Abstract
Purpose
In 1995, the Health Professionals Follow-up Study published an isolated report of lower diverticular disease risk in physically active men, particularly among those who ran. The purpose of this article was to assess whether this finding can be verified among older men and women of the National Runners’ Health Study.
Methods
Survival analyses were applied to incident disease occurring during 7.7 yr of follow-up in 9072 men and 1664 women, representing 84% follow-up of the original ≥ 50-yr-old cohort. In addition to the usual running distance (km/d), 80% of the baseline respondents included 10-km footrace performance times (a measure of cardiorespiratory fitness). Results were adjusted for age, sex, and reported intakes of meat, fish, fruit, and alcohol.
Results
A total of 127 men and 21 women reported clinically diagnosed diverticular disease since baseline. The risk for incident diverticular disease decreased 6.2% per km/d run (P = 0.04). Relative to men and women who ran ≤ 2 km/d, those who ran an average of > 8 km/d had 48% lower risk (P = 0.05). Each meter-per-second increment in the 10-km performance was associated with a 36% risk reduction (P = 0.04). Men and women who ran > 4 m/s had 70% lower risk for diverticular disease than those who ran ≤ 2.8 m/s (P = 0.01), which persisted when adjusted for baseline body mass index (69% risk reduction, P = 0.02) or usual running distance (36% risk reduction, P = 0.03).
Conclusion
These results demonstrate an inverse association between vigorous physical activity and incident diverticular disease among older men and women but are limited by their reliance on self-reported physician diagnosis.
INTRODUCTION
Diverticular disease affects one third of Americans older than 45 yr and two thirds of those older than 80 yr [15]. Diverticula are herniations of the mucosa through the colon’s muscular wall, which may be primarily asymptomatic with colicky pain or altered bowel habits (diverticulosis), or the diverticula may become inflamed causing constant pain, fever, nausea, vomiting, bleeding, and localized or generalized tenderness, in addition to altered bowel habits (diverticulitis) [15]. Because diverticular disease was rare before the 20th century in developed countries [14,24], and remains uncommon in nondeveloped countries [16], etiological studies have focused on lifestyle or environmental characteristics of western societies. Current consensus ascribes the disease’s emergence to decreased dietary fiber intake [24], which is supported by case control [6,17,21], prospective [2], and animal studies [9]. However, the 20th century was also characterized by a reduction in physical activity levels of both moderate (requiring three- to sixfold the energy expenditure of sitting at rest) and vigorous intensities (more than sixfold the resting energy expenditure) [1,32].
In 1995, the Health Professionals Follow-up Study reported that incident symptomatic diverticular disease was inversely related to physical activity in 47,678 men followed prospectively for 4 yr [3]. The association was attributed exclusively to vigorous physical activity. Running, in particular, was associated with reduced risk of diverticular disease. To the best of our knowledge, the relationship has not been observed in other large cohort studies. This may be because other institutionally, geographically, and occupationally based studies tend to include relatively few highly active individuals and thus have limited statistical power to detect relationships between vigorous physical activity and health and disease. The only other relevant citation is by Manousos et al. [22] of more prevalent diverticular disease in people with sedentary versus active occupations.
The National Runners’ Health Study is distinguished in targeting vigorously active men and women engaged in one specific activity, that is, running, whose energy expenditure may be quantified by weekly distance run [33–39]. In addition, many runners have participated in 10-km footraces providing a standardized measure of performance reflecting cardiorespiratory fitness [7]. We hypothesized that if physical activity affects diverticular disease risk as described in the Health Professionals Follow-up Study, then this should be evident in our runners’ cohort.
METHODS
Design
The design and methods of the National Runners’ Health Study are described elsewhere [33–39]. Briefly, cohort recruitment occurred between 1991 and 1994 (primarily 1993) by distributing two-page questionnaires nationwide to runners identified through the Runner’s World magazine subscription lists and among participants of footrace events. The questionnaire solicited information on demographics, running history, weight history, smoking habits, prior history of heart attacks and cancer, and use of medications. We estimate that approximately 15% of the participants who received questionnaires responded to our survey (the number is approximate because we do not know the number of survey questionnaires actually distributed and the proportion of individuals who received multiple questionnaires). The study protocol was approved by the University of California Berkeley Committee for the Protection of Human Subjects, and all participants signed committee-approved informed consents.
