Abstract
Study Objectives:
Sleep deeply affects cardiac autonomic control, the impairment of which is associated with cardiovascular mortality. Obesity entails increased cardiovascular risk and derangements in sleep and cardiac autonomic control. We investigated whether cardiac autonomic control is impaired during sleep in ob/ob mice with morbid obesity caused by congenital leptin deficiency.
Design:
Indexes of cardiac autonomic control based on spontaneous cardiovascular fluctuations were compared between ob/ob and lean wild-type (+/+) mice during wakefulness, non-rapid eye movement sleep (NREMS), and rapid eye movement sleep (REMS).
Setting:
N/A
Patients or Participants:
7 ob/ob and 11 +/+ male mice.
Interventions:
Instrumentation with electrodes for sleep recordings and a telemetric transducer for measuring blood pressure and heart period.
Measurements and Results:
In ob/ob mice, the variability of heart period and cardiac baroreflex sensitivity (sequence technique) were significantly lower than in +/+ mice during each wake-sleep state. The vagal modulation of heart period was significantly weaker in ob/ob than in +/+ mice during NREMS and REMS. In ob/ob mice, the cross-correlation function between heart period and blood pressure suggested that the baroreflex contribution to cardiac control was lower than in +/+ mice during wakefulness and NREMS, whereas the contribution of central autonomic commands was lower than in +/+ mice during NREMS and REMS.
Conclusions:
These data indicate a dysregulation of cardiac autonomic control during sleep in ob/ob mice. Ob/ob mice may represent a useful tool to understand the molecular pathways that lead to cardiac autonomic dysregulation during sleep in obesity.
Citation:
Silvani A; Bastianini S; Berteotti C; Franzini C; Lenzi P; Lo Martire V; Zoccoli G. Dysregulation of heart rhythm during sleep in leptin-deficient obese mice. SLEEP 2010;33(3):355-361.
Keywords: Obesity, leptin, sleep, autonomic nervous system, heart rate, blood pressure, baroreflex
IMPAIRMENTS IN CARDIAC AUTONOMIC CONTROL AS REVEALED BY INDEXES OF HEART RATE VARIABILITY OR THE CARDIAC BAROREFLEX ARE POWERFUL negative prognostic factors in patients with cardiovascular disease.1–3 Derangements in these indexes may be a marker of other cardiovascular risk factors4 and also play a pathogenic role because vagal activation may protect against life-threatening cardiac arrhythmias.5
Sleep exerts a deep physiological impact on cardiovascular regulation.6 In particular, cardiac vagal tone and modulation7,8 and the baroreflex contribution to cardiac control9,10 are enhanced during non-rapid eye movement sleep (NREMS) with respect to wakefulness and rapid eye movement sleep (REMS). The physiological enhancement of cardiac vagal modulation in NREMS may help explain the reduced nocturnal incidence of sudden cardiac death.11 Conversely, patients with recent myocardial infarction are at high risk for sudden death and lose the capability for physiological activation of vagal activity during NREMS.12 On the other hand, the effects of sleep on cardiac autonomic control may differ between physiological and pathological conditions.9,13 Thus, assessment of pathological impairments in cardiac autonomic control may be improved by taking into account the wake-sleep behavior.
Obesity is a challenging threat to health care because it is rapidly rising in prevalence14 and entails a burden of metabolic15 and cardiovascular complications, which prominently include sudden cardiac death.16 Obesity entails impairments in cardiac vagal modulation, which at night tend to resist improvement following weight loss.17–19
The availability of animal models of obesity with impairment in cardiac vagal modulation during sleep would accelerate the understanding of the mechanisms and the possible pathogenic role of such impairment. Mice are the species of choice to study cardiovascular functional genomics and the pathophysiology of the metabolic syndrome at the molecular level.15 An impairment in cardiac vagal modulation has recently been demonstrated in db/db mice with morbid obesity caused by congenital leptin resistance.20 This impairment was proven to be largely functional rather than structural,20 but no evidence was provided on its amelioration or persistence during sleep. We have recently shown that sleep modulates hypertension in ob/ob mice, which are morbidly obese because of congenital leptin deficiency.21 In the present study, we investigated whether ob/ob mice represent a model of impaired cardiac autonomic control during sleep in obesity.
METHODS
The study protocol was approved by the Bologna University ethics committee on animal experimentation and complied with the National Institutes of Health guide for the care and use of laboratory animals.
Animals
Experiments were performed on male B6.V-Lepob/ob/OlaHsd mice (ob/ob, n = 7; Harlan Italy, S. Pietro al Natisone, Italy), which are homozygous for a nonsense mutation in the leptin gene, and on their male B6.V-Lep+/+/OlaHsd littermates with 2 functional leptin alleles (+/+, n = 11). The genotype of +/+ mice was assessed by PCR in the Centre for Applied Biomedical Research – CRBA, S. Orsola University Hospital, Bologna, Italy. Mice were kept on a 12:12 hour light-dark cycle with ambient temperature set at 25°C and free access to water and food (2018 diet, Harlan Italy).
