Abstract
Study Objectives:
To investigate the hypothesis that day/night patterns of prothrombotic activity differ between patients with obstructive sleep apnea (OSA) and individuals with no OSA.
Design:
Prothrombotic markers' day/night rhythms recorded over one 24-h period.
Setting:
General clinical research center.
Patients:
38 untreated OSA patients as verified by polysomnography (apnea-hypopnea index ≥10/h sleep) and 22 non-OSA controls.
Measurements and Results:
Blood samples were collected every 2 h to measure plasma levels of fibrinolysis-inhibiting plasminogen activator inhibitor (PAI)-1 and the primary fibrin degradation product D-dimer. Day/night variation in hemostasis factors was examined using a cosinor analysis. Mesor (mean) PAI-1 over the 24-h period was higher (P = 0.015), and mesor of D-dimer was lower (P = 0.001) in patients with OSA than in the non-OSA controls. These group differences stayed significant when controlling for age and gender. After further adjustment for body mass index, mean arterial pressure, and smoking, the relationship between OSA and PAI-1 became non-significant, but the relationship between OSA and D-dimer continued to be significant (P = 0.006). In the fully adjusted analysis, the amplitude (peak) for D-dimer was lower in OSA patients than in non-OSA controls (P = 0.048). The acrophase (time of the peak) for PAI-1 and D-dimer did not significantly differ between groups.
Conclusions:
The relatively higher average level of PAI-1 and lower average level of D-dimer across the 24-h in OSA patients might reflect decreased fibrinolytic capacity and fibrin degradation, respectively. The findings provide some evidence for a prothrombotic state in OSA, but were only partially independent of metabolic variables.
Citation:
von Käanel R; Natarajan L; Ancoli-Israel S; Mills PJ; Loredo JS; Dimsdale JE. Day/night rhythm of hemostatic factors in obstructive sleep apnea. SLEEP 2010;33(3):371-377.
Keywords: Blood coagulation, cardiovascular disease, day/night rhythm, fibrinolysis, obstructive sleep apnea
OBSTRUCTIVE SLEEP APNEA (OSA) IS A COMMON SLEEP DISORDER CHARACTERIZED BY REPEATED UPPER AIRWAY OBSTRUCTION DURING SLEEP. OSA IS about twice as prevalent in patients with coronary artery disease (CAD) as in those without,1 and contributes to the initiation, progression, and poor outcome of CAD.1–5 The link between OSA and CAD might partly be explained by a prothrombotic state that contributes to atherosclerosis and coronary thrombus formation after plaque rupturing.1,6 Patients with OSA show increased levels of clotting factors7–11 and hyperactive platelets.12–14 Reduced fibrinolytic activity in the form of increased plasminogen activator inhibitor (PAI)-1 is also found in patients with OSA and is associated with apnea severity as defined by an elevated apnea-hypopnea index (AHI).15–18 Several studies find that elevated PAI-1 levels predict atherothrombotic events such as myocardial infarction.19 However, as stated in a recent consensus paper, there is need for further studies on hemostasis factors at large to more definitively confirm that OSA confers a prothrombotic state because hemostatic findings in OSA are not unequivocal.1,6
One reason for this inconsistency might be that previous OSA studies did not consider day/night variation in hemostatic activity.6,20 Moreover, day/night rhythm variation of hypercoagulability with its associated atherothrombotic risk might differ between individuals with and those without OSA. Hypercoagulability after awakening explains some of the increased prevalence of myocardial infarction onset between 6:00 AM and 12:00 PM in the general population.21 In contrast, infarct onset in OSA commonly occurs between midnight and 06:00 AM,22 the time of greatest electrocardiographic signs of myocardial ischemia during sleep in OSA.23
The primary function of PAI-1 is to inhibit fibrinolysis in plasma. Specifically, PAI-1 is the most important inhibitor of circulating tissue-type plasminogen activator (t-PA). A decrease in active t-PA will result in attenuated conversion of inactive plasminogen to plasmin.19 Plasmin splits fibrin, whereby fibrin D-dimer – the primary degradation product of cross-linked fibrin – is formed. Reduced plasma levels of D-dimer thus may reflect decreased global fibrinolytic capacity of an individual at a given time point.24 Day/night rhythmicity of fibrinolysis-inhibiting PAI-1 is characterized by a peak in the early morning (between 7:00 AM and 9:00 AM) and a nadir in the afternoon (between 3:00 PM and 6:00 PM), which contributes to the gradual increase in overall fibrinolytic activity from the early morning to the afternoon.19,20 Congruent with this, D-dimer levels increased from 10:00 AM to a peak level at 2:00 PM in one large epidemiological study25 and from 6:00 AM throughout the day in a smaller study in healthy subjects.26 In other words, the increase in D-dimer parallels the decrease in PAI-1. This suggests that the increase in fibrinolytic activity (as a result of the decrease in PAI-1) goes along with an increase in fibrin degradation and D-dimer formation, respectively.
Given that day/night variation in hemostasis has not previously been investigated in OSA and that one-point measurements show a fairly reliable increase in PAI-1 in OSA, we compared the day/night variation of PAI-1 and D-dimer plasma concentration between patients with OSA and non-OSA controls. Our goal in studying PAI-1 and D-dimer measurements over a 24-h period was to understand, as a first step, the novel and potentially important area of research on day/night variations of complex hemostatic abnormalities in OSA.