Follow-up questionnaires were sent by mail between 1999 and 2002 requesting information on current running levels, body weight, and medical conditions. Multiple follow-up survey questionnaires were sent, and telephone calls were made until an a priori determined response rate of 80% was obtained. Participants were asked, “Since 1991, have you been diagnosed by a physician for any of the following conditions (provide year of diagnosis if yes)” with diverticular disease as one of the listed conditions. We excluded from the analyses patients who gave their baseline survey year, or before, as their year of diagnosis.
Measurements
Running distances were reported in average miles run per week at baseline. Although other leisure time physical activities were not recorded for this cohort, data from runners recruited after 1998 (when the question was added to the survey) showed that running represented (±SD) 91.5 ± 19.1% and 85.2 ± 24.0% of all vigorous activity in men and women, respectively, and 73.5 ± 23.7% and 69.4 ± 25.7% of total leisure time physical activity, respectively. For this report, baseline cardiorespiratory fitness was defined as speed in meters per second (m/s) of the participant’s best 10-km race during the previous 5 yr (reported as finish time in min). Published data support the use of running performance to reflect maximal oxygen consumption (VO2max) [7].
Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Self-reported waist and chest circumferences were elicited by the question, “Please provide, to the best of your ability, your body circumference in inches” without further instruction. There are strong correlations between repeated questionnaires for self-reported running distances (r = 0.89) and between self-reported and clinically measured heights (r = 0.96) and weights (r = 0.96) [33].
Intakes of meat, fish, and fruit were based on the questions “During an average week, how many servings of beef, lamb, or pork do you eat?,” “…servings of fish do you eat?,” and “…pieces of fruit do you eat?.” Alcohol intake was estimated from the corresponding questions for 4-oz (112 mL) glasses of wine, 12-oz (336 mL) bottles of beer, and mixed drinks and liqueurs and was computed as 14.2 mL per 4-oz glass of wine, 14.2 mL per 12-oz. bottle of beer, and 17.7 mL per mixed drink. Correlations between these responses and values obtained from 4-d diet records in 110 men were r = 0.65 for alcohol intake, r = 0.46 for red meat, r = 0.38 for fruit, and r = 0.19 for fish. These values agree favorably with published correlations between food records and more extensive food frequency questionnaires for red meat (r = 0.50), wine (r = 0.66), beer (r = 0.70), and mixed drinks (r = 0.72), somewhat less favorably for fruit (r = 0.50) and fish intake (r = 0.51) [13].
Statistical analyses
The analyses were restricted to men and women aged 50 yr and older to maximize statistical power, i.e., the incidence of diverticular disease was more than threefold greater in this age group than in younger subjects aged 40–49 yr. Cox proportional hazard model (JMP software version 5.0; SAS Institute, Cary, NC) was used to estimate the dose response relationships of incident diverticular disease to baseline body weight and circumferences, average distances run per day, and cardiorespiratory fitness. Reported weekly intakes of alcohol, meat, fish, and fruit, along with, age, sex, and pack-years of cigarette consumption were used as covariates. Results are presented as relative risks (hazard ratios) per kilometer-per-day (km/d) increment in distance run and per meter-per-second (m/s) increment in 10-km performance from survival analyses. In addition, relative risks are presented for categories of running distance (< 2, 2–3.9, 4–5.9, 6–7.9, and ≥ 8 km/d) relative to the < 2 km/d category and for categories of 10-km performance (< 2.8, 2.8–3.1, 3.2–3.5, 3.6–3.9, and ≥ 4 m/s) relative to the < 2.8 m/s category.
RESULTS
The original baseline survey included 10,776 men and 1939 women aged 50 yr and older, and of these, 84.8% and 86.7% provided follow-up questionnaires, respectively. There were 71 men and 9 women excluded for preexisting diverticular disease, leaving 9072 men and 1664 women for analyses. The participants lost to follow-up were similar to those providing follow-up surveys in age (mean ± SE: 56.9 ± 0.1 vs. 57.2 ± 0.1 yr, P = 0.11) and usual running distance (4.9 ± 0.1 vs. 5.0 ± 0.0 km/d, P = 0.08) but were less educated (15.8 ± 0.1 vs. 16.4 ± 0.0 yr, P < 0.0001).
There were 127 men and 21 women who reported clinically diagnosed diverticular disease since baseline on their follow-up questionnaires. Table 1 presents the sample’s baseline characteristics by running distance. Those who ran further each week had lower BMI, ran faster 10-km performance times, and ate less meat and more fruit.