Experimental Procedures
Experimental procedures for surgery, physiological recordings, and sleep scoring have been published in detail.21 Mice underwent surgery under general anesthesia for the implantation of a telemetric blood pressure (BP) transducer (TA11PAC10, DSI, Tilburg, the Netherlands), with the catheter inserted through the femoral artery into the abdominal aorta. Pairs of electrodes were positioned in contact with the dura mater and with nuchal muscles to obtain electroencephalographic (EEG) and electromyographic (EMG) signals, respectively, which were transmitted by conventional cable transmission. After an 11-day period of recovery and habituation, BP (sampling rate 1024 Hz), EEG, and EMG were simultaneously and continuously measured on mice undisturbed in their own cages for 3 days. An electrical swivel and a balanced suspensor arm prevented the cable from twisting and counterbalanced its weight, allowing unhindered movements to the mice. At the end of recordings, mice were aged 15.0 ± 0.5 weeks and ob/ob mice weighted approximately twice as much as +/+ mice (48.6 ± 0.8 versus 25.5 ± 0.7 g). The states of wakefulness, NREMS, and REMS were visually scored off-line on 4-s epochs based on raw EEG and EMG signals. In 4 +/+ mice, failure of the EEG electrodes limited the analysis of wake-sleep episodes to the first 25, 47, 57, and 59 hours of recordings, respectively.
Data Analysis
The main analysis was performed on all artifact-free episodes of wakefulness, NREMS, and REMS of duration ≥ 60 s, which occurred during the 3 days of recordings. The duration requirement of ≥ 60 s allowed to compute validated indexes of autonomic control in the frequency domain.22 Ancillary analyses were performed to assess the replicability of significant findings across days as well as across the light and dark periods.
Beat-to-beat values of heart period (HP) and of systolic, diastolic, and mean BP were computed from the raw BP signal. Values of HP were computed as the time intervals between the onset of successive systolic BP upstrokes, i.e., between the minimum value of a pulse wave and the minimum value of the following one. A semiautomated procedure was performed to identify and exclude from subsequent analyses each 4-s epoch, in which errors in the automatic detection of the minima and maxima of the pulse waves produced artifactual values of HP or of systolic, diastolic, and mean BP greater than twice the respective median values or lower than 10% of the respective median values within wake-sleep episode.21
The total variability of HP was quantified by the standard deviation of HP values (SDHP). The vagal modulation of HP was assessed in the time domain with the index pNN8, which is the % of HP values that differ from the following ones by > 8 ms,23 and in the frequency domain with the index RSA, which corresponds to respiratory sinus arrhythmia and is based on the spectral power of HP in the frequency range 2.5–5.0 Hz.22 A validated index of the sympathetic modulation of BP (SYM) was also computed based on the spectral power of systolic BP in the frequency range 0.15–0.6 Hz.22
The cardiac baroreflex sensitivity (BRS) and the baroreflex effectiveness index (BEI) were computed within each wake-sleep episode with the sequence technique.24 In correspondence with short spontaneous sequences of increases or decreases in beat-to-beat BP values, the indexes BRS and BEI estimate the gain (i.e., the change in HP, which is associated with a unit change in systolic BP) and degree of engagement (i.e., the fraction of BP sequences, which are associated with baroreflex changes in HP) of the baroreflex, respectively. To investigate the contributions of the baroreflex and central autonomic commands to cardiac control at a longer time scale, we analyzed the cross-correlation function (CCF) between HP and systolic BP.9,10,25 The CCF yields the correlation coefficient between HP and systolic BP as a function of the time shift between these variables. Negative values of time shift indicate that changes in HP follow those in BP.
Cardiac autonomic control during the phasic hypertensive events (BP surges) of REMS was investigated with a coherent averaging procedure.13,25 The BP surges in REMS were automatically detected with a 5 mm Hg threshold for peak increase in systolic BP. After subtraction of their respective baseline values, time series of HP and systolic BP during the BP surges were synchronized at the peak increase in systolic BP and averaged.
The indexes SDHP, pNN8, BRS, and BEI were computed based on beat-to-beat values of HP and systolic BP. The other analyses were performed after re-sampling the time series of HP and systolic BP at 20 Hz with a piecewise cubic Hermite interpolation. The CCF and the BP surges in REMS were analyzed after low-pass filtering the time series of HP and BP below 0.8 Hz (3-pole Butterworth filter) to focus the analysis on fluctuations slower than the breathing rate.9,10,13,25 The CCF analysis and the indexes SDHP, pNN8, RSA, and SYM were averaged over consecutive data subsets of 60-s duration overlapped for 45 s. Data analysis was performed with custom software written in Matlab (the Mathworks, Inc., Natick, MA, USA).
Statistical Analysis
With the exception of CCF time shifts, which were not normally distributed, data were reported as mean ± SEM and analyzed with 2-way mixed model analysis of variance (ANOVA, GLM procedure) and t-tests. The CCF time shifts were reported as median and interquartile range and analyzed with the binomial single-sample sign test (cutoff value of 0; probability parameter of 0.5) to evaluate whether they significantly clustered at negative or positive values.10 Data were reported with n = 7 for ob/ob mice and n = 11 for +/+ mice. Analyses were performed with SPSS software (SPSS Inc., Chicago, IL, USA) with P < 0.05 considered statistically significant.
RESULTS
Table 1 shows the percentage of recording time spent in each wake-sleep state and the characteristics of the wake-sleep episodes, on which the analysis of cardiovascular variables was performed. The mean values of HP and BP in these episodes are reported in Table 2.
Table 1.
