Abstract
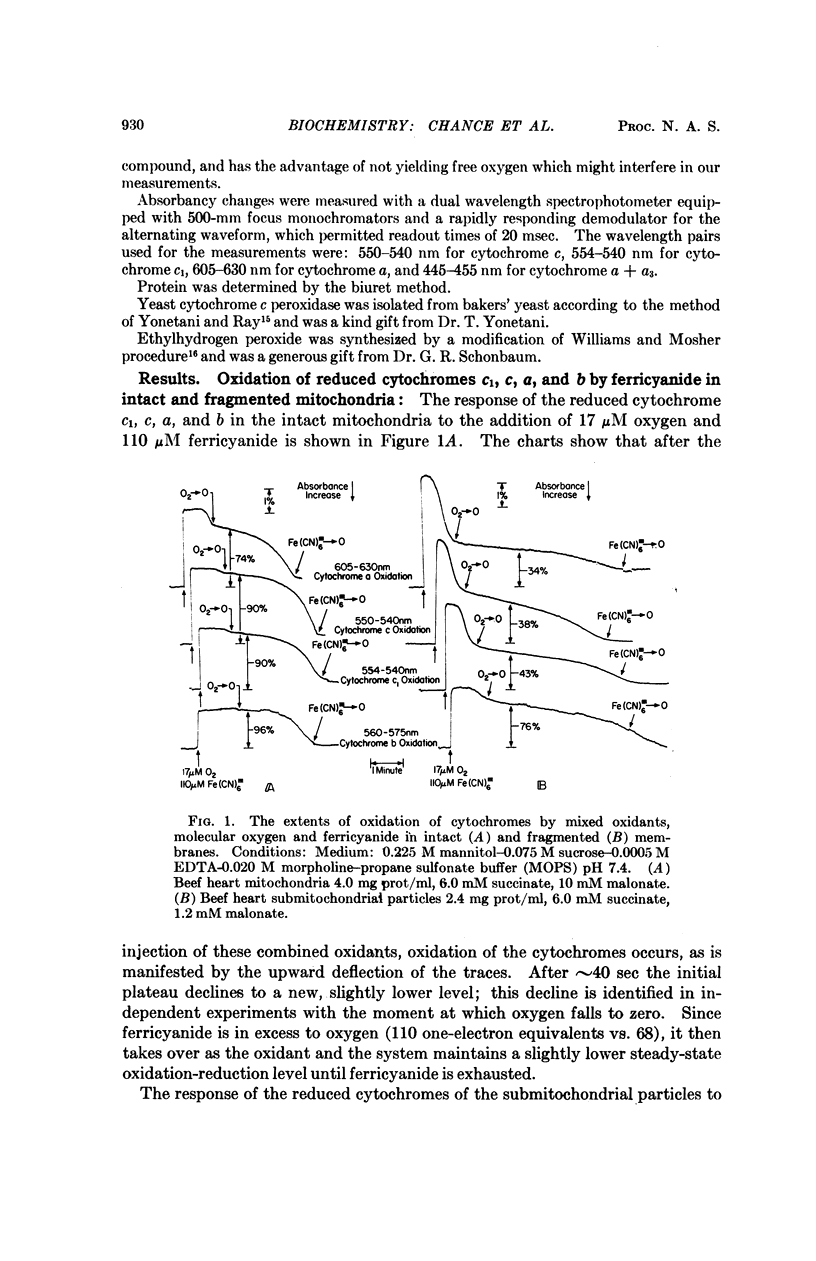
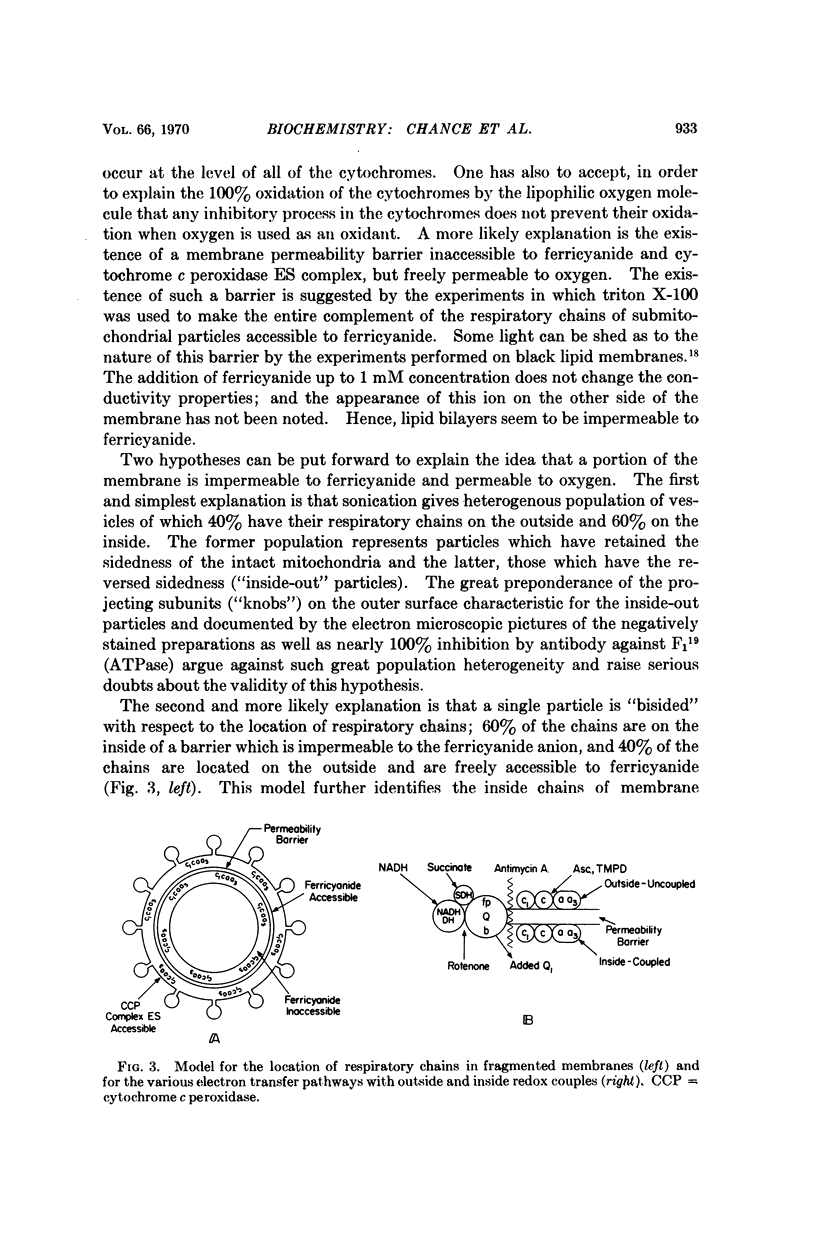
Differences in the reactivity of cytochrome c toward external oxidants ferricyanide and cytochrome c peroxidase enzyme-substrate complexes in the intact and fragmented membranes may be easily explained on the basis of different locations of cytochrome c. The oxidation of cytochrome c is nearly 100% in the intact mitochondria, suggesting its location to be on the outside of the membrane permeability barrier and freely accessible to the external oxidants. In the sonicated membrane fragments, only 40% of cytochrome c reacts with ferricyanide or ES complex. Cytochromes c, c1, a, and a3 are oxidized roughly in the same proportion by the external oxidants. These data suggest the existence of complete cytochrome chains on both sides of the membrane permeability barrier in the submitochondrial particles, and serve to explain a number of hitherto unresolved complexities concerning their electron transfer and energy coupling reactions. Energy coupling and controlled electron flow occurs in sets of cytochromes c1, a, a3 on the same side of the membrane permeability barrier.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A., Chance B., Radda G. K., Lee C. P. A fluorescence probe of energy-dependent structure changes in fragmented membranes. Proc Natl Acad Sci U S A. 1969 Feb;62(2):612–619. doi: 10.1073/pnas.62.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K. G., Parks P. C., Czerlinski G. H., Hess G. P. On the elucidation of the pH dependence of the oxidation-reduction potential of cytochrome c at alkaline pH. J Biol Chem. 1966 Sep 25;241(18):4180–4185. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- ESTABROOK R. W. Studies of oxidative phosphorylation with potassium ferricyanide as electron acceptor. J Biol Chem. 1961 Nov;236:3051–3057. [PubMed] [Google Scholar]
