Abstract
In addition to its key role in complex motor function, the cerebellum is increasingly recognized to have a role in cognition. Songbirds are particularly good models for the investigation of motor and cognitive processes but little is known about the role of the songbird cerebellum in these processes. To explore cerebellar function in a songbird, we lesioned the cerebellum of adult female zebra finches and examined the effects on a spatial working memory task and on motor function during this task. There is evidence for steroid synthesis in the songbird brain and neurosteroids may have an impact on some forms of neural plasticity in adult songbirds. We therefore hypothesized that neurosteroids would affect motor and cognitive function after a cerebellar injury. We found that cerebellar lesions produced deficits in motor and cognitive aspects of a spatial task. In line with our prediction, birds in which estrogen synthesis was blocked had impaired performance in our spatial task compared with those that had estrogen synthesis blocked but estrogen replaced. There was no clear effect of estrogen replacement on motor function. We also found that lesions induced expression of the estrogen synthetic enzyme aromatase in reactive astrocytes and Bergmann glia around a cerebellar lesion. These data suggest that the cerebellum of songbirds mediates both motor and cognitive function and that estrogens may improve the recovery of cognitive aspects of cerebellar function after injury.
Keywords: aromatase, cerebellum, estradiol, spatial cognition, zebra finch
Introduction
The cerebellum participates in the coordination of complex vertebrate motor functions (Paula-Barbosa & Sobrinho-Simões, 1976; Tohyama, 1976; Paula-Barbosa et al., 1980; Smith et al., 1993; Rodriguez et al., 2005; Molinari et al., 2007; Yoshida et al., 2007). Congenital, injury- or toxicity-induced cerebellar abnormalities have long been known to be associated with motor deficits (Levisohn et al., 2000). Strikingly, cognitive functions also diminish with cerebellar deficiency (Levisohn et al., 2000). Subjects with cerebellar injury often display ataxia as well as decreased spatial processing, learning and memory (Gandhi et al., 2000; Gaytán-Tocavén & Olvera-Cortés, 2004; Andreescu et al., 2007; Gordon, 2007; Willson et al., 2007).
Songbirds may be excellent animal models to explore cerebellar involvement in motor and cognitive processes. Motor tasks with considerable cognitive support, such as some types of speech, are known to require cerebellar input (Gordon, 2007). Songbirds possess an exceptional capacity to perform complex behaviors including song, a behavior that requires intricate and well-timed fine motor function as well as significant cognitive input (Williams et al., 1989; Clayton & Dickinson, 1998). The avian cerebellum shares much anatomical similarity with that of mammals so results from songbirds will probably generalize to other species (Paula-Barbosa & Sobrinho-Simões, 1976; Tohyama, 1976; Paula-Barbosa et al., 1980; Smith et al., 1993).
Our first objective was to determine if cerebellar lesions produce deficits in songbird cognitive function as measured by a spatial working memory task. A second objective of this study was to explore the possible involvement of the sex steroid estradiol (E2) in songbird cerebellar recovery of function. We have previously documented the expression of steroidogenic factors (the cholesterol transporter StAR and the enzymes CYP11A1, 3B-HSD and CYP17) in the adult songbird cerebellum (London et al., 2003, 2006). These transporters and enzymes act to convert cholesterol into steroid hormones that, when formed in brain, are termed neurosteroids (Baulieu & Robel, 1990). Cerebellar Purkinje neurons of rats also express neurosteroid-ogenic factors (Sakamoto et al., 2003), suggesting that steroid actions in the cerebellum may be widespread and conserved. Cerebellar Purkinje cells express estrogen receptors α and β in rats (Shughrue et al., 1997; Price & Handa, 2000; Tsutsui, 2006) and low levels of estrogen receptor β have been reported in the songbird cerebellum (Bernard et al., 1999). Estrogens have numerous documented effects on cognitive function and recently such studies have included estrogen action within the cerebellum (Ghidoni et al., 2006; Andreescu et al., 2007; Henderson, 2007).
Importantly, in some brain regions, injury upregulates the estrogen synthetic enzyme aromatase to protect against neural damage and to facilitate neural repair (Garcia-Segura et al., 1999a, b, 2003; Peterson et al., 2001; Lavaque et al., 2006). If the cerebellum has the capacity to synthesize androgens de novo and if injury upregulates aromatase, then estrogens might be available to the injured cerebellum to assist with neural repair and recovery of function (Lavaque et al., 2006). Therefore, we sought to determine if aromatase is upregulated by injury in the songbird cerebellum and whether estrogens could impact recovery of motor and/or cognitive function after cerebellar lesion.
Materials and methods
Animals
All experiments were conducted in accordance with the UCLA Chancellor's Animal Committee and with IACUC rules and regulations. All subjects were adult female zebra finches. Birds were housed in same-sex groups in an aviary on a 14/10 h light/dark cycle. Food and water were freely available unless otherwise described.
