Abstract
Normal-appearing white matter has been shown via diffusion tensor imaging to be affected in tuberous sclerosis complex. Under the hypothesis that some systems might be differentially affected, including the visual pathways and systems of social cognition, diffusion properties of various regions of white matter were compared. For 10 patients and 6 age-matched control subjects, 3 T magnetic resonance imaging was assessed using diffusion tensor imaging obtained in 35 directions. Three-dimensional volumes corresponding to the geniculocalcarine tracts were extracted via tractography, and two-dimensional regions of interest were used to sample other regions. Regression analysis indicated lower fractional anisotropy in the splenium of corpus callosum and geniculocalcarine tracts in tuberous sclerosis complex group, as well as lower axial diffusivity in the internal capsule, superior temporal gyrus, and geniculocalcarine tracts. Mean and radial diffusivity of the splenium of corpus callosum were higher in the tuberous sclerosis complex group. The differences in diffusion properties of white matter between tuberous sclerosis complex patients and control subjects suggest disorganized and structurally compromised axons with poor myelination. The visual and social cognition systems appear to be differentially involved, which might in part explain the behavioral and cognitive characteristics of the tuberous sclerosis complex population.
Introduction
Tuberous sclerosis complex, a neurocutaneous autosomal dominant disorder involving mutations of the TSC1 or TSC2 genes, is characterized by hamartomas in multiple organ systems [1]. Its prevalence is 1 in 6000 live births [2]. Close to 45% of patients have mild-to-profound intellectual disabilities [3], and 25–60% have autism [4]. Most patients with tuberous sclerosis complex have cortical tubers, which are dysplastic lesions composed of giant cells, maloriented dysmorphic neurons, and atypical astrocytes [1]. The association between tuber load and location and neurologic outcomes remains unclear [5–7]. Consequently, efforts are underway to understand what determines clinical phenotype and to identify prognostic indicators, so that targeted interventions can be developed.
Mouse models of tuberous sclerosis complex indicate decreased myelination throughout the cortex, as well as aberrant topographic projections of axon pathways in the reticulogeniculate tract [8,9]. Disruption of white matter may contribute to the high incidence of behavioral and cognitive impairments in tuberous sclerosis complex.
Diffusion tensor imaging is a type of magnetic resonance imaging that examines the direction and magnitude of average water diffusion, allowing inferences about the underlying tissue structure. Water in a biological system does not diffuse equally in all directions, and anisotropy is a measure of directional preference of diffusion. Cortical tubers with higher apparent diffusion coefficient and lower fractional anisotropy seem to have a greater epileptogenic potential [10,11], and in tuberous sclerosis complex patients normal-appearing white matter differs from that of control subjects [12].
Study aims were to test the application of tractography and to survey the condition of white matter in tuberous sclerosis complex, focusing on the visual system and areas of social cognition.
Study Design and Methods
Subjects
Ten patients (age range, 1.5–25 years) with an established diagnosis of tuberous sclerosis complex were imaged with 3 T magnetic resonance imaging (Siemens TrioTim) as part of their routine care. Six control subjects (age range, 1.1–25 years) had the same type of imaging. All the patients fulfilled the clinical criteria for definite tuberous sclerosis complex, as defined by the Tuberous Sclerosis Consensus Conference [13]. All patients with tuberous sclerosis complex were monitored in the Multidisciplinary Tuberous Sclerosis Program at Children’s Hospital Boston. Through this clinic, each patient received full neurologic assessment. Recruitment of the control subjects and data acquisition was conducted with informed consent from patients or guardians, using a protocol approved by the Children’s Hospital Institutional Review Board.
Data Acquisition
Both structural and diffusion images were acquired. Sedation was used to avoid motion artifact during image acquisition for all the tuberous sclerosis complex patients and for the youngest control subject. Sedation is routinely used in pediatric imaging and has not been associated with detectable signal alterations. The potential effects of sedation on diffusion tensor imaging measurements to date have been minimal [14].
