Abstract
Background:
Sleep disordered breathing in children is associated with severity-dependent increases in excessive daytime sleepiness (EDS). TNF-α is an inflammatory cytokine that has been implicated in EDS. Since, at any given level of apnea-hypopnea index, there is significant variability in EDS, we hypothesized that morning tumor necrosis factor (TNF)-α plasma levels may provide a biologic correlate of EDS.
Methods:
Children being evaluated for sleep disordered breathing underwent a blood draw after nocturnal polysomnography, and TNF-α plasma concentrations were assayed using ELISA. In a subset of 15 children with sleep disordered breathing and in 15 matched control subjects, whole blood cultures in the presence of lipopolysaccharide and Multiple Sleep Latency Test were conducted. Furthermore, 22 children with obstructive sleep apnea had TNF-α levels assayed and underwent nocturnal polysomnography and Multiple Sleep Latency Test before and after adenotonsil-lectomy.
Results:
In 298 children, morning TNF-α levels were globally increased in the presence of obstructive sleep apnea, particularly in more severe cases, and correlated with obstructive apnea-hypopnea index and sleep pressure score, a measure of respiratory-induced sleep fragmentation, but not with nadir SaO2. A stepwise logistic regression analysis revealed that sleep pressure score and body mass index accounted for 36.2% of the adjusted variance in TNF-α levels (P < 0.0001). Furthermore, multiple sleep latencies were correlated with whole blood culture-derived TNF-α levels (n = 15), and morning TNF-α levels decreased after adenotonsillectomy in 22 children.
Conclusions:
TNF-α levels are increased in pediatric obstructive sleep apnea, are primarily driven by sleep fragmentation and body mass index, and are closely associated with the degree of sleepiness, as measured by Multiple Sleep Latency Test. Furthermore, surgical treatment of obstructive sleep apnea results in significant reductions in TNF-α levels with reciprocal prolongations in sleep latency.
Citation:
Gozal D; Serpero LD; Kheirandish-Gozal L; Capdevila OS; Khalyfa A; Tauman R. Sleep measures and morning plasma TNF-α levels in children with sleep-disordered breathing. SLEEP 2010;33(3):319-325.
Keywords: Cytokines, sleepiness, sleep apnea, children, inflammation
OBSTRUCTIVE SLEEP APNEA (OSA) IS A RELATIVELY HIGHLY PREVALENT CONDITION IN CHILDREN AND HAS RECENTLY EMERGED AS MAJOR CAUSE OF neurobehavioral dysfunction and cardiovascular morbidity. In adults affected with OSA, excessive daytime sleepiness (EDS) is extremely frequent and accounts for one of the most important symptoms prompting medical referral for evaluation and treatment. However, the prevalence of EDS in children with OSA is somewhat unclear and probably depends on the perceptions of caretakers, since children are unlikely to verbalize such symptoms. In a 1995 study, parental reports in children being evaluated for suspected OSA initially indicated that only a small minority of these children (7%) present symptoms compatible with EDS.1 In contrast, more recent studies using questionnaires that included more specific questions on behaviors associated with EDS suggested that the frequency of EDS may revolve around the 20% to 50% of all children.2 When sleepiness was measured using the Multiple Sleep Latency Test (MSLT), approximately 13 to 20% of nonobese children fulfilling the criteria for OSA displayed EDS, whereas 40% to 60% of obese children with OSA fulfilled MSLT criteria of EDS.3–5
One of the major questions emanating from the aforementioned studies was whether polysomnographic measures could provide insights and identify increased risk for having EDS in children with OSA. To this effect, the magnitude of sleep fragmentation induced by OSA was quantified in both children and adults and allowed for development of a numerical algorithm, i.e., the sleep pressure score (SPS), that would theoretically indicate a putative threshold for disruption of sleep homeostasis such that further sleep disruption would then be theoretically met with increased EDS.6,7 Of note, the SPS derived from the arousal indices in each individual overnight sleep study seemed to confer improved prediction for the occurrence of both cognitive and behavior disturbances in snoring children.8 Notably, working under similar assumptions, Chervin and colleagues showed the presence of respiratory cycle-related electroencephalographic spectral changes in patients with OSA that seemed to correlate with EDS measures.9
Tumor necrosis factor (TNF)-α is one of the most important cytokines involved in sleep regulation.10 For example, injection of TNF-α induces physiologic sleep and increases the time spent in non-rapid eye movement phase. In addition, TNF-α levels follow a circadian variation and are increased after sleep deprivation; inhibition of TNF-α receptors in the brain is accompanied by the suppression of spontaneous non-rapid eye movement sleep. It is now well established that TNF-± levels are elevated in adults patients with OSA, and, in fact, circulating TNF-α levels have been proposed as a biologic marker of EDS. Indeed, Vgontzas and colleagues demonstrated that neutralizing TNF-α activity by administration of etanercept is associated with significant reductions in EDS in adult patients with OSA.11
We therefore conducted the present study to assess whether TNF-α levels are elevated in children with OSA and, if so, to determine whether specific demographic characteristics or polysomnographic measures would account for the variance in morning TNF-α plasma concentrations. We also examined whether the presence of EDS, as determined by MSLT, is associated with increased TNF-α levels in children with OSA and whether improved MSLT and TNF-α levels would emerge after surgical treatment of OSA.
