Abstract
Using a hormone dependent xenograft model, we established that loss of response to letrozole was accompanied by up-regulation of Her-2/MAPK pathway and downregulation of ERα and aromatase activity. In our previous study, we showed that stopping letrozole treatment or adding trastuzumab could reverse acquired resistance. In this study, we compared the effect of intermittent letrozole treatment and switching treatment between letrozole and trastuzumab on tumor growth in an attempt to optimize discontinuous letrozole treatment. The mice were treated with letrozole until the tumors developed resistance and then divided into 3 groups, (i) letrozole, (ii) trastuzumab (iii) off (Δ4A supplement only); tumors were collected every week to examine changes in tumor protein expression and activity. In “off” group tumors, Her-2/p-MAPK activation gradually decreased and ERα, aromatase protein (and activity) increased. Within the first week of trastuzumab treatment, Her-2 and MAPK were downregulated and ERα was upregulated. When letrozole resistant MCF-7Ca tumors were taken “off” treatment for 4-weeks, the second course of letrozole treatment provided a much longer duration of response (p=0.02). However, switching treatment to trastuzumab for 4 weeks did not provide any inhibition of tumor growth. Our studies revealed that the adaptation of cells to a low estrogen environment by upregulation of Her-2/MAPK and down-regulation of ERα/aromatase was reversed upon letrozole withdrawal. The tumors once again became responsive to letrozole for a significant period. These results suggest that response to letrozole can be prolonged by a short “break” in the treatment.
Keywords: aromatase inhibitors, estrogen, trastuzumab, ERα, Her-2, breast cancer
Introduction
The knowledge that steroids play a critical role in growth of hormone dependent tumors is channeled towards development of endocrine therapy of breast cancer, which includes antiestrogens and aromatase inhibitors (1). Since, the development of aromatase inhibitors, the treatment of hormone responsive postmenopausal breast cancer has made significant advances (1-4). However, not all patients respond and some may eventually acquire resistance and relapse. Our study focuses on the mechanisms of acquisition of resistance to AIs and strategies for reversing the resistance to possibly delay the use of chemotherapy.
To study the effects of AIs, we have developed a mouse model system that utilizes tumors of human ERα positive beast cancer cells (MCF-7) that are stably transfected with human placental aromatase gene (MCF-7Ca) grown in ovariectomized female nude mice (5-7). This model simulates postmenopausal breast cancer, where the non-ovarian source of estrogen is through conversion of supplemented androstenedione (Δ4A) by the intratumoral aromatase. Using this model, we have established that AIs are more effective than AE tamoxifen in the treatment of hormone responsive postmenopausal breast cancer (8-11). However, the tumors eventually developed resistance despite continued treatment (10-12). To determine the mechanisms of resistance to AI letrozole, we developed a novel model, where a cell line was isolated from the MCF-7Ca xenografts treated with letrozole (10μg/day) for 56 weeks (12). This cell line was designated LTLT-Ca. We evaluated the changes in protein expression compared to parental MCF-7Ca cells and established that the key adaptive changes in this cell line was upregulation of Her-2/MAPK pathway and down regulation of ERα (12). We also determine that inhibition of Her-2 using trastuzumab (humanized monoclonal antibody against extracellular domain of Her-2) can reverse this resistant phenotype and restore response of LTLT-Ca cells to letrozole, in addition to other AIs and estrogen (13). Discontinuing the treatment of mice with resistant tumors for a few weeks also reversed this resistance (14). Upon discontinuation of treatment, the expression of Her-2 and p-MAPK was downregulated and ERα and aromatase were upregulated. Aromatase activity within the tumors was also upregulated (14).
In order to determine the optimal treatment plan to use for the intermittent treatment strategy, we examined changes in protein expression within the tumors as they were taken “off” treatment or switched to trastuzumab.
Materials and Methods
Materials
Dulbecco’s Modified Eagle Medium (DMEM), Modified Improved Minimum Essential medium (IMEM), penicillin/streptomycin solution (10,000IU each), 0.25% trypsin-1 mM EDTA solution, Dulbecco’s phosphate-buffered saline (DPBS), and geneticin (G418) were obtained from Invitrogen (Carlsbad, CA). Androstenedione (Δ4A), and Matrigel were obtained from Sigma Chemical Company (St. Louis, MO). Antibodies against Her-2, p-Her-2 were purchased from Upstate (now Millipore, Billerica, MA) antibodies against p-MAPK, MAPK, p-Elk-1, and p-p90RSK were purchased from Cell Signaling Technology, (Beverly, MA). Antibodies against ERα, and aromatase (CYP 19) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Radioactive ligand for aromatase assay 3H-Δ4A (23.5 Ci/mmole) was purchased from Perkin Elmer (Boston, MA).
MCF-7 human breast cancer cells stably transfected with the human aromatase gene (MCF-7Ca) were kindly provided by Dr. S. Chen (City of Hope, Duarte, CA). Letrozole was kindly provided by Dr. D. Evans (Novartis Pharma, Basel, Switzerland).