Characteristics of the wake-sleep episodes recorded and analyzed
| All episodes | W |
NREMS |
REMS |
|||
|---|---|---|---|---|---|---|
| ob/ob | +/+ | ob/ob | +/+ | ob/ob | +/+ | |
| % recording time | 40 ± 2 | 45 ± 2 | 50 ± 1 | 44 ± 1 | 7 ± 1 | 7 ± 1 |
| Episodes > 60 s analyzed | ||||||
| % recording time | 27 ± 3 | 29 ± 3 | 33 ± 2 | 31 ± 1 | 4 ± 1 | 5 ± 1 |
| Number | 343 ± 23 | 383 ± 33 | 824 ± 30 | 645 ± 56 | 115 ± 12 | 101 ± 10 |
| Mean duration (s) | 210 ± 20 | 172 ± 20 | 104 ± 4 | 109 ± 4 | 100 ± 3 | 106 ± 1 |
| Total duration (h) | 19.9 ± 1.9 | 17.9 ± 2.3 | 23.8 ± 1.2 | 19.7 ± 2.0 | 3.2 ± 0.4 | 3.0 ± 0.3 |
W, wakefulness; NREMS, non-rapid eye movement sleep; REMS, rapid eye movement sleep.
Data are means ± SEM in ob/ob (n = 7) and +/+ (n = 11) mice.
Table 2.
Mean values of cardiovascular variables
| W |
NREMS |
REMS |
||||
|---|---|---|---|---|---|---|
| ob/ob | +/+ | ob/ob | +/+ | ob/ob | +/+ | |
| SBP (mm Hg) | 144 ± 3 | 138 ± 2 | 123 ± 3 | 120 ± 2 | 121 ± 3 | 122 ± 2 |
| DBP (mm Hg) | 109 ± 2 | 101 ± 1* | 93 ± 3 | 84 ± 1* | 90 ± 3 | 86 ± 1 |
| MBP (mm Hg) | 126 ± 2 | 119 ± 1* | 108 ± 3 | 101 ± 1* | 105 ± 3 | 103 ± 1 |
| HP (ms) | 98 ± 1 | 98 ± 2 | 116 ± 2 | 122 ± 3 | 112 ± 1 | 113 ± 3 |
SBP, DBP, and MBP indicate systolic, diastolic, and mean blood pressure, respectively. HP, heart period. W, wakefulness; NREMS, non-rapid eye movement sleep; REMS, rapid eye movement sleep. Data are means ± SEM in ob/ob (n = 7) and +/+ (n = 11) mice. Analysis of variance: state, P <0.001 for all variables; strain, P = 0.397 (SBP), P = 0.010 (DBP), P = 0.049 (MBP), P = 0.465 (HP); state × strain interaction, P <0.001 (SBP, DBP, MBP), P = 0.019 (HP),
P <0.05 vs. ob/ob mice (t-test).
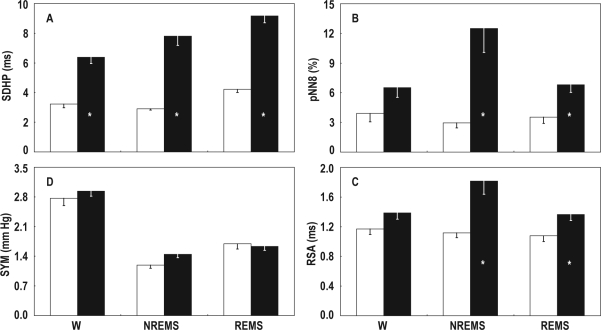
The total variability of HP, as assessed with the index SDHP, was significantly lower in ob/ob than in +/+ mice in each wake-sleep state (P < 0.001; Figure 1, panel A).
Figure 1.
A, total variability of heart period (SDHP); B and C, indexes of cardiac vagal modulation computed in the time domain (pNN8) and frequency domain (RSA); D, index of sympathetic modulation of blood pressure (SYM). Data are means - SEM during wakefulness (W), non-rapid eye movement sleep (NREMS), and rapid eye movement sleep (REMS) in ob/ob mice (open bars, n = 7) and +/+ mice (filled bars, n = 11). Analysis of variance: state, P < 0.001 (SDHP and SYM), P = 0.078 (pNN8), P = 0.029 (RSA); strain, P < 0.001 (SDHP), P = 0.005 (pNN8), P = 0.006 (RSA), P = 0.403 (SYM); state × strain interaction, P = 0.042 (SDHP), P = 0.022 (pNN8), P = 0.025 (RSA), P = 0.135 (SYM). *P < 0.05 vs. ob/ob mice (t-test).
The indexes of cardiac vagal modulation computed in the time (pNN8) and frequency (RSA) domain are reported in panels B and C of Figure 1, respectively. The indexes pNN8 and RSA were significantly lower in ob/ob than in +/+ mice in NREMS (P = 0.007 and P = 0.008, respectively) and REMS (P = 0.008 and P = 0.030, respectively), but not in wakefulness (P = 0.085 and P = 0.100, respectively).
The sympathetic modulation of BP, as assessed with the index SYM, did not differ significantly between ob/ob and +/+ mice (Figure 1, panel D).
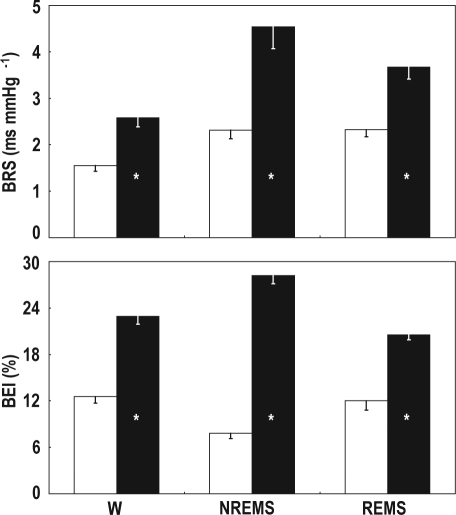
The results of the assessment of the spontaneous cardiac baroreflex with the sequence technique are shown in Figure 2. The indexes BRS and BEI were significantly lower in ob/ob than in +/+ mice in each wake-sleep state (P < 0.002).