A T1-weighted image was acquired in the sagittal plane with slice thickness of 1 mm. Diffusion-weighted imaging was performed using slice thickness of 2.2 mm and 35 diffusion gradient encoding directions.
Data Processing
All scans were corrected for inhomogeneity of signal intensity, aligned, and resampled to match the structural T1-weighted scan. Nonbrain matter was excluded by applying a threshold to the diffusion-weighted image.
Tractography is a diffusion tensor imaging method that permits the tracking of macroscopic water displacement along pathways in the brain, yielding robust identification of three-dimensional volumes of interest in a reproducible way [15–17]. Probabilistic methods allow robust identification of large three-dimensional segments of white matter fiber bundles, rather than only two-dimensional cross sections at specific points, and allow improved sampling of tracts in areas of low anisotropy [18].
The white matter underlying the primary visual cortex was manually drawn on a fractional anisotropy map overlain on a T1-weighted image. The selected region included white matter directly adjacent to the calcarine sulcus in each hemisphere (Fig 1). The lateral geniculate nucleus on each side was identified on color by orientation maps (Fig 2), according to previously described landmarks [19].
Figure 1.
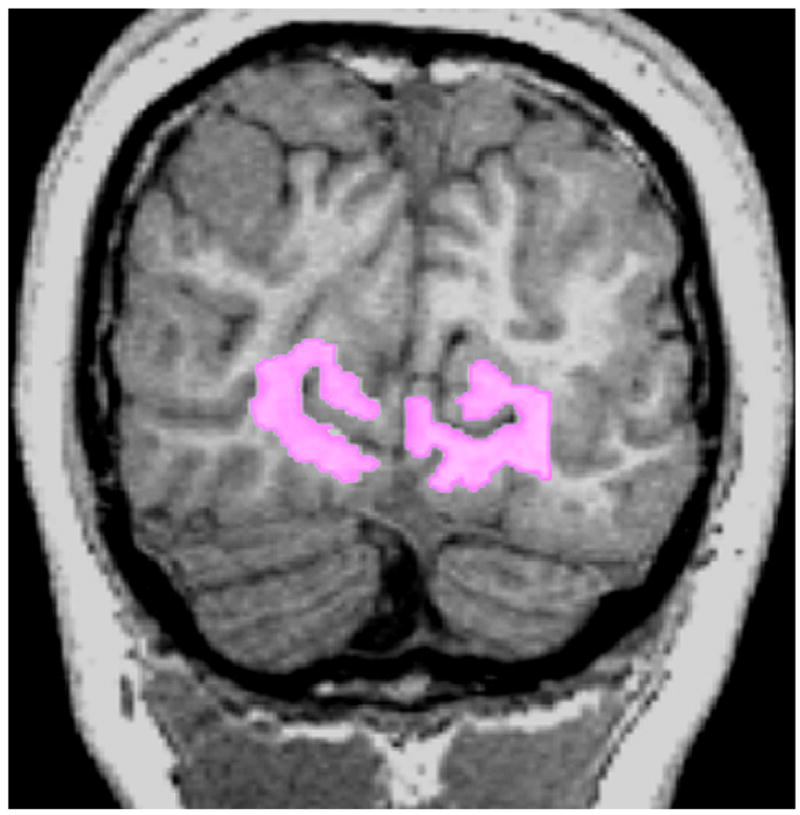
T1-weighted magnetization-prepared rapid gradient-echo magnetic resonance image with manually drawn region of interest in visual cortex, including white matter adjacent to calcarine sulcus.
Figure 2.
