Abstract
Background
Obstructive sleep apnea (OSA) is associated with coronary risk factors, but it is unknown if OSA is associated with development of coronary disease. We evaluated the association between OSA and the presence of subclinical coronary disease assessed by coronary artery calcification (CAC).
Methods
Consecutive patients with no history of coronary disease, who underwent electron-beam computed tomography within 3 years of polysomnography between March 1991 and December 2003, were included. OSA was defined by an apnea-hypopnea index (AHI) ≥ 5, and patients were grouped by quartiles of AHI severity. Logistic regression modeled the association between OSA severity and presence of CAC.
Results
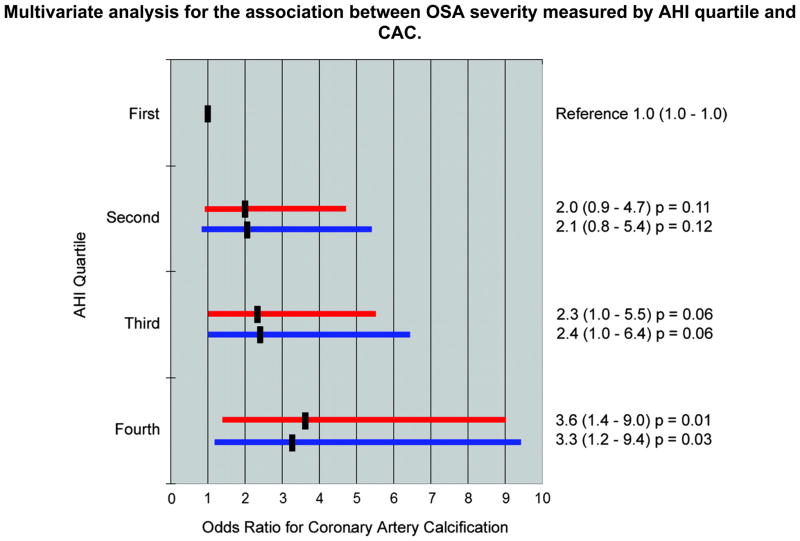
There were 202 patients (70% male, median age 50, mean body mass index 32, 8% diabetic, 9% current smokers, 60% hypercholesterolemic, and 47% hypertensive). OSA was present in 76%. CAC was present in 67% of OSA patients and 31% of non-OSA patients (p<0.001). The median CAC score (Agatston Units) was 9 in OSA patients and 0 in non-OSA patients (p<0.001). The median CAC score was higher as OSA severity increased (p for trend by AHI quartile<0.001). With multivariate adjustment, the odds ratio for CAC increased with OSA severity. Using the first AHI quartile as reference, the adjusted odds ratio for the second, third, and fourth quartiles were 2.1 (p=0.12), 2.4 (p=0.06), and 3.3 (p=0.03) respectively.
Conclusions
In patients without clinical coronary disease, the presence and severity of OSA is independently associated with the presence and extent of CAC. OSA identifies patients at risk for coronary disease and may represent a highly prevalent modifiable risk factor.
Keywords: Calcium, Coronary artery disease, Obstructive sleep apnea, Risk factors
Introduction
Obstructive sleep apnea (OSA) is a common medical condition, with an estimated prevalence of 20% middle-aged adults having at least mild OSA and 4 to 9% having OSA symptoms. The prevalence of OSA and its cardiovascular consequences are becoming increasingly recognized.1,2
OSA causes repetitive acute hypoxemic episodes and sleep deprivation, leading to abnormalities in cardiovascular regulation. Apneic spells lead to sympathetic activation, increased blood pressure, and endothelial dysfunction.3, 4 A proinflammatory state is also present in patients with OSA, evidenced by increased C-reactive protein levels.5, 6 In addition, OSA is associated with metabolic dysregulation, including insulin resistance and elevated leptin levels,7, 8 as well as diabetes, and obesity.9–11
OSA may cause systemic hypertension,12–19 and is associated with a higher incidence of myocardial infarction and cardiovascular mortality.20, 21 While OSA has been linked to subclinical carotid atherosclerosis, no previous studies have directly evaluated the relationship between OSA and measures of subclinical coronary atherosclerotic disease.22, 23 Electron beam computed tomography (EBCT) is an effective tool to quantify the magnitude of coronary artery calcification, which is a marker of coronary atherosclerotic burden,24 and associated with coronary events and asymptomatic myocardial ischemia.25–32 Coronary artery calcification has also shown to predict coronary artery stenoses as defined by angiography, intracoronary ultrasound, and histology.33–36 The goal of the present study was to determine the presence and magnitude of any association between OSA and subclinical coronary disease, as measured by coronary artery calcification.