Figure 2.
BRS, cardiac baroreflex sensitivity; BEI, baroreflex effectiveness index. Data are means - SEM during wakefulness (W), non-rapid-eye-movement sleep (NREMS), and rapid-eye-movement sleep (REMS) in ob/ob mice (open bars, n = 7) and +/+ mice (filled bars, n = 11). Analysis of variance: state, P < 0.001 (BRS), P = 0.159 (BEI); strain, P = 0.001 (BRS), P < 0.001 (BEI); state × strain interaction, P = 0.038 (BRS), P < 0.001 (BEI). *P < 0.05 vs. ob/ob mice (t-test).
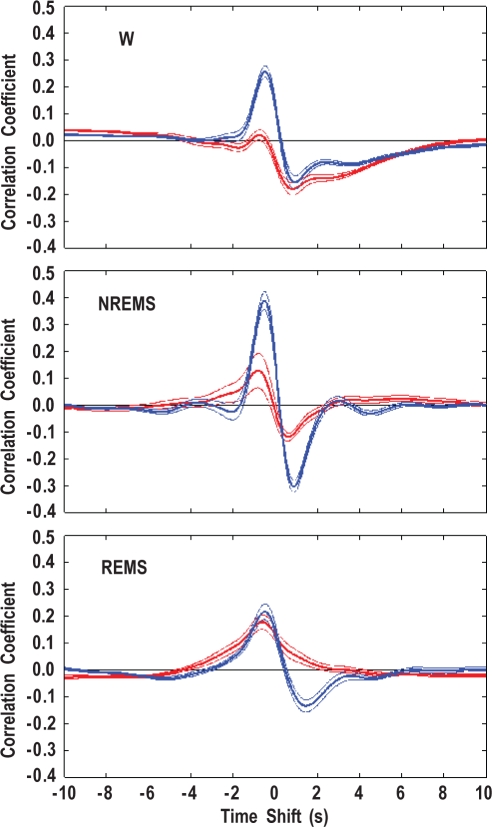
The average CCFs between HP and systolic BP are shown in Figure 3. The maximum (ρMAX) and minimum (ρMIN) values of the CCFs and their corresponding time shifts (τMAX and τMIN) are reported in Table 3. A CCF maximum at negative time shifts (i.e., a positive correlation between HP and the previous BP values) occurred in all wake-sleep states in +/+ mice, whereas in ob/ob mice, it was evident only in REMS. Accordingly, the values of τMAX in wakefulness and NREMS showed a wide dispersion in ob/ob mice. Moreover, ρMAX was significantly lower in ob/ob than in +/+ mice in wakefulness and NREMS (P < 0.001) but not in REMS (P = 0.276). A CCF minimum at positive time shifts (i.e., a negative correlation between HP and the following BP values) occurred in all wake-sleep states in +/+ mice, whereas in ob/ob mice, it was not evident in REMS. Accordingly, the values of τMIN in REMS showed a wide dispersion in ob/ob mice. In ob/ob mice, ρMIN was significantly lower in magnitude than in +/+ mice in NREMS (P < 0.001) and REMS (P = 0.002) but not in wakefulness (P = 0.582).
Figure 3.
Cross-correlation functions between low-frequency (< 0.8 Hz) fluctuations of heart period and systolic blood pressure. Data are means ± SEM during wakefulness (W), non-rapid eye movement sleep (NREMS), and rapid eye movement sleep (REMS) in ob/ob mice (red lines, n = 7) and +/+ mice (blue lines, n = 11).
Table 3.
Maximum and minimum values of the cross-correlation functions between heart period and systolic blood pressure and their corresponding time shifts
| Maximum and minimum CCF values | W |
NREMS |
REMS |
|||
|---|---|---|---|---|---|---|
| ob/ob | +/+ | ob/ob | +/+ | ob/ob | +/+ | |
| ρMAX | 0.05 ± 0.01 | 0.26 ± 0.02* | 0.18 ± 0.04 | 0.40 ± 0.03* | 0.18 ± 0.03 | 0.23 ± 0.03 |
| ρMIN | −0.19 ± 0.02 | −0.17 ± 0.02 | −0.12 ± 0.02 | −0.31 ± 0.02* | −0.04 ± 0.01 | −0.14 ± 0.02* |
| CCF time shifts | ||||||
| τMAX (s) | −8.7 (9.2)† | −0.5 (0.1)† | −0.7 (5.6) | −0.5 (0.1)† | −0.6 (0.1)† | −0.4 (0.2)† |
| τMIN (s) | 0.9 (0.4)† | 0.9 (0.6)† | 0.6 (0.3)† | 0.9 (0.2)† | −5.9 (11.8) | 1.4 (0.3)† |
Maximum and minimum values (ρMAX and ρMIN, respectively) and corresponding time shifts (τMAX and τMIN, respectively) of the cross-correlation functions (CCF) between low-frequency (<0.8 Hz) fluctuations of heart period and systolic blood pressure. W, wakefulness; NREMS, non-rapid eye movement sleep; REMS, rapid eye movement sleep. Data are expressed as means ± SEM (ρMAX, ρMIN) or as median values and interquartile range (τMAX, τMIN) in ob/ob (n = 7) and +/+ (n = 11) mice. Analysis of variance: state and strain, P <0.001 (ρMAX, ρMIN); state × strain interaction, P = 0.002 (ρMAX), P <0.001 (ρMIN),
P <0.05 vs. ob/ob mice (ρMAX, ρMIN; t-test),
P <0.05 (τMAX, τMIN; binomial test).