METHODS
The study was approved by the University of Louisville Human Research Committee, and informed consent was obtained from the legal caretaker of each participant. Assent was also obtained from children if they were older than 6 years of age.
Consecutive habitually snoring children being evaluated for the presence of OSA were enrolled in the study. Exclusion criteria were the presence of genetic disorders, cerebral palsy, neuromuscular diseases, or any underlying systemic diseases or acute infectious processes. In addition, we included 28 control children who were age-, sex-, and ethnicity-matched and who had no history of snoring and in addition had normal polysomnographic findings (see below). Blood was drawn between 07:00 and 08:00 the morning after the child underwent a standard polysomnographic evaluation in the sleep laboratory at the University of Louisville Pediatric Sleep Laboratory.
In a second phase of the study, we identified 15 children with OSA and 15 age-, sex-, ethnicity-, and body mass index (BMI)-matched healthy children who agreed to undergo a blood draw and an MSLT; (see below).
In the third and final phase of the study, 22 children diagnosed with OSA underwent a blood draw and MSLT and were then referred for treatment consisting of surgical adenotonsillectomy and then repeated the same protocol within 6 to 12 weeks after the adenotonsillectomy.
Whole-Blood Cell Cultures
Within 30 minutes after sampling, 40-μL aliquots of heparinized whole blood were cultured for measurement of cytokine production. Heparinized blood was diluted 6.25 × in RPMI 1640 medium with 0.1% fetal calf serum and 30 kIU/L Na-heparin. Diluted whole-blood cultures were set up in 96-well culture and stimulated with a low concentration of lipopolysaccharide (from Escherichia coli O26:B6; Sigma L2654; final concentration 0.001 mg/L) in quadruplicate. Two control wells contained medium alone. After 22 hours (range 21.5-24.5 h) of culture at 37°C, 5% CO2 supernatants were harvested and frozen at −80°C.
TNF-α Assay
Blood was centrifuged within 5 minutes in a cooled centrifuge, and plasma was then immediately stored in multiple aliquots at −80°C until assay within 2 weeks from collection. Plasma or supernatant TNF-α levels were examined using commercial ELISA kits (R&D systems, Minneapolis, MN; cat # QTA00B). This method has a minimum detection level of 0.74 pg/mL, with intraassay and interassay coefficients of variability of 7.4% and 7.8%, respectively, and a dynamic linear range between 2.2 and 7500 pg/mL. To ensure consistency and to prevent protein degradation, particular care was taken to standardize all steps of plasma sample processing and to minimize thawing more than once for each aliquot.
Overnight Polysomnography
A standard overnight multichannel polysomnographic evaluation was performed in the sleep laboratory, as has been previously described.12 Briefly, chest and abdominal wall movement were monitored by respiratory impedance or inductance plethysmography and heart rate by electrocardiogram; air flow with a sidestream end-tidal capnograph, which also provided breath-by-breath assessment of end-tidal carbon dioxide levels (PETCO2; BCI SC-300, Menomonee Falls, WI), nasal pressure catheter, and a oronasal thermistor. Arterial oxygen saturation (SaO2) was assessed by pulse oximetry (Nellcor N 100; Nellcor Inc., Hayward, CA), with simultaneous recording of the pulse waveform. The bilateral electrooculogram, 8 channels of electroencephalogram, chin and anterior tibial electromyograms, and analog output from a body-position sensor (Braebon Medical Corporation, Ogdensburg, NY) were also monitored. All measures were digitized using a commercially available polysomnography system (Rembrandt, MedCare diagnostics, Amsterdam, the Netherlands). Tracheal sound was monitored with a microphone sensor (Sleepmate Technologies, Midlothian,VA), and a digital time-synchronized video recording was performed.