We hypothesized that patients with OSA would show different PAI-1 and D-dimer day/night rhythm patterns from non-OSA controls. Specifically, we predicted in OSA patients, compared to non-OSA controls, there would be more day/night variation in PAI-1 with higher mesor (mean), greater amplitude (peak) and earlier (during the night; i.e., between midnight and 6:00 AM) acrophase (timing of the peak), while in D-dimer there would be lower mesor, smaller amplitude, and later acrophase (in the morning hours; i.e. between 6:00 and 12:00 PM). We additionally explored whether day/night variation differences in PAI-1 and D-dimer would be independent of demographic and metabolic factors.19,26
MATERIALS AND METHODS
Study Participants
The University of California San Diego (UCSD) Human Subjects Committee approved the study protocol. All participants provided written informed consent. Patients with untreated OSA and healthy non-OSA controls were recruited from the community by advertisement, word-of-mouth, or referral from local medical practitioners. Specific exclusion criteria were a history of major medical illnesses other than OSA and hypertension, current psychiatric diagnoses, and intake of psychotropic medication. Patients who were receiving antihypertensive medications had these tapered for 3 weeks before they underwent the study protocol. No participants received any other medication on a regular basis (including anticoagulant medication and aspirin). In this prospective study, data from 38 patients with OSA and 22 non-OSA controls who had measures of both PAI-1 and D-dimer over a 24-h period were included.
Demographic and Metabolic Factors
Data were collected on age and gender. Subjects who currently smoked ≥ 1 cigarette per day were termed smokers. Smoking status was missing for 4 participants. The body mass index (BMI) was computed as the ratio of body weight in kilograms divided by the square of height in meters (kg/m2). Blood pressure data were collected and averaged over 3 seated resting measurements. The mean arterial pressure (MAP) was computed by the formula 2/3 diastolic blood pressure plus 1/3 systolic blood pressure.
Study Protocol
All participants arrived at the UCSD General Clinical Research Center Gillin Laboratory of Sleep and Chronobiology at 5:00 PM, at which time a venous catheter was inserted. Starting at 6:00 PM, a blood sample was collected every 2 h for the next 24 h. The catheter was kept patent with normal saline. During the 24-h blood draws, participants were asked to avoid any vigorous activities. Subjects were not required to remain at bed rest throughout the sampling interval. However, in order to achieve activity restriction, all participants were encouraged to stay in a non-active position, either in bed or a chair. There was no direct instruction on posture, although the participants were encouraged to be supine as much as possible while they slept. Lighting and room temperature were not restricted, and the participants were able to select food items from a standard menu list. Smoking, alcoholic beverages, and caffeine consumption were not allowed during the 24-h blood draws.
Beginning at 8:00 PM the next day, participants were instrumented for standard polysomnography (PSG) with the Grass Heritage (model PSG 36-2, West Warwick, RI). Lights out occurred at 10:00 PM, and PSG recording continued until 7:00 AM. Rechtschaffen and Kales' criteria were used to score sleep recordings.27 Apneas were defined as decrements in airflow ≥ 90% from baseline ≥ 10 sec. Hypopneas were defined as decrements in airflow of ≥ 50% but < 90% from baseline ≥ 10 sec with an accompanying desaturation of 4%. The numbers of apneas and hypopneas per hour of sleep were calculated to obtain the AHI. A diagnosis of OSA was given if AHI ≥ 10.
Biochemical Analysis
Venous blood was drawn into plastic tubes containing 3.8% sodium citrate (ratio 9:1). Samples were spun in a refrigerated centrifuge between 4°C and 8°C for 10 min at 3,000g. Plasma was immediately frozen in polypropylene tubes at −80°C until analyzed. Plasma levels of D-dimer and PAI-1 antigen were determined by enzyme-linked immunosorbent assay following the instructions of the manufacturer (Asserachrom; Diagnostica Stago; Asnièeres, France). To minimize intra-assay variance, all samples from each participant were analyzed in the same run. Because of occasional assay problems and sample availability, of the potential 720 total data points assessed (12 time points × 60 participants), 33 (4.6%) and 53 (7.4%) measurements were missing for D-dimer and PAI-1, respectively. More precisely, for the D-dimer analysis, one subject had 5 (of 12) measures; all other subjects had ≥ 8 measures. For the PAI-1 analysis, one subject had 2 (of 12) measures, one had 4, two had 5, and the rest all had ≥ 8 measures. Inter- and intra-assay coefficients of variation were < 10% for D-dimer and PAI-1.
Statistical Methods
Data were analyzed using SPSS 15.0 for Windows and the public domain software package R v. 2.7.1 (http://cran.stat.ucla.edu/). Level of significance was set at P ≤ 0.05 (2-tailed). To better approximate a Gaussian distribution, D-dimer and PAI-1 values were logarithmically transformed before performing analyses. Student t-test and Fisher exact test were applied to test for differences in continuous and categorical data, respectively, between patients with OSA and non-OSA controls.
A cosinor analysis was used to examine the day/night rhythmicity of prothrombotic markers with the model specified as y = mes + amp * cos (2π (t-phi)/24) where y was the (log) marker value, t represented time-of-day, mes represented the mesor, amp the amplitude and phi the acrophase of the day/night rhythm. This cosinor curve was fitted using mixed-effects models allowing for subject-specific intercept and rhythm-slopes (i.e., slopes for the cos and sin terms in the model) for each individual.28–30 Day/night rhythm parameters (i.e., mesor, amplitude, acrophase) and their standard errors were estimated. These parameters were derived from the output of the cosinor models. In particular, using the Law of Cosines, the model can be reparametrized as follows: y = mes + amp * cos (2π (t-phi)/24) = mes + amp [cos(2π t/24)* cos(2 π phi /24) + sin(2π t/24)* sin(2 π phi)/24)] = mes + β1*cos(2π t/24) + β2*sin(2π t/24), where β1= amp*cos(2 π phi /24) and β2 = amp*sin(2 π phi /24). The mixed model was used to estimate β1 and β2,and these coefficients were then used to derive the amplitude, amp = sqrt(β12 +β22) and acrophase phi = 24*arctan(β2/β1)/2π. Standard errors were calculated using a delta-method (i.e., a Taylor's series expansion of the above functions for amplitude, and acrophase).
OSA status was included in the models as a binary variable (OSA patients versus non-OSA controls) to test for differences in mesor between patients with and without OSA. Interactions between OSA status and cos and sin terms were included to test if rhythm patterns varied between OSA patients and non-OSA controls. Unadjusted and adjusted cosinor models were fitted with the adjusted models controlling for a maximum of five key covariates to guard against model overfitting31: age, gender, BMI, MAP, and smoking status (yes versus no). Likelihood ratio tests were used to compare the means-only model (with an intercept and OSA status terms alone) to the cosinor models, to test the presence of a diurnal pattern in these markers.
We used Cohen's d to estimate effect sizes of the observed differences in day/night rhythm parameters between OSA patients and non-OSA controls.32 Cohen's d was inferred from the Wald statistic testing for differences in rhythm parameters between OSA and non-OSA patients. According to Cohen, an effect size (d) of 0.20 implies a small effect, 0.50 a medium effect, and ≥ 0.80 a large effect.32
RESULTS
Characteristics of Participants
A total of 60 subjects (38 OSA patients and 22 non-OSA controls) were included in this analysis. Demographic and medical characteristics of the study sample are presented in Table 1. Briefly, the proportion of men was greater and blood pressure was higher in patients with OSA than in the non-OSA controls, whereas there were no significant group differences in age, BMI, or smoking status.
Table 1.
Mean (SD) of demographic and metabolic characteristics of OSA vs. non-OSA participants
| Variable | OSA patients (n = 38) | Non-OSA controls (n = 22) | P-value |
|---|---|---|---|
| Age (y) | 49.7 ± 8.7 | 48.0 ± 8.7 | 0.472 |
| Men / Women (%) | 90 / 10 | 55 / 45 | 0.004 |
| Body mass index (kg/m2) | 30.1 ± 5.7 | 27.7 ± 4.4 | 0.092 |
| Current smokers (%) | 10 | 18 | 0.423 |
| Systolic blood pressure (mm Hg) | 131.5 ± 18.4 | 119.1 ± 14.3 | 0.009 |
| Diastolic blood pressure (mm Hg) | 79.3 ± 9.6 | 75.4 ± 10.0 | 0.138 |
| Mean arterial pressure (mm Hg) | 96.7 ± 11.8 | 90.0 ± 10.6 | 0.030 |
| Apnea-hypopnea index | 40.4 ± 28.9 | 5.0 ± 2.9 | < 0.001 |
Day/Night Pattern of Prothrombotic Factors
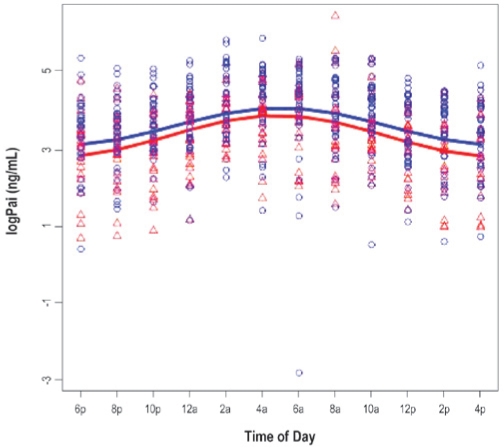
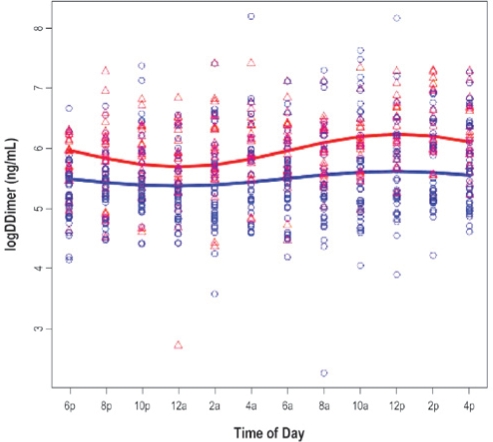
The results for 3 cosinor models are presented in Tables 2a and 2b. Model 1 shows the unadjusted cosinor model testing for a difference in the day/night pattern of PAI-1 (Table 2a) and D-dimer (Table 2b) between patients with OSA and their non-OSA counterparts. Model 2 augmented Model 1 by further adjusting for age and gender, while Model 3 added BMI, MAP, and smoking status (yes versus no) to Model 2. Figures 1 and 2 show the fitted day/night pattern with individual data points of PAI-1 and D-dimer in patients with OSA and non-OSA controls.
Table 2a.
Cosinor model for (log) plasminogen activator inhibitor-1 (ng/mL)
| Parameter | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Cost | 0.12 ± 0.09 | 0.12 ± 0.09 | 0.16 ± 0.10 |
| Sint | 0.48 ± 0.11*** | 0.48 ± 0.11*** | 0.50 ± 0.12*** |
| Cost*OSA | 0.00 ± 0.11 | 0.01 ± 0.11 | −0.04 ± 0.12 |
| Sint*OSA | −0.02 ± 0.14 | −0.03 ± 0.14 | −0.04 ± 0.15 |
| Age | 0.01 ± 0.01 | −0.00 ± 0.01 | |
| Gender | 0.01 ± 0.22 | 0.07 ± 0.22 | |
| Body mass index | 0.04 ± 0.02* | ||
| Mean arterial pressure | 0.02 ± 0.01* | ||
| Smoking status | 0.01 ± 0.24 |
Columns show the unstandardized β-coefficient ± SE
P < 0.05
P < 0.01
P < 0.001
Coding of parameters: Obstructive sleep apnea (OSA) status: 1 = OSA patients, 0 = non-OSA controls; gender: women = 1, men = 0; smoking status: 1 = smoker, 0 = non-smoker
Table 2b.
Cosinor model for (log) fibrin D-dimer (ng/mL)
| Parameter | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Cost | −0.23 ± 0.06*** | −0.23 ± 0.06*** | −0.28 ± 0.06*** |
| Sint | 0.04 ± 0.06 | 0.04 ± 0.06 | −0.01 ± 0.06 |
| Cost*OSA | 0.11 ± 0.07 | 0.11 ± 0.07 | 0.15 ± 0.08* |
| Sint*OSA | −0.03 ± 0.07 | −0.03 ± 0.07 | 0.01 ± 0.07 |
| Age | 0.01 ± 0.01 | 0.01 ± 0.01 | |
| Gender | 0.12 ± 0.16 | 0.16 ± 0.17 | |
| Body mass index | 0.01 ± 0.01 | ||
| Mean arterial pressure | −0.00 ± 0.01 | ||
| Smoking status | −0.41 ± 0.18* |
Columns show the unstandardized β-coefficient ± SE
P < 0.05
P < 0.01
P < 0.001
Coding of parameters: Obstructive sleep apnea (OSA) status: 1 = OSA patients, 0 = non-OSA controls; gender: women = 1, men = 0; smoking status: 1 = smoker, 0 = non-smoker
The significant main effect for the “sin t” term in all the PAI-1 models was indicative of a periodic pattern. Interactions between the “cos t” and “sin t” terms with OSA status were non-significant, indicating that the shape of the day/night pattern did not vary significantly between those with and without OSA.
For D-dimer, there was a significant main effect for “cos t” in all the models, suggesting a periodic pattern. Interactions between the “cos t” and “sin t” terms with OSA status were non-significant in Models 1 and 2; however, the “cos t” by OSA status interaction was significant (P = 0.049) in Model 3, indicating that the shape of the day/night pattern varied between OSA patients and non-OSA controls after adjusting for confounders.
A likelihood ratio test comparing the means-only mixed model (which included a subject-specific intercept and OSA status terms only) to Model 1 indicated that the fit of the cosinor model was far superior to that of the simpler model (likelihood ratios were 72.1 for D-dimer (P < 0.001) and 183.5 for PAI-1 (P < 0.001).
Effects of Covariates
BMI and MAP were significantly associated with (log)PAI-1 levels (Table 2a, Model 3). Each unit increase in BMI corresponded to a 0.04 ng/mL increase in (log)PAI-1 (P = 0.020), and each unit increase in MAP resulted in a 0.02 ng/mL increase in (log)PAI (P = 0.016). Smokers had on average 0.41 ng/mL lower (log) D-dimer values than non-smokers (P = 0.029) (Table 2b, Model 3). Age and gender were not significantly associated with either PAI-1 or D-dimer.
Mesor Analysis
Table 3 shows that the PAI-1 mesor, i.e., overall mean of the (log)PAI-1 rhythm over the 24-h period, was significantly higher in OSA patients than in non-OSA controls without adjustment for covariates in Model 1 (P = 0.015). This effect maintained significance after adjustment for age and gender in Model 2 (P = 0.026), but it became non-significant after additional adjustment for metabolic covariates and smoking in Model 3. The effect size of the group difference in PAI-1 mesor was medium-to-large in Models 1 and 2 and became small-to-medium in Model 3. The mesor of (log)D-dimer was significantly lower in patients with OSA than in non-OSA controls in the unadjusted and adjusted analyses (all P-values < 0.007). The effect size of this group difference was large in all three models.
Table 3.
Cosinor model parameters for hemostatic factors by OSA categorization
| Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| Parameter | OSA patients | Non-OSA controls | OSA patients | Non-OSA controls | OSA patients | Non-OSA controls |
| Mesor (ng/mL) | ||||||
| PAI-1 | 3.70 ± 0.12* | 3.23 ± 0.15 | 3.70 ± 0.12* | 3.22 ± 0.18 | 3.60 ± 0.12 | 3.36 ± 0.19 |
| d = 0.7 | d = 0.6 | d = 0.3 | ||||
| D-dimer | 5.48 ± 0.09 | 5.97 ± 0.11** | 5.46 ± 0.09 | 5.93 ± 0.13** | 5.51 ± 0.09 | 5.98 ± 0.15** |
| d = 0.9 | d = 0.9 | d = 0.8 | ||||
| Amplitude (ng/mL) | ||||||
| PAI-1 | 0.47 ± 0.08 | 0.49 ± 0.10 | 0.47 ± 0.08 | 0.49 ± 0.10 | 0.48 ± 0.08 | 0.53 ± 0.11 |
| d = 0.05 | d = 0.05 | d = 0.1 | ||||
| D-dimer | 0.12 ± 0.04 | 0.23 ± 0.06 | 0.12 ± 0.04 | 0.23 ± 0.06 | 0.12 ± 0.04 | 0.28 ± 0.06* |
| d = 0.4 | d = 0.4 | d = 0.6 | ||||
| Acrophase (t) | ||||||
| PAI-1 | 4.99 ± 0.60 | 5.08 ± 0.74 | 4.99 ± 0.60 | 5.09 ± 0.75 | 5.02 ± 0.60 | 4.83 ± 0.77 |
| d = 0.03 | d = 0.03 | d = 0.06 | ||||
| D-dimer | 11.73 ± 1.39 | 11.40 ± 0.93 | 11.73 ± 1.39 | 11.41 ± 0.93 | 11.89 ± 1.33 | 12.08 ± 0.83 |
| d = 0.05 | d = 0.05 | d = 0.04 | ||||
Values are given as means ± SE; d = effect size of group difference
P < 0.05
P < 0.01
OSA, obstructive sleep apnea; PAI-1, plasminogen activator inhibitor-1
Model 1 controlled for intercept, OSA status, cost, sint, cost*OSA, and sint*OSA
Model 2 controlled for all covariates in Model 1 plus age and gender
Model 3 controlled for all covariates in Model 2 plus body mass index, mean arterial pressure, and smoking status
Amplitude Analysis
There were no significant differences in amplitude of PAI-1 between OSA patients and non-OSA controls in the unadjusted and adjusted analysis (Table 3 and Figure 1a). As shown in Table 3 and Figure 1b, patients with OSA appeared to have flatter D-dimer rhythm. The amplitude was significantly smaller in OSA patients than in non-OSA controls adjusting for age, gender, BMI, MAP, and smoking status in Model 3 (P = 0.048). While the group difference in amplitude of D-dimer showed a medium effect size, there was no meaningful effect size in terms of the group difference in amplitude of PAI-1.
Figure 1a.
Day/Night pattern of PAI-1 by obstructive sleep apnea status.
Figure 1b.
Day/night pattern of D-dimer by obstructive sleep apnea status.
The figures show the fitted day/night pattern with individual data points for plasminogen activator-inhibitor-1 (PAI-1; Figure 1a) and fibrin D-dimer (Figure 1b) in 38 patients with obstructive sleep apnea (blue) and 22 non-OSA controls (red).
Acrophase Analysis
The acrophase (i.e., the time at which the maximum values occurred) for PAI-1 and D-dimer was not significantly different between OSA patients and non-OSA controls in unadjusted and adjusted models (Table 3). Acrophase for PAI-1 occurred at 4:59 AM in patients with OSA and at 5:04 AM in non-OSA controls based on Models 1 and 2. For D-dimer, acrophase occurred at 11:44 AM in patients with OSA and at 11:24 AM in non-OSA controls. The effect size of the group difference in acrophase for PAI-1 and D-dimer was not meaningful.
Secondary Exploratory Analyses
Because different AHI cut-offs have previously been used to define OSA in hemostasis research,6,16 we performed an exploratory analysis, in which we categorized subjects into those with AHI ≥ 15 (“OSA patients”, n = 35) versus those with AHI < 15 (“non-OSA controls”, n = 25). We found that results were qualitatively consistent with the previous OSA definition (i.e., AHI ≥ 10). For the D-dimer analysis, the significance of results was maintained in the unadjusted analysis (Model 1). In Model 3, age (0.01 ± 0.01, P < 0.09) and the previously significant amplitude difference (Cost*OSA; 0.13 ± 0.8, P < 0.10) were both marginally significant. The significance of PAI-1 results did not change.
In a further exploratory analysis we performed a sensitivity analysis restricted to 48 non-smokers with (n = 33) and without (n = 15) OSA (i.e., we excluded 4 smokers with OSA, 4 smokers without OSA, and 4 subjects with unknown smoking status). For the D-dimer and PAI-1 analysis, the significance of OSA status did not change compared to previous models. For the D-dimer analysis, in Model 3, age was marginally significant (0.015 ± 0.01, P < 0.06) and the previously significant difference in amplitude (Cost*OSA) disappeared (P = 0.73).
DISCUSSION
The day/night variation of two hemostatic factors, PAI-1 and D-dimer, was examined to further characterize the prothrombotic state in OSA. We corroborated the previously described day/night pattern in PAI-1 and diurnal pattern in D-dimer.19,25,26 While the overall PAI-1 day/night pattern was not different between those with and without OSA, PAI-1 mesor was significantly higher in OSA patients relative to their non-OSA counterparts, even after controlling for age and gender. This may suggest impaired fibrinolysis in OSA since PAI-1 is the main inhibitor of endogenous fibrinolysis and determines net fibrinolytic activity.19 However, the relationship between OSA and increased PAI-1 may not be independent of metabolic factors, since it was attenuated when controlling for BMI and MAP which emerged as significant correlates of day/night variation in PAI-1. A positive relationship between BMI and PAI-1 is a consistent finding, probably because PAI-1 is overexpressed in human adipose tissue.33 Elevated PAI-1 is also viewed as a component of the metabolic syndrome that is further characterized by obesity and elevated blood pressure.34,35 The metabolic syndrome is prevalent in OSA,36 and we previously showed that the metabolic syndrome was more strongly related to PAI-1 than OSA.16 Moreover, a previous study showed that PAI-1 assessed at one time point was significantly higher in OSA patients with hypertension than in those with normal blood pressure.17
We further found that OSA patients had lower D-dimer mesor than their non-OSA counterparts. Moreover, after adjusting for covariates, the diurnal pattern of D-dimer differed by OSA status; patients with OSA had a smaller D-dimer amplitude than non-OSA controls. Reduced basal levels of D-dimer have been proposed as a global screening method for decreased fibrinolytic potential, given that attenuated fibrinolytic activity results in reduced fibrin splitting and, consequently, in lower levels of the fibrin-splitting product D-dimer.24 Taken together, our data may suggest that OSA patients have impaired day/night variation in fibrinolytic capacity, which is partially affected by metabolic variables.
Single time-point assessments of elevated PAI-1 levels are prospectively associated with an increased risk of ischemic cardiovascular events in a number of studies.19 In contrast, interpretation of the hemostatic meaning of plasma D-dimer concentration with regard to thrombogenic risk is not straightforward.26 Alterations in D-dimer levels are regrettably equivocal in that elevations are associated with excess procoagulant activity, whereas decreases in D-dimer can be a marker for impaired fibrinolytic capacity.24 The notion in cardiovascular research is to designate D-dimer a marker of fibrin formation and thus coagulation activation.37 In agreement with this conceptualization, increased plasma D-dimer levels assessed at one time point are predictive of incident CAD in different populations.38 However, increase in D-dimer level is not only caused by de novo formation of cross-linked fibrin by the action of thrombin, but also through proteolysis of cross-linked fibrin or other fibrin derivatives.39 The latter process makes D-dimer also a marker of fibrinolytic activity because its concentration also reflects the extent to which cross-linked fibrin is cleaved by plasmin.40 Some authors accordingly concluded that the association of D-dimer with increased risk of myocardial infarction in initially healthy individuals is evidence for enhanced fibrinolytic activity in the presence of increased fibrin formation several years in advance of coronary occlusion.41 Moreover, low PAI-1 levels appear to be required to increase circulating D-dimer in subjects with atherosclerosis, such that increased D-dimer reflects increased fibrinolysis in these patients.42 It is possible that some of our patients with OSA had occult atherosclerotic disease that was accompanied by elevated PAI-1 giving raise to reduced D-dimer formation. All of this might explain why low D-dimer levels (i.e., reflecting decreased overall fibrinolytic potential) as well as high D-dimer levels (reflecting enhanced fibrinolysis because of greater fibrin production) both indicate enhanced atherothrombotic risk.43 Given this literature, we feel it more accurate to discuss the finding of lower average levels of D-dimer across the 24 hours in our patients with OSA relative to non-OSA controls to be indicative for reduced fibrinolytic capacity rather than for attenuated coagulation activity.
Our finding concurs with several previous investigations using a single assessment of PAI-1 in the morning, which showed that OSA patients had higher PAI-1 than non-OSA individuals.15–18 However, the day/night variability of hemostatic factors has not been assessed in OSA. While we have not applied constant routine, measuring multiple time points at least gives some more information beyond those other studies. In this regard, our study is a first important step concerning exploring these complex hemostatic factors in OSA. Our data support the clinical meaning of increased PAI-1 in OSA.6 Specifically, the effect size of the difference in PAI-1 mesor between patients with OSA and non-OSA controls was clinically meaningful, even after adjustment for metabolic variables and smoking; a clinically meaningful effect was also observed for the group difference in D-dimer.
Although not significantly different from controls, we found that PAI-1 concentration peaked at about 5:00 AM (acrophase). While this peak in PAI-1 during the night is unlikely to harm healthy controls, it is of potential interest for cardiovascular health in OSA. Myocardial infarction onset in OSA occurs predominately during the night, likely consequent to the hypoxia-induced increase in blood pressure fostering plaque rupture.22 A concurrent increase in the thrombogenic potential through an elevation in PAI-1 might hamper lysis of a coronary thrombotic occlusion that builds up after plaque rupturing.19,44 In support of this, transgenic mice that overexpress PAI-1 spontaneously develop coronary thrombosis and myocardial infarction.45 We did not find a significant difference for D-dimer acrophase which occurred at noon in both OSA patients and non-OSA controls.
From a clinical point of view, therapy with continuous positive airway pressure (CPAP) has been associated with significant benefits to cardiovascular morbidity and mortality.46 We showed in OSA patients that two weeks of treatment with CPAP significantly decreased morning PAI-1 levels compared to placebo-CPAP.47 Various other studies have reported that CPAP treatment leads to restoration of perturbed levels of other hemostasis factors and reduction of platelet hyperactivity was also achieved through CPAP treatment.6 However, whether treatment with CPAP may restore the day/night variation in PAI-1 and D-dimer awaits further investigations.
Our study had several strengths and limitations. Studying unmedicated OSA patients, who, except for comorbid hypertension, were otherwise healthy, prevented potential confounding of hemostatic factor levels. The findings seem robust because two sensitivity analyses, one using a different AHI cut-off to define OSA, and one using only non-smoking participants, showed both results that allowed for the same inferences as did the primary analysis. Assessment of hemostatic activity at 12 time points over the 24-h cycle facilitated identifying day/night variation in plasma levels of PAI-1 and D-dimer, but collecting data over several days or in a constant behavioral and environmental routine, might have yielded more reliable day/night rhythms. Loss of individual hemostatic data points due to technical problems was minimal, yielding reliable statistical models. The study might have had limited power to detect a relationship between OSA status and PAI-1 over the 24-h period that was significant independent of covariates. Even though there is no statistical difference between groups and in the model there could be a confounding effect of gender because only 10% of OSA patients (but 45% of non-OSA controls) were women. We did not assess additional measures of fibrinolysis such as t-PA activity which did not previously differ between OSA patients and non-OSA controls; however this might be because day/night change in t-PA was not assessed.15 We assessed the concentration of PAI-1 (i.e., antigen level) but not PAI-1 activity. However, PAI-1 antigen and PAI-1 activity show a similar day/night change,48 and were both predictive for first-time myocardial infarction in middle-aged men and women.49 Our findings might not generalize to OSA patients with diabetes or cardiac diseases, such as CAD and chronic heart failure, or to OSA populations differing in terms of gender and age.
In sum, we found differences in day/night rhythm of PAI-1 and D-dimer between OSA patients and non-OSA controls, whereby metabolic factors may account for the group difference in PAI-1. The findings seem clinically meaningful and provide some evidence for decreased fibrinolytic capacity in patients with OSA over the 24-h period.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Ancoli-Israel has received research support from Sepracor and Litebook and has consulted or advised for Arena, Ferring, Orphagen, Pfizer, Respironics, Sanofi-Aventis, Sepracor, and Schering-Plough. Dr. Dimsdale has received research support from Sepracor. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was financially supported by grants HL44915, HL073355, AG08415, M01 RR00827 and CA23100 from the National Institutes of Health.
REFERENCES
- 1.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Sorajja D, Gami AS, Somers VK, Behrenbeck TR, Garcia-Touchard A, Lopez-Jimenez F. Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest. 2008;133:927–33. doi: 10.1378/chest.07-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990;336:261–4. doi: 10.1016/0140-6736(90)91799-g. [DOI] [PubMed] [Google Scholar]
- 4.Peker Y, Hedner J, Kraiczi H, Löth S. Respiratory disturbance index: an independent predictor of mortality in coronary artery disease. Am J Respir Crit Care Med. 2000;162:81–6. doi: 10.1164/ajrccm.162.1.9905035. [DOI] [PubMed] [Google Scholar]
- 5.Mooe T, Franklin KA, Holmström K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med. 2001;164:1910–3. doi: 10.1164/ajrccm.164.10.2101072. [DOI] [PubMed] [Google Scholar]
- 6.von Känel R, Dimsdale JE. Hemostatic alterations in patients with obstructive sleep apnea and the implications for cardiovascular disease. Chest. 2003;124:1956–67. doi: 10.1378/chest.124.5.1956. [DOI] [PubMed] [Google Scholar]
- 7.Steiner S, Jax T, Evers S, Hennersdorf M, Schwalen A, Strauer BE. Altered blood rheology in obstructive sleep apnea as a mediator of cardiovascular risk. Cardiology. 2005;104:92–6. doi: 10.1159/000086729. [DOI] [PubMed] [Google Scholar]
- 8.Wessendorf TE, Thilmann AF Wang YM, Schreiber A, Konietzko N, Teschler H. Fibrinogen levels and obstructive sleep apnea in ischemic stroke. Am J Respir Crit Care Med. 2000;162:2039–42. doi: 10.1164/ajrccm.162.6.2001048. [DOI] [PubMed] [Google Scholar]
- 9.Nobili L, Schiavi G, Bozano E, De Carli F, Ferrillo F, Nobili F. Morning increase of whole blood viscosity in obstructive sleep apnea syndrome. Clin Hemorheol Microcirc. 2000;22:21–7. [PubMed] [Google Scholar]
- 10.Reinhart WH, Oswald J, Walter R, Kuhn M. Blood viscosity and platelet function in patients with obstructive sleep apnea syndrome treated with nasal continuous positive airway pressure. Clin Hemorheol Microcirc. 2002;27:201–7. [PubMed] [Google Scholar]
- 11.Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR. Circulating cardiovascular risk factors in obstructive sleep apnoea: data from randomised controlled trials. Thorax. 2004;59:777–82. doi: 10.1136/thx.2003.018739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu M, Kamio K, Haida M, et al. Platelet activation in patients with obstructive sleep apnea syndrome and effects of nasal-continuous positive airway pressure. Tokai J Exp Clin Med. 2002;27:107–12. [PubMed] [Google Scholar]
- 13.Geiser T, Buck F, Meyer BJ, Bassetti C, Haeberli A, Gugger M. In vivo platelet activation is increased during sleep in patients with obstructive sleep apnea syndrome. Respiration. 2002;69:229–34. doi: 10.1159/000063625. [DOI] [PubMed] [Google Scholar]
- 14.Hui DS, Ko FW, Fok JP, et al. The effects of nasal continuous positive airway pressure on platelet activation in obstructive sleep apnea syndrome. Chest. 2004;125:1768–75. doi: 10.1378/chest.125.5.1768. [DOI] [PubMed] [Google Scholar]
- 15.Rangemark, C, Hedner, JA, Carlson, JT, et al. Platelet function and fibrinolytic activity in hypertensive and normotensive sleep apnea patients. Sleep. 1995;18:188–94. doi: 10.1093/sleep/18.3.188. [DOI] [PubMed] [Google Scholar]
- 16.von Känel R, Loredo JS, Ancoli-Israel S, Mills PJ, Dimsdale JE. Elevated plasminogen activator inhibitor 1 in sleep apnea and its relation to the metabolic syndrome: an investigation in 2 different study samples. Metabolism. 2007;56:969–76. doi: 10.1016/j.metabol.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Zamarrón C, Ricoy J, Riveiro A, Gude F. Plasminogen activator inhibitor-1 in obstructive sleep apnea patients with and without hypertension. Lung. 2008;186:151–6. doi: 10.1007/s00408-008-9076-8. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa J, Hoshide S, Eguchi K, et al. Increased low-grade inflammation and plasminogen-activator inhibitor-1 level in nondippers with sleep apnea syndrome. J Hypertens. 2008;26:1181–7. doi: 10.1097/HJH.0b013e3282fd9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaughan DE. PAI-1 and atherothrombosis. J Thromb Haemost. 2005;3:1879–83. doi: 10.1111/j.1538-7836.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- 20.Haus E. Chronobiology of hemostasis and inferences for the chronotherapy of coagulation disorders and thrombosis prevention. Adv Drug Deliv Rev. 2007;59:966–84. doi: 10.1016/j.addr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Chasen E, Muller JE. Cardiovascular triggers and morning events. Blood Press Monit. 1998;3:35–42. [PubMed] [Google Scholar]
- 22.Kuniyoshi FH, Garcia-Touchard A, Gami AS, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52:343–6. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanly P, Sasson Z, Zuberi N, Lunn K. ST-segment depression during sleep in obstructive sleep apnea. Am J Cardiol. 1993;71:1341–5. doi: 10.1016/0002-9149(93)90552-n. [DOI] [PubMed] [Google Scholar]