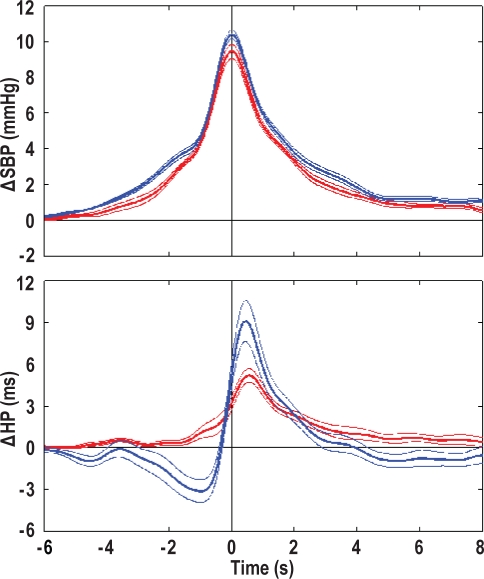
The results of the coherent averaging of BP surges during REMS are shown in Figure 4 and Table 4. With respect to +/+ mice, ob/ob mice had a similar rate of occurrence of BP surges in REMS (P = 0.536) and a slight reduction in the peak increase in BP during the surges (P = 0.050). The peak increase in BP was preceded by a decrease in HP in +/+ mice but not in ob/ob mice (P = 0.001 vs. +/+ mice). The peak increase in BP was followed by an increase in HP, the peak value of which was significantly lower in ob/ob than in +/+ mice (P = 0.026).
Figure 4.
Coherent averaging of blood pressure surges during rapid-eye-movement sleep. Data are means ± SEM in ob/ob mice (red lines, n = 7) and +/+ mice (blue lines, n = 11) synchronized at the peak increase of systolic blood pressure (SBP, time 0). From top to bottom, the panels show the normalized changes in SBP (Δ SBP) and heart period (Δ HP).
Table 4.
Analysis of blood pressure surges in rapid-eye-movement sleep
| ob/ob | +/+ | |
|---|---|---|
| Number | 180 ± 36 | 176 ± 21 |
| Frequency (number in 10 min REMS) | 9 ± 1 | 10 ± 1 |
| ΔBP (mm Hg) | 9.5 ± 0.4 | 10.4 ± 0.3* |
| ΔHPtrough (ms) | 0.0 ± 0.1 | -3.5 ± 0.8* |
| ΔHPpeak (ms) | 5.2 ± 0.5 | 9.2 ± 1.5* |
REMS, rapid eye movement sleep; ΔBP, peak increase in systolic blood pressure (BP) associated with the BP surges in REMS; ΔHPtrough, maximum decrease in heart period (HP) in the 4 s before the BP peak; ΔHPpeak, maximum increase in HP in the 4 s after the BP peak. Data are means ± SEM in ob/ob (n = 7) and +/+ (n = 11) mice,
P <0.05 vs. ob/ob mice (t-test).
The significant differences, which occurred between ob/ob and +/+ mice on the whole 3-day recording period, generally showed a complete replicability across recording days as well as across the light and dark periods in ancillary analyses (indexes SDHP, BRS, BEI, ρMAX, ρMIN, and the HP decrease during BP surges in REMS; P < 0.050). Differences in pNN8 and RSA between ob/ob and +/+ mice also showed replicability across recording days as well as across the light and dark periods (P < 0.050) with the exceptions of differences in REMS in the third recording day (P = 0.067 and P = 0.158, respectively) and the dark period (P = 0.161 and P = 0.224, respectively). The reduction in the HP rise during the BP surges in REMS in ob/ob mice lost statistical significance in the ancillary analyses restricted to the second (P = 0.215) or the third (P = 0.075) recording day or to the dark period (P = 0.357).
DISCUSSION
We tested the hypothesis that cardiac autonomic control is impaired during sleep in ob/ob mice. This hypothesis was fully supported by our findings.
To our knowledge, our study was the first to investigate cardiac autonomic control during sleep in a mouse model of human disease. We assessed cardiac autonomic control based on spontaneous cardiovascular variability. This approach is inherently indirect and cannot discriminate between derangements in cardiac autonomic nerve activity and derangements in the cardiac response to such activity. On the other hand, this approach informs on real-life cardiac regulation and lends itself optimally to clinical translation, yielding indexes with prognostic significance in human patients.1–3
The vagal modulation of HP is specifically targeted by the indexes pNN8 and RSA in mice.22,23 However, the indexes SDHP and BRS also depend on the vagal modulation of HP in this species.23,26 In ob/ob mice, all of these indexes were lower than in +/+ mice during NREMS and REMS (Figure 1 and Figure 2), although the replicability of the difference in pNN8 and RSA during REMS was limited to the light period. On the other hand, the indexes SDHP (Figure 1) and BRS (Figure 2) were also lower in ob/ob than in +/+ mice during wakefulness. These findings indicate a dramatic impairment in the vagal modulation of HP in ob/ob mice, which is particularly prominent in NREMS and, during the light period, also in REMS. Moreover, reductions in spontaneous BRS estimates may effectively reflect reductions in the classical pharmacological estimates of BRS in mice.27
To our knowledge, no validated index of cardiac sympathetic modulation is available in mice. However, a validated index of the sympathetic modulation of BP had similar values in ob/ob and +/+ mice in each wake-sleep state (Figure 1, index SYM). The mean value of HP, which reflects the balance among intrinsic HP and the effects of cardiac vagal and sympathetic tone,26 was also similar in ob/ob and +/+ mice in each wake-sleep state (Table 2). These data argue against any significant impairment in cardiac autonomic tone or sympathetic modulation during sleep and wakefulness in ob/ob mice.