Sleep architecture was assessed by standard techniques.13 The proportion of time spent in each sleep stage was expressed as percentage of total sleep time (TST). Central, obstructive, and mixed apneic events were counted. Obstructive apnea was defined as the absence of airflow with continued chest wall and abdominal movement for duration of at least 2 breaths.12 Hypopneas were defined as a decrease in oronasal flow of at least 50% on either the thermistor or nasal pressure transducer signal with a corresponding decrease in SaO2 of at least 4% and/or arousal.12 The obstructive apnea-hypopnea index (OAHI) was defined as the number of apnea and hypopneas per hour of TST. Children with snoring and an AHI less than 1 were considered to have habitual snoring, those with an AHI of at least 1 but less than 5 were considered to have mild OSA; an AHI of at least 5 but less than 10 was categorized as moderate OSA, and an AHI of 10 or greater was considered as severe OSA. Control subjects were defined as nonsnoring children with an AHI less than 1.
Arousals were defined as recommended by the American Sleep Disorders Association Task Force report using the 3-second rule and/or the presence of movement arousal.14Arousals were further subdivided as respiratory, spontaneous, or technician-induced, and the SPS was calculated, as previously reported.6,7
The MSLT
The morning following the sleep study, an MSLT was conducted, as previously reported.3 Briefly, 5 nap opportunities of 30-minute durations were allowed every 2 hours, starting at 08:00. Parents were requested to stay in the room with their child to eliminate any external apprehension. Each latency test was abrogated after 3 successive 30-second epochs of stage 1 sleep or 1 epoch of any other stage sleep (i.e., stages 2 or higher of non-rapid eye movement sleep), and, as such, this approach slightly differs from the procedures recommended by the American Academy of Sleep Medicine.15The sleep latency for each trial was calculated as the time elapsed from “lights out” to the first epoch of sleep. If no sleep occurred during a nap, the sleep latency for that nap was assigned a value of 30 minutes. The mean latency derived from all 5 nap opportunities was defined as the mean sleep latency (MSL). A, MSL of 12 minutes or less was considered as indicative of EDS (i.e., 4 standard deviations beyond the mean in healthy children).5
Body Mass Index
Height and weight were obtained using standard techniques from each child. BMI was then calculated (body mass/height2). BMI Z scores for age and sex were determined based on Centers of Disease Control and Prevention growth charts (National Center for Health & Statistics. CDC Growth Charts. US Department of Health & Human Services, 2000). Children with BMI z scores exceeding 1.65 were classified as fulfilling the criteria for obesity.
Data Analysis
Data are presented as means ± SEM unless otherwise indicated. All analyses were conducted using SPSS software (version 17.5; SPPS Inc., Chicago, IL.). Comparisons of demographics according to OSA-severity group assignment were made with independent t tests or analysis of variance followed by posthoc Bonferroni comparisons, with P values adjusted for unequal variances when appropriate (Levene test for equality of variances), or χ2 analyses with Fisher exact test (dichotomous outcomes). Correlations of log TNF-α levels with SPS, obstructive AHI (OAHI), SaO2 nadir, and BMI, as well as other demographic and polysomnographic measures were performed initially using linear regression and then followed by a sigmoidal logistic regression analysis using the following equation: y = A2 + (A1-A2)/(1 + (x/x0)p) with no weighting assigned to y values, and subsequent calculation of the goodness of fit, expressed as r2. Stepwise logistic regression analyses were also performed to assess the specific contribution of all potential sleep and demographic variables to TNF-α levels. Paired t tests were used to compare preadenotonsillectomy and postadenotonsillectomy changes. All P values reported are 2 tailed, with statistical significance set at < 0.05.
RESULTS
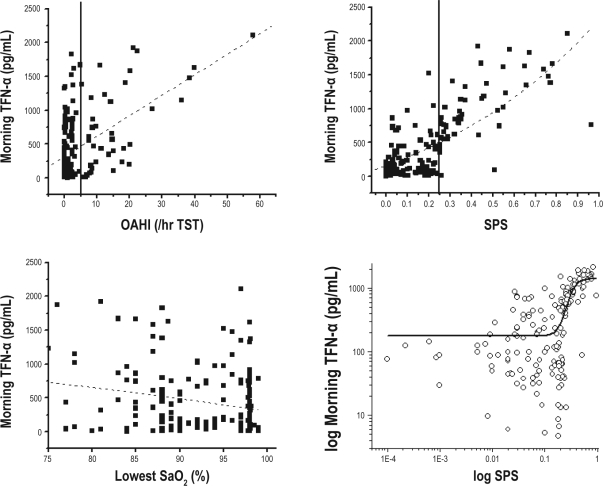
A total of 298 children including 28 control subjects were enrolled in the study out of 319 potential candidates. The 21 children who refused to participate did not differ from the other participants and did not wish to have blood drawn. Table 1 shows the major demographic and polysomnographic characteristics of the participant cohort based on the categorical severity of their sleep disordered breathing (SDB). Table 1 also shows the mean morning TNF-α levels for children with an OAHI of 1 or less (including both snoring and nonsnoring children whose data were merged, since there were no differences between these 2 groups), as well as for the other sleep disordered breathing severity categories. Significant differences emerged among morning plasma TNF-α levels in children with an OAHI of 1 or less and children in whom the OAHI was greater than 5 (P < 0.001). As shown in Figure 1, significant linear correlations emerged between morning TNF-α levels and OAHI (r2: 0.24; P < 0.001) and SPS (r2: 0.52; P < 0.000001) but not with nadir SaO2 (r2: 0.05; P > 0.05). Furthermore, sigmoidal regression analysis revealed further improvement in the relationship between log SPS and log TNF-α levels (r2: 0.64; P < 0.00000001). Stepwise logistic regression further showed that log SPS accounted for 32.1% of the adjusted variance in log TNF-α levels, with an additional 4.2% of the variance being contributed by the BMI z score (P < 0.0001). However, neither age, sex, ethnicity, nadir SaO2, nor any other sleep measure including non-rapid eye movement sleep, slow wave sleep, or rapid eye movement sleep was retained in the model.