Cell culture
MCF-7Ca cells were routinely cultured in DMEM supplemented with 5% FBS, 1% penicillin/streptomycin, 700μg/mL G418. LTLT-Ca cells were developed from MCF-7Ca cells as described earlier (12, 14) from tumors of mice treated with letrozole for 56 weeks and cultured in steroid-depleted medium, containing 1μM of letrozole. Cell proliferation assays were performed using the MTT assay as described earlier (13, 14). The results were expressed as a percentage of the cell number in the Δ4A-treated control wells. IC50 values for inhibitors were calculated from the linear regression line of the plot of percentage inhibition versus log inhibitor concentration.
Tumor Growth in Ovariectomized Female Athymic Nude Mice
All animal studies were performed according to the guidelines and approval of the Animal Care Committee of the University Of Maryland, Baltimore. Female ovariectomized athymic nude mice 4–6 weeks of age were obtained from the National Cancer Institute - Frederick Cancer Research and Development Center (Frederick, MD). The mice were housed in a pathogen-free environment under controlled conditions of light and humidity; received food, and water ad libitum.
The tumor xenografts of MCF-7Ca cells were grown in the mice as previously described (6, 9, 12-14). Each mouse received subcutaneous (sc) inoculations in one site per flank with 100μL of cell suspension containing ~ 2.5×107 cells. The mice were injected daily with supplemental Δ4A (100μg/day). Weekly tumor measurements and treatments began when the tumors reached ~ 300 mm3. Mice were assigned to groups for treatment so that there was no statistically significant difference in tumor volume among the groups at the beginning of treatment. Letrozole and Δ4A for injection were prepared using 0.3% HydroxyPropylCellulose (HPC) in 0.9% NaCl solution. Trastuzumab for injection was prepared as 20 mg/ml stock solution in bacteriostatic water for injection which was then diluted in 0.9% NaCl solution to obtain the required concentration. Mice were then injected sc 5 times weekly with the indicated drugs (except trastuzumab was injected intra-peritoneally (ip) twice a week). The doses of trastuzumab (5mg/kg/wk divided in two doses), letrozole (10μg/day) and Δ4A (100μg/day) used are as previously determined and reported (13).
Western blotting
The protein extracts from tumor tissues were prepared by homogenizing the tissue in ice-cold DPBS containing protease inhibitors. Cellular protein extracts were made as described earlier. Total 50μg of protein from each sample was analyzed by SDS-PAGE as described previously (13, 14). Bands were quantitated by densitometry using Molecular Dynamics Software (ImageQuant®, Sunnyvale, CA). The densitometric values are corrected for loading control.
3H2O release assay for aromatase activity measurement
For measuring aromatase activity in tumor samples, the tumors were homogenized in ice-cold DPBS. The resulting homogenate was used for aromatase activity assay. The radiometric 3H2O release assay was performed as described previously (13, 14) using [1-β3H] Δ4A as substrate. The activity of the enzyme is corrected for protein concentration in the tumor homogenates.
Her-2 activity ELISA
Activity of Her-2 was measured by photometric ELISA assay as per manufacturer’s instructions (Cell Signaling Technologies).
Statistics
For in vivo studies, mixed-effects models were used. The tumor volumes were analyzed with S-PLUS (7.0, Insightful Corp.) to estimate and compare an exponential parameter (βi) controlling the growth rate for each treatment groups. The original values for tumor volumes were log transformed. The spline model with a single knot at time = week-22 weeks was used to accommodate the non-linearity with a piece-wise linear model. All p values less than 0.05 were considered statistically significant. The graphs are represented as mean ± standard error of the mean (SEM).
Results
Intermittent letrozole treatment in letrozole responsive tumors
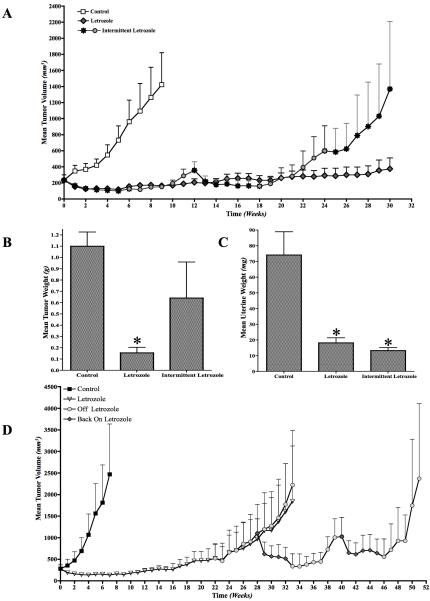
Mice with tumors of MCF-7Ca cells were given intermittent letrozole treatment of 6 weeks on and 6 weeks off. In this case, we observed that the tumors acquired resistance quicker than those in the continuous letrozole group (Figure 1A). All mice were sacrificed on week 30 (intermittent and continuous). The tumor weights were significantly different. The group receiving continuous letrozole treatment had significantly lower mean tumor weight (p<0.01) than control and intermittent letrozole group (Figure 1B). The uterine weight in both the groups (intermittent or continuous letrozole) were not significantly different (p=0.8), although they were significantly lower than control (p<0.001) (Figure 1C). This suggests than letrozole was able to maintain suppression of estrogen synthesis, even though the tumors grew. This suggests that intermittent letrozole treatment may be detrimental compared to continuous treatment in hormone responsive breast cancers.