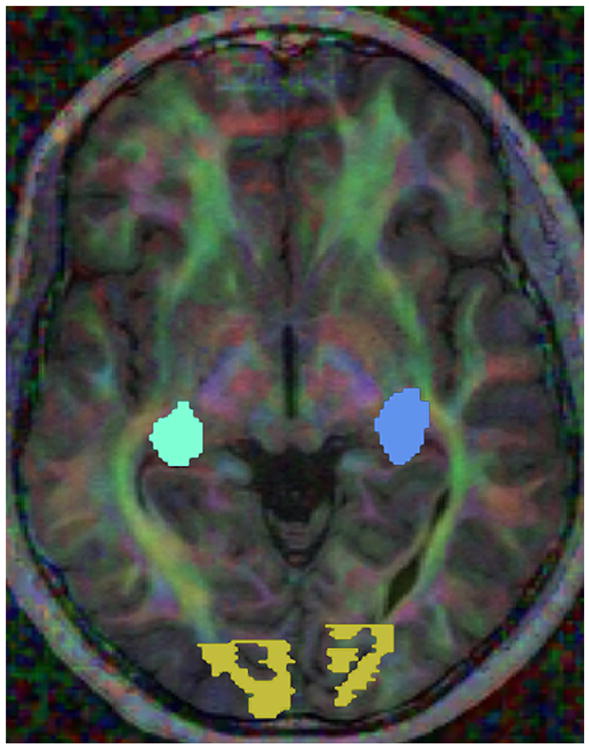
Visualization of white matter connectivity with color coding based on the orientation of the primary eigenvector of each diffusion tensor, with intensity proportional to the fractional anisotropy (red for left-right, blue for superior-inferior, and green for anterior-posterior). Regions of interest in the lateral geniculate nuclei (blue tones) and visual cortex (yellow) are also shown.
White matter fiber tracts were identified with a stochastic tractography algorithm [20,21]. Briefly, this algorithm models the path of the fiber tract as a sequence of vectors, wherein steps are taken along the path with an orientation that depends only upon the previous vector and the local diffusion information. Five tracts were initiated per voxel in the starting region of interest, with a maximum path length of 200 steps allowed, and were terminated in regions with extremely low fractional anisotropy.
Tracts consistent with the optic radiation were generated by first seeding all the tracts from the white matter underlying the primary visual cortex. Subsequent filtering by a second region of interest retained only those tracts that also passed through the lateral geniculate nucleus on each side. The probability that a voxel was present in a fiber pathway was then determined by counting the total number of sampled paths that included the voxel and then dividing by the total number of sampled paths. Images of the resultant probability distributions were generated, and a 7% threshold was set to isolate anatomically consistent fibers bundles and produce three-dimensional volume of interest masks. These masks were overlain on aligned fractional anisotropy and mean diffusivity maps, to sample volumes of interest (Fig 3). The mean fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity were extracted for each tract as a whole at each time point.
Figure 3.
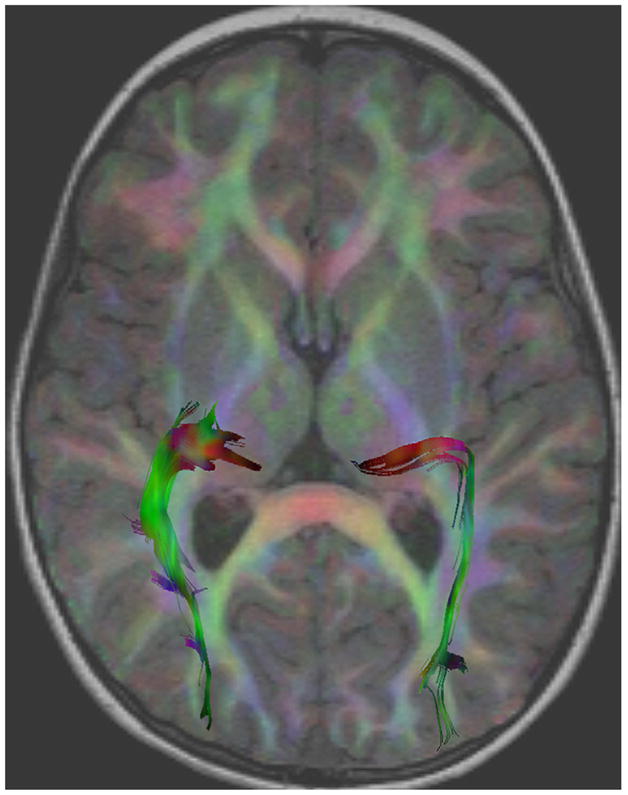
Visualization of tractography of the geniculocalcarine tract in the left and right hemisphere. The diffusion tensor image is color-coded based on the local orientation and blended with the underlying T1-weighted image (red for left-right, blue for superior-inferior, and green for anterior-posterior). Individual trajectories are also shown color-coded based on the local orientation of the underlying diffusion tensor.