Table 1.
Demographic, polysomnographic, and TNF-α levels in children with different severity levels of SDB
| OAHI | No. | Age, y | BMI, z score | OAHI, no/h | Lowest SaO2, % | SPS | TNF-α, pg/mL |
|---|---|---|---|---|---|---|---|
| ≤1 | 75 | 7.2 ± 0.2 | 1.03 ± 0.06 (25.3) | 0.2 ± 0.0 | 95.9 ± 0.4 | 0.09 ± 0.02 | 330.3 ± 40.0 |
| > 1 & < 5 | 68 | 7.4 ± 0.3 | 1.14 ± 0.09 (21.8) | 2.2 ± 0.1 | 92.9 ± 0.6 | 0.21 ± 0.02 | 409.4 ± 63.4 |
| ≥ 5 & < 10 | 82 | 7.2 ± 0.3 | 1.24 ± 0.08 (46.3) | 7.9 ± 0.4 | 84.9 ± 0.8 | 0.30 ± 0.04 | 452.6 ± 113.4a |
| ≥10 | 73 | 7.5 ± 0.5 | 1.29 ± 0.11 (50.6) | 21.8 ± 2.4 | 81.6 ± 1.5 | 0.41 ± 0.05 | 1002.4 ± 136.1a |
Data are presented as mean ± SEM unless otherwise indicated and include the percentage of children in each group who are classied as being obese in parentheses.
TNF refers to tumor necrosis factor; OAHI, obstructive apnea-hypopnea index expressed as the number of events per hour of total sleep time; BMI, body mass index; SPS, sleep pressure score.
P < 0.01 vs. children with OAHI ≤ 1
Figure 1.
Scattergrams of individual tumor necrosis factor (TNF)-α morning plasma levels plotted against corresponding obstructive apnea-hypopnea index (OAHI), nadir SaO2, and sleep pressure score (SPS) in 270 habitually snoring children and 28 nonsnoring control subjects. Linear regression lines are shown and were highly signicant for OAHI (r2: 0.24; P < 0.001) and SPS (r2: 0.52; P < 0.000001) but not with nadir SaO2 (r2: 0.05; p > 0.05). The vertical lines show OAHI = 5 and SPS = 0.25 as cutoffs for disease severity. The right bottom panel shows log TNF-α plotted against log SPS, with the vertical line representing SPS = 0.25 whereas the curvilinear line represents the sigmoidal t function (r2: 0.64; P < 0.00000001). Please note the marked take off of TNF-α morning plasma concentrations once SPS exceeds the 0.25 cutoff value (7).
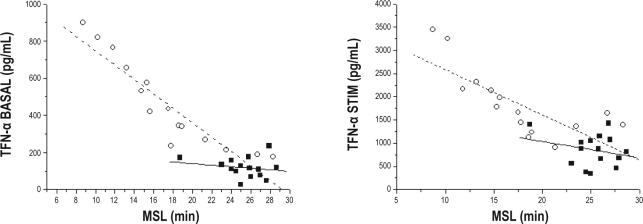
TNF-α levels measured in the supernatants of whole-blood cultures were highly correlated with MSLs in the MSLT among 15 children with OSA and also with MSLT-derived MSLs in 15 age-, sex-, and ethnicity-matched control nonsnoring children in both in basal and lipopolysaccharide-stimulated culture conditions (Figure 2). However, the slopes of these linear relationships were markedly steeper in children with OSA (Figure 2).
Figure 2.
Scattergrams of individual basal and after lipopolysaccharide stimulation tumor necrosis factor (TNF)-α supernatant levels obtained by ex vivo blood cultures and plotted against corresponding mean sleep latencies (MSL) in 15 children with obstructive sleep apnea and 15 age-, sex-, ethnicity-, and body mass index-matched control subjects.