Experiments 1 and 2: behavioral testing
We performed two separate behavioral experiments. In both experiments, birds were pre-trained to eat food from cups at the end of the four arms of a plus maze. Birds were then randomly assigned to treatment groups and given bilateral cerebellar lesions or sham lesions. Birds were then trained in the plus maze with only one arm having a cup with accessible seed. This allowed us to assess spatial learning of this goal. Importantly, it has previously been shown that zebra finches can learn a spatial task by using distal cues to locate a goal (Patel et al., 1997; Watanabe & Bischof, 2004).
Treatment groups
In experiment 1, we compared a sham-lesioned group that received no lesion (n = 7) with a group that received bilateral cerebellar lesions as described below (n = 7). In experiment 2, we compared zebra finches that received bilateral lesions plus 20 μL of the aromatase inhibitor Fadrozole (10 mg/mL) per day for 8 days post-surgery (Fadrozole, n = 6) with birds that received this Fadrozole treatment and a 5 mm silastic E2 (Fadrozole/E2) implant on the day of surgery (n = 6). The 5 mm silastic implant had an internal diameter of 0.78 mm and an outer diameter of 1.6 mm. These Fadrozole and E2 treatments are known to effectively inhibit aromatase and elevate plasma E2 levels within the physiological range, respectively, in zebra finches (Adkins-Regan et al., 1990; Lee et al., 2007). Behavioral testing was identical for both experiments.
Apparatus
We used a custom-built four-arm plus maze manufactured with a white wooden frame covered by aluminum screen. The maze was 101.6 cm in total length and width and 45.7 cm in height. Each arm was 10.2 cm in width (Fig. 1). Although the birds could fly in the maze subjects typically hopped through the maze in search of food. Removable cardboard flaps were inserted about 10 cm into the arms to prevent the bird's entry into the arms of the maze at the start of each trail. These were removed to initiate trials. Cups (medium-sized plastic weighing boats, 6 cm diameter, 3 cm high) filled with seed were placed at the end of each arm. During spatial testing a parafilm cover was used to block access to seed in the cups at the end of three of the arms. The maze was placed on the floor and was not moved throughout testing. In the room there were several objects that birds could use as distal cues to orient themselves to the cup with accessible seed.
Fig. 1.
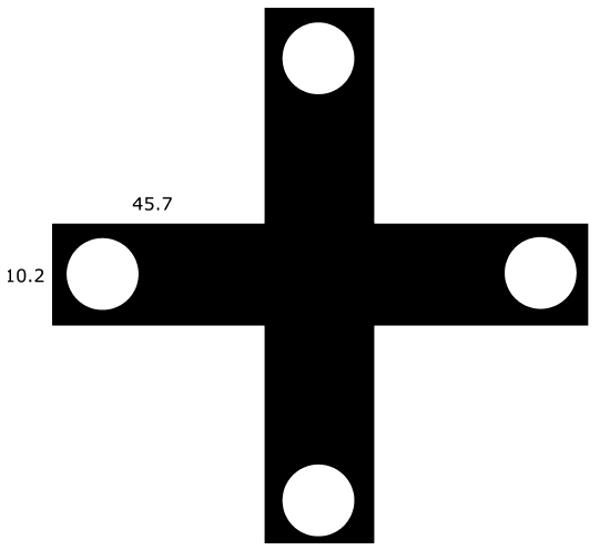
Top view of the maze drawn to scale. Circles are seed cups. Trials were initiated with birds in the center. During pre-training all cups had accessible seed. Throughout spatial training three cups were covered with parafilm to prevent access.
Pre-training
Prior to assignment to experimental treatment groups, zebra finches were pre-trained with two trials per day for 4–6 days to go down the arms of the maze to access food. Birds were deprived of food for 6 h prior to being placed in the maze. To initiate a trial, subjects were placed in the center of the maze with the flaps blocking entry into the arms of the maze. The lights were then turned off, the flaps removed and then the lights were turned back on. The bird was allowed to freely explore any arm of the maze. The trial ended either 2 min after the bird found food or when 25 min had passed. The intertrial interval was no less than 60 min and no more than 90 min. If, after 6 days, a bird was still unable to locate and eat seed from the cups within 25 min on both trials of the day it was not included in the experiment.
Spatial training
After pre-training, birds were randomly assigned to experimental groups; for experiment 1, the sham-lesioned or lesioned group and for experiment 2, the Fadrozole or Fadrozole/E2 group. We initiated the spatial memory test at 48 h post-surgery. Birds were tested in a random order. All methods were the same as during pre-training except that only one arm contained a cup of seed that was accessible. The other three arms contained cups with seed but these were covered with parafilm. An observer behind a blind recorded the bird's behavior. Observers were not aware of the bird's treatment group. The trial stopped either 2 min after a bird had found the cup of seed without parafilm or when 25 min had passed. We ran two trails per day for 6 days. Birds that did not attempt to search for food in two daily test trials were removed from the study.