We performed an array of analyses to test whether cardiac regulation by baroreflex and non-baroreflex mechanisms is impaired in ob/ob mice in the different wake-sleep states. Over a time scale of few (i.e., 3-4) heart beats, the values of index BEI (Figure 2) indicate that the contribution of the baroreflex to HP control was significantly and substantially lower in ob/ob than in +/+ mice in each wake-sleep state. On a longer time scale, a positive CCF peak at negative time shifts indicates, e.g., a pattern of cardiac slowing after an increase in BP, which is consistent with the operation of the arterial baroreflex.9,10,25 In +/+ mice in each wake-sleep state, such positive peak was clearly evident in the average CCFs (Figure 3) and was highly reproducible among mice, as shown by the small dispersion (interquartile range) of the corresponding time shifts (τMAX, Table 3). In ob/ob mice, the CCFs did not show any reproducible positive peak in wakefulness and NREMS, as demonstrated by the wide dispersion of the τMAX time shifts in these states. Moreover, the values of the CCF maxima (ρMAX, Table 3) were significantly lower in ob/ob than in +/+ mice during wakefulness and NREMS, whereas they did not differ between ob/ob and +/+ mice in REMS. These findings suggest that at a longer time scale than that explored by the BEI index, the baroreflex contribution to HP control is impaired in ob/ob mice in wakefulness and NREMS, but not in REMS. On the other hand, the negative CCF trough at positive time shifts indicates, e.g., a pattern of cardiac acceleration before an increase in BP, which is consistent with the operation of central autonomic commands.9,10 In REMS, this negative CCF trough was not detectable in ob/ob mice (Figure 3 and Table 3). In ob/ob mice, moreover, the CCF minimum values (ρMIN) had significantly lower magnitude than in +/+ mice during NREMS (Table 3). These findings suggest that the contribution of central autonomic commands to HP control over a time scale of 1 minute was lower in ob/ob than in +/+ mice during the sleep states (NREMS and REMS), whereas it was maintained in wakefulness. During wakefulness and REMS, the pattern of impairment in cardiac regulation, which was evidenced by the CCF analysis in ob/ob mice, was the same as that previously reported in spontaneously hypertensive rats,9 which are a widely studied model of human hypertension. Notably, spontaneously hypertensive rats also show sleep related derangements in cardiac vagal modulation.28
In spontaneously hypertensive rats, the impaired contribution of central commands to HP control during REMS9 was supported by the analysis of the phasic hypertensive events (BP surges),13 which are a physiological feature of this state.6,25,29 Similarly, in the present study, the impaired contribution of central commands to HP control during REMS in ob/ob mice was supported by the analysis of the BP surges in REMS. A cardiac acceleration before the peak of BP surges is consistent with the central autonomic commands on HP prevailing upon the baroreflex.6,13,25 This cardiac acceleration was observed in +/+ mice, whereas it was absent in ob/ob mice (Figure 4 and Table 4). Conversely, the cardiac slowing after the peak of BP surges, which is consistent with baroreflex control of HP prevailing upon central autonomic commands,6,25 clearly occurred in ob/ob mice (Figure 4 and Table 4), although it tended to have smaller magnitude than in +/+ mice particularly during the light period.
Our findings compare favorably with the limited evidence available on obese humans. During quiet wakefulness, obese women without hypertension or diabetes were found to have lower values of BRS, total HP variability, and cardiac vagal modulation (high-frequency spectral power of HP) than lean controls.19 In obese subjects, reductions in the total variability of HP and cardiac vagal modulation with respect to lean controls were also observed on 24-h recordings, but the latter finding fell short of statistical significance at night.17 At night, however, the total variability of HP and cardiac vagal modulation in obese humans are more resistant to improvement following weight loss than during the day.17,18 The impairment in cardiac vagal modulation, which we documented during sleep states in ob/ob mice, bears a notable resemblance to the complete loss of sleep related vagal activation in human patients with a recent myocardial infarction, which are at risk of lethal arrhythmic events.12 This similarity emphasizes the need for clinical investigation on differences in cardiac autonomic control between obese and lean human subjects during specific sleep states.