Two-Dimensional White Matter Regions of Interest
Two-dimensional regions of interests were drawn bilaterally in the anterior and posterior limbs of the internal capsule, superior temporal gyrus, inferior temporal gyrus, and splenium of the corpus callosum (Fig 4). The mean of two measures on different slices was taken. Diffusion parameters extracted were fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity.
Figure 4.
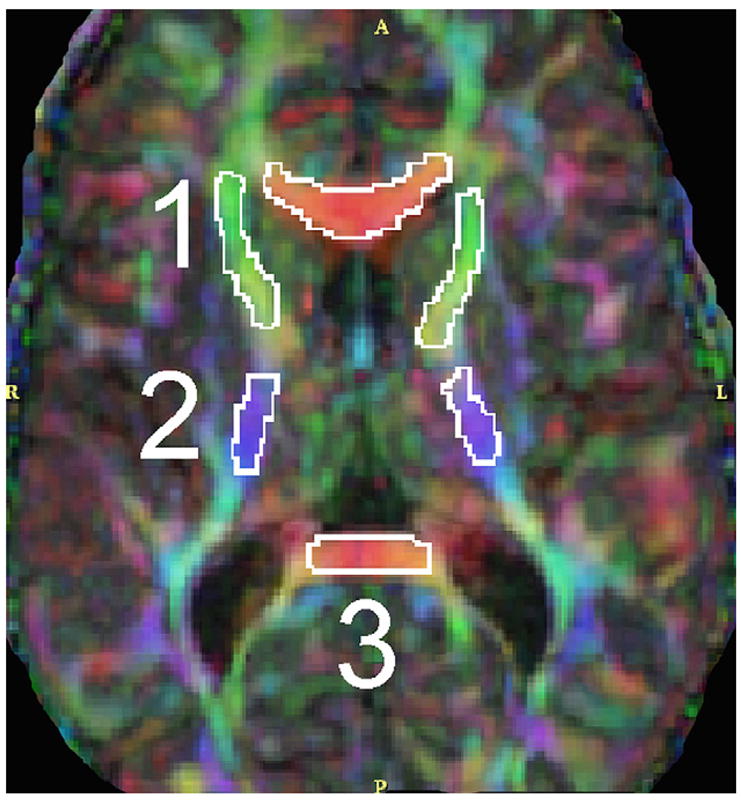
Color by orientation map showing two-dimensional regions of interest in deep white matter structures: (1) anterior limb of internal capsule; (2) posterior limb of internal capsule; (3) splenium of corpus callosum.
Results
All 16 cases (i.e., both patients and control subjects) yielded excellent quality diffusion tensor imaging and structural scans that were successfully registered. Seeding of tracts between the primary visual cortex and lateral geniculate nucleus bilaterally produced anatomically plausible conditioned-probability maps and masks (Fig 3). Although clinically the tuberous sclerosis complex patients were characterized as either typically developing (n = 3), developmentally delayed (n = 2), or autistic (n = 5), the number of subjects was too small to analyze diffusion tensor imaging results by phenotype.
For data analysis, diffusion values for tuberous sclerosis complex patients were compared with those for control subjects. All tuberous sclerosis complex patients and control subjects were above 1.1 years of age. The rate of change of diffusion parameters in white matter is expected to decrease from this age onward, because the fastest increase in maturation occurs during the first year of life [22]. Age was included as a covariate, and its effect was adjusted for in a simple linear regression model.