In each behavioral trial we recorded three dependent variables: falls, errors and time to reach the goal. The number of times that a bird lost balance and fell during a trial was considered to be a pure motor deficit. An incorrect choice or error was marked when a bird went into an arm that contained a cup covered with parafilm whether or not it continued all the way to the cup. We considered this to be an error of spatial reference memory. Birds often went down the same incorrect arm more than once. We thought that repeat errors down the same incorrect arm might be more indicative of working memory deficits, whereas initial errors might be more indicative of spatial reference memory errors. Therefore, in addition to the total number of errors we separately examined initial travel into incorrect arms and repeat errors into incorrect arms during a single trial. Lastly, we measured the time required for each bird to find the correct feeder. We believed that this variable would reflect both motor and cognitive function as falls or poor hopping might slow the time to the feeder.
Probe trial
At the end of training we conducted a single probe trial to examine whether visual or olfactory cues associated with the goal-feeding cup could have guided birds to the goal rather than distal spatial cues. The probe trial was conducted on the day after spatial testing ended. For each experimental group, we randomly chose two birds to use in the probe trial. During the probe trial, all four cups were without seed and uncovered. All birds were allowed to explore the maze until they found the site of the previously correct cup. We recorded errors prior to identifying the previously correct cup and the time required to find the cup. If the number of errors and latencies to find the correct cup were similar to or better than those seen during the spatial trials it suggests that the birds had not previously used odors or visual cues associated with the goal cup but had instead learned the location of the goal cup presumably by using distal spatial cues.
Statistical analysis
Data were analysed using Statview 5.0 (SAS Inst.) on Windows 2000. The two trials per day were averaged for each bird. Trials in which a bird took longer than 25 min to reach the goal were not included in the averages. This happened eight separate times, so that eight data points only had one trial for that bird for the day. This occurred in all four experimental groups and was usually a result of the bird not moving from the central area during a trial, suggesting insufficient motivation to search for food. Time to reach the cup, errors and falls were compared between groups in both experiments 1 and 2 using repeated-measures anova. Fisher's PLSD post-hoc analysis was used to examine between-group differences for individual days. When overall anovas were significant, paired t-tests were used to compare the first and last day of training to determine whether learning had occurred. We used linear regression analysis to determine if there was a linear improvement in measured variables over days for each group. To improve normality, errors were square root transformed prior to analyses. The level for statistical significance was P < 0.05.
Lesions
The lesion site targeted for the behavioral experiments and immunocytochemical assays described below in experiments 3–5 was the medial deep cerebellar nucleus as well as the overlying folia of the cerebellar cortex. We targeted these regions because pilot studies showed that this lesion produced obvious deficits in motor function, allowing us to examine both motor and cognitive deficits, whereas several other lesion locations tested resulted in no obvious motor deficit.
Lesions were performed as follows. Birds were deprived of food, but not water, for 6 h prior to surgery. A deep plane of anesthesia was induced with Equithesin (3.2 mL/kg, i.m.; 0.85 g chloral hydrate, 0.21 g pentobarbitol, 0.42 g MgSO4, 2.2 mL 100% ethanol and 8.6 mL propylene glycol brought to a 20 mL final volume with dH2O and then filtered) and birds were placed in a stereotaxic frame (Herb Adams Engineering, Glendora, CA, USA) at an 80° angle inferior to the horizontal under a binocular microscope (Zeiss). Feathers from the caudal region of the skull were plucked and a small dorsal incision through the skin was made at the base of the skull. A craniotomy was made over the cerebellum. Mechanical lesions were made bilaterally for behavioral studies (experiments 1 and 2) and unilaterally for immunocytochemistry (experiments 3–5) with a 26 G needle at coordinates lateral (± 0.97 mm) and rostral (−1.35 mm) to the bifurcation of the central sinus and ventral (−4.9 mm) to the surface of the brain. Penetrating injury produced in this manner is known to upregulate aromatase in the zebra finch hippocampus and whole cerebellum (Peterson et al., 2001, 2004, 2007). The injured volume is roughly 2.25 mm3 of the cerebellum in each hemisphere. Studies of cerebellar function in other species have involved larger lesions. In goldfish (Rodriguez et al., 2005) the whole cerebellum was removed and in rats (Mandolesi et al., 2001) hemicerebellectomy has been used. Our smaller lesion will allow us to isolate functionality of various regions in future work. After lesion, the skin was carefully placed over the skull and sealed with ethyl cyanoacrylate. Sham-lesioned birds underwent all of the same procedures except for needle penetration. Post-surgery, birds were monitored until their breathing and activity appeared normal. Behavioral testing for birds in experiments 1 and 2 was initiated at 48 h after surgery.
