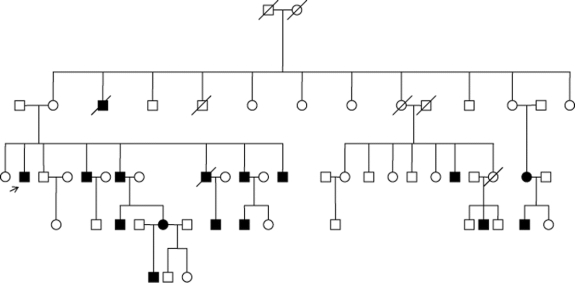
Mirror movements (MM) are synkinesias occurring in the opposite side during the intentional use of a limb. They are occasionally present in children, but persistence after age 10 is abnormal.1,2 We have found a large 4-generation family with congenital mirror movements not associated with other neurologic abnormalities.
Methods.
Nineteen members (11 affected and 8 unaffected) of a 4-generation family with individuals affected with congenital MM were interviewed in a systematic questionnaire that queried perinatal history, development, presence of learning disabilities, and other medical history. Information regarding the onset, distribution, suppressibility, and functional and social impact of MM was obtained. A videotaped examination was performed, and subjects underwent diadochokinesimeter measurement of rapid alternating pronation-supination movements at 2 different velocities. The diadochokinesimeter consisted of 2 hand-held plastic spheres connected to optical encoders, which recorded amplitude and velocity of movement in each hand.3 This project was approved by the Institutional Review Board of the Centre Hospitalier de l’Université de Montréal.
Results.
This French Canadian family originates from the Lanaudière region of Quebec. There is no known consanguinity. Transmission is autosomal dominant with high but incomplete penetrance. Penetrance is higher in males (figure).
Figure Pedigree of the family with congenital mirror movements illustrating autosomal dominant inheritance with incomplete penetrance
The index case first sought neurologic attention for episodes of fatigue, and MM were incidentally noted on examination. They had been present since childhood, and had never resulted in functional or social impairment. The remainder of the neurologic examination was normal. MRI of the brain and cervical cord was normal.
Questionnaire results and clinical features are presented in tables e-1 and e-2 on the Neurology® Web site at www.neurology.org. More men than women were affected (M:F = 9:2). Perinatal and developmental histories did not differ between affected and unaffected individuals. MM were present in hands, fingers, and forearms of all affected individuals: upon voluntary activation of one hand, the contralateral hand would mirror both simple and complex movements such as writing, typing, and tapping. Three individuals reported MM in the toes and feet, which were observable during foot tapping and movement of the toes. In most, MM were noted at birth or infancy, and persisted unchanged throughout life. Half could partially suppress the movements. Despite often high amplitude and easily observed movements, patients functioned essentially normally. One patient worked successfully as an electrician performing high precision bimanual tasks, and another worked as a secretary and could type rapidly. Three reported mild clumsiness during childhood. Several reported social impairment, stating they felt conspicuous or embarrassed by their MM.
Neurologic examinations were otherwise normal in all. Specifically, there was no anosmia, parkinsonism, motor asymmetry, or dysmetria. An example of the MM is shown on the video. Results of the quantitative testing confirmed clinical diagnosis. In affected members, MM showed amplitudes and velocities ranging from 4% to 26% and 5% to 43% of the values observed in the voluntary hand at normal and fast pace. There were no differences between left and right hand MM. Unaffected members did not show any MM movements.
Discussion.
This report of a large 4-generation family with familial congenital MM not associated with neurologic abnormalities provides definite evidence that MM can be familial, with an autosomal dominant inheritance with incomplete penetrance. There have been sporadic case reports of congenital MM in 2 or 3 members of a family, and a recent report suggested potential for multigeneration involvement, although a very limited cohort was examined.2,4
The pathophysiology of familial congenital MM is uncertain. MRI of brain and upper cervical spine shows no abnormalities of the corpus callosum, brainstem, or upper cervical cord, as seen in Klippel-Feil syndrome, Joubert syndrome, or Chiari type 1. There have been no pathology reports of familial congenital MM.