The response was one of four measures (fractional anisotropy, mean diffusivity, radial diffusivity, or axial diffusivity) in each of six regions: posterior limb of the internal capsule, anterior limb of the internal capsule, superior temporal gyrus, inferior temporal gyrus, splenium of the corpus callosum, and geniculocalcarine tract. Although these are certainly multivariate data, there were not enough observations for a multivariate response model, and thus 24 univariate response models were applied. The predictor was age at the time of scan, and a simple variable, TSC. For each of the 24 models, the model fit was
where TSC = 0 for control and 1 otherwise. It was then tested whether b1, the coefficient for TSC, was equal to zero adjusted for age.
The responses are listed in Table 1. Fractional anisotropy in the splenium of the corpus callosum and geniculocalcarine tract regions had a negative coefficient, signifying that the values in the tuberous sclerosis complex group were lower than in the control group. Axial diffusivity in the anterior limb of the internal capsule, superior temporal gyrus, and geniculocalcarine tract was also lower for tuberous sclerosis complex groups. Mean diffusivity and radial diffusivity of the splenium of the corpus callosum region were higher in the tuberous sclerosis complex group than in the control group.
Table 1.
Linear regression responses and level of significance adjusting for age, for four measures in six regions of interest
| Measure | Response Estimate | P Value |
|---|---|---|
| Splenium of the Corpus Callosum | ||
| Axial diffusivity | 0.0000 | 0.7005 |
| Fractional anisotropy | −0.1723 | 0.0001 |
| Mean diffusivity | 0.0002 | 0.0051 |
| Radial diffusivity | 0.0003 | 0.0001 |
| Geniculocalcarine Tract | ||
| Axial diffusivity | −0.0003 | 0.0038 |
| Fractional anisotropy | −0.0904 | 0.0584 |
| Mean diffusivity | −0.0001 | 0.0864 |
| Radial diffusivity | 0.0000 | 0.7317 |
| Superior Temporal Gyrus | ||
| Axial diffusivity | −0.0001 | 0.0160 |
| Fractional anisotropy | −0.0327 | 0.2497 |
| Mean diffusivity | −0.0001 | 0.0813 |
| Radial diffusivity | 0.0000 | 0.1842 |
| Inferior Temporal Gyrus, Fusiform Gyrus | ||
| Axial diffusivity | 0.0000 | 0.6819 |
| Fractional anisotropy | 0.0092 | 0.7816 |
| Mean diffusivity | −0.0001 | 0.1082 |
| Radial diffusivity | −0.0001 | 0.1611 |
| Posterior Limb of the Internal Capsule | ||
| Axial diffusivity | −0.0001 | 0.0902 |
| Fractional anisotropy | −0.0186 | 0.3681 |
| Mean diffusivity | 0.0000 | 0.1470 |
| Radial diffusivity | 0.0000 | 0.6108 |
| Anterior Limb of the Internal Capsule | ||
| Axial diffusivity | −0.0001 | 0.0210 |
| Fractional anisotropy | −0.0244 | 0.3697 |
| Mean diffusivity | 0.0000 | 0.5825 |
| Radial diffusivity | 0.0000 | 0.6965 |
Discussion
Diffusion tensor imaging and tractography methods were applied to the analysis of white matter in tuberous sclerosis complex. Differences in diffusion characteristics were found predominantly in the geniculocalcarine tract and splenium of the corpus callosum, as well as in the anterior limb of the internal capsule and the superior temporal gyrus. Lower fractional anisotropy in the tuberous sclerosis complex group suggests the presence of disorganized and poorly myelinated axons [23–25], whereas lower axial diffusivity implies poor integrity of the axons themselves [26]. Higher mean diffusivity could indicate differences in the extracellular environment of this region between groups [27], and higher radial diffusivity might signify impaired myelination [28]. These findings are in accord with reports indicating a diffuse abnormality of normal-appearing white matter examined with diffusion tensor magnetic resonance imaging [12]. Loss of TSC1 or TSC2 function has been shown to have detrimental effects on the regulation of axonal growth, particularly neuronal polarity and axon formation [29]. No differences were found between the patient and control groups in the region pertaining to the motor system (posterior limb of the internal capsule). Some systems might therefore be differentially affected, despite the widespread nature of the white matter involvement.