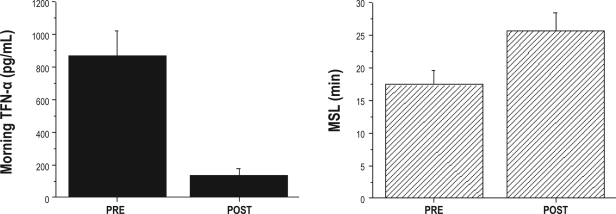
Sleep studies and MSLT were further obtained in 22 children with OSA both before (mean OAHI: 16.9 ± 3.9) and after surgical removal of enlarged adenoids and tonsils (mean OAHI: 2.3 ± 2.1; P < 0.001; Table 2). Normalization of sleep studies occurred in 16 of 22 children (i.e., OAHI ≤ 1), with 4 children showing an OAHI between 1 and 5 after surgery, and 2 children having residual OSA, with an OAHI of 6.7 and 8.5, respectively. MSL was significantly increased, and mean TNF-α levels were reduced after surgical treatment (P < 0.001; Figure 3).
Table 2.
Demographic and polysomnographic characteristics of 22 children with OSA before and after adenotonsillectomy
| Before adenotonsillectomy (n = 22) | After adenotonsillectomy (n = 22) | P value | |
|---|---|---|---|
| Age, y | 5.7 ± 0.8 | 6.1 ± 0.8 | |
| Boys, no. | 12 | ||
| African American, no. | 10 | ||
| BMI z score | 0.48 ± 0.7 | 0.49 ± 0.7 | NS |
| TST, min | 479 ± 42 | 481 ± 45 | NS |
| Sleep efciency, % | 88 ± 6 | 90 ± 7 | NS |
| Sleep stage, % TST85 | |||
| NREM | |||
| 1 | 7.6 ± 5.6 | 5.9 ± 3.7 | NS |
| 2 | 48.1 ± 6.2 | 47.4 ± 6.9 | NS |
| 3+4 | 25.5 ± 4.3 | 27.4 ± 4.4 | NS |
| REM | 18.8 ± 4.1 | 19.3 ± 4.4 | NS |
| OAHI, no/h | 16.9 ± 3.9 | 2.3 ± 2.1 | < 0.001 |
| SpO2nadir, % | 86.7 ± 2.2 | 91.1 ± 2.8 | < 0.01 |
| RAI, no/h | 6.7 ± 1.3 | 1.1 ± 0.9 | < 0.001 |
| % PETCO2 > 55 mm | |||
| Hg | 34.8 ± 5.1 | 26.3 ± 5.1 | < 0.05 |
| MSL, min | 17.4 ± 2.2 | 26.7 ± 2.7 | < 0.001 |
Data are presented as mean ± SEM. OSA refers to obstructive sleep apnea; BMI z, body mass index, Z score; NS, not signicant; TST, total sleep time; NREM, non rapid eye movement; REM, rapid eye movement; OAHI, obstructive apnea hypopnea index; SpO2, oxygen saturation measured by pulse oximetry; RAI, respiratory arousal index; PETCO2, end tidal carbon dioxide tension; MSL, mean sleep latency.
Figure 3.
Tumor necrosis factor (TNF)-α morning plasma levels and mean sleep latencies (MSL) in 22 children with obstructive sleep apnea before (PRE) and after (POST) surgical adenotonsillectomy. (PRE vs POST for both TNF-α levels and MSL: P < 0.001)
DISCUSSION
The present study shows that moderate to severe OSA is associated with significant elevations in the circulating morning concentrations of TNF-α in children. The latter were primarily correlated with the severity of SDB and, more particularly with sleep fragmentation, although BMI also contributed to the variance in plasma TNF-α. Furthermore, there is a correlation between basal or stimulated TNF-α levels and sleep propensity, as evidenced from sleep latencies during MSLT. However, the slope of such a relationship is clearly steeper in children with SDB. Finally, treatment of OSA by surgical extirpation of enlarged adenoids and tonsils not only resulted in improved MSL, but also yielded reductions in TNF-α levels. Based on such ndings, morning plasma TNF- measurements may provide a reliable marker for habitually snoring sleepy children.