Materials and Methods
Subjects
We performed a historical cross-sectional study of consecutive patients at our institution who underwent polysomnography from March 1, 1991 to December 31, 2003, and who also underwent EBCT for coronary artery calcification quantification within 36 months of polysomnography. Using administrative records, we identified patients that were referred by their caring physicians to the Mayo Clinic Sleep Disorders Clinic and underwent polysomnography for clinically suspected sleep disorders and were also referred for EBCT for coronary artery disease risk stratification. Patients were asymptomatic for coronary artery disease prior to EBCT. For patients with multiple EBCTs or polysomnograms, the first such study was used for analysis. We excluded patients who prior to EBCT or polysomnography had documented coronary artery disease by angiography, prior coronary artery bypass, or history of myocardial infarction. Patients were also excluded if the EBCT report did not quantify the amount of coronary artery calcification, if any, in Agatston units. Diabetes was defined as a fasting blood glucose level >126 mg/dL and/or use of antidiabetic medication. Hypercholesterolemia was defined as total cholesterol >240 mg/dL and/or use of a lipid-lowering medication. Hypertension was defined as systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg, or use of an antihypertensive medication.
Polysomnography
Polysomnography was performed using the standard clinical protocol at the Mayo Clinic Sleep Disorders Center, which utilized a digital polygraph (NCI, Wisconsin) that measured three electroencephalograms, two electro-oculograms, submental and tibialis electromyograms, rib cage and abdominal respiratory inductance plethysomnography (RIP, Ambulatory Monitoring, Inc., New York), nasal pressure transducer (PTAF Pressure Transduce, Pro-Tech Inc., Washington), pulse oximetry (Ohmeda 3740, Wisconsin), sonography via decibel meter (Tandy Corp, Texas), and body position measurements. Sleep staging and arousals were scored using 30-s epochs with criteria by Rechtschaffen, Kales, and the American Sleep Disorders Association.37, 38 OSA was diagnosed by standard criteria, requiring an apnea-hypopnea index (AHI) ≥ 5 events per hour.37
Electron Beam Computed Tomography
EBCT imaging was performed using a GE Imatron-150 with contiguous 3-mm slice thickness and 100 msec scanning time, with a total of 40 slices extending from carina to diaphragm. Tomographic imaging was triggered through three-lead electrocardiography at 80% of the R-to-R interval during end-inspiration to minimize artifact. The degree of coronary artery calcification was determined using a standard protocol. An automated system was utilized to score the tomograms after scan acquisition. Lesion area in square millimeters was obtained, and the peak CT scan density of each lesion was calculated. Lesion area was defined as a plaque of 4 consecutive pixels (area of 1.0 mm2) with a density of >130 Hounsfield units. A score for each lesion was generated by multiplying the measured area of any lesion >1.0 mm2 by an attenuation coefficient based on its peak CT number. The summation of the scores for each lesion in each vessel was used to determine the overall calcium score, and a percentile score adjusted for age and gender, according to the Agatston quantification algorithm.24
Informed consent was obtained from all study participants, and this study was approved by the Mayo Foundation Institutional Review Board.
Statistical Analysis
OSA was defined by an AHI ≥ 5. The severity of OSA was determined using quartiles of the AHI distribution. Patients were classified as having subclinical coronary disease if the coronary artery calcification score was > 0. Chi-square and independent-sample t-test were used to compare demographics and clinical characteristics. We compared the median value of coronary artery calcification among patients with increasing severity of OSA, based on AHI quartiles, with the non-parametric Kruskall-Wallis test because of the non-Gaussian distribution of coronary calcification scores. We compared the mean percentile coronary calcification among AHI quartiles using one-way ANOVA. Step-wise logistic regression models tested the independent association between measures of obstructive sleep apnea and the presence of coronary calcification after adjusting for sex, body-mass index, current smoking status, hypertension, diabetes mellitus and dyslipidemia. OSA was used as a categorical variable and the second, third and highest quartiles were compared to the first quartile. Different measures of OSA (AHI, lowest overnight oxygen saturation and average overnight oxygen saturation) were included in the models separately. Statistical significance was defined as a p-value < 0.05 for all analyses.
RESULTS
The study sample comprised 202 patients. The average time between EBCT and polysomnography was 16 months (0 to 35.5 months). Of the 103 OSA patients having EBCT after polysomnography, 38 patients initiated CPAP therapy for an average of 18 months. Tables 1 and 2 provide a comparison of characteristics for patients with and without OSA and when grouped by OSA severity, respectively. Patients with OSA were more likely to be older, male, obese, and had more traditional risk factors than non-OSA patients except for active smoking status. Coronary artery calcification was present in 67% of OSA patients and 31% of non-OSA patients (p<0.001). The median coronary artery calcification score was significantly greater in OSA (9 Agatston units) compared to non-OSA patients (0 Agatston units, p<0.001). The mean amount of coronary artery calcification was significantly higher in patients with OSA (144 Agatston units) compared to patients without OSA (26 Agatston units, p=0.001) and OSA patients had higher percentile coronary artery calcification scores than non-OSA patients, 46th versus 20th percentile (p<0.001) respectively. Coronary artery calcification had a strong direct correlation with the severity of OSA, based on the AHI (Table 3). The median and mean score of coronary artery calcification as well as the percentile score increased as OSA worsened (all with a p<0.001). The strength of the association remained essentially unchanged after adjustment for age and sex and after multivariate adjustment for traditional coronary risk factors (Figure 1). Coronary calcification was not associated with lowest or average overnight oxygen saturation.
Table 1.
Comparison of Baseline Characteristics Between Patients With and Without Obstructive Sleep Apnea
| Variables | Patients with OSA* (n=154) | Patients without OSA (n=48) | P-value |
|---|---|---|---|
| Age, y | 51 | 46 | <0.001 |
| Male sex, % | 72 | 63 | 0.21 |
| Diabetes mellitus, % | 9 | 4 | 0.46 |
| Hypercholesterolemia, % | 64 | 48 | 0.03 |
| Hypertension, % | 49 | 40 | 0.44 |
| Active smoking, % | 8 | 15 | 0.07 |
| Past smoking, % | 48 | 46 | 0.20 |
| Body Mass Index mean, kg/m2 | 35 | 31 | 0.15 |
| Fasting blood glucose mean, mg/dL | 105 | 97 | 0.01 |
| LDL mean, mg/dL | 132 | 131 | 0.83 |
| Systolic blood pressure mean, mmHg | 132 | 126 | 0.02 |
| Diastolic blood pressure mean, mmHg | 82 | 79 | 0.03 |
| Presence of coronary artery calcification | 67 | 31 | <0.001 |
OSA = obstructive sleep apnea
Table 2.
Comparison of Baseline Characteristics Between Patients With Increasing Obstructive Sleep Apnea Severity Measured by AHI* Quartile
| Variables | First Quartile (n=53) | Second Quartile (n=50) | Third Quartile (n=49) | Fourth Quartile (n=50) | P-value |
|---|---|---|---|---|---|
| Age, y | 46 | 51 | 51 | 52 | 0.001 |
| Male sex, % | 64 | 58 | 76 | 82 | 0.04 |
| Diabetes mellitus, % | 4 | 8 | 16 | 4 | 0.12 |
| Hypercholesterolemia, % | 49 | 66 | 65 | 62 | 0.39 |
| Hypertension, % | 40 | 36 | 49 | 62 | 0.09 |
| Active smoking, % | 13 | 10 | 4 | 10 | 0.48 |
| Past smoking, % | 47 | 52 | 35 | 56 | 0.24 |
| Body Mass Index mean, kg/m2 | 30 | 32 | 33 | 40 | 0.03 |
| Fasting blood glucose mean, mg/dL | 97 | 106 | 108 | 104 | 0.22 |
| LDL mean, mg/dL | 131 | 129 | 131 | 137 | 0.67 |
| Systolic blood pressure mean, mmHg | 127 | 130 | 131 | 134 | 0.11 |
| Diastolic blood pressure mean, mmHg | 80 | 82 | 80 | 84 | 0.13 |
AHI = apnea hypopnea index
Table 3.
Mean AHI* and Coronary Artery Calcification Score by Obstructive Sleep Apnea Severity Measured by AHI Quartile
| Variable | First AHI Quartile | Second AHI Quartile | Third AHI Quartile | Fourth AHI Quartile | P-value for trend |
|---|---|---|---|---|---|
| Mean AHI (Range) | 2.2 (0–5) | 8.9 (6–13) | 20.5 (14–32) | 63.4 (≥33) | <0.001 |
| Presence of Coronary Artery Calcification, % | 36 | 58 | 65 | 76 | <0.001 |
| Median Coronary Artery Calcification Score, Agatston Units (Range) | 0 (0–500) | 4 (0–2300) | 6 (0–245) | 44 (0–2196) | <0.001 |
| Mean Coronary Artery Calcification Score, Agatston Units (Standard deviation) | 32 (97) | 109 (340) | 38 (60) | 286 (569) | <0.001 |
| Mean Coronary Artery Calcification Percentile for age and sex (Standard deviation) | 22 (33) | 43 (41) | 41 (35) | 54 (38) | <0.001 |
AHI = apnea hypopnea index
Figure 1.
Multivariate Analysis for the Association Between Obstructive Sleep Apnea Severity Measured by AHI Quartile and Coronary Artery Calcification
 Adjusted for age and gender
Adjusted for age and gender
 Adjusted for age, gender and traditional risk factors
Adjusted for age, gender and traditional risk factors
Traditional risk factors = diabetes mellitus, hypercholesterolemia, active smoking, hypertension, and body mass index
DISCUSSION
The novel finding of the present study is that OSA is associated with subclinical coronary artery disease, independent of traditional coronary risk factors. Not only was coronary artery calcification more likely to be present in patients with OSA, but the amount of coronary artery calcification increased with increasing severity of OSA.
Similar to previous studies that have shown OSA to be associated with coronary risk factors, our patients with OSA had more comorbidities than the non-OSA group.9–19, 39 While associations between OSA and coronary events have been reported,20, 21 no prior study has identified a relationship between OSA and subclinical coronary disease in patients without established coronary artery disease. Prior studies have shown an association of OSA and atherosclerosis in mice and patients with known symptomatic CAD by angiography have been evaluated for sleep disordered breathing.40,41 The present findings may represent an intermediate step from the pathophysiology of OSA to the development of clinical outcomes.
Our findings highlight not only an association between coronary artery calcification and increasing OSA severity, but also suggest a high prevalence of subclinical coronary artery disease in patients with OSA. Detection of subclinical coronary artery calcification is important for risk stratification and treatment decisions. Traditional cardiac risk factors have been shown to correlate with the severity of coronary artery calcification.42–44 However, several studies have pointed out that Framingham risk scores may under-recognize patients with subclinical coronary artery calcification who are at higher risk.45–47 In one study, patients with diabetes had the same survival rate as non-diabetics if no calcification was present but had increased mortality if any calcification was present.48 Coronary artery calcification is associated with silent ischemia and coronary events.25–32 Our data further show that an AHI >15 is associated with a mean coronary artery calcification score of 162 Agatston Units that is suggestive of significant coronary lesions or extensive atherosclerotic heart disease, and increased risk of ischemia and coronary events.20, 21, 28, 32, 49–53
Our findings are strengthened by the use of the gold standard test, polysomnography, to diagnose OSA, and inclusion of patients without prior polysomnography, thereby eliminating the possibility of prior OSA therapy. Also, patients had no clinical history of coronary disease, and data used were from each patient’s first EBCT. All EBCT and polysomnograms were performed under standardized protocols at a single facility minimizing variability of methodology and interpretation of these measures.
Our findings confirm and extend the results of other studies assessing the potential interaction between OSA and coronary artery disease The AHI, which can be considered a composite measure of hypoxia severity and apneic episode frequency, correlated to the coronary atherosclerosis present.40–41 The magnitude of the association between OSA and coronary artery calcification we identified is similar to that shown in two longitudinal studies that reported associations between OSA and incidental cardiovascular disease, stroke, and death.20, 21
Interestingly, the amount of coronary calcification did not correlate to the average overnight oxygen saturation or with the lowest overnight oxygen desaturation. Potential explanations for this include the possibility that mechanisms linking CAD and OSA relate more to the hyperadrenergic state generated by apneic episodes, more than to the hypoxia itself. Other studies have suggested different stresses and disease mechanisms for different comorbidities linked to OSA. These various stresses include hypoxia, but also carbon dioxide retention, sleep disruption, truncated total sleep time, and strenuous respiratory efforts. The effect of these stressors is manifested through the neuro-humoral, vascular, and endocrine responses, but the individual or collective contribution of these in OSA is unknown. One study showed that the frequency of respiratory related arousals most strongly correlated with higher blood pressure, more so than nocturnal hypoxemia in OSA patients.54 Similarly, in a study by Norman the treatment of OSA with CPAP lowers AHI and blood pressure, but treatment with supplemental nocturnal oxygen did not affect blood pressure despite improved oxyhemoglobin saturation.55
Potential limitations of the present study include the inherent limitations of a cross-sectional design, which cannot identify causal or temporal relationships between OSA and subclinical coronary artery calcification. Given the established effects of OSA on cardiovascular disease mechanisms, the most plausible explanation is that OSA contributes to coronary atherosclerosis and not vice versa. Another potential limitation is the time been the polysomnography and EBCT, up to 3 years in some cases. The interval was variable since referrals for these studies were unrelated to one another. However, the effects of OSA on vascular health take many years to develop and coronary calcifications scores should not significantly change within 3 years. Furthermore, any bias incorporated by time delay between the studies would be toward the null hypothesis. Selection bias is another limitation, since the study sample was comprised of patients referred for polysomnography and cardiac EBCT. This may have resulted in a study sample with higher risk individuals than the average individual with OSA in the community. However, the number of males in the OSA group fit with prevalence data previously published.1 Gender is an important factor and possible confounder, and an analysis stratified by gender would be ideal. With our number of patients, however, the statistical power to do this analysis would be very low and the results difficult to interpret. Thus, we cannot say with our current data if the association applies to both sexes or if it is limited to men or women. Our use of multivariate regression analysis included sex as a covariate and therefore most of the potential confounding effect by sex and the other confounders should have been accounted for. However, the results likely are not directly applicable to individuals from the community who would not have been otherwise referred for polysomnography or cardiac risk assessment.56 But, importantly, our results should be applicable to the large population of patients who are seen in medical clinics who are at risk for sleep disorders or coronary artery disease. A prospective study of individuals from the community was outside the scope of our research. It is not clear if the results in this study can be extended to other ethnic groups, since our study cohort comprised mostly white Caucasian patients.
In conclusion, we found a strong association between OSA and subclinical coronary disease, as measured by coronary artery calcification. This association was independent of traditional risk factors and correlated with the severity of OSA. The presence and severity of OSA should be considered for coronary artery disease risk stratification, and in general, OSA should be an important consideration in the practice of preventive cardiology.
Abbreviations
- AHI
apnea-hypopnea index
- CAC
coronary artery calcification
- EBCT
electron beam computed tomography
- OSA
obstructive sleep apnea
Footnotes
Disclosures: Dr. Lopez-Jimenez is a recipient of a Clinical Scientist Development Award from the American Heart Association. Dr. Somers is supported by NIH grants HL-65176, HL-70302, HL-73211 and M01-RR00585.
Contributor Information
Dan Sorajja, Email: sorajja.dan@mayo.edu.
Apoor S Gami, Email: gami.apoor@mayo.edu.
Virend K Somers, Email: somers.virend@mayo.edu.
Thomas R Behrenbeck, Email: behrenbeck.thomas@mayo.edu.
Arturo Garcia-Touchard, Email: agtouchard@gmail.com.
Francisco Lopez-Jimenez, Email: lopez@mayo.edu.
