortality of the Extreme Aged”. Demography. 1981;18:257–66. [PubMed] [Google Scholar]
- Rosero-Bixby L. “Infant Mortality in Costa Rica: Explaining the Recent Decline”. Studies in Family Planning. 1986;17:57–65. [PubMed] [Google Scholar]
- Rosero-Bixby L. “Socioeconomic Development, Health Interventions, and Mortality Decline in Costa Rica”. Scandinavian Journal of Social Medicine. 1991;46(Suppl):33–42. [PubMed] [Google Scholar]
- Rosero-Bixby L. “The Decline in Adult Mortality in Costa Rica.”. In: Chackiel J, Ruzicka L, Timoeus I, editors. Adult Mortality in Latin America. Oxford, United Kingdom: Oxford University Press; 1996. pp. 166–95. [Google Scholar]
- Rosero-Bixby L. “Evaluación del impacto de la reforma del sector salud en Costa Rica” [Impact evaluation of the health sector reform in Costa Rica] Revista Panamericana de salud Pública. 2004;15:94–103. doi: 10.1590/s1020-49892004000200004. [DOI] [PubMed] [Google Scholar]
- Rosero-Bixby L, Brenes-Camacho G, Collado-Chaves A.2004“Tablas de vida para cálculo actuarial de rentas vitalicias y retiro programado. Costa Rica circa 2000”[Life tables for actuarial computations of life-long rents and programmed retirement. Costa Rica circa 2000]Población y Salud En Mesoamérica(Revista Electrónica) Vol. 1, article 4. Available online at http://ccp.ucr.ac.cr/revista/index.htm
- Rosero-Bixby L, Dow WH, Lacle A. “Insurance and Other Determinants of Elderly Longevity in a Costa Rican Panel”. Journal of Biosocial Sciences. 2005;37:705–20. doi: 10.1017/S0021932004006996. [DOI] [PubMed] [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. “Biomarkers of Caloric Restriction May Predict Longevity in Humans”. Science. 2002;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- Shryock HS, Siegel JS. The Methods and Materials of Demography. New York: Academic Press; 1976. [Google Scholar]
- Statacorp . Stata Statistical Software: Release 9.0. College Station, TX: Stata Corporation; 2005. [Google Scholar]
- United Nations . Demographic Yearbook. New York: United Nations; 1961. [Google Scholar]
- United Nations Population Division . Trends in Total Migrant Stock: The 2005 Revision. New York: United Nations; 2006. [Google Scholar]
- Vaupel JW, Carey JR. “Compositional Interpretations of Medfly Mortality”. Science. 1993;260:1666–67. doi: 10.1126/science.8503016. [DOI] [PubMed] [Google Scholar]
- Vaupel JW, Carey JR, Christensen K, Johnson TE, Yashin AI, Holm NV, Iachine IA, Kannisto V, Khazaeli AA, Liedo P, Longo VD, Zeng Y, Manton KG, Curtsinger JW. “Biodemographic Trajectories of Longevity”. Science. 1998;280:855–60. doi: 10.1126/science.280.5365.855. [DOI] [PubMed] [Google Scholar]
- Vaupel JW, Manton KG, Stallard E. “The Impact of Heterogeneity in Individual Frailty on the Dynamics of Mortality”. Demography. 1979;16:439–54. [PubMed] [Google Scholar]
- Vincent P. “La mortalité des Vieillards” [The mortality of old people] Population. 1951;6:181–204. [Google Scholar]
- Wedderburn RWM. “Quasi-Likelihood Functions, Generalized Linear Models, and the Gauss-Newton Method”. Biometrika. 1974;61:439–47. [Google Scholar]
- Whitehead J. “Fitting Cox’s Regression Model to Survival Data Using GLIM”. Applied Statistics. 1980;29:268–75. [Google Scholar]
- World Bank . New York: Oxford University Press; 2006. “World Development Report 2006: Equity and Development”. [Google Scholar]
- Yashin AI, Iachine IA. “How Frailty Models Can Be Used for Evaluating Longevity Limits: Taking Advantage of an Interdisciplinary Approach”. Demography. 1997;34:31–48. [PubMed] [Google Scholar]