TABLE 1.
Baseline characteristics (mean ± SD) of the men and women 50 yr and older.
| Baseline Distance Run per Week (km/d) | |||||
|---|---|---|---|---|---|
| < 2 | 2–3.9 | 4–5.9 | 6–7.9 | ≥ 8 | |
| Males | |||||
| Sample (N) | 1050 | 2589 | 2809 | 1090 | 1534 |
| Incidence (N) | 21 | 38 | 43 | 13 | 12 |
| Age (yr) | 57.8 ± 6.5 | 57.7 ± 6.3 | 57.2 ± 5.9 | 57.4 ± 6.0 | 56.4 ± 5.6 |
| BMI (kg/m2) | 25.4 ± 3.2 | 24.4 ± 2.4 | 23.9 ± 2.3 | 23.2 ± 2.0 | 22.8 ± 2.1 |
| 10-km performance (m/s) | 3.4 ± 0.5 | 3.5 ± 0.4 | 3.6 ± 0.4 | 3.8 ± 0.4 | 4.0 ± 0.4 |
| Cigarettes (pack-yr) | 9.7 ± 17.3 | 10.3 ± 17.6 | 10.5 ± 18.5 | 10.7 ± 17.9 | 10.7 ± 18.8 |
| Alcohol (mL/d) | 12.8 ± 17.3 | 14.4 ± 18.4 | 13.8 ± 18.3 | 13.6 ± 18.0 | 13.2 ± 18.8 |
| Meat (servings/d) | 0.4 ± 0.4 | 0.4 ± 0.3 | 0.4 ± 0.3 | 0.3 ± 0.3 | 0.3 ± 0.3 |
| Fish (servings/d) | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.2 ± 0.2 |
| Fruit (pieces/d) | 1.5 ± 1.3 | 1.7 ± 1.3 | 1.7 ± 1.3 | 1.8 ± 1.3 | 1.9 ± 1.5 |
| Females | |||||
| Sample (N) | 212 | 530 | 509 | 188 | 225 |
| Incidence (N) | 2 | 10 | 5 | 2 | 4 |
| Age (yr) | 56.4 ± 5.6 | 56.6 ± 5.9 | 56.1 ± 5.5 | 56.0 ± 5.2 | 55.8 ± 5.8 |
| BMI (kg/m2) | 23.1 ± 3.3 | 22.0 ± 2.6 | 21.7 ± 2.5 | 21.2 ± 2.4 | 20.7 ± 2.4 |
| 10-km performance (m/s) | 2.9 ± 0.5 | 3.0 ± 0.5 | 3.2 ± 0.4 | 3.3 ± 0.4 | 3.4 ± 0.4 |
| Cigarettes (pack-yr) | 7.0 ± 13.3 | 7.9 ± 16.8 | 7.7 ± 14.3 | 6.8 ± 12.2 | 6.7 ± 13.4 |
| Alcohol (mL/d) | 6.8 ± 9.4 | 8.9 ± 12.0 | 8.4 ± 12.1 | 10.1 ± 13.5 | 6.7 ± 11.1 |
| Meat (servings/d) | 0.3 ± 0.4 | 0.3 ± 0.3 | 0.3 ± 0.3 | 0.2 ± 0.3 | 0.2 ± 0.3 |
| Fish (servings/d) | 0.2 ± 0.2 | 0.2 ± 0.2 | 0.2 ± 0.2 | 0.2 ± 0.2 | 0.2 ± 0.2 |
| Fruit (pieces/d) | 1.7 ± 1.0 | 1.9 ± 1.2 | 1.9 ± 1.2 | 1.9 ± 1.4 | 2.1 ± 1.2 |
There was no significant difference in the percentage of males (1.4%) and females (1.4%, P = 0.79) affected, but those reporting incident diverticular disease were slightly older (58.0 ± 0.5 vs. 57.1 ± 0.1 yr, P = 0.08). When adjusted for age and sex, those diagnosed had significantly greater BMI (24.1 ± 0.2 vs. 23.6 ± 0.0 kg/m2, P = 0.02), greater waist circumference (84.7 ± 0.5 vs. 83.6 ± 0.1 cm, P = 0.03), greater chest circumference (101.1 ± 0.6 vs. 99.6 ± 0.1 cm, P = 0.01), had smoked more pack-years (13.9 ± 1.4 vs. 10.3 ± 0.2, P = 0.01), and had eaten more servings of meat per week than the undiagnosed men and women (2.8 ± 0.2 vs. 2.4 ± 0.0 servings, P = 0.03). Differences in waist and chest circumferences became nonsignificant when adjusted for BMI. The diagnosed and undiagnosed subjects did not differ with respect to age- and sex-adjusted education (16.2 ± 0.2 vs. 16.4 ± 0.0 yr, P = 0.36) or with weekly intakes of alcohol (99.7 ± 10.0 vs. 92.4 ± 1.5 mL, P = 0.47), fish (1.8 ± 0.2 vs. 1.7 ± 0.0 servings, P = 0.32), or fruit (11.6 ± 0.9 vs. 11.1 ± 0.1 pieces, P = 0.08).