- 24.Seljeflot I, Eritsland J, Andersen P, Arnesen H. Global fibrinolytic capacity assessed by the serum D-dimer test. Correlation between basal and stimulated values. Thromb Res. 1994;75:157–62. doi: 10.1016/0049-3848(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 25.Rudnicka AR, Rumley A, Lowe GD, Strachan DP. Diurnal, seasonal, and blood processing patterns in levels of circulating fibrinogen, fibrin D-dimer, C-reactive protein, tissue plasminogen activator, and von Willebrand factor in a 45-year-old population. Circulation. 2007;115:996–1003. doi: 10.1161/CIRCULATIONAHA.106.635169. [DOI] [PubMed] [Google Scholar]
- 26.MacCallum PK, Cooper JA, Martin J, Howarth DJ, Meade TW, Miller GJ. Haemostatic and lipid determinants of prothrombin fragment F1.2 and D-dimer in plasma. Thromb Haemost. 2000;83:421–6. [PubMed] [Google Scholar]
- 27.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. NIH, Washington, DC: US Government Printing Office; 1968. Publication No. 204. [Google Scholar]
- 28.Marler MR, Gehrman P, Martin JL, Ancoli-Israel S. The sigmoidally transformed cosine curve: The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med. 2006;25:3893–904. doi: 10.1002/sim.2466. [DOI] [PubMed] [Google Scholar]
- 29.Tong YL. Parameter estimation in studying circadian rhythms. Biometrics. 1976;32:85–94. [PubMed] [Google Scholar]
- 30.Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford Science Publication; 1994. [Google Scholar]
- 31.Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004;66:411–21. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical power analysis for the behavioral sciences (2nd ed.) Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 33.Bastelica D, Morange P, Berthet B, et al. Stromal cells are the main plasminogen activator inhibitor-1-producing cells in human fat: evidence of differences between visceral and subcutaneous deposits. Arterioscler Thromb Vasc Biol. 2002;22:173–8. doi: 10.1161/hq0102.101552. [DOI] [PubMed] [Google Scholar]
- 34.Alessi MC, Juhan-Vague I. PAI-1 and the metabolic syndrome: links, causes, and consequences. Arterioscler Thromb Vasc Biol. 2006;26:2200–7. doi: 10.1161/01.ATV.0000242905.41404.68. [DOI] [PubMed] [Google Scholar]
- 35.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, American Heart Association; National Heart, Lung and Blood Institute Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 36.Lam JC, Ip MS. An update on obstructive sleep apnea and the metabolic syndrome. Curr Opin Pulm Med. 2007;13:484–9. doi: 10.1097/MCP.0b013e3282efae9c. [DOI] [PubMed] [Google Scholar]
- 37.Lowe GD. Fibrin D-dimer and cardiovascular risk. Semin Vasc Med. 2005;5:387–98. doi: 10.1055/s-2005-922485. [DOI] [PubMed] [Google Scholar]
- 38.Danesh J, Whincup P, Walker M, et al. Fibrin D-dimer and coronary heart disease: prospective study and meta-analysis. Circulation. 2001;103:2323–7. doi: 10.1161/01.cir.103.19.2323. [DOI] [PubMed] [Google Scholar]
- 39.Fassbender K, Dempfle CE, Mielke O, et al. Changes in coagulation and fibrinolysis markers in acute ischemic stroke treated with recombinant tissue plasminogen activator. Stroke. 1999;30:2101–4. [PubMed] [Google Scholar]
- 40.Lip GY, Lowe GD. Fibrin D-dimer: a useful clinical marker of thrombogenesis? Clin Sci (Lond) 1995;89:205–14. doi: 10.1042/cs0890205. [DOI] [PubMed] [Google Scholar]
- 41.Ridker PM, Hennekens CH, Cerskus A, Stampfer MJ. Plasma concentration of cross linked fibrin degradation product (D-dimer) and the risk of future myocardial infarction among apparently healthy men. Circulation. 1994;90:2236–40. doi: 10.1161/01.cir.90.5.2236. [DOI] [PubMed] [Google Scholar]
- 42.van der Bom JG, Bots ML, Haverkate F, Meyer P, Hofman A, Grobbee DE, Kluft C. Fibrinolytic activity in peripheral atherosclerosis in the elderly. Thromb Haemost. 1999;81:275–80. [PubMed] [Google Scholar]
- 43.Folsom AR. Hemostatic risk factors for atherothrombotic disease: an epidemiologic view. Thromb Haemost. 2001;86:366–73. [PubMed] [Google Scholar]
- 44.Selwyn AP. Prothrombotic and antithrombotic pathways in acute coronary syndromes. Am J Cardiol. 2003;91:3H–11H. doi: 10.1016/s0002-9149(03)00428-4. [DOI] [PubMed] [Google Scholar]
- 45.Eren M, Painter CA, Atkinson JB, Declerck PJ, Vaughan DE. Age-dependent spontaneous coronary arterial thrombosis in transgenic mice that express a stable form of human plasminogen activator inhibitor-1. Circulation. 2002;106:491–6. doi: 10.1161/01.cir.0000023186.60090.fb. [DOI] [PubMed] [Google Scholar]
- 46.McNicholas WT. Cardiovascular outcomes of CPAP therapy in obstructive sleep apnea syndrome. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1666–70. doi: 10.1152/ajpregu.00401.2007. [DOI] [PubMed] [Google Scholar]
- 47.von Känel R, Loredo JS, Ancoli-Israel S, Dimsdale JE. Association between sleep apnea severity and blood coagulability: treatment effects of nasal continuous positive airway pressure. Sleep Breath. 2006;10:139–46. doi: 10.1007/s11325-006-0060-3. [DOI] [PubMed] [Google Scholar]
- 48.Andreotti F, Kluft C. Circadian variation of fibrinolytic activity in blood. Chronobiol Int. 1991;8:336–51. doi: 10.3109/07420529109059170. [DOI] [PubMed] [Google Scholar]
- 49.Thögersen AM, Jansson JH, Boman K, et al. High plasminogen activator inhibitor and tissue plasminogen activator levels in plasma precede a first acute myocardial infarction in both men and women: evidence for the fibrinolytic system as an independent primary risk factor. Circulation. 1998;98:2241–7. doi: 10.1161/01.cir.98.21.2241. [DOI] [PubMed] [Google Scholar]