Diffusion abnormalities were found primarily in the splenium of the corpus callosum and the geniculocalcarine tract, both of which are heavily involved in the transmission of basic visual information to the cortex. Of the areas involved in higher level processing of sensory information (inferior temporal gyrus, fusiform gyrus, and superior temporal gyrus), only the superior temporal gyrus showed significant differences between groups. The superior temporal gyrus and anterior limb of the internal capsule of the tuberous sclerosis complex group had significantly lower axial diffusivity values than did the control group. The superior temporal gyrus has been associated with auditory processing, social cognition, regulation of behavior and neural mechanisms of imitation [30,31]. The anterior limb of the internal capsule is the principal white matter tract providing reciprocal connections among the frontal cortex, striatum, and thalamus and is involved in long-range communication. Functionally, this may indicate a combination of disturbed primary sensory processing, with some associated higher level and long range disturbances, as hinted at in previous magnetoencephalography findings [32].
In the present study, regions involved in the processing of visual auditory and social stimuli were different in the tuberous sclerosis complex group and the control group, indicating more disorganized and dysmyelinated axons with impaired structural integrity in the patient group. Given the high frequency of autism and behavioral problems in tuberous sclerosis complex, this finding raises the question of whether this abnormality in white matter tracts is correlated with behavioral and cognitive phenotype. In other words, are there specific diffusion tensor imaging characteristics that may differentiate tuberous sclerosis complex patients with autism from those with global delay, or from those with typical development? Identification and description of such imaging characteristics may shed light on the mechanisms of clinical heterogeneity in tuberous sclerosis complex.
There were some practical limitations to the present study, the first being the small number of tuberous sclerosis complex cases included. The 10 cases in the tuberous sclerosis complex group included a variety of clinical phenotypes, ranging from typical development to autism spectrum disorder and severe developmental delay. Such a range is a known feature of the tuberous sclerosis complex population, and larger data sets could allow a focus on each of these behavioral subtypes individually.
The presence or absence of tubers along the path of the visual tract was not included in the analysis. The tractography algorithm used in the present study terminates tracts in regions of extremely low fractional anisotropy, such as is exhibited by tubers. In this analysis, the tracts that pass through the regions of interest of the lateral geniculate nuclei and the white matter adjacent to the calcarine sulcus were considered, and the tracts passed through normal-appearing white matter. The white matter signal may be altered by the presence of small tubers at or below the resolution of the imaging, or by disruption of white matter at the periphery of larger tubers.
Given that even normal-appearing white matter has been found to be abnormal in tuberous sclerosis complex [12], and that tuber number has not been shown to correlate very closely with clinical outcome [7], it is likely that the microscopic effects of tubers extend well beyond what can be subjectively identified. Furthermore, electrophysiologic studies have shown that electrodes embedded in cortical tubers detect little or no epileptogenic activity while the surrounding tissue is highly active [33]. One of the aims of the present study was to find a more reliable way of identifying and quantifying abnormalities in the white matter, and the focus was therefore on analysis of generated tracts. The relationship between the tuber pathology and the nontuber pathology in tuberous sclerosis complex is likely to be an interesting area of research in the future.
Rapid maturation occurs in the first year of life, and the subjects were all over 1 year of age. The regression model used to test for a difference in white matter microstructure between tuberous sclerosis complex and control subjects used age as a dependent variable to account for the differing ages of the subjects, with a dichotomous dependent variable describing the group (i.e., tuberous sclerosis complex or control). Analysis using this regression model identified a statistically significant association between the group indicator and white matter microstructure in particular brain regions.