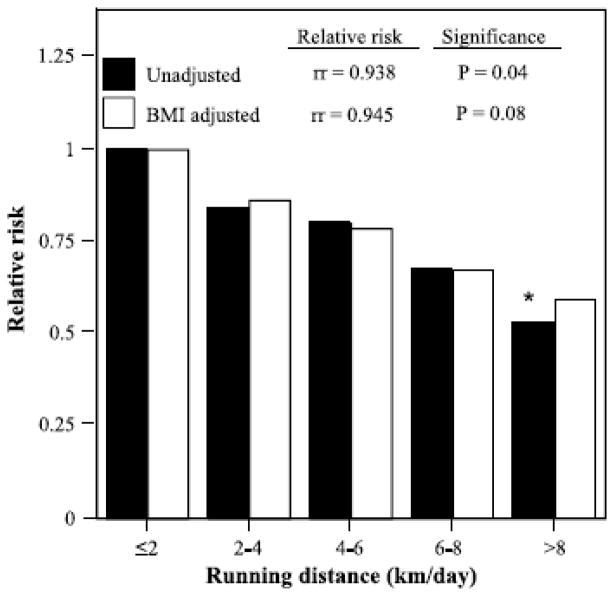
Each kilometer-per-day increment in running distance was associated with a 6.2% risk reduction for diverticular disease (i.e., relative risk = 0.938, P = 0.04). Figure 1 shows that relative to men and women who ran < 2 km/d, those who ran an average of ≥ 8 km/d had 48% lower risk (P = 0.05). Figure 1 also shows that the decline in risk with running distance was linear. Adjustment for baseline BMI slightly diminished the significance of the risk reduction with running distance (P = 0.08); however, Figure 1 suggests that the effect of the adjustment was minor.
FIGURE 1.
Relative risk of self-reported physician-diagnosed diverticular disease by usual running distance relative to the least active runners, adjusted for age, sex, pack-years of cigarette consumption, and intake of meat, fish, fruit, and alcohol. Additional adjustment for BMI where indicated. *P ≤ 0.05 for the reduction in risk relative to the least active runners. Relative risks (rr) per kilometer-per-day increment in daily running distance (as a continuous variable) presented at the top.
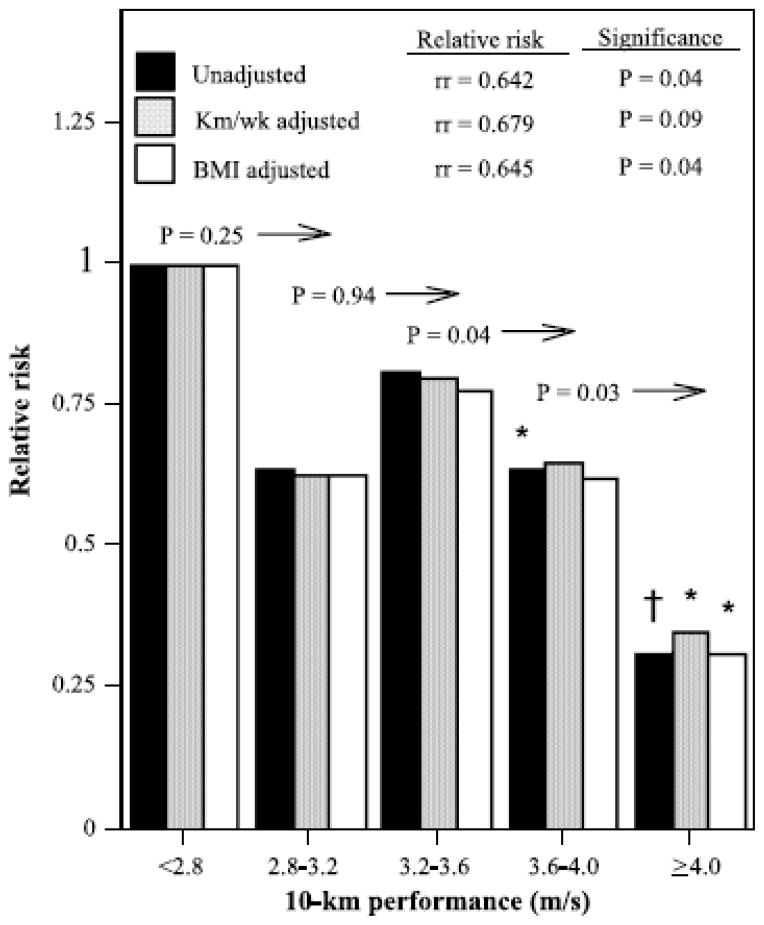
Figure 2 shows that each meter-per-second increment in 10-km performance was associated with a 36% risk reduction (i.e., relative risk = 0.64, P = 0.04), which was largely unaffected by adjustment for BMI (35% risk reduction, P = 0.04), but reduced moderately when adjusted for running distance (32% risk reduction). Men and women whose 10-km pace was ≥ 4 m/s had 70% lower risk for diverticular disease than those who ran < 2.8 m/s (relative risk = 0.30, P = 0.01), which persisted when adjusted for kilometer-per-day of running distance (relative risk = 0.35, P = 0.03) and baseline BMI (relative risk = 0.31, P = 0.02).
FIGURE 2.
Relative risk of self-reported physician-diagnosed diverticular disease by cardiorespiratory fitness (10-km race performance time) relative to the least-fit (slowest) runners, adjusted for age, sex, pack-years of cigarette consumption, and intake of meat, fish, fruit, and alcohol. Additional adjustment for usual running distance (km/d) and BMI where indicated. Significance levels relative to the least fit runners are coded: *P < 0.05, †P < 0.01. Significance levels relative to all faster men are presented above the bars and to the left of the arrows (e.g., men who ran faster than 3.6 m/s were significantly less likely to report physician-diagnosed diverticular disease than those who ran 3.2–3.6 m/s, P = 0.04). Relative risks (rr) per meter-per-second increment in the 10-km performance (as a continuous variable) are presented at the top.