Ob/ob mice develop massive obesity even when fed a standard diet because of congenital lack of leptin. These mice show metabolic syndrome traits, including hypertension,21 dyslipidemia and type 2 diabetes,30 as well as sleep disturbances,21,31 which resemble excessive daytime sleepiness in obese humans.32 A functional impairment in cardiac vagal modulation also occurs in massively obese db/db mice with dysfunctional leptin receptors.20 Conversely, cardiac vagal modulation is not reduced either in C57BL/6J mice with mild diet-induced obesity33 or in non-obese diabetic mice with overt type 1 diabetes and autonomic neuropathy.34 However, cardiac autonomic control was investigated in these mouse models of obesity and/or diabetes without controlling for wake-sleep states. In the present study, we found that indexes of cardiac autonomic control either differed significantly among wake-sleep states or displayed significant interaction effects between the wake-sleep state and the mouse strain (Figs. 1 and 2, Tables 2 and 3). These results demonstrate that the effects of disease or mutations on cardiac autonomic control may vary significantly as a function of the wake-sleep state in mice. Based on our findings in the different wake-sleep states, ob/ob mice may be a useful model to clarify which of the metabolic, endocrine, and inflammatory changes associated with massive obesity is involved in causing impairment in cardiac autonomic control during sleep. The study of ob/ob mice before the development of full-blown obesity, with food restriction or with leptin replacement therapy may also shed light on the role of leptin signaling on cardiac autonomic control in sleep. Accordingly, in leptin-resistant db/db mice, altered sleep regulation has been attributed to the deleterious effects of impaired leptin signaling.35 Finally, sleep disordered breathing may contribute to cardiovascular dysregulation in obesity.16 Ob/ob mice do not show signs of upper airway obstruction during sleep but have a depressed chemosensitivity to carbon dioxide, which mirrors a condition of obesity hypoventilation syndrome in humans.36
In conclusion, the results of the present study provide compelling evidence of a dramatic dysregulation of heart rhythm in ob/ob mice, with a prominent impairment in cardiac vagal modulation during the sleep states of NREMS and REMS. By establishing ob/ob mice as a mouse model of cardiac autonomic dysregulation in sleep, our results may open the way to a deeper understanding of the molecular pathways, which lead to cardiac autonomic impairment during sleep in obesity. Moreover, our results support the view that objective discrimination of wake-sleep states improves cardiovascular phenotyping in pre-clinical research on mice.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work has been funded by the University of Bologna (RFO 06 and Strategic Project 2006) and by the Fondazione Cassa di Risparmio di Bologna (grant 100, May 27, 2007).
ABBREVIATIONS
- W
wakefulness
- NREMS
non-rapid eye movement sleep
- REMS
rapid eye movement sleep
- HP
heart period
- BP
blood pressure
- SDHP
standard deviation of the values of heart period
- pNN8
index of cardiac vagal modulation computed in the time domain.
- RSA
index of cardiac vagal modulation computed in the frequency domain
- SYM
index of the sympathetic modulation of blood pressure
- BRS
cardiac baroreflex sensitivity
- BEI
baroreflex effectiveness index
- CCF
cross-correlation function
- ρMAX, ρMIN
maximum and minimum values of the cross-correlation function
- τMAX, τMIN
time shifts corresponding to the maximum and minimum values of the cross-correlation function
REFERENCES
- 1.Tsuji H, Larson MG, Venditti FJJ, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–5. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 2.La Rovere MT, Bigger JTJ, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–84. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 3.Johansson M, Gao SA, Friberg P, et al. Baroreflex effectiveness index and baroreflex sensitivity predict all-cause mortality and sudden death in hypertensive patients with chronic renal failure. J Hypertens. 2007;25:163–8. doi: 10.1097/01.hjh.0000254377.18983.eb. [DOI] [PubMed] [Google Scholar]
- 4.Lantelme P, Khettaba F, Custaud M-A, et al. Spontaneous baroreflex sensitivity: toward an ideal index of cardiovascular risk in hypertension? J Hypertens. 2002;20:935–44. doi: 10.1097/00004872-200205000-00029. [DOI] [PubMed] [Google Scholar]
- 5.Billman GE. A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: implications for future anti-arrhythmic drug development. Pharmacol Ther. 2006;111:808–35. doi: 10.1016/j.pharmthera.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Silvani A. Physiological sleep-dependent changes in arterial blood pressure: central autonomic commands and baroreflex control. Clin Exp Pharmacol Physiol. 2008;35:987–94. doi: 10.1111/j.1440-1681.2008.04985.x. [DOI] [PubMed] [Google Scholar]
- 7.Zemaityte D, Varoneckas G. Heart rhythm control during sleep. Psychophysiology. 1984;21:279–89. doi: 10.1111/j.1469-8986.1984.tb02935.x. [DOI] [PubMed] [Google Scholar]
- 8.Monti A, Medigue C, Nedelcoux H, Escourrou P. Autonomic control of the cardiovascular system during sleep in normal subjects. Eur J Appl Physiol. 2002;87:174–81. doi: 10.1007/s00421-002-0597-1. [DOI] [PubMed] [Google Scholar]
- 9.Berteotti C, Asti V, Ferrari V, et al. Central and baroreflex control of heart period during the wake-sleep cycle in spontaneously hypertensive rats. Am J Physiol. 2007;293:R293–R298. doi: 10.1152/ajpregu.00086.2007. [DOI] [PubMed] [Google Scholar]