In individuals with familial congenital MM, there is evidence of fast-conducting corticospinal pathways connecting M1 with both sides of the spinal cord. Transcranial magnetic stimulation (TMS) studies have shown that stimulation of either M1 can elicit bilateral motor evoked potentials of normal and symmetric latency in resting hand muscles in MM patients.5,6 This is in contrast to physiologic MM in children, where evoked responses are not simultaneous and have a long latency, probably reflecting the spread of excitation across the corpus callosum.1,5 Therefore, the pathophysiology of physiologic and congenital MM are distinct, and there exists an abnormal ipsilateral corticospinal projection in familial congenital MM.
The origin of these abnormal ipsilateral corticospinal pathways is unclear. Hypotheses include abnormal branching of crossed corticospinal fibers to ipsilateral spinal motor neurons or presence of a separate ipsilateral corticospinal projection.1,7 Dysfunctional inhibitory transcallosal projections (i.e., from premotor regions) affecting M1 contralateral to the MM may also contribute to the MM. Further genetic studies are underway in this family to clarify the underlying genetic basis of this disorder.
Supplementary Material
Supplemental data at www.neurology.org
Disclosure: Dr. Richer receives research support from the Natural Sciences & Engineering Research Council of Canada (PI) and the Society & Culture Research Fund of Quebec (PI). Dr. Srour received educational funding through a provincial FRSQ grant. Her husband, Dr. Ronald Postuma, has received research support from FSRQ, and has received speaker fees and has served on the scientific advisory board of Teva Neurosciences. M. Philibert received a Doctoral Research Award of the Canadian Institutes of Health Research for research on attentional mechanisms in Huntington disease. Dr. Dion reports no disclosures. Dr. Duquette is supported by Parkinson Society Canada through a Basic Research Fellowship. Dr. Rouleau reports no disclosures. Dr. Chouinard has served on the scientific advisory boards of Novartis and Teva; receives funding for travel from Allergan, Teva, and Novartis; receives speaker’s honoraria from Novartis and Teva; and receives research support from the Parkinson’s Society of Canada (outreach program) and for clinical trials from Allergan, Teva, Novartis, and Schering.
Received January 15, 2009. Accepted in final form April 10, 2009.
Address correspondence and reprint requests to Dr. Sylvain Chouinard, Hôpital Notre Dame, Unité des Troubles du Mouvement André Barbeau, 1560 rue Sherbrooke Est, Montréal QC H2L 4M1, Canada; sylvain.chouinard@umontreal.ca
&NA;
- 1.Cincotta M, Ziemann U. Neurophysiology of unimanual motor control and mirror movements. Clin Neurophysiol 2008;119:744–762. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen P. Persistent mirror movements: a clinical study of 17 children, adolescents and young adults. Dev Med Child Neurol 1993;35:699–707. [DOI] [PubMed] [Google Scholar]
- 3.Beuter A, de Geoffroy A, Edwards R. Analysis of rapid alternating movements in Cree subjects exposed to methylmercury and in subjects with neurological deficits. Environ Res 1999;80:64–79. [DOI] [PubMed] [Google Scholar]
- 4.Sharafaddinzadeh N, Bavarsad R, Yousefkhah M, Aleali AM. Familial mirror movements over five generations. Neurol India 2008;56:482–483. [DOI] [PubMed] [Google Scholar]
- 5.Reitz M, Muller K. Differences between ‘congenital mirror movements’ and ‘associated movements’ in normal children: a neurophysiological case study. Neurosci Lett 1998;256:69–72. [DOI] [PubMed] [Google Scholar]
- 6.Kanouchi T, Yokota T, Isa F, Ishii K, Senda M. Role of the ipsilateral motor cortex in mirror movements. J Neurol Neurosurg Psychiatry 1997;62:629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayston MJ, Harrison LM, Quinton R, Stephens JA, Krams M, Bouloux PM. Mirror movements in X-linked Kallmann’s syndrome: I: a neurophysiological study. Brain 1997;120:1199–1216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.