Figure 1.
A: Effect of intermittent (6-weeks-on; 6-weeks-off) letrozole treatment on the growth of the MCF-7Ca xenografts: Xenografts of MCF-7Ca cells were grown in mice as described in the Materials and Methods. After the tumors reached 300mm3, mice were treated with either continuous letrozole or intermittent (6-weeks-on; 6-weeks-off) letrozole. The tumor volumes were measured weekly.
B: Effect of intermittent (6-weeks-on; 6-weeks-off) letrozole treatment on the mean tumor weights of mice bearing MCF-7Ca xenografts: Xenografts of MCF-7Ca cells were grown in mice as described in the Materials and Methods. After the tumors reached 300mm3, mice were treated with either continuous letrozole or intermittent (6-weeks-on; 6-weeks-off) letrozole. The tumor weights were measured at autopsy. The continuous letrozole group had significantly lower (*p<0.01) mean tumor weight.
C: Effect of intermittent (6-weeks-on; 6-weeks-off) letrozole treatment on the mean uterine weights of mice bearing MCF-7Ca xenografts: Xenografts of MCF-7Ca cells were grown in mice as described in the Materials and Methods. After the tumors reached 300mm3, mice were treated with either continuous letrozole or intermittent (6-weeks-on; 6-weeks-off) letrozole. The uterine weights were measured at autopsy. The mean uterine weights of mice in both continuous and intermittent letrozole were significantly lower than control (*p<0.001).
D: Effect of intermittent (6-weeks-on; 6-weeks-off) letrozole treatment on the growth of the MCF-7Ca xenografts that have acquired resistance to letrozole: Xenografts of MCF-7Ca cells were grown in mice as described in the Materials and Methods. After the tumors reached 300mm3, mice were treated with letrozole at 10μg/day for 22 weeks. At this time mice were divided into 2 groups; one group received continuous letrozole and the other received intermittent (6-weeks-on; 6-weeks-off) letrozole. The tumor volumes were measured weekly.
Intermittent letrozole treatment in letrozole resistant tumors
The tumors of mice treated with letrozole for 22 weeks were withdrawn from letrozole treatment for 6 weeks and put back on letrozole for 6 weeks. This cyclic treatment was continued for 2 additional cycles. The tumor volume was maintained at 500 mm3 until week 45. However, there was no substantial reduction in tumor volume (Figure 1D).
Analysis of changes in protein expression and activity in response to discontinuation letrozole treatment
Next we examined the time dependent changes that occurred in letrozole resistant tumors upon discontinuation of treatment. The mice were treated with letrozole until the mean tumor volume reached double the initial volume. This was assigned as week 0. At this time, the mice were randomized into three groups, one continued on letrozole, second group received trastuzumab and third group was taken “off” treatment. Three mice were sacrificed each week (assigned as weeks 1–7); tumors were collected and analyzed by western blotting. In addition, uteri were weighed and collected.
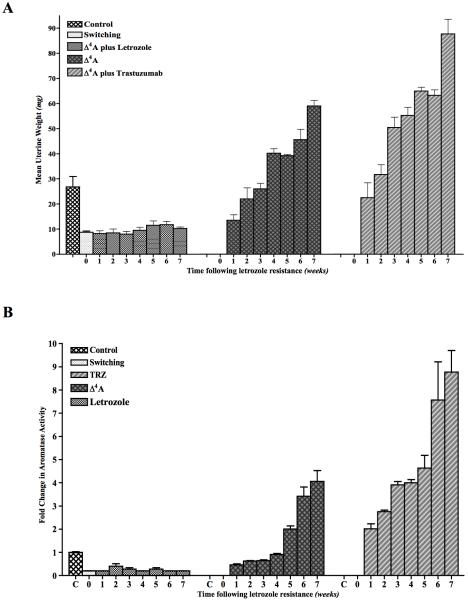
The uteri of mice in the letrozole group did not show any change in the mean uterine weight from that of week 0 (Figure 2A). The mean uterine weights of mice in the “off” group increased gradually over the next 7 weeks. The mean uterine weights of the mice in the trastuzumab group also increased weekly, but this increase was significantly higher than that of the “off” group. This suggests that estrogen synthesis is resumed after stopping letrozole in “off” group. Trastuzumab however, enhances the estrogen synthesis as confirmed by rapid increase in uterine weight with trastuzumab treatment. This increase in uterine weight also correlates with increase in aromatase activity (Figure 2B).
Figure 2.
A: Mean uterine weights of mice bearing MCF-7Ca xenografts that were switched to Δ4A or TRZ treatment after acquisition of letrozole resistance: Xenografts of MCF-7Ca cells were grown in mice as described in the Materials and Methods. After the tumors reached 300mm3, mice were treated with either letrozole at 10μg/day for 22 weeks. At this time mice were randomized into 3 groups; one group continued on letrozole and the second group received Δ4A and the third group received TRZ, after which three mice were sacrificed each week from each group. The uterine weights were measured at autopsy.
