Abstract
Background:
A genetic deficiency in sepiapterin reductase leads to a combined deficit of serotonin and dopamine. The motor phenotype is characterized by a dopa–responsive fluctuating generalized dystonia–parkinsonism. The non–motor symptoms are poorly recognized. In particular, the effects of brain serotonin deficiency on sleep have not been thoroughly studied.
Objective:
We examine the sleep, sleep–wake rhythms, CSF neurotransmitters, and melatonin profile in a patient with sepiapterin reductase deficiency.
Patient:
The patient was a 28–year–old man with fluctuating generalized dystonia–parkinsonism caused by sepiapterin reductase deficiency.
Methods:
A sleep interview, wrist actigraphy, sleep log over 14 days, 48–h continuous sleep and core temperature monitoring, and measurement of CSF neurotransmitters and circadian serum melatonin and cortisol levels before and after treatment with 5–hydroxytryptophan (the precursor of serotonin) and levodopa were performed.
Results:
Before treatment, the patient had mild hypersomnia with long sleep time (704 min), ultradian sleep–wake rhythm (sleep occurred every 11.8 ± 5.3 h), organic hyperphagia, attention/executive dysfunction, and no depression. The serotonin metabolism in the CSF was reduced, and the serum melatonin profile was flat, while cortisol and core temperature profiles were normal. Supplementation with 5–hydroxytryptophan, but not with levodopa, normalized serotonin metabolism in the CSF, reduced sleep time to 540 min, normalized the eating disorder and the melatonin profile, restored a circadian sleep–wake rhythm (sleep occurred every 24±1.7 h, P < 0.0001), and improved cognition.
Conclusion:
In this unique genetic paradigm, the melatonin deficiency (caused by a lack of its substrate, serotonin) may cause the ultradian sleep–wake rhythm.
Citation:
Leu–Semenescu S; Arnulf I; Dicaix C; Moussa F; Clot F; Boniol C; Touitou Y; Levy R; Vidailhet M; Roze E. Sleep and rhythm consequences of a genetically induced loss of serotonin. SLEEP 2010;33(3):307–314.
Keywords: Sepiapterin reductase deficiency, hypersomnia, melatonin, ultradian sleep–wake rhythm, tetrahydrobiopterin, serotonin
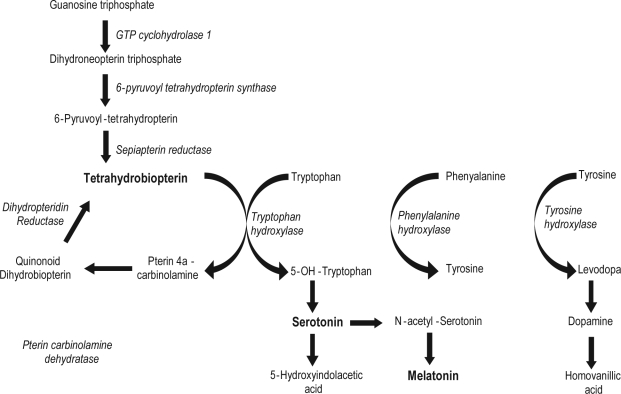
SEPIAPTERIN REDUCTASE DEFICIENCY (SRD) IS A RARE, AUTOSOMAL, RECESSIVE, METABOLIC DISORDER THAT RESULTS FROM MUTATIONS IN THE SPR gene, which is located on chromosome 2p14–p12.1,2 The diagnosis is suspected by pediatricians in infants with hypotonia and early psychomotor delay. The typical phenotype associated with SRD is early–onset dystonia with marked diurnal fluctuations and dramatic dopa–responsiveness, axial hypotonia, oculogyric crisis, and mild mental retardation.1,3,7The dystonia is usually generalized, and early bulbar involvement (hyperkinetic dysarthria and swallowing difficulties) is frequent. It may be either isolated or associated with other movement disorders, including chorea and parkinsonism. Pyramidal signs, seizures, and excessive sweating are also occasionally observed. The SRD mutation leads to altered tetrahydrobiopterin (BH4) biosynthesis, and thus abnormal biogenic amine metabolism (Figure 1). In particular, SRD patients have defects in the synthesis of dopamine and serotonin, as the metabolites of these neurotransmitters are decreased in CSF. The diagnosis can be confirmed by molecular analysis of the SPR gene or a measurement of sepiapterin reductase activity in skin fibroblasts.
Figure 1.
Biosynthesis of tetrahydrobiopterin, dopamine, serotonin
Several reports have mentioned sleep disturbances in SRD patients, namely “diurnal sleepiness,”5 “short sleep, frequent awakenings, irregular movements,”7 “hypersomnolence,”6 and problems initiating and maintaining sleep with daytime sleepiness.8 However, sleep disturbances in this setting have not been investigated in detail, and the underlying pathophysiological mechanisms (especially regarding serotonin, dopamine, and sleep systems) are not clear. The serotonergic pathway is a key contributor to the regulation of circadian rhythm, sleep, and wakefulness. Serotonergic axonal release is high during wakefulness, decreased during NREM sleep, and absent during REM sleep.9 Serotonin helps maintain wakefulness, but also conditions later sleep episodes, as blockade of serotonin synthesis causes long–lasting, complete insomnia in animal models. In addition, melatonin is synthesized from serotonin in the pineal gland (Figure 1). In humans, endogenous depression is associated with a dysfunction of serotonin transmission with concomitant sleep signs including insomnia and a shortening of REM sleep latency.10However, there is only a partial serotonin brain deficiency in these patients. In contrast, genetic tetrahydrobiopterin deficiencies (including sepiapterin reductase deficiency), which are a key factor for 5–hydroxytryptophan synthesis, lead to marked decreases in serotonin degradation product levels in the CSF and also to a decreased dopamine transmission.