DISCUSSION
Consistent with the Health Professionals Follow-up Study [3], we found that the risk for diverticular disease decreased in proportion to the distance run per week in older men and women. Clinically, the risk reduction was substantial, 6.2% per km/d run, resulting in a 48% lower incidence in those who ran ≥ 8 km/d relative to those who averaged between 0 and 2 km/d (Fig. 1). The minimum guideline level currently recommended by government and nongovernment institutions (495 METmin or 5 d of 30 min of brisk walking per week [10]) corresponds to running 1.2 km/d. Thus, our findings demonstrate an important benefit of exceeding guideline levels and add to the list of reasons why those who meet guideline levels should increase their exercise, i.e., lower risk for hypertension [38], diabetes [38], hypercholesterolemia [38], gallbladder disease [34], benign prostatic hyperplasia [35], gout [36], cataracts [37], and macular degeneration [39]. The decrease in diverticular disease risk with physical activity may be consistent with its characterization as a disease of colonic motility, given that jogging and running are reported to dramatically accelerate colon transit time in at least one study [23]. Other studies also suggest that physical activity acutely affects colon transit time [8,20] and others do not [27,40]; their differences have been attributed to methodological problems [25].
Diverticular disease risk was also inversely related to cardiorespiratory fitness as measured by the 10-km race performance (Fig. 2). Although adjustment for weekly running distance weakened the significance of the risk reduction, Figure 2 suggests that the effect of the adjustment was relatively minor. Moreover, the figure shows that the dose response relationship was most pronounced within the upper one half of the fitness distribution, i.e., lower risk for exceeding 3.6 m/s than running a 10-km race at 3.2–3.6 m/s (P = 0.04), and exceeding 4 m/s vis-à-vis running a 3.6- to 4.0- m/s race (P = 0.03). Cardiorespiratory fitness reflects in part the effects of training, particularly when vigorous, but there is also a substantial inherited component affecting even the improvement in fitness with training [28]. Thus, the observed association with fitness may involve effects that are inherited in addition to training effects.