B: Aromatase activity in MCF-7Ca xenografts that were switched to Δ4A or TRZ treatment after acquisition of letrozole resistance: Xenografts of MCF-7Ca cells were grown in mice as described in the Materials and Methods. After the tumors reached 300mm3, mice were treated with either letrozole at 10μg/day for 22 weeks. At this time mice were randomized into 3 groups; one group continued on letrozole and the second group received Δ4A and the third group received TRZ, after which three mice were sacrificed each week from each group. The tumors were collected at autopsy. Aromatase activity was measured by radiometric 3H2O release assay.
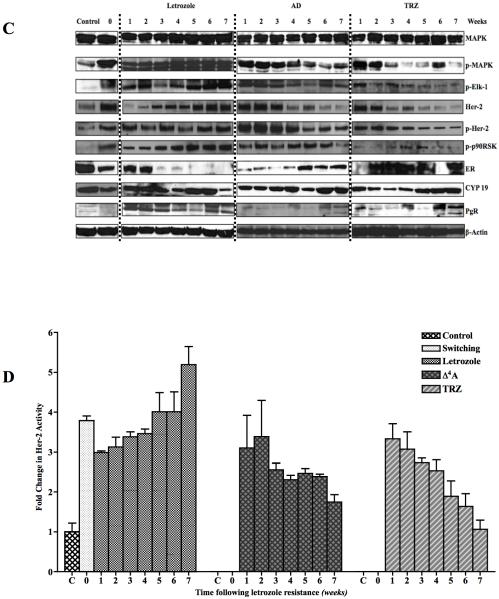
C: Western blot analysis of MCF-7Ca xenografts that were switched to Δ4A or TRZ treatment after acquisition of letrozole resistance: Xenografts of MCF-7Ca cells were grown in mice as described in the Materials and Methods. After the tumors reached 300mm3, mice were treated with letrozole at 10μg/day for 22 weeks. At this time mice were assigned to 3 groups; one group continued on letrozole and the second group received Δ4A and the third group received TRZ, after which three mice were sacrificed each week from each group. The tumors were collected at autopsy and protein expression was analyzed by western immunoblotting.
D: Her-2 activity in MCF-7Ca xenografts that were switched to Δ4A or TRZ treatment after acquisition of letrozole resistance: Xenografts of MCF-7Ca cells were grown in mice as described in the Materials and Methods. After the tumors reached 300mm3, mice were treated with letrozole at 10μg/day for 22 weeks. At this time mice were assigned to 3 groups; one group continued on letrozole and the second group received Δ4A and the third group received TRZ, after which three mice were sacrificed each week from each group. The tumors were collected at autopsy and Her-2 activity was measured by ELISA.
We next examined the changes in protein expressions in the tumors (Figure 2C). Consistent with our previous results (12-14), letrozole resistant tumors (week 0) show upregulation of Her-2/MAPK pathway. This was also accompanied by down-regulation of ERα and aromatase. In the letrozole group from week 1–7, the p-MAPK expression showed gradual increase and by week 7, it had increased significantly. Similar pattern was seen with p-Elk, p-p90RSK, Her-2 and p-Her-2. Her-2 activity (Figure 2D) also increased gradually until week 7. ERα expression decreased during letrozole treatment from week 1–7. When the mice were taken “off”, p-MAPK, p-p90RSK, Her-2 and p-Her-2 expression and Her-2 activity decreased while ERα and aromatase increased. A similar effect of trastuzumab was observed, however, a marked difference was seen at week 2. In off group, by week 4, the protein expression had changed to levels similar in Control tumors. We next examined the effect of 4-weeks “off” treatment and trastuzumab switch on tumor growth.
Mechanism of increase in Her-2 in letrozole resistant tumors
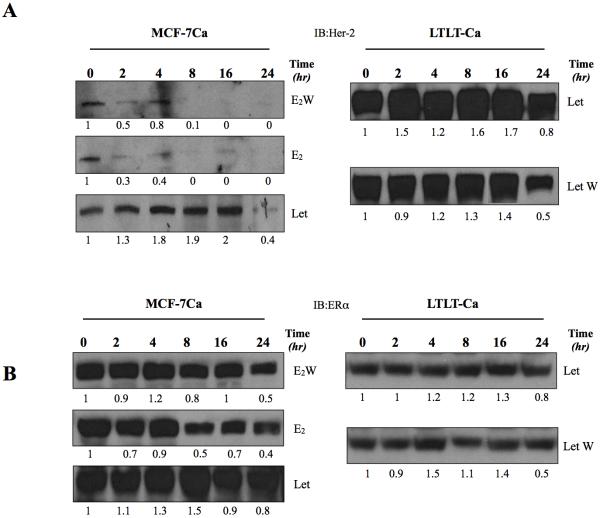
To evaluate the mechanism of Her-2 upregulation in letrozole resistant tumors, we performed fluorescence in situ hybridization (FISH) analysis on the tumors treated with letrozole (Figure 1D) and compared with Δ4A treated control and off groups. As shown in supplemental figure 1, letrozole resistant tumors did not have amplification of the Her-2 gene. We next evaluated the stability and half-life of the Her-2 protein in LTLT-Ca cells and compared it to the parental MCF-7Ca cells. The cells were treated with actinomycin D and cyclohexamide (5μM each) to inhibit all new protein synthesis and half-life of ERα and Her-2 were measured by western blotting. As shown in Figure 3A, when supplemented with E2, Her-2 protein in MCF-7Ca cells has a short half-life. The levels dropped to below 30% in the first 2 hours, whereas in E2W condition, the levels stayed higher longer (10% at 8 hours). In addition, when treated with letrozole the Her-2 protein levels stayed up for 16 hours and 40% by 24 hours. In LTLT-Ca cells, Her-2 protein has a long half-life (80% by 24 hours). When letrozole was withdrawn, Her-2 half-life in LTLT-Ca cells was shorter (50% in 24-hours), but still longer than MCF-7Ca cells. This suggests that low levels of estrogen (as achieved by AIs) inhibit degradation of Her-2, causing increased levels.