We took the opportunity of a complete drug withdrawal in an adult with SRD to study sleep mechanisms with long term sleep monitoring and to investigate the circadian system with wrist actigraphy, a sleep log, and melatonin and cortisol circa–dian secretion profiles.
METHODS
Patient Case Report
A 28–year–old man (ITD613) was born to consanguineous French parents. There was no familial history of neurological disease, except for his sister, who was thought to have writer's cramp since adolescence but declined to be seen in our department. The pregnancy, delivery, and neonatal period were normal. He was a floppy baby with slow psychomotor development and had episodes of his eyes rolling up from the first month of life. He walked at age 36 months with marked postural instability and abnormal posture of the upper limbs. Clumsiness and dysarthria were also noted in childhood. At this time, his parents already noted that his motor function was clearly better in the morning, and that sleeping during daytime could relieve most of the symptoms. At age five, based on a presumptive diagnosis of atypical dopamine–responsive dystonia, he started L–dopa at a daily dose of 5 mg/kg, which resulted in a dramatic improvement in his condition (including the motor fluctuations), but also triggered choreic movements of the 4 limbs and face. With a decrease of the daily dose of L–dopa to 2 mg/kg, the abnormal movements disappeared, but the benefits with regard to motor function persisted. Between the ages of 5 and 27 years, he was treated with a daily dose of L–dopa ranging from 1 to 2 mg/kg. Under this treatment, he completed high school, had no motor complaints, and had only mild bradykinesia on examination. Within this period, he would nap 1 or 2 times each day, whereas he was awake for more than 2 hours on most nights. In addition, from adolescence, he had excessive eating with an uncontrollable need to eat frequently during the day and night, which caused mild obesity. There was no binge eating, pica, food selection, purging behavior, guilt, or alteration of his body image. The sleep and motor phenotype of this patient did not change with age. At age 27, he had a global cognitive efficiency in the low normal range with an attention deficit. A psychiatric examination was normal (in particular, there was no mood disorder). At this stage, the following features of this patient prompted us to look for an unusual cause of dopa–responsive dystonia: (1) the sleep and eating disorders; (2) an incomplete, although dramatic, response of the motor symptoms to L–dopa; (3) a history of oculogyric crisis in childhood; (4) an absent GTP cyclohydrolase 1 mutation; and (5) the consanguinity of the parents. For this examination, the patient was withdrawn from drugs for 10 days, resulting in the reappearance of a generalized dystonia–parkinsonism with major diurnal fluctuations. The neurotransmitters and pterins measurements in the CSF were highly suggestive of SRD.
The molecular analysis confirmed he had a homozygous pathogenic mutation (p.Arg150Gly) of the SPR gene.11 This mutation has already been described and results in an inactive protein.1
The patient was studied for motor and sleep symptoms and circadian rhythm before and after supplementation with 5–OH–tryptophan and L–dopa.
Sleep and Rhythm Measures
The patient underwent a semi–standardized medical interview on his sleep habits, including the Epworth sleepiness scale. He completed a sleep log and wore a wrist actigraphy (Actiwatch–Mini, Cambridge Neurotechnology Ltd, UK), which measured the intensity of movements at 1–min intervals for 7 days. The patient slept at home and was given no instruction regarding sleep, except to sleep at his preferred time. His schedule was free, as he was not working and had no family life. At the end of this week, he was hospitalized for 3 days and nights. He underwent a continuous 48–h sleep recording in the sleep disorders unit, followed by a 24–h sampling of hourly serum melatonin and cortisol levels and urinary sampling, with the normal ambient lights. Sleep was monitored from lights off (ad libitum) to lights on (ad libitum). The nighttime sleep recordings were followed by a continuous daytime sleep recording, with the ad libitum opportunity to nap in the morning and in the afternoon. Polysomnographic recording was performed, and the sleep stages, arousal, respiratory events, and legs movements were scored according to standard international criteria.12 In addition, core body temperature was measured using an ingested pill containing a thermal sensor and a transmitter, which sent a measure every minute for around 48 h (depending on the transit time) to an external Holter receptor (Vitalsense Ltd, UK). The melatonin and cortisol were measured in the 24 serum samples, using a radioimmunoassay.
DNA, Blood, and CSF Analysis
The molecular analysis of the SPR gene was performed on the patient DNA as previously described.11Plasma melatonin was measured by radioimmunoassay with a specific rabbit antiserum (INRA, Nouzilly, France) and 125I–labeled melatonin tracer (Perkin–Elmer, France). The intra–assay coefficient of variation was 4.7% for melatonin concentration of 50 pg/mL. The inter–assay coefficients of variation were 10.9% and 15% for the same concentration. Plasma cortisol was measured using the Beckman DXI 800 (Beckman, Margency, France). The intra–assay coefficient of variation was 4.5% at 6.5 μg/dL. Biogenic amines (5–hydroxyindolacetic acid and homovanillic acid), neopterin, total biopterins, and sepiapterin were analyzed using high–performance liquid–chromatography following previously reported procedures.13–16
Treatment
The supplementation treatment was started the next week. The patient received 5–OH–tryptophan (200 mg/d) in the morning, in addition to ongoing L–dopa (150 mg/d) and benserazide (37.5 mg/d). The same procedure of sleep and circadian measurements was repeated 8 months after starting therapy. The neurotransmitter and pterin levels in the CSF were measured before and after treatment. All of the investigations were performed with the informed consent of the patient.
Neuropsychological Tests
A standardized neuropsychological evaluation was performed before and one year after treatment. The intelligence quotient (IQ) was assessed using the Wechsler Adult Intel–ligence Scale–Third Edition17for verbal IQ; Colored Progressive Matrices18 were used to determine performance IQ. Executive and attentional functioning were tested using direct and indirect methods including: (1) the Digit Span subtest of the WAIS–III, which assesses attention, short term memory, and working memory; (2) the Wisconsin Card Sorting Test (WCST),19 a rule finding and set shifting test; (3) the Stroop test,20 which measures the ability to resist interferences; and (4) the Trail Making test forms A & B,21,22 which test the ability to shift from one set to another.
RESULTS
Sleep Interview
The patient reported sleep problems since childhood. He would sleep 1 or 2 times every day since childhood and was awake during more than 2 hours most nights since adolescence. At the time of the first interview, the night sleep was irregular with a sleep onset at 22:00 and offset between 02:00 and 03:00. He often needed 1 or 2 spontaneous, long (2– to 5–h) naps during the daytime. The usual sleep duration varied from 7 to 14 hours per day. He complained of excessive daytime sleepiness and scored 18/24 on the Epworth sleepiness scale. He had no symptoms of restless legs syndrome, snoring, apnea, nocturia, parasomnia, cataplexy, sleep paralysis, or sleep drunkenness. He experienced occasional nightmares and auditory hypnagogic hallucinations (voices). He ate frequently during the day and night during periods of wake with clear consciousness of what he was eating. His weight was 88 kg (body mass index of 31.2 kg/m2; obese range).
The patient received 5–OH–tryptophan in addition to L–dopa and benserazide as a treatment for SRD. After treatment, the sleep disturbances totally disappeared. He was able to fall asleep around 23:00 and wake up around 09:00, with no need for a daytime nap. The Epworth sleepiness score dropped to 9/24. In addition, his eating behavior normalized. As a consequence, he lost 12 kg within 8 months without voluntary dieting, leading to a body mass index of 26.3 kg/m2(slightly overweight) at the time of the second sleep interview.
Sleep Log and Actigraphy
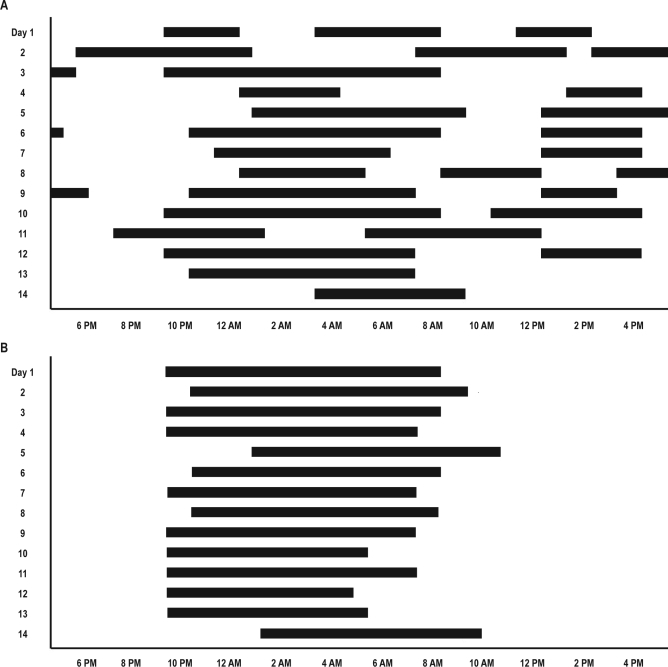
Before treatment, the sleep–wake activity was irregular and ultradian (grossly bi–circadian), as illustrated by the variable sleep onset (from 20:00 to 02:00) and offset (01:00 to 13:00) and the presence of 1 to 3 long bouts of sleep (lasting 3 to 11 h each) per 24 h (Figure 2A). The total sleep time varied between 6.5 and 14 h per 24–h period. The sleep/wake period was 11.8 ± 5.3 h (Table 1). Actigraphy showed a marked decrease in activity between 19:00 and 21:00 (sleep onset), and an increased activity between 13:00 and 15:00 for 1 to 3 h (sleep offset). The daytime naps started between 10:00 and 12:00 and ended between 13:00 and 16:00. The assumed sleep duration varied between 9 h 49 min and 12 h 47 min. After treatment, there was a dramatic change towards more regular sleep onset (21:30 to 23:00) and offset (04:00 to 10:00) times, with sleep being a single continuous episode per 24 h and no more naps on the sleep log (Figure 2B) and actigraphy. The sleep/wake period was 24.0 ± 1.7 h, which was very different (P < 0.000001) from the 11.8 ± 5.3 h period before treatment.
Figure 2.
Sleep log before (A) and after treatment with 5–0H–tryptophan (B), with x axis for clock time, and y axis for week days. Note that the sleep/wake rhythm is ultradian (periodicity: 11.8 h) before treatment, and circadian (periodicity: 24 h) after treatment. Black bars = sleep period.
Table 1.
Biological and sleep markers before and after treatment with 5–OH tryptophan
| Before Treatment | After Treatment | |
|---|---|---|
| Epworth sleepiness score, 0–24 | 18 | 9 |
| Sleep duration/24 h log, min | 704 ± 180 | 540 ± 76 |
| Sleep/wake period, h | 11.8 ± 5.3 | 24 ± 1.7 |
| Body mass index, kg/m2 | 31.2 | 26.3 |
| Intestinal transit time, h | 22h22 | 10h48 |
| Polysomnography (2 nights/period) | ||
| Sleep onset latency, min | 56–75 | 23–39 |
| REM sleep latency, min | 51–65 | 113–232 |
| Night–time total sleep time, min | 452–489 | 531–533 |
| Sleep efficiency, % | 76.6–87.8 | 68.2–81.7 |
| Total sleep time/24 h, min | 533–700 | 531–603 |
| Stage N1, % | 11–l;11.5 | 10.5–14.6 |
| Stage N2, % | 48.5–53.5 | 54.7–60.9 |
| Stage N3, % | 14.5–14.6 | 10.6–11.2 |
| REM sleep, % | 20.5–26 | 18–20.2 |
| Arousal, No/h | 11.7–11.8 | 6.4–7.2 |
| Apnea–hypopnea, No/h | 0.9 | 1.6 |
| Periodic leg movements, No/h | 0 | 0.1 |
| CSF biological markers | ||
| 5–hydroxyindolacetic acid, nmol/L Range: 65–200, NV: 130 | 45 | 100 |
| Homovanillic acid, nmol/L Range: 150–400, NV: 250 | 30 | 170 |
| Neopterin, nmol/L Range: 9–28, NV: 18 | 67 | 43 |
| Total biopterins, nmol/L Range: 12–35, NV: 17 | 47 | 77 |
| Sepiapterin, nmol/L NV: not detected, < 0.3 nmol/L | 6 | 11 |
NV, normal value; ND, not detected (< 0.3 nmol/L);
major differences in bold
Sleep Monitoring
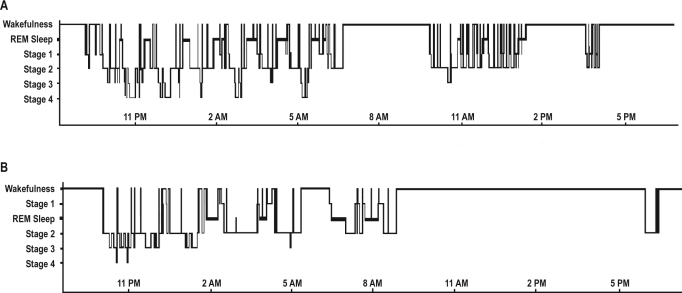
Before treatment, the total sleep time was 700 min (11 h 39 min), with nighttime sleep lasting 489 min and 2 naps in the morning and afternoon lasting 211 min (Figure 3A). The sleep structure was normal (Table 1). REM sleep latency was 65 min on Night 1 and 51 min on Night 2 before treatment and 232 min on Night 1 and 113 min on Night 2 after treatment. There was no abnormal sleep fragmentation, sleep apnea, or periodic leg movements. After treatment, the total sleep time was reduced to 603 min per 24 h (10 h), with a nighttime sleep lasting 533 min and one end–afternoon nap lasting 30 min (Figure 3B).
Figure 3.
Hypnogram before (upper panel) and after treatment with 5–OH–tryptophan (lower panel) with x axis for clock time, and y axis for sleep stages
Melatonin and Cortisol Circadian Profiles
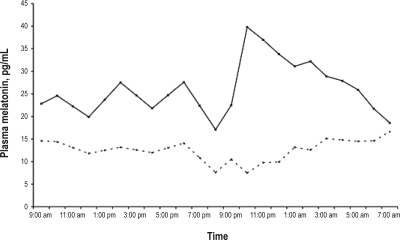
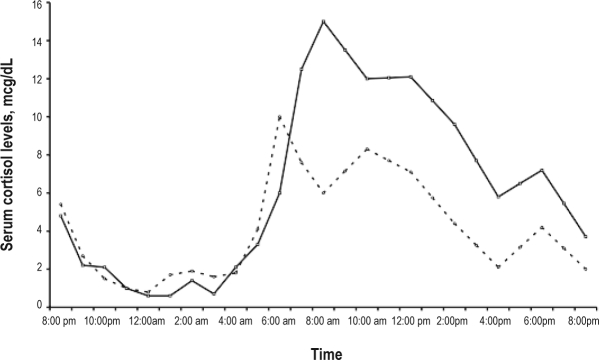
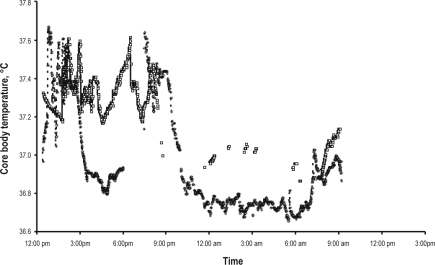
Before treatment, the plasma melatonin circadian profile was at (Figure 4A), ranging from a minimum serum level of 7.5 pg/mL at 21:00 to a maximum of 16.6 pg/mL at 07:00, when the expected normal values range between 5 pg/mL during daytime to 60 pg/mL at night with a peak between 23:00 and 07:00. After treatment with 5–OH–tryptophan in the morning, the plasma melatonin levels doubled during nighttime, compared to daytime, ranging from a minimum of 19.9 pg/mL at 21:00 to a maximum of 39.8 pg/mL at 22:00. The plasma cortisol profile showed circadian variations within the normal ranges. Before treatment, the timing of the morning cortisol peak was at 06:00 and the amplitude was 10 μg/dL. After treatment, the secretory profile was similar (Supplemental Figure 1 available online at www.journalsleep.org). As for the core body temperature, 3 capsules were necessary to monitor 48–h temperature, versus one before treatment, as the transit time was much increased. The temperature circadian profile (although some points were missing) was grossly circadian in both periods (Supplemental Figure 2 available online at www.journalsleep.org).
Figure 4.
Plasma melatonin profile (pg/mL vs. clock time) before (dotted line) and after (plain line) treatment with 5–OH–tryptophan
Neurotransmitters and Pterins CSF Analysis
Before treatment, the patient had a severe decrease in the 5–hydroxyindolacetic acid and homo–vanillic acid concentrations with increased total biopterin and neopterin concentrations (Table 1). As much as 80% to 90% of the biopterin was composed of dihydrobiopterin, which is the biopterin form accumulating upstream of the sepiapterin reductase.18 The elevated levels of sepiapterin confirmed the diagnosis. In keeping with the clinical improvement, the neurotransmitter levels in the CSF returned to normal values under treatment (Table 1).
Neuropsychological Tests
The results of the neuropsychological tests are presented in Table 2. Before treatment, the patient had a global cognitive efficiency in the low normal range with a mild but consistent dysexecutive syndrome. In particular, he had mild difficul–ties in rule finding and marked difficulties in set shifting as demonstrated by the number of perseverative errors in the WCST. By contrast, his ability to resist interferences and his working and short term memory were relatively preserved. The post–treatment results demonstrate a normalization of his WCST results.
Table 2.
Neuropsychological scores before and after treatment with 5–OH–tryptophan
| Scores | Before Treatment | After Treatment | Norms(Mean ± SD) |
|---|---|---|---|
| Colored Progressive Matrices | 85 | 85 | 100 ± 15 |
| WAIS–III –Verbal IQ | 84 | 90 | 100 ± 15 |
| Direct digit span | 6 | 6 | 7.6 ± 2 |
| Reverse digit span | 4 | 4 | 6.3 ± 2 |
| Processing speed | |||
| Trail Making Test A, s | 47 | 35 | 31 ± 12 |
| Trail Making Test B, s | 75 | 65 | 66 ± 24 |
| Directed attention | |||
| STROOP (raw scores for age corrected) | T–Scored | T–Scored | |
| Words | 42 | 46 | 50 ± 10 |
| Colors | 40 | 58 | 50 ± 10 |
| Colors | of | words | 37 41 50 ± 10 |
| Reasoning and concept | |||
| Wisconsin Card Sorting Test | 5 criteria | 6 criteria | 5.9 ± 0.36 |
| Perseverative errors | 66% | 0% | normal < 50% |
DISCUSSION
This patient with SRD has a dopa–responsive generalized dystonia–parkinsonism, as previously reported in other SRD patients. In addition, he has non–motor symptoms, including an abnormal (ultradian) sleep–wake rhythm, a mild hypersomnia, an eating behavior disorder, and an executive/attention dys–function. Supplementation with 5–hydroxy tryptophan, but not levodopa, improved the non–motor symptoms, with normalization of sleep and circadian sleep–wake rhythm. The change in melatonin profile (flat before treatment, normalized after) parallels the change from ultradian to circadian sleep–wake rhythm, suggesting that this observation provides a unique paradigm of melatonin deficiency caused by lack of its substrate, serotonin.
Before treatment, the patient slept a mean of 11 h 44 min, which is higher than in controls,23 and suggests hypersomnia with a long sleep time. Although little is known about the mechanisms of sleep excess, a deficiency of the arousal systems or an increased homeostatic drive to sleep are suspected. Here the serotonin (5HT) arousal system is probably altered as a result of a genetically decreased 5HT synthesis. We cannot totally exclude that other arousal systems (namely hypocretin and histamine in the lateral hypothalamus, acetylcholine neurons in the basal forebrain noradrenaline in the coeruleus nucleus and dopamine in the periaqueductal gray matter, the two latter being possibly affected by SRD) are deficient, except that we have indirect evidence (such as an almost–complete motor response and a persistence of sleep problems while treated with levodopa alone) that the patient is adequately supplemented with dopamine. In a series of 22 patients with Segawa syndrome (a dopamine responsive dystonia caused by GTP cyclohydroxylase deficiency also resulting in BH4 deficiency and, in turn, in dopamine and serotonin deficiency), several sleep disturbances have been described, including sleep onset and maintenance insomnia (but sleep time was long in two patients), nightmares in 22% of patients, and no daytime sleepiness. During polysomnog–raphy, sleep was normal except for reduced REM sleep percentage.24 The sleep differences between these patients and our SRD patient may relate to a different methodology (no study of circadian sleep wake–rhythm, no long–term sleep monitoring in Segawa syndrome), and use of dopaminergic with/out serotonergic agents in all patients with Segawa syndrome. In addition, the severity of the serotonin deficiency may be lower in Segawa syndrome than in SRD. The evidence for a role of 5–HT as an arousal system comes only from animal studies. In the rat and cat, the 5HT neurons are active during wakefulness, while the stimulation of post–synaptic 5HT1A, 5HT1B, 5HT2A–C, 5HT3, and 5HT7 receptors increases wakefulness.25 SRD patients, including our patient, have a unique condition with a proven deficit of serotonin synthesis. The sleep disturbances in these patients have been previously described as “fidiurnal sleepiness,” 5 “frequent awakenings,”6 “hypersomnolence,”7 and problems initiating and maintaining sleep with daytime sleepiness,8 but have not been fully documented. The sleep excess and circadian aspects have not been studied before. We provide a detailed demonstration that our patient had both a mild hypersomnia and altered circadian rhythm. As these disorders were present with adequate L–dopa supplementation and lessened with additional 5–OH–tryptophan treatment, we infer that they are related to the serotonin deficiency in our patient. Patients with major depression also have a form of serotonin dysfunction (although this is not a major defect as in SRD) that can be reproduced by a rapid tryptophan depletion.26,27 Major depression or rapid tryptophan depletion are usually associated with insomnia,28 but occasionally with daytime sleepiness; true excess of sleep, as with our patient, is exceptional.29 More frequently, the REM sleep latency is shortened, although this sign is not specific to depression. Interestingly, our patient (who had no depression) had a short REM sleep latency (51 and 65 min) before treatment, which doubled with treatment, suggesting that the change was linked to serotonin deficiency. No other sleep markers frequent in depression (early morning awakening, more disrupted sleep, or prolonged first REM sleep episode) were found in this patient. The biological mechanisms of depression are however incompletely elucidated and may include dysfunction in several neurotransmitters, possibly unaffected in our patient.
This patient had an ultradian sleep–wake rhythm. His sleep period occurred every 11.8 h, following a grossly bi–circadian rhythm, with large (5.3 h) variability. This pattern of sleep, with long daytime naps, may have contributed to the feeling of hypersomnia before treatment (with an elevated Epworth score) and the improvement after treatment. As his serum melatonin profile was simultaneously flat, without any physiological nighttime increase, it suggests that SRD causes, via the reduced serotonin release, a low, flat melatonin secretion. In the human model of rapid tryptophan depletion, the subsequent decreased brain serotonin turnover also results in a decreased nocturnal melatonin secretion.30 With quasi–absence of melatonin secretion, this patient lost his major endogenous marker of darkness. Since the other circadian markers such as the body temperature and cortisol were normal, the patient was, before treatment, in a state of internal desynchronization between sleep and melatonin on one hand, and cortisol and temperature on the other hand. Interestingly, the sleep–wake rhythm of our patient was close to 12 h (half of 24 h, hence possibly bi–circadian). The cortisol/temperature increases may have entrained the bi–circa–dian rhythm, at least as zeitgebers for the morning awakening.
Before 5–OH–tryptophan supplementation, the patient had a mild obesity related to an eating behavior disorder that clearly improved with this treatment. His excessive eating does not share the typical characteristics of bulimia nervosa as the psychiatric items are absent, including binge eating, a purging behavior, guilt, or altered body image. Together, these findings are suggestive of an organic hyperphagia related to the serotonin deficiency. Several lines of investigation indicate that eating disturbances are associated with an abnormal hypothalamic serotonergic neurotransmission.31–33 Its modulation may promote weight variations by mediating satiety and thus regulating food intake. Numerous drugs acting as serotonin agonists or serotonin reuptake inhibitors decrease food intake, induce weight loss, and advance the behavioral satiety sequence in rodents.34 Recent studies emphasize the role of the 5–HT2C receptor activation in this process.35–37 Such drugs, particularly fenfluramine and sibutramine, reduce the food intake and weight in obese patients.38–40 An increased hypothalamic serotonin tone (in animals) and an increase of CSF tryptophan (in humans) are associated with cancer anorexia.41 The clinical characteristics of our patient (hyperphagic when 5HT–depleted, normalized after 5–OH–tryptophan supplementation) provide further evidence for an important role of serotonin neurotransmission in satiety in humans.
Before treatment, our patient had a mild dysexecutive syndrome mostly affecting the mental flexibility (as assessed by the WSCT), which normalized with serotonin supplementation. We cannot exclude the possibility that the initial dysexecutive disorder was sleepiness related and improved when the sleepiness disappeared after the serotonin supplementa–tion. Alternatively, the improvement may have resulted from the serotonin supplementation. None of the previous reports on SRD mutation focused on the executive functions, and the effect of therapy on cognition was controversial. On levodopa treatment alone, the cognition or school level was improved in two patients7,5 and unchanged in seven patients.6 In contrast, the re–establishment of both dopamine and serotonin transmission markedly improved the school level and intellectual abilities in two patients,5,8 although the cognitive benefit was sustained in only one patient.5
It is now well established from animal and human studies that the serotonin neurotransmission plays a role in cognition, particularly in executive functions.42 The primate prefrontal cortex receives strong serotonin inputs, while 5HT receptors are largely distributed in the prefrontal neurons over different cortical layers.43 Interestingly, animals depleted of prefrontal serotonin have an altered flexibility.44 In keeping with this experimental data, our patient had a specific pattern of alteration with a predominant defect of the set–shifting ability rather than a global alteration of the attention/executive functions. This report may then provide further evidence that serotonin deficiency more specifically affects the prefrontal functions and that prefrontal serotonin stimulation can modulate the mental flexibility.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Arnulf has participated in speaking engagements for UCB Pharma. The other authors have indicated no financial conflicts of interest. The tests were performed as the normal follow–up of any in–hospital patient covered by the national healthcare system. Levodopa is commercially available, while 5–hydroxytryptophan (not available in France) is produced and furnished by the national central pharmacy.
ACKNOWLEDGMENTS
The authors wish to thank David Grabli and Constance Flamand–Rouvière for the helpful suggestions on the article.
Supplemental Figure 1.
Serum cortisol profile (mcg/dL vs. clock time) before (dotted line) and after (plain line) treatment with 5-OH-tryptophan
Supplemental Figure 2.
Core body temperature before (circles) and after (square points) treatment with 5–OH–tryptophan
REFERENCES
- 1.Bonafé L, Thö B, Penzien JM, Czarnecki B, Blau N. Mutations in the sepiapterin reductase gene cause a novel tetrahydrobiopterin–dependent monoamine–neurotransmitter deficiency without hyperphenylalaninemia. Am J Hum Genet. 2001;69:269–77. doi: 10.1086/321970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blau N, Bonafé L, Thö B. Tetrahydrobiopterin deficiencies without hyperphenylalaninemia: diagnosis and genetics of dopa–responsive dystonia and sepiapterin reductase deficiency. Mol Genet Metab. 2001;74:172–85. doi: 10.1006/mgme.2001.3213. [DOI] [PubMed] [Google Scholar]
- 3.Neville BG, Parascandalo R, Farrugia R, Felice A. Sepiapterin reductase deficiency: a congenital dopa–responsive motor and cognitive disorder. Brain. 2005;128:2291–6. doi: 10.1093/brain/awh603. [DOI] [PubMed] [Google Scholar]
- 4.Abeling NG, Duran M, Bakker HD, et al. Sepiapterin reductase deficiency an autosomal recessive DOPA–responsive dystonia. Mol Genet Metab. 2006;89:116–20. doi: 10.1016/j.ymgme.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Echenne B, Roubertie A, Assmann B, et al. Sepiapterin reductase deficiency: clinical presentation and evaluation of long–term therapy. Pediatr Neurol. 2006;35:308–13. doi: 10.1016/j.pediatrneurol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Friedman J, Hyland K, Blau N, MacCollin M. Dopa–responsive hyper–somnia and mixed movement disorder due to sepiapterin reductase deficiency. Neurology. 2006;12:2032–5. doi: 10.1212/01.wnl.0000247274.21261.b4. [DOI] [PubMed] [Google Scholar]
- 7.Verbeek MM, Willemsen MA, Wevers RA, et al. Two Greek siblings with sepiapterin reductase deficiency. Mol Genet Metab. 2008;94:403–9. doi: 10.1016/j.ymgme.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Kusmierska K, Jansen EE, Jakobs C, et al. Sepiapterin reductase deficiency in a 2–year–old girl with incomplete response to treatment during short–term follow–up. J Inherit Metab Dis. 2009 doi: 10.1007/s10545-008-1009-4. in press. [DOI] [PubMed] [Google Scholar]
- 9.Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;6:55–69. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- 10.Adrien J. Neurobiological bases for the relation between sleep and depression. Sleep Med Rev. 2002;6:341–51. [PubMed] [Google Scholar]
- 11.Clot F, Grabli D, Cazeneuve C, et al. Exhaustive analysis of BH4 and dopamine biosynthesis genes in patients with dopa–responsive dystonia. Brain. 2009;132:1753–63. doi: 10.1093/brain/awp084. [DOI] [PubMed] [Google Scholar]
- 12.Morgenthaler TI, Lee-Chiong T, Alessi C, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007;30:1445–59. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ormazabal A, Garcia-Cazorla A, Fernandez Y, Fernandez-Alvarez E, Campistol J, Artuch R. HPLC with electrochemical and fluorescence detection procedures for the diagnosis of inborn errors of biogenic amines and pterins. J Neurosci Methods. 2005;142:153–8. doi: 10.1016/j.jneumeth.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Fekkes D, Voskuilen-Kooijman A. Quantitation of total biopterin and tetrahydrobiopterin in plasma. Clin Biochem. 2007;40:411–3. doi: 10.1016/j.clinbiochem.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Zorzi G, Redweik U, Trippe H, Penzien JM, Thoë B, Blau N. Detection of sepiapterin in CSF of patients with sepiapterin reductase deficiency. Mol Gen Metab. 2002;75:174–7. doi: 10.1006/mgme.2001.3273. [DOI] [PubMed] [Google Scholar]
- 16.Ormazabal A, García-Cazorla A, Pérez-Dueñas B, et al. Determination of 5–methyltetrahydrofolate in cerebrospinal fluid of paediatric patients: Reference values for a paediatric population. Clin Chim Acta. 2006;371:159–62. doi: 10.1016/j.cca.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D. Wechsler Adult Intelligence Scale. 3rd Edition. San Antonio, TX: Harcourt Assessment; 1997. WAIS-3. [Google Scholar]
- 18.Raven JC. Coloured progressive matrices. Oxford, UK: Oxford Psychologists Press Ltd; 1947. [Google Scholar]
- 19.Nelson HE. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976;12:313–24. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- 20.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;28:643–62. [Google Scholar]
- 21.Davies A. The influence of age on trails making test performance. J Clin Psychol. 1968;24:96–8. doi: 10.1002/1097-4679(196801)24:1<96::aid-jclp2270240131>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Godefroy O. Evaluation en pratique clinique. Paris, France: Editions Solal; 2008. Fonctions exécutives et pathologies neurologiques et psychiatriques. [Google Scholar]
- 23.Vernet C, Arnulf I. Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients. Sleep. 2009;32:753–759. doi: 10.1093/sleep/32.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Hove JL, Steyaert J, Matthijs G, et al. Expanded motor and psychiatric phenotype in autosomal dominant Segawa syndrome due to GTP cyclohydrolase deficiency. J Neurol Neurosurg Psychiatry. 2006;77:18–23. doi: 10.1136/jnnp.2004.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monti JM, Jantos H. The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking. Prog Brain Res. 2008;172:625–46. doi: 10.1016/S0079-6123(08)00929-1. [DOI] [PubMed] [Google Scholar]
- 26.Voderholzer U, Hornyak M, Thiel B, et al. Impact of experimentally induced serotonin deficiency by tryptophan depletion on sleep EEG in healthy subjects. Neuropsychopharmacol. 1998;18:112–24. doi: 10.1016/S0893-133X(97)00094-8. [DOI] [PubMed] [Google Scholar]
- 27.Arnulf I, Quintin P, Alvarez JC, et al. Mid-morning tryptophan depletion delays REM sleep onset in healthy subjects. Neuropsychopharmacol. 2002;27:843–51. doi: 10.1016/S0893-133X(02)00358-5. [DOI] [PubMed] [Google Scholar]
- 28.Van Moffaert MM. Sleep disorders and depression: the ’chicken and egg’ situation. J Psychosom Res. 1994;38:9–13. doi: 10.1016/0022-3999(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 29.Vgontzas AN, Bixler EO, Kales A, Criley C, Vela-Bueno A. Differences in nocturnal and daytime sleep between primary and psychiatric hypersomnia: diagnostic and treatment implications. Psychosom Med. 2000;62:220–6. doi: 10.1097/00006842-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Zimmermann RC, McDougle CJ, Schumacher M, et al. Effects of acute tryptophan depletion on nocturnal melatonin secretion in humans. J Clin Endocrinol Metab. 1993;76:1160–4. doi: 10.1210/jcem.76.5.8496306. [DOI] [PubMed] [Google Scholar]
- 31.Blundell JE, Halford JCG. Serotonin and appetite regulation. CNS Drugs. 1998;9:473–95. [Google Scholar]
- 32.Simansky KJ. Serotonergic control of the organization of feeding and satiety. Behav Brain Res. 1996;73:37–42. doi: 10.1016/0166-4328(96)00066-6. [DOI] [PubMed] [Google Scholar]
- 33.Meguid MM, Fetissov SO, Varma M, et al. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16:843–57. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- 34.Tallett AJ, Blundell JE, Rodgers RJ. Sibutramine–induced anorexia: potent, dose–dependent and behaviourally–selective profile in male rats. Behav Brain Res. 2009;198:359–65. doi: 10.1016/j.bbr.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Somerville EM, Horwood JM, Lee MD, Kennett GA, Clifton PG. 5–HT(2C) receptor activation inhibits appetitive and consummatory components of feeding and increases brain c–fos immunoreactivity in mice. Eur J Neurosci. 2007;25:3115–24. doi: 10.1111/j.1460-9568.2007.05567.x. [DOI] [PubMed] [Google Scholar]
- 36.Nonogaki K, Ohba Y, Sumii M, Oka Y. Serotonin systems upregulate the expression of hypothalamic NUCB2 via 5–HT2C receptors and induce anorexia via a leptin–independent pathway in mice. Biochem Biophys Res Commun. 2008;372:186–90. doi: 10.1016/j.bbrc.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Asarian L. Loss of cholecystokinin and glucagon–like peptide–1–induced satiation in mice lacking serotonin 2C receptors. Am J Physiol Regul Integr Comp Physiol. 2009;296:R51–6. doi: 10.1152/ajpregu.90655.2008. [DOI] [PubMed] [Google Scholar]
- 38.Weintraub M, Sundaresan PR, Madan M, Schuster B, Balder A, Lasagna L, Cox C. Long–term weight control study. I (weeks 0 to 34). The enhancement of behavior modification, caloric restriction, and exercise by fenfluramine plus phentermine versus placebo. Clin Pharmacol Ther. 1992;51:586–94. doi: 10.1038/clpt.1992.69. [DOI] [PubMed] [Google Scholar]
- 39.Guy-Grand B, Apfelbaum M, Crepaldi G, Gries A, Lefebvre P, Turner P. International trial of long–term dexfenfluramine in obesity. Lancet. 1989;2:1142–5. doi: 10.1016/s0140-6736(89)91499-2. [DOI] [PubMed] [Google Scholar]
- 40.James WP, Astrup A, Finer N, et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. STORM Study Group. Sibutramine Trial of Obesity Reduction and Maintenance. Lancet. 2000;356:2119–25. doi: 10.1016/s0140-6736(00)03491-7. [DOI] [PubMed] [Google Scholar]
- 41.Laviano A, Inui A, Meguid MM, Molfino A, Conte C, Rossi Fanelli F. NPY and brain monoamines in the pathogenesis of cancer anorexia. Nutrition. 2008;24:802–5. doi: 10.1016/j.nut.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Robbins TW, Roberts AC. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb Cortex. 2007;17:i151–60. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
- 43.Goldman-Rakic PS, Lidow MS, Gallager DW. Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. J Neurosci. 1990;10:2125–38. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boulougouris V, Tsaltas E. Serotonergic and dopaminergic modulation of attentional processes. Prog Brain Res. 2008;172:51. doi: 10.1016/S0079-6123(08)00925-4. [DOI] [PubMed] [Google Scholar]