In addition to the disease’s association with running distance and cardiorespiratory fitness, we also found that men and women who developed diverticular disease during follow-up ate significantly more meat at baseline. This is consistent with the Health Professionals Follow-up Study, which reported that baseline red meat consumption predicted greater incidence of symptomatic diverticular disease [2]. Greater beef and lamb consumption were specifically identified in one case control study of diverticulitis [21]. It has been hypothesized that red meat may cause bacteria to produce a metabolite or spasmogen that weakens the colon wall and causes diverticula to form [11]. Our survey questionnaire provided only limited dietary assessment (weekly intake of meat, fish, fruit, and alcohol) and thus provides few data on fiber intake. Diets characterized by high meat intake are also expected to include low fiber and high fat content, which have been shown to increase risk prospectively [2]. High-fiber diets and bulking agents are effective treatment of patients diagnosed with diverticular disease. Fiber increases fecal output, decreases fecal transit time, and reduces fecal bile acid output [30]. Although exercise is neither prescribed as part of the prevention nor treatment of diverticular disease, the reported relative risk for the highest versus the lowest quintile of total fiber intake, relative risk = 0.58 [2], was no greater than the relative risk we observed in running between ≥ 8 and < 2 km/d (Fig. 1), suggesting a possible benefit of prescribing vigorous exercise in addition to high-fiber diets.
The primary limitation of our analyses is that incident diverticular disease was ascertained via self-report of physician diagnosis. Diverticular disease is often asymptomatic; less than one quarter of those with the disease develop symptoms [26], and it is not known from our questionnaire which diverticular cases were identified because of diverticulitis versus endoscopy, barium enema examination, or operation performed for other reasons. The Health Professionals Follow-up Study reported that medical record review confirmed 95% of self-reported diverticular disease, 23% of self-reported cases were asymptomatic (diverticula discovered during routine screening), and that 63% of symptomatic cases involved abdominal pain, 18% involved bleeding, and 8% involved change in bowel habits [3]. The aforementioned percentages may not necessarily apply to the runners. Given their training and professional experience, it is reasonable to assume that participants of the Health Professionals Follow-up Study provided more reliable self-reported incident data than can be expected from the runners. We did not collect information on endoscopies to determine whether their rates were related to running distance or 10-km performance. However, there are consistencies in relationships observed in our study and in the Health Professionals Follow-up Study, which support at least some degree of comparability of the outcome measures. The Health Professionals Follow-up Study also reported that the risk for diverticular disease increased with BMI [2] and increased moderately with smoking [4], which agrees with our own results. We found that alcohol intake did not differ between runners who developed and remained free of diverticular disease, which agrees with their observation that there was no significant increase in risk associated with alcohol [4]. Finally, the Health Professionals Follow-up Study showed that the relationship between diverticular disease and exercise in men who had endoscopies was consistent in all self-reported cases, suggesting that the relationship was not due to an association between the rate of endoscopies and the activity level [3]. We do not expect that our findings are due to a difference in the frequency of medical checkup by fitness or activity level. The Health Professionals Follow-up Study reported that their more vigorously active participants had more routine medical checkups than less active men [18], and there was no difference in routine medical checkup by activity level in the Nurses’ Health Study [19].
Our data do not address the possible misclassification of diverticular disease with irritable bowel syndrome [31], which share the common presentation of abdominal pain. The commonness of both diverticula and irritable bowel syndrome suggest that their chance concurrence may, in some cases, be misclassified as diverticulitis [12]. The Health Professionals Follow-up Study reported that the risk reduction with physical activity was also observed in the limited number of diverticular disease cases only presenting bleeding [5] and in men who had endoscopies [3], although these lacked statistical significance owing to the limited statistical power. We also had no adjustment for fiber intake, other than weekly intake of fruit, and therefore cannot directly rule out higher fiber intake among the higher mileage, more physically fit runners. However, data presented by the Health Professionals Follow-up Study suggest a weaker contribution of dietary fiber intake to diverticular disease risk at higher physical activity levels [3].