Histology
Birds used in behavioral tests were killed at 72 h after testing. Birds used for immunocytochemistry (experiments 3–5 described below) were killed at 48 h after surgery. Birds were killed by halothane overdose and perfused with saline followed by 10% formalin. Following perfusion, the brain was removed from the skull and post-fixed in 20% sucrose formalin for 24 h. Brains were fast frozen in optimum cutting temperature on dry ice and stored at −80°C. Brains were sectioned on a cryostat at 40 μm and free-floating sections were placed in 0.1% phosphate-buffered saline. Sections from birds used in behavioral tests were then mounted, dehydrated through graded alcohols and stained with 0.1% thionin to confirm lesion location. Sections from brains used for immunocytochemistry were held in 0.1% phosphate-buffered saline at 4°C until used in assays. Identification of lesioned areas was performed under bright-field microscopy (Axioskop 20; Carl Zeiss) using objectives from 2× to 10× with reference to a zebra finch brain atlas (Nixdorf-Bergweiler & Bischof, 2007). Lesion volume was reconstructed in all birds used for behavioral testing. This was done by measuring the outline of the lesion in serial sections using ImageJ (NIH software).
Aromatase expression: experiments 3–5
To determine whether the upregulation of aromatase in glia or neurons around the site of the cerebellar lesion is a possible mechanism for repair and recovery of function in the zebra finch cerebellum we performed immunocytochemistry for aromatase and for aromatase in combination with the neuron-specific marker glutamic acid decarboxylase (GAD)67 (courtesy of Dr Niranjala Tillakaratne, University of California, Los Angeles) and the glial-specific marker vimentin (University of Iowa Hybridoma Bank).
Aromatase immunocytochemistry
Zebra finches (n = 5) were lesioned unilaterally as described in the Lesions section above. Brain sections were collected at 48 h post-lesion as described in the Histology section above. Tissues were transferred from 0.1% phosphate-buffered saline and incubated (60 min) in normal goat serum in 0.3% Triton X-100 in 0.1M PB (PBT) (Vector Laboratories) before incubation (72 h) with an antibody to zebra finch aromatase (AZAC; Saldanha et al., 2000) at a concentration of 1: 2000 in 0.3% PBT. Sections were washed (3 × 15 min) in 0.1% PBT followed by secondary antibody incubation (60 min) with biotinylated goat anti-rabbit IgG in 0.3% PBT (Vector Laboratories) at a dilution of 1: 200. Sections were washed (3 × 15 min) in 0.1% PBT followed by incubation with Vectastain ABC (Vector Laboratories) as per the manufacturer's instructions. Sections were washed (3 × 15 min) in 0.1% PBT and then placed in 0.03% 3′3-diaminobenzidine tetrahydrochloride catalysed with 1% hydrogen peroxide. The reaction was stopped by adding 0.1% phosphate-buffered saline. Slices were then dehydrated through graded alcohols, cleared with Hemo-De and coverslipped.
Aromatase and glutamic acid decarboxylase immunocytochemistry
Zebra finches (n = 2) were lesioned unilaterally through the cerebellum and sections were collected as previously described. Staining against both proteins was performed sequentially in the order listed below. Pre-incubation was for 60 min using 10% normal goat serum in 0.3% PBT followed by primary antibody incubation using the anti-aromatase antibody (AZAC; Saldanha et al., 2001) at a 1: 2000 concentration in 0.3% PBT for 72 h. Secondary antibody incubation was for 60 min using a 1: 200 dilution of biotinylated goat anti-rabbit IgG in 0.3% PBT (Vector Laboratories). Tissue was washed three times for 10 min in 0.1% PBT and followed with an incubation for 60 min of avidin fluorescein (10 μg/mL) in 0.3% PBT (Vector Laboratories). Tissue was then washed for an additional 24 h at 4°C in 0.1% PBT. Sections were washed with Avidin/Biotin Blocking Kit solution A for 15 min followed by two 10 min 0.1% PBT washes and then 15 min in solution B (Vector Laboratories). Sections were washed twice for 10 min in 0.1% PBT and then washed for 60 min in 10% normal donkey serum in 0.3% PBT (Vector Laboratories). Primary antibody incubation against the protein GAD67 occurred with a 60 min wash with 1: 2000 anti-GAD67 in 0.3% PBT and then storage for 24 h at 4°C. Sections were washed three times for 15 min in 0.1% PBT. Secondary antibody incubation was for 60 min with 1: 200 donkey anti-rabbit indocarbocyanine (Cy3) IgG in 0.3% PBT (Jackson Laboratories). Sections were washed three times for 15 min in 0.1% PBT and then five times for 15 min in 0.1 m phosphate buffer (PB). