Abstract
Purpose
We have previously reported increased levels of Osteonectin/SPARC transcript in age-related cataractous compared to normal human lenses. The purpose of the present study was to evaluate the corresponding levels of osteonectin/SPARC protein in age-related cataractous relative to normal lenses and to evaluate the levels of osteonectin/SPARC transcript in specific types of age-related human cataracts. The spatial expression of osteonectin/SPARC was also evaluated in normal human lenses.
Methods
Specific types of age-related cataracts were collected and graded. Normal human lenses were microdissected into epithelia and fibers. Osteonectin/SPARC protein levels were monitored by Western immunoblotting, and transcript levels were evaluated by reverse transcriptase polymerase chain reaction (RT-PCR). Osteonectin/SPARC expression patterns were examined by RT-PCR and by immunostaining.
Results
Higher levels of osteonectin/SPARC protein were detected in age-related cataractous relative to normal human lenses. Increased levels of osteonectin/SPARC transcript were also detected in posterior-subcapsular and nuclear cataractous lenses relative to normal lenses. Osteonectin/SPARC transcripts were detected in the lens epithelium but not fibers. Osteonectin/SPARC protein levels were highest in the peripheral lens epithelium.
Conclusions
Consistent with our previous studies on osteonectin/SPARC mRNA levels, osteonectin/SPARC protein levels were also elevated in cataractous compared to normal human lenses. Increased levels of osteonectin/SPARC mRNA were also found in nuclear and posterior-subcapsular cataracts relative to normal lenses. Osteonectin/SPARC expression is confined to the lens epithelium, and osteonectin/SPARC levels are highest in the peripheral lens epithelium.
Age-related cataract is a multifactorial disease with a poorly understood etiology [1–3]. One key player in the development of age-related cataract is the lens epithelium [4,5] which contains most of the enzymes in the lens [1–5] and can communicate with the underlying fiber cells [6–8]. Damage to the lens epithelium is associated with cataract formation, and numerous gene expression changes in the lens epithelium are associated with lens opacity [9–15]. We are interested in identifying genes of the human lens epithelium which respond to the presence of age-related cataract through an increase or decrease in their expression levels. Isolation of these genes points to specific components and functions of the lens epithelium that might be important for the maintenance of lens transparency. To this end, we have previously used reverse transcriptase differential display and semi-quantitative RT-PCR to detect multiple gene expression differences between age-related cataractous and normal human lens epithelia [14,15]. One of the genes identified in these studies was osteonectin (also called secreted protein acidic and rich in cysteine [SPARC]) [16,17]. In this study [15], differential display was used to identify increased levels of osteonectin/SPARC transcript between 50 epithelia from age-related cataractous lenses and 25 epithelia from normal lenses. Increased expression of SPARC was confirmed by RT-PCR analysis of an additional 19 epithelia from cataractous lenses and 8 epithelia from normal lenses. RT-PCR was also used to demonstrate high levels of SPARC mRNA in 6 epithelia from individual cataractous lenses and to analyze the expression of SPARC mRNA between whole and central normal human lens epithelium.
Osteonectin/SPARC, also termed BM-40 [18], is a 43 kDa Ca+2-binding protein with diverse biological functions [19]. In addition to the increased levels of mRNA in human age-related cataract [15], deletion of the SPARC gene results in cataract formation in mice [20,21].
In this report we show that SPARC protein and mRNA are increased in cataractous compared to normal human lenses. This finding is particularly important with regard to our previous work on cataract-specific gene expression, since it provides confirmation that the transcript levels detected by differential-display and RT-PCR are consonate with the corresponding protein levels. This report also provides evidence that SPARC levels are increased in both nuclear and posterior-subcapsular age-related cataracts, relative to normal lenses and that expression of SPARC in the human lens is not only epithelium-specific but might in fact differ between the central and peripheral lens epithelium.
METHODS
Microdissection of human lenses
Cataractous epithelial tags were obtained within minutes of surgery and were extracted and graded by K.M.M. The LOCS III system was used with slit lamp imaging for cataract classification with the exception that no photos were taken and no reference photos were used. For normal human lenses, the lens epithelium was microdissected away from the underlying fiber cells. For both normal and cataractous lens epithelia, contaminating fiber cells were removed and the resulting tissues were washed to remove potential contaminates as previously described [22,23].
Isolation of RNA and preparation of protein extracts
Total RNA was prepared from pooled epithelia by phenol/guanidinium isothiocyanate extraction [24]. Lens protein extracts were prepared as previously described [23].
Reverse Transcriptase-PCR
RT-PCR was performed by modification of established procedures [25]. Indicated amounts of RNA were examined using the One Step RT-PCR system (Gibco-BRL, Gaithersburg, MD) as recommended by the manufacturer. The primer concentration used in these studies (200 nM) was chosen to insure that primers would not be limiting and was determined by performing control reactions at different primer concentrations (data not shown). Control reactions employed primers specific for glyceraldehyde phosphate dehydrogenase (GAPDH) were 5′-CCACCCATGGCAAATTCCATGGCA-3′ and 5′-CCACCTGGACTGGACGGCAGATCT-3′. PCR-cycling parameters (30–35 cycles) were derived from preliminary experiments ensuring linear product formation over the amounts of RNA and other reagents indicated (data not shown). Controls lacking reverse transcriptase were performed under identical conditions with heat-inactivated RT. The sequences of the osteonectin primers were 5′-CCTGAGGCTGTAACTGAGAGAAAG-3′ and 5′-GTGGGAGGGGAAACAAGAAGATAA-3′. Products were separated on agarose gels and visualized by ethidium bromide staining. In order to ensure that RT-PCR products represented authentic SPARC, separate control reactions were performed and the products cloned and sequenced.
Western Analysis
Indicated amounts of protein were denatured by boiling in 10% SDS buffer (10% w/v SDS; 0.5 M Tris-HCl, pH 6.8; 5% [v/v] 2-mercaptoethanol; 5% [v/v] glycerol) and were resolved by electrophoresis on 12.5% SDS-polyacrylamide gels. The proteins were subsequently transferred (30 V for 1 h in 12 mM Tris-HCl, 96 mM glycine, 15% methanol) to nitrocellulose filters. The resulting blots were fixed in 25% isopropanol/10% acetic acid for 1 h, washed with phosphate buffered saline (PBS) for 30 min, and blocked with 3% BSA in PBS for 1 h. The blot was washed thee times in TBS (10 mM Tris-HCl, 50 mM NaCl, pH 7.5) over 15 min. and incubated overnight with a 1:500 dilution of osteonectin/SPARC antibody in 1% BSA in tris-buffered saline (TBS). The blot was subsequently washed three times in TBS over 1 h and immunoreactive osteonectin/SPARC was visualized using ECL western blotting reagents (Amersham-Pharmacia, Piscataway, NJ) as specified by the manufacturer. Identical procedures were used for immunoblot analysis of αB-crystallin with a 1:400 dilution of antibody.
Immunostaining
A 16 year-old female human lens (less than 18 hours post-mortem) was micodissected and the lens epithelium was fixed in 4% paraformaldehyde in PBS overnight, followed by cryoprotection overnight in 30% sucrose in PBS prior to embedding. 14 μm frozen sections were prepared and air-dried. Sections were blocked for one hour at room temperature in DMEM, 10% fetal calf serum, 1% goat serum, and 0.1% Triton X-100. Sections were incubated overnight with a 1:600 dilution of anti-SPARC antibody in blocking solution at 4 °C overnight. After five washes with 0.1% Tween 20 in PBS, sections were incubated with streptavidinconjugated secondary antibody (Vector Laboratories, Burlingame, CA) and were visualized by use of the Vectastain Elite kit as specified by the manufacturer. Photographs were taken with a Nikon E800 microscope equipped with a SPOTII digital camera. Identical procedures were used for immunocytochemical analysis of control αB-crystallin (1:400 dilution of antibody) and pre-immune serum (1:600 dilution) of the same lens.
RESULTS
Analysis of Osteonectin/SPARC protein levels in age-related cataractous and normal human lenses
Levels of SPARC protein in cataractous and normal human lens epithelia were monitored by immunoblotting. The data are shown in Figure 1. Fifteen age-related cataractous lenses were combined in the preparation of the cataractous lens extract, and 4 normal lenses (average age 72 years) were combined in the preparation of the normal lens extract. The types and ages of cataracts pooled in the preparation of the protein extract for immunoblotting are shown in Table 1. Cataractous or normal lenses from donors exposed to steroids were excluded. Whereas equal amounts of αB-crystallin were detected between cataractous (Figure 1, lane 4, 2 μg) and normal lens extract (Figure 1, lane 5, 2 μg), osteonectin/SPARC was detected only in the cataractous lens extract (Figure 1, lane 2, 4 μg). The osteonectin/SPARC band detected with the lens extract migrated slightly above the band detected with purified control osteonectin/SPARC. The slight differences in migration between the lens band and the purified osteonectin/SPARC band are most likely due to differences in glycosylation which have been reported for osteonectin/SPARC [19]. No osteonectin/SPARC was detected in up to 20 μg of normal lens extract (Figure 1, lane 3). As much as 35 μg of extract was required to detect osteonectin/SPARC in the normal lens extract (data not shown).
Figure 1.
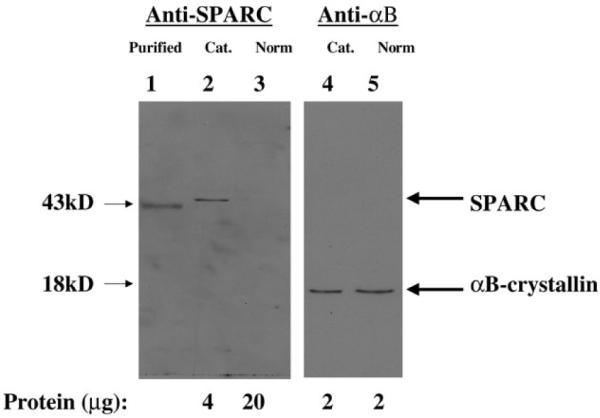
Western immunoblotting of osteonectin/SPARC in lens extracts. Extracts were prepared and polypeptides were separated and blotted as described in Materials and Methods. Shown is the autoradiogram of the corresponding blot. Lane 1 contains purified osteonectin/SPARC, lane 2 contains cataractous lens extract (cat), and lane 3 contains normal lens extract (norm) probed with anti-SPARC antibody. Lane 4 contains cataractous lens extract (cat) and lane 5 contains normal lens extract (norm) probed with anti-αB-crystallin antibody. Also shown are the osteonectin/SPARC and the αB-crystallin bands with their corresponding molecular weights. The protein added to each well is shown below the blot.
Table 1.
Cataract types used to prepare protein extracts
| Number of lenses |
Average age |
Cataract type |
|---|---|---|
| 7 | 75.6 | mixed |
| 6 | 69.8 | nuclear |
| 1 | 73 | cortical |
| 1 | 72 | posterior subcapsular |
RT-PCR of osteonectin/SPARC in specific types of cataracts
Osteonectin/SPARC mRNA levels were evaluated in pooled RNAs isolated from 7 nuclear (average age 75), 5 posterior subcapsular (average age 47), and 10 normal lenses (average age 58). Osteonectin/SPARC levels were increased in both nuclear (Figure 2, lane 1) and posterior-subcapsular (Figure 2, lane 2) lenses relative to normal lenses (Figure 2, lane 3). In control reactions, GAPDH was detected at equal levels in nuclear cataracts and normal lenses (Figure 2, compare lanes 1 and 3). GAPDH was found at consistently lower levels in posterior subcapsular (Figure 2, lane 2) comparison to normal or nuclear cataractous RNA preparations in multiple experiments (data not shown). GAPDH was chosen as a control in the present study because it is highly expressed in lens epithelium and has previously been used as a control for gene expression studies in this tissue [14,15]. Regardless of the absolute levels of GAPDH, osteonectin/SPARC expression was consistently increased in nuclear and posterior-subcapsular cataracts relative to normal human lenses.
Figure 2.
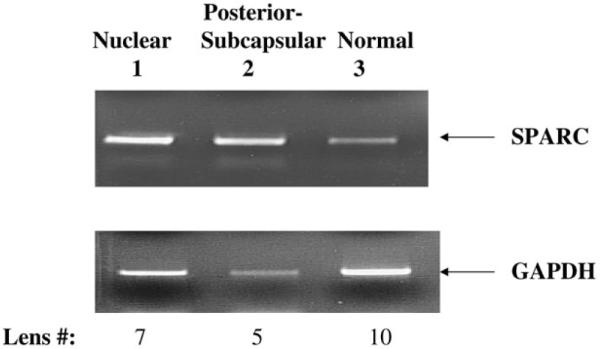
RT-PCR analysis of osteonectin/SPARC in specific cataracts. Ethidium bromide stained gel showing the levels of osteonectin/SPARC and GAPDH transcripts after 30 PCR cycles in nuclear cataracts (lane 1), posterior subcapsular cataracts (lane 2), and normal lenses (lane 3). The osteonectin/SPARC (419 bp) and GAPDH (600 bp) bands and the number of lenses used for each sample are indicated.
Spatial Expression of osteonectin/SPARC transcripts in normal human lenses
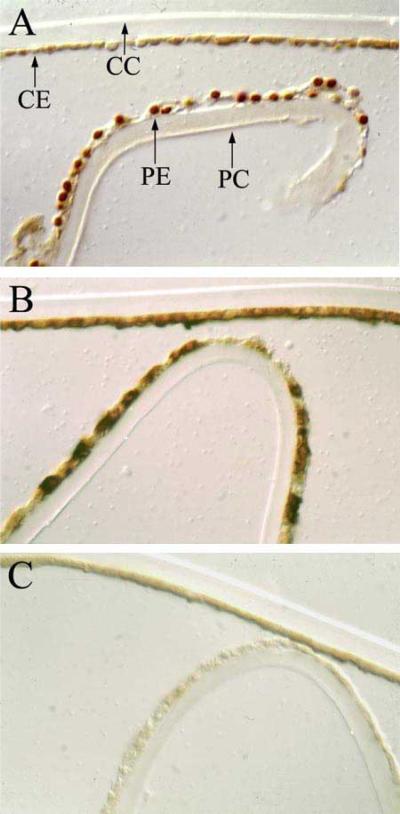
Osteonectin/SPARC transcript levels were monitored in combined extracts of 4 microdissected epithelia (average age 60) by RT-PCR. Osteonectin/SPARC transcript expression was restricted to the lens epithelium (Figure 3, compare lanes 3 and 4). As a control for RNA quality, equal amounts of GAPDH transcript were detected between the epithelium and the fibers (Figure 3, compare lanes 1 and 2). Osteonectin/SPARC protein expression was also examined in a normal human lens epithelium from a 16 year old female. Osteonectin/SPARC was detected at high levels in peripheral epithelium (PE, Figure 4A) and at levels barely above background in the central epithelium (CE, Figure 4A). No osteonectin/SPARC staining was observed in the lens capsule (CC) and (PC, Figure 4A). Osteonectin/SPARC may be present in the nucleus in the peripheral epithelium (PE) but not the central epithelium (CE, Figure 4A). However, it is not possible to distinguish nuclear from perinuclear staining in the present study, and further experiments will be required to pinpoint the subcellular location of osteonectin/SPARC. As a control, uniform staining of αB-crystallin was detected throughout the lens epithelium (Figure 4B). By contrast, αB-crystallin is known to be expressed abundantly thorughout the lens epithelium [5]. No staining was observed with pre-immune serum (Figure 4C).
Figure 3.
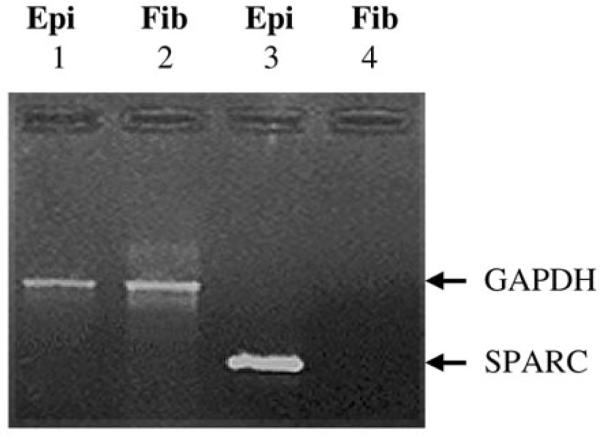
RT-PCR analysis of osteonectin/SPARC in specific cataracts. Ethidium bromide stained gel showing the levels of osteonectin/SPARC and GAPDH in microdissected normal lens epithelia (0.5 μg) and fiber cells (0.5 μg) after 35 PCR cycles. GAPDH transcript (600 bp) levels for lens epithelia (Epi) and fiber cells (Fib) are shown in lanes 1 and 2, respectively. Osteonectin/SPARC transcript (419 bp) levels for lens epithelia (Epi) and fiber cells (Fib) are shown in lanes 3 and 4, respectively.
Figure 4.
Immunostaining of human lens with anti-osteonectin/SPARC. A normal lens epithelium examined with (A) anti-osteonectin/SPARC antibody, (B) anti-αB-crystallin antibody, and (C) pre-immune serum. The central lens capsule (CC), central lens epithelium (CE), peripheral lens capsule (PC), and peripheral lens epithelium (PE) are marked with arrows.
DISCUSSION
In this report we have shown that osteonectin/SPARC protein and mRNA are increased in cataractous compared to normal human lenses (Figure 1). This result is important to our studies on cataract gene expression since it demonstrates that gene expression changes detected at the mRNA level are also detected at the protein level. Based on the amount of osteonectin/SPARC detected in equal amounts of protein extracts prepared from age-related cataractous or normal lenses, we estimate that osteonectin/SPARC protein is increased at least 5-fold in cataractous lenses. This level of SPARC is consistent with that reported for osteonectin/SPARC transcript levels between age-related cataractous and normal human lenses [15]. We are confident that the single osteonectin/SPARC band is authentic since a single band within the reported molecular weight range of osteonectin/SPARC [26] is recognized by the osteonectin/SPARC antibody. The osteonectin/SPARC antibody used in the present work is specific for human osteonectin/SPARC [26] despite its similarity in epitope to a related protein called SC-1/hevin [27]. We are confident that the protein detected in the lens epithelium is not SC-1 because we detected no band of 65,000 Da or greater, as reported for SC-1 [27]. In other studies, we have failed to detect SC1 transcripts in normal human lens epithelia by RT-PCR (Kantorow and Zhang, unpublished).
In addition to providing evidence for increased expression of osteonectin/SPARC protein between age-related cataractous and normal human lenses, we also found increased expression of osteonectin/SPARC in pooled samples of nuclear and posterior-subcapsular cataracts (Figure 2). Although the present data do not address the levels of osteonectin/SPARC in other subtypes of age-related cataract, they nevertheless provide evidence that increased expression of osteonectin/SPARC is not restricted to specific sub-types of human age-related cataracts. Further experiments, with larger numbers of lenses, will be required to produce the amounts of extract needed to examine the levels of osteonectin/SPARC protein in specific types of cataracts.
Osteonectin/SPARC mRNA expression was found to be restricted to the lens epithelium (Figure 3). Whereas relatively low amounts of osteonectin/SPARC protein were detected in the central lens epithelium of a 16 year old normal lens, relatively high amounts were detected in the peripheral lens epithelium (Figure 4). Interestingly, the protein may be present in the nucleus in the peripheral lens epithelium (Figure 4). Although other studies have reported nuclear localization of osteonectin/SPARC [28], further experiments including subcellular fractionation and electron microscopy will be required to evaluate the intercellular location of osteonectin/SPARC in the human lens.
At present, the exact function of osteonectin/SPARC in the maintenance of lens transparency is not known. Increased expression of osteonectin/SPARC by the cataractous lens epithelium is likely a response to stress caused by the underlying cataract. Thus, SPARC is likely to have a protective and/or repair function in the lens. It is clear that osteonectin/SPARC is important for the maintenance of lens transparency since deletion of the osteonectin/SPARC gene results in late-onset cataract [20,21]. The collective properties of SPARC are consistent with its having an important protective role in the lens. Osteonectin/SPARC is a Ca+2 -binding protein whose functions include among others: regulation of cell growth [29]; increased expression with cell injury [30]; and growth factor control [31]. It is widely expressed in human tissues [32,33], including most ocular tissues. It has been found in: bovine cornea [34]; bovine [34,35], mouse [20,21] and human [15] lens; quail [36], chicken [37] and monkey [38] retina; and human [39] trebecular meshwork. Further analysis of SPARC function in the lens should provide insight into those mechanisms that the lens has evolved to maintain its transparent function.
ACKNOWLEDGEMENTS
This work was supported grants EY13022 to MK and EY3897 to JH and QLH. The authors wish to express their gratitude to Paula Ousley and Rory Dunaway of the Lions Eye Bank of Oregon for the normal human lenses used in this work and to Lin-lin Ding, John Hawse, and Ashley Halstead for their expert support. No commercial interests or relationships are involved in this study.
REFERENCES
- 1.Spector A. Aging of the lens and cataract formation. In: Sekuler R, Kline D, Dismukes K, editors. Aging and human visual function. A.R. Liss; New York: 1982. pp. 27–43. [Google Scholar]
- 2.Brown NAP, Bron AJ. Lens disorders: a clinical manual of cataract diagnosis. Butterwoth-Heinemasnn; Oxford: 1996. pp. 53–133. [Google Scholar]
- 3.Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995;9:1173–82. [PubMed] [Google Scholar]
- 4.Hightower KR. The role of the lens epithelium in development of UV cataract. Curr Eye Res. 1995;14:71–8. doi: 10.3109/02713689508999916. [DOI] [PubMed] [Google Scholar]
- 5.Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–53. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 6.Rae JL, Bartling C, Rae J, Mathias RT. Dye transfer between cells of the lens. J Membr Biol. 1996;150:89–103. doi: 10.1007/s002329900033. [DOI] [PubMed] [Google Scholar]
- 7.Goodenough DA. The crystalline lens. A system networked by gap junctional intercellular communication. Semin Cell Biol. 1992;3:49–58. doi: 10.1016/s1043-4682(10)80007-8. [DOI] [PubMed] [Google Scholar]
- 8.Rae JL, Kuszak JR. The electrical coupling of epithelium and fibers in the frog lens. Exp Eye Res. 1983;36:317–26. doi: 10.1016/0014-4835(83)90114-8. [DOI] [PubMed] [Google Scholar]
- 9.Dovrat A, Weinreb O. Recovery of lens optics and epithelial enzymes after ultraviolet A radiation. Invest Ophthalmol Vis Sci. 1995;36:2417–24. [PubMed] [Google Scholar]
- 10.Delamere NA, Dean WL, Stidam JM, Moseley AE. Differential expression of sodium pump catalytic subunits in the lens epithelium and fibers. Ophthalmic Res. 1996;28(Suppl 1):73–6. doi: 10.1159/000267975. [DOI] [PubMed] [Google Scholar]
- 11.Andley UP, Walsh A, Kochevar IE, Reddan JR. Effect of ultraviolet-B radiation on protein synthesis in cultured lens epithelial cells. Curr Eye Res. 1990;9:1099–106. doi: 10.3109/02713689008997583. [DOI] [PubMed] [Google Scholar]
- 12.Li WC, Spector A. Lens epithelial cell apoptosis is an early event in the development of UVB-induced cataract. Free Radic Biol Med. 1996;20:301–11. doi: 10.1016/0891-5849(96)02050-3. [DOI] [PubMed] [Google Scholar]
- 13.Sidjanin D, Grdina D, Woloschak GE. UV-induced changes in cell cycle and gene expression within rabbit lens epithelial cells. Photochem Photobiol. 1996;63:79–85. doi: 10.1111/j.1751-1097.1996.tb02995.x. [DOI] [PubMed] [Google Scholar]
- 14.Kantorow M, Kays T, Horwitz J, Huang Q, Sun J, Piatigorsky J, Carper D. Differential display detects altered gene expression between cataractous and normal human lenses. Invest Ophthalmol Vis Sci. 1998;39:2344–54. [PubMed] [Google Scholar]
- 15.Kantorow M, Horwitz J, Carper D. Up-regulation of osteonectin/SPARC in age-related cataractous human lens epithelia. Mol Vis. 1998;4:17. [PubMed] [Google Scholar]
- 16.Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26:99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 17.Mason IJ, Murphy D, Munke M, Francke U, Elliott RW, Hogan BL. Developmental and transformation-sensitive expression of the Sparc gene on mouse chomosome 11. EMBO J. 1986;5:1831–7. doi: 10.1002/j.1460-2075.1986.tb04434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dziadek M, Paulsson M, Aumailley M, Timpl R. Purification and tissue distribution of a small protein (BM-40) extracted from a basement membrane tumor. Eur J Biochem. 1986;161:455–64. doi: 10.1111/j.1432-1033.1986.tb10466.x. [DOI] [PubMed] [Google Scholar]
- 19.Yan Q, Sage EH. SPARC, A matricellular glycoprotein with important biological functions. J Histochem Cytochem. 1999;47:1495–506. doi: 10.1177/002215549904701201. [DOI] [PubMed] [Google Scholar]
- 20.Gilmour DT, Lyon GJ, Carlton MB, Sanes JR, Cunningham JM, Anderson JR, Hogan BL, Evans MJ, Colledge WH. Mice deficient for the secreted glycoprotein SPARC/osteonectin/BM40 develop normally but show severe age-onset cataract formation and disruption of the lens. EMBO J. 1998;17:1860–70. doi: 10.1093/emboj/17.7.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norose K, Clark JI, Syed NA, Basu A, Heber-Katz E, Sage EH, Howe CC. SPARC deficiency leads to early-onset cataractogenesis. Invest Ophthalmol Vis Sci. 1998;39:2674–80. [PubMed] [Google Scholar]
- 22.Straatsma BR, Horwitz J, Takemoto LJ, Lightfoot DO, Ding LL. Clinicobiochemical correlations in aging-related human cataract. The Pan American Association and American Journal of Ophthalmology lecture. Am J Ophthalmol. 1984;97:457–69. doi: 10.1016/s0002-9394(14)76129-x. [DOI] [PubMed] [Google Scholar]
- 23.Horwitz J, Dovrat A, Straatsma BR, Revilla PJ, Lightfoot DO. Glutathione reductase in human lens epithelium: FAD-induced in vitro activation. Curr Eye Res. 1987;6:1249–56. doi: 10.3109/02713688709025235. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch EF, Maniatis T. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 1989. Molecular cloning: a laboratory manual. [Google Scholar]
- 25.Nordqvist K, Tohonen V. An mRNA differential display strategy for cloning genes expressed during mouse gonad development. Int J Dev Biol. 1997;41:627–38. [PubMed] [Google Scholar]
- 26.Lane TF, Sage EH. Functional mapping of SPARC: peptides from two distinct Ca+(+)-binding sites modulate cell shape. J Cell Biol. 1990;111:3065–76. doi: 10.1083/jcb.111.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soderling JA, Reed MJ, Corsa A, Sage EH. Cloning and expression of murine SC1, a gene product homologous to SPARC. J Histochem Cytochem. 1997;45:823–35. doi: 10.1177/002215549704500607. [DOI] [PubMed] [Google Scholar]
- 28.Gooden MD, Vernon RB, Bassuk JA, Sage EH. Cell cycle-dependent nuclear location of the matricellular protein SPARC: association with the nuclear matrix. J Cell Biochem. 1999;74:152–67. [PubMed] [Google Scholar]
- 29.Sage EH. Secretion of SPARC by endothelial cells transformed by polyoma middle T oncogene inhibits the growth of normal endothelial cells in vitro. Biochem Cell Biol. 1992;70:579–92. doi: 10.1139/o92-089. [DOI] [PubMed] [Google Scholar]
- 30.Sage H, Decker J, Funk S, Chow M. SPARC: a Ca+-binding extracellular protein associated with endothelial cell injury and proliferation. J Mol Cell Cardiol. 1989;21(Suppl 1):13–22. doi: 10.1016/0022-2828(89)90833-x. [DOI] [PubMed] [Google Scholar]
- 31.Raines EW, Lane TF, Iruela-Arispe ML, Ross R, Sage EH. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci U S A. 1992;89:1281–5. doi: 10.1073/pnas.89.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swaroop A, Hogan BL, Francke U. Molecular analysis of the cDNA for human SPARC/osteonectin/BM-40: sequence, expression, and localization of the gene to chomosome 5q31–q33. Genomics. 1998;2:37–47. doi: 10.1016/0888-7543(88)90107-3. [DOI] [PubMed] [Google Scholar]
- 33.Young MF, Day AA, Dominquez P, McQuillan CI, Fisher LW, Termine JD. Structure and expression of osteonectin mRNA in human tissue. Connect Tissue Res. 1990;24:17–28. doi: 10.3109/03008209009152419. [DOI] [PubMed] [Google Scholar]
- 34.Maillard C, Malaval L, Delmas PD. Immunological screening of SPARC/Osteonectin nonmineralized tissues. Bone. 1992;13:257–64. doi: 10.1016/8756-3282(92)90206-c. [DOI] [PubMed] [Google Scholar]
- 35.Sawhney RS. Identification of SPARC in the anterior lens capsule and its expression by lens epithelial cells. Exp Eye Res. 1995;61:645–8. doi: 10.1016/s0014-4835(05)80060-0. [DOI] [PubMed] [Google Scholar]
- 36.Guermah M, Crisanti P, Laugier D, Dezelee P, Bidou L, Pessac B, Calothy G. Transcription of a quail gene expressed in embryonic retinal cells is shut off sharply at hatching. Proc Natl Acad Sci U S A. 1991;88:4503–7. doi: 10.1073/pnas.88.10.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SY, Ondhia N, Vidgen D, Malaval L, Ringuette M, Kalnins VI. Spatiotemporal distribution of SPARC/osteonectin in developing and mature chicken retina. Exp Eye Res. 1997;65:681–9. doi: 10.1006/exer.1997.0377. [DOI] [PubMed] [Google Scholar]
- 38.Kantorow M, Sage EH, Moreira E, Rodriquez IR. Increased expression of SPARC in cataractous human lenses and monkey macula RPE. Invest Opthalmol Vis Sci. 1999;40:S580. [Google Scholar]
- 39.Wirtz MK, Bradley JM, Xu H, Domreis J, Nobis CA, Truesdale AT, Samples JR, Van Buskirk EM, Acott TS. Proteoglycan expression by human trabecular meshworks. Curr Eye Res. 1997;16:412–21. doi: 10.1076/ceyr.16.5.412.7040. [DOI] [PubMed] [Google Scholar]