In conclusion, our observation reaffirms the association between vigorous physical activity and reduced risk for diverticular disease. Running and jogging were the only activities specifically identified by Health Professionals Follow-up Study that significantly reduced diverticular disease risk [3]. Elsewhere, we have shown that usual running intensity was associated with lower prevalence of hypertension, hypercholesterolemia, and diabetes independent of the total distance run [38]. Others have shown that when total energy is held constant, vigorous exercise produces greater reductions in diastolic blood pressure and greater improvements in glucose control than moderate exercise does [29]. Vigorous exercise is also more effective in improving fitness than moderately intense exercise [28]. Current physical activity guidelines mainly ascribe the health benefits of exercise in the total volume, and discuss greater exercise intensity primarily as a means for obtaining the same total volume within a shorter duration [10]. Reductions in diverticular disease risk with vigorous, but not moderate, exercise may represent yet another benefit to exercising more intensely.
Acknowledgments
This research was supported in part by grants AG032004 and HL72110 from the National Heart Lung and Blood Institute and by DK066738 from the Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health and was conducted at the Ernest Orlando Lawrence Berkeley National Laboratory (Department of Energy DE-AC02-05CH11231 to the University of California).
References
- 1.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 suppl):S498–S516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 2.Aldoori WH, Giovannucci EL, Rimm EB, Wing AL, Trichopoulos DV, Willett WC. A prospective study of diet and the risk of symptomatic diverticular disease in men. Am J Clin Nutr. 1994;60:757–64. doi: 10.1093/ajcn/60.5.757. [DOI] [PubMed] [Google Scholar]
- 3.Aldoori WH, Giovannucci EL, Rimm EB, et al. Prospective study of physical activity and the risk of symptomatic diverticular disease in men. Gut. 1995;36:276–82. doi: 10.1136/gut.36.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldoori WH, Giovannucci EL, Rimm EB, Wing AL, Trichopoulos DV, Willett WC. A prospective study of alcohol, smoking, caffeine, and the risk of symptomatic diverticular disease in men. Ann Epidemiol. 1995;5:221–8. doi: 10.1016/1047-2797(94)00109-7. [DOI] [PubMed] [Google Scholar]
- 5.Aldoori WH, Willett WC. Reply, Diverticular distraction. Gut. 1995;37:299. [Google Scholar]
- 6.Brodribb AJM, Humphreys DM. Diverticular disease: three studies. Part I Relation to other disorders and fibre intake. Br Med J. 1976;1:424–5. doi: 10.1136/bmj.1.6007.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper KH. A means of assessing maximal oxygen intake: correlation between field and treadmill testing. JAMA. 1968;203:201–4. [PubMed] [Google Scholar]
- 8.Cordain L, Latin RW, Behnke JJ. The effects of an aerobic running program on bowel transit time. J Sports Med Phys Fitness. 1986;26:101–4. [PubMed] [Google Scholar]
- 9.Fisher N, Berry CS, Feam T, Gregory IA, Hardy I. Cereal dietary fiber consumption and diverticular disease: a lifespan study in rats. Am J Clin Nutr. 1985;42:788–804. doi: 10.1093/ajcn/42.5.788. [DOI] [PubMed] [Google Scholar]
- 10.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 11.Heaton KW. Diet and diverticulosis: new leads. Gut. 1985;26:54l–3. doi: 10.1136/gut.26.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heaton KW, Thompson WG. Exercise and diverticular disease. BMJ. 1995;310:1332. doi: 10.1136/bmj.310.6990.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69:243–9. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 14.Hughes LE. Postmortem survey of diverticular disease of the colon. I. Diverticulosis and diverticulitis. Gut. 1969;10:336–44. doi: 10.1136/gut.10.5.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones JD. ABC of colorectal diseases. Diverticular disease. BMJ. 1992;304:1435–7. doi: 10.1136/bmj.304.6839.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyle J, Adesola AO, Tinckler LF, et al. Incidence of diverticulitis. Scand J Gastroenterol. 1967;2:77–80. doi: 10.3109/00365526709180050. [DOI] [PubMed] [Google Scholar]
- 17.Leahy AL, Ellis RM, Quill DS, Peel AL. High fibre diet in symptomatic diverticular disease of the colon. Ann R Coll Surg Engl. 1985;67:173–4. [PMC free article] [PubMed] [Google Scholar]
- 18.Leitzmann MF, Giovannucci EL, Rimm EB, et al. The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med. 1998;128:417–25. doi: 10.7326/0003-4819-128-6-199803150-00001. [DOI] [PubMed] [Google Scholar]