In 2 previous studies, we have shown that sleep apnea in children is associated with severity-dependent reductions in MSL and that the presence of obesity is associated with marked exacerbations in EDS.3,5The results of the current study reinforce previous ndings by Chervin and colleagues that treatment of OSA with surgical adenotonsillectomy leads to significant improvements in MSL, suggesting that the deleterious effects of OSA on daytime sleep propensity appear to be reversible.16 Of interest, in an animal model of OSA consisting of intermittent hypoxic exposures during sleep, neuronal cellular losses in the locus coeruleus were associated with increased sleepiness and did not completely resolve after discontinuation of the intermittent hypoxic exposures.17
Sleep apnea in children is associated with increased inflammatory responses, and increased plasma levels of C-reactive protein, interleukin-6, and many other inflammatory mediators have been consistently reported by the majority of the investigators.18–26 However, in the few studies in which plasma TNF-α concentrations were measured in pediatric OSA, increased levels of this proinflammatory cytokine were not consistently present.27–29 Although the discrepancies between such studies and the current study cannot be fully explained, several factors may underlie such inconsistent ndings. First and foremost, TNF-α assays are extremely sensitive to the time elapsed between sample collection and actual ELISA assay, with substantial degradation occurring over time as well as during thawing and refreezing procedures (Gozal D, 2007, unpublished observations). Therefore, it is possible that differences in the handling of the blood samples may be responsible for the unpredictability of TNF-α levels in previous studies. As indicated in the Methods section, particular attention to the processing and assaying of all of the samples was taken.
Second, only more severe forms of pediatric OSA appear to be associated with increased TNF-α morning plasma concentrations (Table 1). However, we did not quantify the duration of waking prior to the night in which the sleep study occurred, and this factor could modulate TNF-α responses. Third, even when OSA is moderate or severe, there is substantial variability in TNF-α levels, suggesting that OAHI, i.e., the primary determinant of severity-category assignment, may not be the foremost contributor to TNF-α changes in the context of pediatric OSA and, thus, explain the inconsistent findings. This assumption was indeed conrmed in the present study, whereby a much stronger association emerged between the degree of respiratory event-induced sleep fragmentation (i.e., SPS) and TNF-α, than the association between OAHI and TNF-α (Figure 1). As has been previously reported, the overall trajectory of SPS in the context of pediatric SDB is not explained by a linear function relative to OAHI but, rather, is dictated by the concomitant compensatory efforts to reduce spontaneous arousals.7 Based on these considerations, we would surmise that morning plasma TNF-α levels in children may provide an excellent biologic correlate with the cumulative effects of sleep fragmentation, particularly considering the clear separation that occurs when SPS reaches the critical value of 0.25, at which time TNF-α clearly shows remarkable increases as a function of SPS (Figure 1, bottom right panel). A recent preliminary study in adults further supports the critical importance of sleep fragmentation in eliciting the recruitment of inflammatory mediators, such as TNF-α.30 We should point out that the lack of any relationship between nadir SaO2 and TNF-α was somewhat surprising, considering the previously reported increases in other proinflammatory cytokines as induced by the severity of hypoxemia.31–35 It is possible that the frequency of oxyhemoglobin desaturations below a particular threshold may be a more critical contributor to the induction of TNF-α gene expression than just the nadir SaO2.
Of note, not all adults with OSA display increased TNF-α concentrations, and, in fact, specific polymorphisms in the TNF-α gene have been implicated in the variance of TNF-α concentrations in the context of obesity and adult OSA.36–40 Notwithstanding, significant reductions in TNF-α levels will occur in effectively treated adult OSA patients,41 and, indeed, treatment of OSA in children resulted in similar plasma TNF-α reductions. However, the role of cytokine gene polymorphisms in pediatric SDB remains completely unexplored.
Ex-vivo blood cultures have been previously extensively used to assess cytokine and immune regulatory mechanisms42–44 and allow for the study of circadian or basal cytokine release, including TNF-α, as well as responses to varying doses of stimuli. The circadian rhythm of TNF-α release is significantly disturbed in adult patients with OSA, whereby nocturnal physiologic peaks almost disappear and an additional daytime peak emerges.45 In the current study, we found a significant association between MSLs and corresponding TNF-α concentrations in the supernatant in basal and stimulated conditions for both children with OSA and matched control subjects. However, the slopes of these linear relationships were more pronounced in children with OSA, suggesting that the presence of OSA may serve as a priming component of immune cell cytokine release and also that the actual degree of in vitro TNF-α production may be closely correlated with the degree of sleepiness.
In summary, pediatric OSA is associated with increases in morning plasma TNF-α concentrations, particularly in the moderate to severe disease categories. The variance in TNF-α levels in the cohort was better explained by the respiratory arousal index, i.e., SPS than by any other polysomnographically derived index and was also affected to some extent by the ponderal index. Interestingly, in vitro blood cultures revealed a significant linear relationship between TNF-α production and the degree of sleepiness measured in the MSLT, and surgical treatment of OSA is associated with decreases in TNF-α plasma levels as well as parallel improvement in sleep propensity. In the context of 5y3 current findings, morning TNF-α levels may provide a reliable and accurate measure of sleepiness in snoring children. Further assessment on the role of TNF-α gene polymorphisms in the context of pediatric OSA and sleepiness appear warranted.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. David Gozal has participated in speaking engagements for Merck. Dr. Leila Kheirandish-Gozal has received research support from Merck. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grant HL-65270 (DG).
REFERENCES
- 1.Carroll JL, McColley SA, Marcus CL, Curtis S, Loughlin GM. Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest. 1995;108:610–8. doi: 10.1378/chest.108.3.610. [DOI] [PubMed] [Google Scholar]
- 2.Chervin RD, Weatherly RA, Ruzicka DL, et al. Subjective sleepiness and polysomnographic correlates in children scheduled for adenotonsillectomy vs other surgical care. Sleep. 2006;29:495–503. [PMC free article] [PubMed] [Google Scholar]
- 3.Gozal D, Wang M, Pope DW., Jr. Objective sleepiness measures in pediat-Objective sleepiness measures in pediatric obstructive sleep apnea. Pediatrics. 2001;108:693–7. doi: 10.1542/peds.108.3.693. [DOI] [PubMed] [Google Scholar]
- 4.Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114:768–75. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- 5.Gozal D, Kheirandish-Gozal L. Obesity and excessive daytime sleepiness in pre-pubertal children with obstructive sleep apnea. Pediatrics. 2009;123:13–8. doi: 10.1542/peds.2008-0228. [DOI] [PubMed] [Google Scholar]
- 6.Tauman R, O'Brien LM, Barbe F, Iyer VG, Gozal D. Reciprocal interac-tions between spontaneous and respiratory arousals in adults with suspected sleep-disordered breathing. Sleep Med. 2006;7:229–34. doi: 10.1016/j.sleep.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Tauman R, O'Brien LM, Holbrook CR, Gozal D. Sleep pressure score: a new index of sleep disruption in snoring children. Sleep. 2004;27:274–8. doi: 10.1093/sleep/27.2.274. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien LM, Tauman R, Gozal D. Sleep pressure correlates of cognitive and behavioral morbidity in snoring children. Sleep. 2004;27:279–82. doi: 10.1093/sleep/27.2.279. [DOI] [PubMed] [Google Scholar]
- 9.Chervin RD, Burns JW, Ruzicka DL. Electroencephalographic changes during respiratory cycles predict sleepiness in sleep apnea. Am J Respir Crit Care Med. 2005;171:652–8. doi: 10.1164/rccm.200408-1056OC. [DOI] [PubMed] [Google Scholar]
- 10.Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des. 2008;14:3408–16. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab. 2004;89:4409–13. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysom-nographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–53. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 13.Rechstschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles: Brain Information Services/Brain Research Institute, University of California; 1968. [Google Scholar]
- 14.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 15.Littner MR, Kushida C, Wise M, et al. Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 16.Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117:e769–78. doi: 10.1542/peds.2005-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veasey SC, Davis CW, Fenik P, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- 18.Tauman R, Ivanenko A, O'Brien LM, Gozal D. Plasma C-reactive protein in children with sleep-disordered breathing. Pediatrics. 2004;113:e564–9. doi: 10.1542/peds.113.6.e564. [DOI] [PubMed] [Google Scholar]
- 19.Kheirandish-Gozal L, Sans Capdevila O, Tauman R, Gozal D. C-reactive protein in non-obese children with obstructive sleep apnea before and after adenotonsillectomy. J Clin Sleep Med. 2006;2:301–4. [PMC free article] [PubMed] [Google Scholar]
- 20.Tauman R, O'Brien LM, Gozal D. Hypoxemia and obesity modulate plasma C-reactive protein and interleukin 6 levels in snoring children. Sleep Breath. 2007;11:77–84. doi: 10.1007/s11325-006-0085-7. [DOI] [PubMed] [Google Scholar]
- 21.Gozal D, McLaughlin Crabtree V, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. 2007;176:188–93. doi: 10.1164/rccm.200610-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med. 2008;9:254–9. doi: 10.1016/j.sleep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalyfa A, Sans Capdevila O, Boazza M, Serpero LD, Kheirandish-Gozal L, Gozal D. Genome-wide gene expression profiling in children with obstructive sleep apnea. Sleep Med. 2009;10:75–86. doi: 10.1016/j.sleep.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Goldbart AD, Tal A. Inflammation and sleep disordered breathing in children: a state-of-the-art review. Pediatr Pulmonol. 2008;43:1151–60. doi: 10.1002/ppul.20943. [DOI] [PubMed] [Google Scholar]
- 25.Larkin EK, Rosen CL, Kirchner HL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–84. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 26.Li AM, Chan MH, Yin J, et al. C-reactive protein in children with obstructive sleep apnea and the effects of treatment. Pediatr Pulmonol. 2008;43:34–40. doi: 10.1002/ppul.20732. [DOI] [PubMed] [Google Scholar]
- 27.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrou-sos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 28.Waters KA, Mast BT, Vella S, et al. Structural equation modeling of sleep apnea, inflammation, and metabolic dysfunction in children. J Sleep Res. 2007;16:388–95. doi: 10.1111/j.1365-2869.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- 29.Li AM, Lam HS, Chan MH, et al. Inflammatory cytokines and childhood obstructive sleep apnoea. Ann Acad Med Singapore. 2008;37:649–54. [PubMed] [Google Scholar]
- 30.Yue HJ, Mills PJ, Ancoli-Israel S, Loredo JS, Ziegler MG, Dimsdale JE. The roles of TNF-alpha and the soluble TNF receptor I on sleep architecture in OSA. Sleep Breath. 2009;13:263–9. doi: 10.1007/s11325-008-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alberti A, Sarchielli P, Gallinella E, et al. Plasma cytokine levels in pa-Plasma patients with obstructive sleep apnea syndrome: a preliminary study. J Sleep Res. 2003;12:305–11. doi: 10.1111/j.1365-2869.2003.00361.x. [DOI] [PubMed] [Google Scholar]
- 32.Imagawa S, Yamaguchi Y, Ogawa K, et al. Interleukin-6 and tumor necrosis factor-alpha in patients with obstructive sleep apnea-hypopnea syndrome. Respiration. 2004;71:24–9. doi: 10.1159/000075645. [DOI] [PubMed] [Google Scholar]
- 33.Minoguchi K, Tazaki T, Yokoe T, et al. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126:1473–9. doi: 10.1378/chest.126.5.1473. [DOI] [PubMed] [Google Scholar]
- 34.Kataoka T, Enomoto F, Kim R, et al. The effect of surgical treatment of obstructive sleep apnea syndrome on the plasma TNF-alpha levels. Tohoku J Exp Med. 2004;204:267–72. doi: 10.1620/tjem.204.267. [DOI] [PubMed] [Google Scholar]
- 35.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006;174:824–30. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- 36.Riha RL, Brander P, Vennelle M, et al. Tumour necrosis factor-alpha (-308) gene polymorphism in obstructive sleep apnoea-hypopnoea syndrome. Eur Respir J. 2005;26:673–8. doi: 10.1183/09031936.05.00130804. [DOI] [PubMed] [Google Scholar]
- 37.Popko K, Gorska E, Potapinska O, et al. Frequency of distribution of inflammatory cytokines IL-1, IL-6 and TNF-alpha gene polymorphism in patients with obstructive sleep apnea. J Physiol Pharmacol. 2008;59:607–14. [PubMed] [Google Scholar]
- 38.Bhushan B, Guleria R, Misra A, Luthra K, Vikram NK. TNF-alpha gene polymorphism and TNF-alpha levels in obese Asian Indians with obstructive sleep apnea. Respir Med. 2009;103:386–92. doi: 10.1016/j.rmed.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Kanbay A, Kokturk O, Ciftci TU, Tavil Y, Bukan N. Comparison of serum adiponectin and tumor necrosis factor-alpha levels between patients with and without obstructive sleep apnea syndrome. Respiration. 2008;76:324–30. doi: 10.1159/000134010. [DOI] [PubMed] [Google Scholar]
- 40.Aouizerat B, Dodd M, Lee K, et al. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biol Res Nurs. 2009;11:27–41. doi: 10.1177/1099800409333871. [DOI] [PubMed] [Google Scholar]
- 41.Steiropoulos P, Kotsianidis I, Nena E, et al. Long-term effect of continuous positive airway pressure therapy on inflammation markers of patients with obstructive sleep apnea syndrome. Sleep. 2009;32:537–43. doi: 10.1093/sleep/32.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aguillón JC, Escobar A, Ferreira V, et al. Daily production of human tumor necrosis factor in lipopolysaccharide (LPS)-stimulated ex vivo blood culture assays. Eur Cytokine Netw. 2001;12:105–10. [PubMed] [Google Scholar]
- 43.Thurm CW, Halsey JF. Measurement of cytokine production using whole blood. Curr Protoc Immunol. 2005 doi: 10.1002/0471142735.im0718bs66. Chapter 7:Unit 7.18B. [DOI] [PubMed] [Google Scholar]
- 44.Ray CA, Dumaual C, Willey M, et al. Optimization of analytical and pre-analytical variables associated with an ex vivo cytokine secretion assay. J Pharm Biomed Anal. 2006;41:189–95. doi: 10.1016/j.jpba.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 45.Entzian P, Linnemann K, Schlaak M, Zabel P. Obstructive sleep apnea syndrome and circadian rhythms of hormones and cytokines. Am J Respir Crit Care Med. 1996;153:1080–6. doi: 10.1164/ajrccm.153.3.8630548. [DOI] [PubMed] [Google Scholar]