- 10.Silvani A, Grimaldi D, Vandi S, et al. Sleep-dependent changes in the coupling between heart period and blood pressure in human subjects. Am J Physiol. 2008;294:R1686–R1692. doi: 10.1152/ajpregu.00756.2007. [DOI] [PubMed] [Google Scholar]
- 11.Portaluppi F, Hermida RC. Circadian rhythms in cardiac arrhythmias and opportunities for their chronotherapy. Adv Drug Deliv Rev. 2007;59:940–51. doi: 10.1016/j.addr.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Vanoli E, Adamson PB, Ba-Lin MBH, Pinna GD, Lazzara R, Orr WC. Heart rate variability during specific sleep stages. A comparison of healthy subjects with patients after myocardial infarction. Circulation. 1995;91:1918–22. doi: 10.1161/01.cir.91.7.1918. [DOI] [PubMed] [Google Scholar]
- 13.Berteotti C, Franzini C, Lenzi P, Zoccoli G, Silvani A. Surges of arterial pressure during rapid-eye-movement sleep in spontaneously hypertensive rats. Sleep. 2008;31:111–17. doi: 10.1093/sleep/31.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 15.Lusis AJ, Attie AD, Reue K. Metabolic syndrome: from epidemiology to systems biology. Nat Rev Genet. 2008;9:819–30. doi: 10.1038/nrg2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 17.Karason K, Mølgaard H, Wikstrand J, Sjöström L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol. 1999;83:1242–7. doi: 10.1016/s0002-9149(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 18.Nault I, Nadreau E, Paquet C, et al. Impact of bariatric surgery-induced weight loss on heart rate variability. Metabolism. 2007;56:1425–30. doi: 10.1016/j.metabol.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Skrapari I, Tentolouris N, Perrea D, Bakoyiannis C, Papazafiropoulou A, Katsilambros N. Baroreflex sensitivity in obesity: relationship with cardiac autonomic nervous system activity. Obesity. 2007;15:1685–93. doi: 10.1038/oby.2007.201. [DOI] [PubMed] [Google Scholar]
- 20.da Costa Goncalves AC, Tank J, Diedrich A, et al. Diabetic hypertensive leptin receptor-deficient db/db mice develop cardioregulatory autonomic dysfunction. Hypertension. 2009;53:387–92. doi: 10.1161/HYPERTENSIONAHA.108.124776. [DOI] [PubMed] [Google Scholar]
- 21.Silvani A, Bastianini S, Berteotti C, et al. Sleep modulates hypertension in leptin-deficient obese mice. Hypertension. 2009;53:251–5. doi: 10.1161/HYPERTENSIONAHA.108.125542. [DOI] [PubMed] [Google Scholar]
- 22.Baudrie V, Laude D, Elghozi J-L. Optimal frequency ranges for extracting information on cardiovascular autonomic control from the blood pressure and pulse interval spectrograms in mice. Am J Physiol. 2007;292:R904–R912. doi: 10.1152/ajpregu.00488.2006. [DOI] [PubMed] [Google Scholar]
- 23.Laude D, Baudrie V, Elghozi J-L. Effects of atropine on the time and frequency domain estimates of blood pressure and heart rate variability in mice. Clin Exp Pharmacol Physiol. 2008;35:454–7. doi: 10.1111/j.1440-1681.2008.04895.x. [DOI] [PubMed] [Google Scholar]
- 24.Laude D, Baudrie V, Elghozi J-L. Applicability of recent methods used to estimate spontaneous baroreflex sensitivity to resting mice. Am J Physiol. 2008;294:R142–R150. doi: 10.1152/ajpregu.00319.2007. [DOI] [PubMed] [Google Scholar]
- 25.Silvani A, Asti V, Bojic T, et al. Sleep-dependent changes in the coupling between heart period and arterial pressure in newborn lambs. Pediatr Res. 2005;57:108–14. doi: 10.1203/01.PDR.0000148065.32413.B0. [DOI] [PubMed] [Google Scholar]
- 26.Swoap SJ, Li C, Wess J, Parsons AD, Williams TD, Overton JM. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. Am J Physiol. 2008;294:H1581–H1588. doi: 10.1152/ajpheart.01000.2007. [DOI] [PubMed] [Google Scholar]
- 27.da Costa Goncalves AC, Tank J, Plehm R, et al. Role of the multidomain protein spinophilin in blood pressure and cardiac function regulation. Hypertension. 2008;52:702–7. doi: 10.1161/HYPERTENSIONAHA.108.114355. [DOI] [PubMed] [Google Scholar]
- 28.Kuo TB, Yang CC. Sleep-related changes in cardiovascular neural regulation in spontaneously hypertensive rats. Circulation. 2005;112:849–54. doi: 10.1161/CIRCULATIONAHA.104.503920. [DOI] [PubMed] [Google Scholar]
- 29.Campen MJ, Tagaito Y, Jenkins TP, Smith PL, Schwartz AR, O'Donnell CP. Phenotypic differences in the hemodynamic response during REM sleep in six strains of inbred mice. Physiol Genomics. 2002;11:227–34. doi: 10.1152/physiolgenomics.00031.2002. [DOI] [PubMed] [Google Scholar]
- 30.Haluzik M, Colombo C, Gavrilova O, et al. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology. 2004;145:3258–64. doi: 10.1210/en.2004-0219. [DOI] [PubMed] [Google Scholar]
- 31.Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin-deficient mice. Am J Physiol. 2006;290:R894–R903. doi: 10.1152/ajpregu.00304.2005. [DOI] [PubMed] [Google Scholar]
- 32.Vgontzas AN, Bixler EO, Tan T-L, Kantner D, Martin LF, Kales A. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med. 1998;158:1333–7. doi: 10.1001/archinte.158.12.1333. [DOI] [PubMed] [Google Scholar]
- 33.Williams TD, Chambers JB, Roberts LM, Henderson RP, Overton JM. Diet-induced obesity and cardiovascular regulation in C57BL/6J mice. Clin Exp Pharmacol Physiol. 2003;30:769–78. doi: 10.1046/j.1440-1681.2003.t01-1-03808.x. [DOI] [PubMed] [Google Scholar]
- 34.Gross V, Tank J, Partke H-J, et al. Cardiovascular autonomic regulation in non-obese diabetic (NOD) mice. Auton Neurosci. 2008;138:108–13. doi: 10.1016/j.autneu.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Laposky AD, Bradley MA, Williams DL, Bass J, Turek FW. Sleep-wake regulation is altered in leptin-resistant (db/db) genetically obese and diabetic mice. Am J Physiol. 2008;295:R2059–R2066. doi: 10.1152/ajpregu.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Donnell CP, Schaub CD, Haines AS, et al. Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med. 1999;159:1477–84. doi: 10.1164/ajrccm.159.5.9809025. [DOI] [PubMed] [Google Scholar]