- 19.Leitzmann MF, Rimm EB, Willett WC, et al. Recreational physical activity and the risk of cholecystectomy in women. N Engl J Med. 1999;341:777–84. doi: 10.1056/NEJM199909093411101. [DOI] [PubMed] [Google Scholar]
- 20.Liu F, Kondo T, Toda Y. Brief physical inactivity prolongs colonic transit time in elderly active men. Int J Sports Med. 1993;14:465–7. doi: 10.1055/s-2007-1021212. [DOI] [PubMed] [Google Scholar]
- 21.Manousos O, Day NE, Tzonou A, et al. Diet and other factors in the aetiology of diverticulosis: an epidemiological study in Greece. Gut. 1985;26:544–9. doi: 10.1136/gut.26.6.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manousos ON, Vrachliotis G, Papaevangelou G, et al. Relation of diverticulosis of the colon to environmental factors in Greece. Am J Dig Dis. 1973;18:174–6. doi: 10.1007/BF01071969. [DOI] [PubMed] [Google Scholar]
- 23.Oettlé GJ. Effect of moderate exercise on bowel habit. Gut. 1991;32:941–4. doi: 10.1136/gut.32.8.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Painter NS, Burkitt DP. Diverticular disease of the colon, a 20th century problem. Clin Gastroenterol. 1975;4:3–21. [PubMed] [Google Scholar]
- 25.Peters HP, De Vries WR, Vanberge-Henegouwen GP, Akkermans LM. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001;48:435–9. doi: 10.1136/gut.48.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts PL, Veidenheimer MC. Diverticular disease of the colon. In: Baylcss TM, editor. Current Therapy in Gastroenterology and Liver Diseases. Philadelphia (PA): BC Decker Inc; 1990. pp. 416–9. [Google Scholar]
- 27.Robertson G, Meshkinpour H, Vandenberg K, et al. Effects of exercise on total and segmental colon transit. J Clin Gastroenterol. 1993;16:300–3. doi: 10.1097/00004836-199306000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Swain DP. Moderate or vigorous intensity exercise: which is better for improving aerobic fitness? Prev Cardiol. 2005;8:55–8. doi: 10.1111/j.1520-037x.2005.02791.x. [DOI] [PubMed] [Google Scholar]
- 29.Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97:141–7. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]
- 30.Tarpila S, Miettinen TA, Mctsaranta L. Effect of bran on serum cholesterol, faecal mass, fat bile acids and neutral sterols, and biliary lipids in patients with diverticular disease of the colon. Gut. 1978;19:137–45. doi: 10.1136/gut.19.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson WG, Heaton KW. Diverticular distraction. Gut. 1995;37:298–9. doi: 10.1136/gut.37.2.298-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.US Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. pp. 1–276. [Google Scholar]
- 33.Williams PT. Vigorous exercise and the population distribution of body weight. Int J Obes Relat Metab Disord. 2004;28:120–8. doi: 10.1038/sj.ijo.0802480. [DOI] [PubMed] [Google Scholar]
- 34.Williams PT. Independent effects of cardiorespiratory fitness, vigorous physical activity, and body weight on gallbladder disease risk during 7.6 years of follow-up. Am J Gastroenterology. 2008;103:2239–47. doi: 10.1111/j.1572-0241.2008.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams PT. Effects of running distance and performance on incident benign prostatic hyperplasia in vigorously active men. Med Sci Sports Exerc. 2008;40:1733–9. doi: 10.1249/MSS.0b013e31817b8eba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams PT. Effects of diet, physical activity and performance and body weight on incident gout in ostensibly healthy, vigorously active men. Am J Clin Nutr. 2008;87:1480–7. doi: 10.1093/ajcn/87.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams PT. Prospective epidemiological cohort study of reduced risk for incident cataract with vigorous physical activity and cardiorespiratory fitness during a 7-year follow-up. Invest Ophthalmol Vis Sci. 2009;50:95–100. doi: 10.1167/iovs.08-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams PT. Vigorous exercise, fitness and incident hypertension, high cholesterol, and diabetes. Med Sci Sports Exerc. 2008;40:998–1006. doi: 10.1249/MSS.0b013e31816722a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams PT. Prospective study of incident age-related macular degeneration in relation to vigorous physical activity during 7-year follow-up. Invest Ophthalmol Vis Sci. 2009;50:1073–9. doi: 10.1167/iovs.08-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yiamouyiannis CA, Martin BJ, Watkins JB. Chronic physical activity alters hepatobiliary excretory function in rats. J Pharmacol Exp Ther. 1993;265:321–7. [PubMed] [Google Scholar]