References
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Namen AM, Dunagan DP, Fleischer A, et al. Increased physician-reported sleep apnea: the National Ambulatory Medical Care Survey. Chest. 2002;121(6):1741–7. doi: 10.1378/chest.121.6.1741. [DOI] [PubMed] [Google Scholar]
- 3.Phillips BG, Narkiewicz K, Pesek CA, et al. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens. 1999;17(1):61–6. doi: 10.1097/00004872-199917010-00010. [DOI] [PubMed] [Google Scholar]
- 4.Schulz R, Schmidt D, Blum A, et al. Decreased plasma levels of nitric oxide derivatives in obstructive sleep apnoea: response to CPAP therapy. Thorax. 2000;55(12):1046–51. doi: 10.1136/thorax.55.12.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105(21):2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 6.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165(7):934–9. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 7.Punjabi NM, Sorkin JD, Katzel LI, et al. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165(5):677–82. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 8.Phillips BG, Kato M, Narkiewicz K, et al. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279(1):H234–7. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 9.Schafer H, Koehler U, Ewig S, et al. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology. 1999;92(2):79–84. doi: 10.1159/000006952. [DOI] [PubMed] [Google Scholar]
- 10.Reichmuth KJ, Austin D, Skatrud JB, et al. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172(12):1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borgel J, Sanner BM, Bittlinsky A, et al. Obstructive sleep apnoea and its therapy influence high-density lipoprotein cholesterol serum levels. Eur Respir J. 2006;27(1):121–7. doi: 10.1183/09031936.06.00131304. [DOI] [PubMed] [Google Scholar]
- 12.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 13.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc. 2004;79(8):1036–46. doi: 10.4065/79.8.1036. [DOI] [PubMed] [Google Scholar]
- 14.Kraiczi H, Peker Y, Caidahl K, et al. Blood pressure, cardiac structure and severity of obstructive sleep apnea in a sleep clinic population. J Hypertens. 2001;19(11):2071–8. doi: 10.1097/00004872-200111000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320(7233):479–82. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 17.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–52. [PubMed] [Google Scholar]
- 18.Grote L, Ploch T, Heitmann J, et al. Sleep-related breathing disorder is an independent risk factor for systemic hypertension. Am J Respir Crit Care Med. 1999;160(6):1875–82. doi: 10.1164/ajrccm.160.6.9811054. [DOI] [PubMed] [Google Scholar]
- 19.Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med. 2000;160(15):2289–95. doi: 10.1001/archinte.160.15.2289. [DOI] [PubMed] [Google Scholar]
- 20.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 21.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 22.Drager LF, Bortolotto LA, Lorenzi MC, et al. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172(5):613–8. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 23.Minoguchi K, Yokoe T, Tazaki T, et al. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172(5):625–30. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- 24.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 25.Margolis JR, Chen JT, Kong Y, et al. The diagnostic and prognostic significance of coronary artery calcification. A report of 800 cases. Radiology. 1980;137(3):609–16. doi: 10.1148/radiology.137.3.7444045. [DOI] [PubMed] [Google Scholar]
- 26.Berman DS, Wong ND, Gransar H, et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol. 2004;44(4):923–30. doi: 10.1016/j.jacc.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 27.Schmermund A, Erbel R. Unstable coronary plaque and its relation to coronary calcium. Circulation. 2001;104(14):1682–7. doi: 10.1161/hc3901.093339. [DOI] [PubMed] [Google Scholar]
- 28.Kondos GT, Hoff JA, Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107(20):2571–6. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 29.Arad Y, Goodman KJ, Roth M, et al. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46(1):158–65. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 30.He ZX, Hedrick TD, Pratt CM, et al. Severity of coronary artery calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation. 2000;101(3):244–51. doi: 10.1161/01.cir.101.3.244. [DOI] [PubMed] [Google Scholar]
- 31.Blumenthal RS, Becker DM, Yanek LR, et al. Comparison of coronary calcium and stress myocardial perfusion imaging in apparently healthy siblings of individuals with premature coronary artery disease. Am J Cardiol. 2006;97(3):328–33. doi: 10.1016/j.amjcard.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 32.Anand DV, Lim E, Raval U, et al. Prevalence of silent myocardial ischemia in asymptomatic individuals with subclinical atherosclerosis detected by electron beam tomography. J Nucl Cardiol. 2004;11(4):450–7. doi: 10.1016/j.nuclcard.2004.06.125. [DOI] [PubMed] [Google Scholar]