Figure 3.
A: Half-life of Her-2 in MCF-7Ca and LTLT-Ca cells: The cells were treated with actinomycin D and cyclohexamide (5μM each) to inhibit all new protein synthesis. The cells were either subjected to E2W or treated with E2 (10nM) or letrozole (1μM). Cell lysates were made as described in Materials and Methods. 50μg of protein was analyzed by western blotting to measure relative Her-2 protein levels. Left panel shows MCF-7Ca cells and right panel shows LTLT-Ca cells. Each lane corresponds to treatment time in hours.
B: Half-life of ERα in MCF-7Ca and LTLT-Ca cells: The cells were treated with actinomycin D and cyclohexamide (5μM each) to inhibit all new protein synthesis. The cells were either subjected to E2W or treated with E2 (10nM) or letrozole (1μM). Cell lysates were made as described in Materials and Methods. 50μg of protein was analyzed by western blotting to measure relative ERα protein levels. Left panel shows MCF-7Ca cells and right panel shows LTLT-Ca cells. Each lane corresponds to treatment time in hours.
The stability of ERα protein was then examined (Figure 3B). The half-life of ERα was not significantly different between MCF-7Ca and LTLT-Ca cells. In presence of E2, 50% of the ERα was degraded by 8 hours. In absence of E2, ERα protein levels were stabilized and significant reduction in ERα protein levels was only observed at 24 hours.
Treatment of letrozole resistant tumors using intermittent “off” or trastuzumab regimen
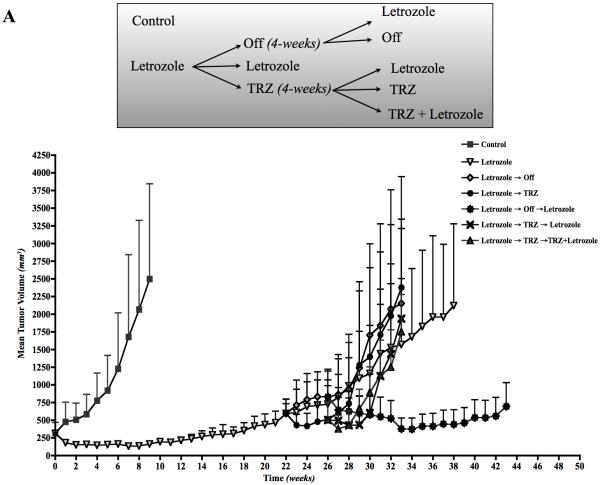
We evaluated the effect of intermittent treatment using MCF-7Ca xenograft model as described in Materials and Methods (13, 14). Figure 4A top-panel shows a schematic of the experimental design. Figure 4A shows the tumor volumes across different groups versus time (weeks). The mice were grouped so that the tumor volumes on week zero were not different across the groups (p=0.76). As expected the growth rate of letrozole (βi = −0.11±0.019) treated tumors was significantly lower than control (βi = 0.24±0.5) tumors (p<0.0001) over first 9 weeks. At this time point, the mice in the control group were sacrificed due to large tumor volumes. However, the tumors of the letrozole treated mice eventually began to grow and had doubled in volume by week 22. At this time, they were assigned to 3 groups; letrozole (Δ4A + letrozole −10μg/day), off (Δ4A-100μg/day) and trastuzumab (Δ4A + trastuzumab − 5mg/kg/wk divided in 2 doses). The tumor volumes were not significantly different across the groups (p=0.86). Based on tumor growth rates, we concluded that the groups did not have different rate of growth through week 26 (letrozole vs. off, p=0.47; letrozole vs. trastuzumab, p=0.93; off vs. trastuzumab, p=0.2).
Figure 4.
A: Tumor volumes of the MCF-7Ca xenografts: Xenografts of MCF-7Ca cells were grown in mice as described in the Materials and Methods. After the tumors reached 300mm3, mice were randomized as shown in the schematic in figure 3A. The tumor volumes were measured weekly. TOP: Schematic representation of the treatment schedule.
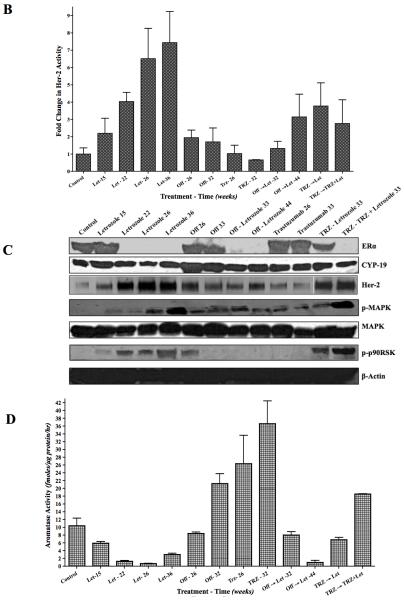
B: Her-2 activity in letrozole resistant MCF-7Ca xenografts of figure 3A: Xenografts of MCF-7Ca cells were grown in mice as described in the Materials and Methods. After the tumors reached 300mm3, mice were treated with either letrozole at 10μg/day for 22 weeks. At this time mice were randomized as per figure 3A. The tumors were collected at autopsy and Her-2 activity was measured by ELISA.
C: Western blot analysis of letrozole resistant MCF-7Ca xenografts of figure 3A: Xenografts of MCF-7Ca cells were grown in mice as described in the Materials and Methods. After the tumors reached 300mm3, mice were treated with either letrozole at 10μg/day for 22 weeks. At this time mice were randomized as per figure 3A. The tumors were collected at autopsy and protein expression was analyzed by western immunoblotting.
D: Aromatase activity in letrozole resistant MCF-7Ca xenografts of figure 3A: Xenografts of MCF-7Ca cells were grown in mice as described in the Materials and Methods. After the tumors reached 300mm3, mice were treated with either letrozole at 10μg/day for 22 weeks. At this time mice were randomized as per figure 3A. The tumors were collected at autopsy and aromatase activity was measured by radiometric 3H2O release assay.
As a continuation of treatment, the “off” group was split into two, one continued without letrozole and other group received letrozole. The growth rates of letrozole and the “off” groups were not significantly different over weeks 26–33 (p=0.37). However, the growth rate of mice switched back to letrozole had significantly lower growth rate compared to continuous letrozole treatment (p=0.02).
On the other hand, the mice receiving trastuzumab were assigned to 3 groups; trastuzumab, trastuzumab plus letrozole, letrozole. The growth rates across these groups were not significantly different over 32 weeks (p=0.42).
This suggests that trastuzumab, as single agent in letrozole resistant tumors does not provide any benefit. In addition, when switched from letrozole to trastuzumab and then back to letrozole or letrozole plus trastuzumab, the tumors continue to grow. In contrast, when given a 4-week break in treatment (“off”) and then switched back to letrozole, the tumors were inhibited for a prolonged period (week 26–44) of 18 weeks, approximately the same as that of the first course of letrozole.
We also examined tumors for protein expression and activity. Consistent with previous results (12, 13), tumors in the letrozole treated group had higher Her-2/MAPK activation and lower ERα and aromatase activity (Figure 4B-D). We have shown that treatment with MAPK inhibitor decreased MAPK but increased ERα. In contrast, when taken “off” treatment or switched to trastuzumab, Her-2 and MAPK were downregulated and ERα was upregulated. Aromatase activity also followed ERα expression. Interestingly, tumors of mice on letrozole at 44 weeks had similar protein expression profile as those on letrozole at 15 weeks (Figure 4C).
Discussions
Despite significant improvement in the outcome of hormone responsive breast cancer following aromatase inhibitor treatment, acquisition of resistance remains a major concern. In our model system that mimics the post-menopausal hormone dependent breast cancer patient, aromatase inhibitor letrozole was more effective than tamoxifen in controlling tumor growth. However, tumors eventually began to re-grow. To understand the mechanisms of this acquired resistance, we developed a cell line from the tumors treated with letrozole for prolonged period. These were designated as Long-Term Letrozole Treated (LTLT-Ca) cells. Using this new model, we established that activation of growth factor receptor mediated pathways such as Her-2 and MAPK was associated with letrozole resistance. Furthermore, inhibition of these pathways with the inhibitor of Her-2 (trastuzumab) (13) or MAPK (PD98059) (12) resulted in reversal of resistance. Thus, we concluded that resistance to letrozole was a result of adaptation of tumor cells to low estrogen environment through upregulation of Her-2 and downregulation of ERα. Following treatment with trastuzumab, Her-2 activation was downregulated and ERα levels were restored. This result suggested that Her-2 is a negative regulator of ERα. Similar reversal of resistant phenotype was observed upon letrozole withdrawal (14). Stopping letrozole treatment for 6 weeks led to restoration of response of tumors to letrozole in MCF-7Ca xenograft model (14). Studies with MAPK inhibitor U0126 however, showed that inhibition of MAPK pathway then leads to activation of PI3K/Akt pathway (data not shown). As such, MAPK appears to be one of the effectors in the Her-2 pathway.
In tumors of mice in the trastuzumab group, these changes occurred at a faster rate. Our previous studies have shown that inhibition of Her-2 (with trastuzumab) can activate ERα and increase aromatase activity in ERα dependent manner (13). As such, inhibition of both ERα and Her-2 pathway is essential to overcome the acquired resistance to letrozole. This study also shows that trastuzumab treatment can reverse resistant phenotype within 1 week. ERα and aromatase are increased to the same levels as in hormone responsive tumors and hence inhibition of ER mediated pathway would be necessary within the first week. This suggests that Her-2 is a negative regulator of ERα. In patient tissue samples, a strong inverse correlation is observed before any endocrine treatment. However, upon resistance, ERα was lost in 17% samples, and Her-2 was increased in 11% of the patients (15, 16). Acquired Her-2 amplification was also observed on patients’ circulating tumor cells (17), but was not evidenced here. However, letrozole appeared to inhibit Her-2 degradation up to 16 hours, which may account for the increase in levels of Her-2. Similarly, conversion of serum Her-2 from negative to positive has been observed in patients with advanced cancer that has progressed on endocrine therapy (16).
Several reports have suggested a role of Her-2 in mediating resistance to hormonal therapy such as antiestrogen tamoxifen and aromatase inhibitors. A bi-directional crosstalk between ERα and Her-2 and/or other members of the EGFR family has been shown to be a key phenomenon in the resistant model systems (18-23). Intracellular kinases such as Akt, MAPK can phosphorylate the Serine residues (such as S118, S167) in the AF-1 domain ERα and activate transcription (24-26). This is consistent with our LTLT-Ca model, where inhibition of Her-2 with trastuzumab restored responsiveness to letrozole (13).
Studies involving tamoxifen resistance have also revealed that cyclic treatment with tamoxifen and E2 leads to longer duration of response to tamoxifen. Jordan and colleagues have shown that tamoxifen stimulates the growth of tumors upon acquisition of resistance as tamoxifen exerts more agonistic effects (27). In these tumors, E2 can inhibit the tumor growth (27, 28). After a few days on E2, tumors once again became sensitive to growth inhibitory effects of tamoxifen (27, 28). The results presented here indicate that once letrozole treatment is stopped, aromatization of Δ4A is resumed and E2 is synthesized. This suggests that “off” treatment slowly reverses resistance whereas switching to trastuzumab forces increase in ERα allowing response to endogenous estrogen production. This strategy presented here could result in longer response and disease stabilization in the patients. However, detailed clinical studies need to be performed to establish correct intermittent scheduling.
Supplementary Material
Acknowledgements
This work was supported by grant CA-62483 to Dr. Brodie from the National Cancer Institute, National Institutes of Health.
Abbreviations used
- ER
Estrogen Receptor
- Δ4A
Androstenedione
- E2
Estradiol
- Her-2
Human Epidermal Growth factor Receptor- 2
- MAPK
Mitogen Activated Protein Kinase
- AIs
Aromatase Inhibitors
- AEs
Antiestrogens
- TRZ
Trastuzumab
- Let
Letrozole
Footnotes
Note: Novartis Pharma (Basel, Switzerland) supplied the letrozole used in this study. This work was presented in part at the IXth International Aromatase Meeting, Shanghai, China, October 2008 and 100th Annual AACR Meeting, Denver CO, USA, April 2009.
References
- 1.Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72:7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goss PE, Muss HB, Ingle JN, Whelan TJ, Wu M. Extended adjuvant endocrine therapy in breast cancer: current status and future directions. Clin Breast Cancer. 2008;8:411–7. doi: 10.3816/CBC.2008.n.049. [DOI] [PubMed] [Google Scholar]
- 3.Swain SM. Aromatase inhibitors--a triumph of translational oncology. N Engl J Med. 2005;353:2807–9. doi: 10.1056/NEJMe058273. [DOI] [PubMed] [Google Scholar]
- 4.Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of Aromatase: Saga of an Important Biologic Mediator and Therapeutic Target. Endocr Rev. 2009 doi: 10.1210/er.2008-0016. [DOI] [PubMed] [Google Scholar]
- 5.Yue W, Brodie A. MCF-7 human breast carcinomas in nude mice as a model for evaluating aromatase inhibitors. J Steroid Biochem Mol Biol. 1993;44:671–3. doi: 10.1016/0960-0760(93)90278-5. [DOI] [PubMed] [Google Scholar]
- 6.Yue W, Zhou D, Chen S, Brodie A. A new nude mouse model for postmenopausal breast cancer using MCF-7 cells transfected with the human aromatase gene. Cancer Res. 1994;54:5092–5. [PubMed] [Google Scholar]
- 7.Zhou DJ, Pompon D, Chen SA. Stable expression of human aromatase complementary DNA in mammalian cells: a useful system for aromatase inhibitor screening. Cancer Res. 1990;50:6949–54. [PubMed] [Google Scholar]
- 8.Brodie AH, Jelovac D, Long B. The intratumoral aromatase model: studies with aromatase inhibitors and antiestrogens. J Steroid Biochem Mol Biol. 2003;86:283–8. doi: 10.1016/s0960-0760(03)00368-6. [DOI] [PubMed] [Google Scholar]
- 9.Jelovac D, Macedo L, Goloubeva OG, Handratta V, Brodie AM. Additive antitumor effect of aromatase inhibitor letrozole and antiestrogen fulvestrant in a postmenopausal breast cancer model. Cancer Res. 2005;65:5439–44. doi: 10.1158/0008-5472.CAN-04-2782. [DOI] [PubMed] [Google Scholar]
- 10.Long BJ, Jelovac D, Handratta V, et al. Therapeutic strategies using the aromatase inhibitor letrozole and tamoxifen in a breast cancer model. J Natl Cancer Inst. 2004;96:456–65. doi: 10.1093/jnci/djh076. [DOI] [PubMed] [Google Scholar]
- 11.Long BJ, Jelovac D, Thiantanawat A, Brodie AM. The effect of second-line antiestrogen therapy on breast tumor growth after first-line treatment with the aromatase inhibitor letrozole: long-term studies using the intratumoral aromatase postmenopausal breast cancer model. Clin Cancer Res. 2002;8:2378–88. [PubMed] [Google Scholar]
- 12.Jelovac D, Sabnis G, Long BJ, et al. Activation of mitogen-activated protein kinase in xenografts and cells during prolonged treatment with aromatase inhibitor letrozole. Cancer Res. 2005;65:5380–9. doi: 10.1158/0008-5472.CAN-04-4502. [DOI] [PubMed] [Google Scholar]
- 13.Sabnis G, Schayowitz A, Goloubeva O, Macedo L, Brodie A. Trastuzumab Reverses Letrozole Resistance and Amplifies the Sensitivity of Breast Cancer Cells to Estrogen. Cancer Res. 2009;69:1416–28. doi: 10.1158/0008-5472.CAN-08-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabnis GJ, Macedo LF, Goloubeva O, Schayowitz A, Brodie AM. Stopping treatment can reverse acquired resistance to letrozole. Cancer Res. 2008;68:4518–24. doi: 10.1158/0008-5472.CAN-07-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez MC, Detre S, Johnston S, et al. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005;23:2469–76. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 16.Lipton A, Leitzel K, Ali SM, et al. Serum HER-2/neu conversion to positive at the time of disease progression in patients with breast carcinoma on hormone therapy. Cancer. 2005;104:257–63. doi: 10.1002/cncr.21202. [DOI] [PubMed] [Google Scholar]
- 17.Meng S, Tripathy D, Shete S, et al. HER-2 gene amplification can be acquired as breast cancer progresses. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9393–98. doi: 10.1073/pnas.0402993101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29:217–33. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massarweh S, Osborne CK, Creighton CJ, et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68:826–33. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 20.Osborne CK, Bardou V, Hopp TA, et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95:353–61. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 21.Osborne CK, Shou J, Massarweh S, Schiff R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005;11:865s–70s. [PubMed] [Google Scholar]
- 22.Sabnis GJ, Jelovac D, Long B, Brodie A. The role of growth factor receptor pathways in human breast cancer cells adapted to long-term estrogen deprivation. Cancer Res. 2005;65:3903–10. doi: 10.1158/0008-5472.CAN-04-4092. [DOI] [PubMed] [Google Scholar]
- 23.Yue W, Wang JP, Conaway MR, Li Y, Santen RJ. Adaptive hypersensitivity following long-term estrogen deprivation: involvement of multiple signal pathways. J Steroid Biochem Mol Biol. 2003;86:265–74. doi: 10.1016/s0960-0760(03)00366-2. [DOI] [PubMed] [Google Scholar]
- 24.Campbell RA, Bhat-Nakshatri P, Patel NM, et al. Phosphatidylinositol 3-Kinase/AKT-mediated Activation of Estrogen Receptor alpha. A NEW MODEL FOR ANTI-ESTROGEN RESISTANCE. J. Biol. Chem. 2001;276:9817–24. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 25.Kato S, Endoh H, Masuhiro Y, et al. Activation of the Estrogen Receptor Through Phosphorylation by Mitogen-Activated Protein Kinase. Science. 1995;270:1491–94. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 26.Rayala SK, Talukder AH, Balasenthil S, et al. P21-Activated Kinase 1 Regulation of Estrogen Receptor-{alpha} Activation Involves Serine 305 Activation Linked with Serine 118 Phosphorylation. Cancer Res. 2006;66:1694–701. doi: 10.1158/0008-5472.CAN-05-2922. [DOI] [PubMed] [Google Scholar]
- 27.Osipo C, Gajdos C, Cheng D, Jordan VC. Reversal of tamoxifen resistant breast cancer by low dose estrogen therapy. J Steroid Biochem Mol Biol. 2005;93:249–56. doi: 10.1016/j.jsbmb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Yao K, Lee ES, Bentrem DJ, et al. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:2028–36. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.