- Fessenden J. M., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XI. Stimulation of oxidative phosphorylation by coupling factors and oligomycin; inhibition by an antibody against coupling factor 1. J Biol Chem. 1966 May 25;241(10):2483–2489. [PubMed] [Google Scholar]
- Lee C. P., Ernster L., Chance B. Studies of the energy-transfer system of submitochondrial particles. Kinetic studies of the effect of oligomycin on the respiratory chain of EDTA particles. Eur J Biochem. 1969 Mar;8(2):153–163. doi: 10.1111/j.1432-1033.1969.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Parsons D. F., Williams G. R., Chance B. Characteristics of isolated and purified preparations of the outer and inner membranes of mitochondria. Ann N Y Acad Sci. 1966 Jul 14;137(2):643–666. doi: 10.1111/j.1749-6632.1966.tb50188.x. [DOI] [PubMed] [Google Scholar]
- SLATER E. C. Phosphorylation coupled with the reduction of cytochrome C by alpha-ketoglutarate in heart muscle granules. Nature. 1950 Dec 9;166(4232):982–984. doi: 10.1038/166982a0. [DOI] [PubMed] [Google Scholar]
- Taurog A., Howells E. M. Enzymatic iodination of tyrosine and thyroglobulin with chloroperoxidase. J Biol Chem. 1966 Mar 25;241(6):1329–1339. [PubMed] [Google Scholar]
- Wilson D. F., Dutton P. L. Energy dependent changes in the oxidation-reduction potential of cytochrome b. Biochem Biophys Res Commun. 1970 Apr 8;39(1):59–64. doi: 10.1016/0006-291x(70)90757-6. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Dutton P. L. The oxidation-reduction potentials of cytochromes a and a3 in intact rat liver mitochondria. Arch Biochem Biophys. 1970 Feb;136(2):583–585. doi: 10.1016/0003-9861(70)90233-x. [DOI] [PubMed] [Google Scholar]
- Wohlrab H. Equilibration of reducing equivalents among the terminal portions of the mitochondrial respiratory chains. Biochemistry. 1970 Feb 3;9(3):474–479. doi: 10.1021/bi00805a004. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Ray G. S. Studies on cytochrome c peroxidase. I. Purification and some properties. J Biol Chem. 1965 Nov;240(11):4503–4508. [PubMed] [Google Scholar]