Future work might concentrate on the first few years of life, with a view to identifying early signs of later compromise and toward targeting therapeutic interventions. The underlying cause for the phenotypical heterogeneity in tuberous sclerosis complex might be elucidated by examining differences in diffusion properties of white matter between different clinical groups. Furthermore, mTOR inhibitor (rapamycin) therapy appears to benefit cell size and myelination in a mouse model in which Tsc1 is ablated in most neurons during development [8], and such therapy may be effective in improving cognitive outcome in tuberous sclerosis complex patients.
Conclusion
The differences in diffusion properties of white matter between the tuberous sclerosis complex group and the control group suggest disorganized and structurally compromised axons with poor myelination. The visual and social cognition systems appear to be differentially involved, and these differences may correlate with the behavioral phenotype.
Acknowledgments
Thanks go to Charles Nelson III and Richard L. Robertson for valuable discussions throughout the project, and to Jonathan Lipton and Kira Dies for critical reading of the manuscript. The authors are grateful to the Kennedy Memorial Trust for research fellowship funding for M.L.K. M.S. is supported in part by the John Merck Fund and a Junior Investigator Award from the Children’s Hospital Boston Translational Research Program. This investigation was supported in part by grant RG 3478A2/2 from the National Multiple Sclerosis Society, and by National Institutes of Health grant R01 RR021885 to S.K.W. Most importantly, the authors are indebted to the children and families who have participated in this study.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit:
References
- 1.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–56. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 2.Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. 1991;615:125–7. doi: 10.1111/j.1749-6632.1991.tb37754.x. [DOI] [PubMed] [Google Scholar]
- 3.Curatolo P, Verdecchia M, Bombardieri R. Tuberous sclerosis complex: a review of neurological aspects. Eur J Paediatr Neurol. 2002;6:15–23. doi: 10.1053/ejpn.2001.0538. [DOI] [PubMed] [Google Scholar]
- 4.Jeste SS, Sahin M, Bolton P, Ploubidis GB, Humphrey A. Characterization of autism in young children with tuberous sclerosis complex. J Child Neurol. 2008;23:520–5. doi: 10.1177/0883073807309788. [DOI] [PubMed] [Google Scholar]
- 5.Jansen FE, Vincken KL, Algra A, et al. Cognitive impairment in tuberous sclerosis complex is a multifactorial condition. Neurology. 2008;70:916–23. doi: 10.1212/01.wnl.0000280579.04974.c0. [DOI] [PubMed] [Google Scholar]
- 6.Walz NC, Byars AW, Egelhoff JC, Franz DN. Supratentorial tuber location and autism in tuberous sclerosis complex. J Child Neurol. 2002;17:830–2. doi: 10.1177/08830738020170111401. [DOI] [PubMed] [Google Scholar]
- 7.Wong V. Study of the relationship between tuberous sclerosis complex and autistic disorder. J Child Neurol. 2006;21:199–204. doi: 10.2310/7010.2006.00046. [DOI] [PubMed] [Google Scholar]
- 8.Meikle L, Talos DM, Onda H, et al. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27:5546–58. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahin M. Neuronal connectivity in TSC. Presented at Tuberous Sclerosis Complex: from Genes to New Therapeutics; International TSC Research Symposium organized by the Tuberous Sclerosis Alliance; September 23–25, 2007; Annapolis, MD. [Google Scholar]
- 10.Chandra PS, Salamon N, Huang J, et al. FDG-PET/MRI coregistration and diffusion-tensor imaging distinguish epileptogenic tubers and cortex in patients with tuberous sclerosis complex: a preliminary report. Epilepsia. 2006;47:1543–9. doi: 10.1111/j.1528-1167.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- 11.Luat AF, Makki M, Chugani HT. Neuroimaging in tuberous sclerosis complex. Curr Opin Neurol. 2007;20:142–50. doi: 10.1097/WCO.0b013e3280895d93. [DOI] [PubMed] [Google Scholar]