- 33.Kajinami K, Seki H, Takekoshi N, et al. Coronary calcification and coronary atherosclerosis: site by site comparative morphologic study of electron beam computed tomography and coronary angiography. J Am Coll Cardiol. 1997;29(7):1549–56. doi: 10.1016/s0735-1097(97)00090-9. [DOI] [PubMed] [Google Scholar]
- 34.Budoff MJ, Georgiou D, Brody A, et al. Ultrafast computed tomography as a diagnostic modality in the detection of coronary artery disease: a multicenter study. Circulation. 1996;93(5):898–904. doi: 10.1161/01.cir.93.5.898. [DOI] [PubMed] [Google Scholar]
- 35.Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92(8):2157–62. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 36.Baumgart D, Schmermund A, Goerge G, et al. Comparison of electron beam computed tomography with intracoronary ultrasound and coronary angiography for detection of coronary atherosclerosis. J Am Coll Cardiol. 1997;30(1):57–64. doi: 10.1016/s0735-1097(97)00147-2. [DOI] [PubMed] [Google Scholar]
- 37.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–89. [PubMed] [Google Scholar]
- 38.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–84. [PubMed] [Google Scholar]
- 39.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290(14):1906–14. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 40.Savransky V, Nanayakkara A, Li J, et al. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175(12):1290–7. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayashi M, Fujimoto K, Urushibata K, et al. Nocturnal oxygen desaturation correlates with the severity of coronary artherosclerosis in coronary artery disease. Chest. 2003;124(3):936–941. doi: 10.1378/chest.124.3.936. [DOI] [PubMed] [Google Scholar]
- 42.Hoff JA, Daviglus ML, Chomka EV, et al. Conventional coronary artery disease risk factors and coronary artery calcium detected by electron beam tomography in 30,908 healthy individuals. Ann Epidemiol. 2003;13(3):163–9. doi: 10.1016/s1047-2797(02)00277-6. [DOI] [PubMed] [Google Scholar]
- 43.Taylor AJ, Arora NS, Bindeman J, et al. Conventional, emerging, heredity, lifestyle, and psychosocial coronary risk factors: relationships to subclinical atherosclerosis. Prev Cardiol. 2006;9(1):25–32. doi: 10.1111/j.1520-037x.2006.04301.x. [DOI] [PubMed] [Google Scholar]
- 44.Wong ND, Sciammarella MG, Polk D, et al. The metabolic syndrome, diabetes, and subclinical atherosclerosis assessed by coronary calcium. J Am Coll Cardiol. 2003;41(9):1547–53. doi: 10.1016/s0735-1097(03)00193-1. [DOI] [PubMed] [Google Scholar]
- 45.Taylor AJ, Feuerstein I, Wong H, et al. Do conventional risk factors predict subclinical coronary artery disease? Results from the Prospective Army Coronary Calcium Project. Am Heart J. 2001;141(3):463–8. doi: 10.1067/mhj.2001.113069. [DOI] [PubMed] [Google Scholar]
- 46.Michos ED, Vasamreddy CR, Becker DM, et al. Women with a low Framingham risk score and a family history of premature coronary heart disease have a high prevalence of subclinical coronary atherosclerosis. Am Heart J. 2005;150(6):1276–81. doi: 10.1016/j.ahj.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 47.Cassidy AE, Bielak LF, Zhou Y, et al. Progression of subclinical coronary atherosclerosis: does obesity make a difference? Circulation. 2005;111(15):1877–82. doi: 10.1161/01.CIR.0000161820.40494.5D. [DOI] [PubMed] [Google Scholar]
- 48.Raggi P, Shaw LJ, Berman DS, et al. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43(9):1663–9. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 49.Gami AS, Howard DE, Olson EJ, et al. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352(12):1206–14. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 50.Rumberger JA, Brundage BH, Rader DJ, et al. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74(3):243–52. doi: 10.4065/74.3.243. [DOI] [PubMed] [Google Scholar]
- 51.Pletcher MJ, Tice JA, Pignone M, et al. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med. 2004;164(12):1285–92. doi: 10.1001/archinte.164.12.1285. [DOI] [PubMed] [Google Scholar]
- 52.Raggi P, Callister TQ, Cooil B, et al. Identification of patients at increased risk of first unheralded acute myocardial infarction by electron-beam computed tomography. Circulation. 2000;101(8):850–5. doi: 10.1161/01.cir.101.8.850. [DOI] [PubMed] [Google Scholar]
- 53.Nasir K, Michos ED, Blumenthal RS, et al. Detection of high-risk young adults and women by coronary calcium and National Cholesterol Education Program Panel III guidelines. J Am Coll Cardiol. 2005;46(10):1931–6. doi: 10.1016/j.jacc.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 54.Sulit L, Storfer-Isser A, Kirchner HL, et al. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep. 2006;29(6):777–83. doi: 10.1093/sleep/29.6.777. [DOI] [PubMed] [Google Scholar]
- 55.Norman D, Loredo JS, Nelesen RA, et al. Effects of continuous positive airway pressure versus supplemental oxygen on 24-hour ambulatory blood pressure. Hypertension. 2006;47(5):840–5. doi: 10.1161/01.HYP.0000217128.41284.78. [DOI] [PubMed] [Google Scholar]
- 56.Walker SH, Duncan DB. Estimation of the probability of an event as a function of several independent variables. Biometrika. 1967;54(1):167–79. [PubMed] [Google Scholar]