- 12.Makki MI, Chugani DC, Janisse J, Chugani HT. Characteristics of abnormal diffusivity in normal-appearing white matter investigated with diffusion tensor MR imaging in tuberous sclerosis complex. AJNR Am J Neuroradiol. 2007;28:1662–7. doi: 10.3174/ajnr.A0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roach ES, Gomez MR, Northrup H. Tuberous Sclerosis Complex Consensus Conference: revised clinical diagnostic criteria. J Child Neurol. 1998;13:624–8. doi: 10.1177/088307389801301206. [DOI] [PubMed] [Google Scholar]
- 14.Lee JE, Bigler ED, Alexander AL, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett. 2007;424:127–32. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 15.Conturo TE, Lori NF, Cull TS, et al. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A. 1999;96:10422–7. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori S, Kaufmann WE, Davatzikos C, et al. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med. 2002;47:215–23. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- 17.Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–44. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behrens TE, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–7. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto T, Yamada K, Nishimura T, Kinoshita S. Tractography to depict three layers of visual field trajectories to the calcarine gyri. Am J Ophthalmol. 2005;140:781–5. doi: 10.1016/j.ajo.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Friman O, Farnebäck G, Westin CFA. Bayesian approach for stochastic white matter tractography. IEEE Trans Med Imaging. 2006;25:965–78. doi: 10.1109/tmi.2006.877093. [DOI] [PubMed] [Google Scholar]
- 21.Ngo T. A stochastic tractography system and applications [master’s thesis] Cambridge, MA: Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology; 2007. [Google Scholar]
- 22.Provenzale JM, Liang L, DeLong D, White LE. Diffusion tensor imaging assessment of brain white matter maturation during the first postnatal year. AJR Am J Roentgenol. 2007;189:476–86. doi: 10.2214/AJR.07.2132. [DOI] [PubMed] [Google Scholar]
- 23.Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magn Reson Med. 1994;31:394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- 24.Gulani V, Webb AG, Duncan ID, Lauterbur PC. Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magn Reson Med. 2001;45:191–5. doi: 10.1002/1522-2594(200102)45:2<191::aid-mrm1025>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Drobyshevsky A, Song SK, Gamkrelidze G, et al. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci. 2005;25:5988–97. doi: 10.1523/JNEUROSCI.4983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J Neurosci. 2009;29:2805–13. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neil JJ, Shiran SI, McKinstry RC, et al. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology. 1998;209:57–66. doi: 10.1148/radiology.209.1.9769812. [DOI] [PubMed] [Google Scholar]
- 28.Larvaron P, Boespflug-Tanguy O, Renou JP, Bonny JM. In vivo analysis of the postnatal development of normal mouse brain by DTI. NMR Biomed. 2007;20:413–21. doi: 10.1002/nbm.1082. [DOI] [PubMed] [Google Scholar]
- 29.Choi YJ, Di Nardo A, Kramvis I, et al. Tuberous sclerosis complex proteins control axon formation. Genes Dev. 2008;22:2485–95. doi: 10.1101/gad.1685008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gernsbacher MA, Kaschak MP. Neuroimaging studies of language production and comprehension. Annu Rev Psychol. 2003;54:91–114. doi: 10.1146/annurev.psych.54.101601.145128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelphrey KA, Viola RJ, McCarthy G. When strangers pass: processing of mutual and averted social gaze in the superior temporal sulcus. Psychol Sci. 2004;15:598–603. doi: 10.1111/j.0956-7976.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 32.Peresson M, Lopez L, Narici L, Curatolo P. Magnetic source imaging and reactivity to rhythmical stimulation in tuberous sclerosis. Brain Dev. 1998;20:512–8. doi: 10.1016/s0387-7604(98)00034-5. [DOI] [PubMed] [Google Scholar]
- 33.Major P, Rakowski S, Simon MV, et al. Are cortical tubers epileptogenic? Evidence from electrocorticography. Epilepsia. 2009;50:147–54. doi: 10.1111/j.1528-1167.2008.01814.x. [DOI] [PubMed] [Google Scholar]


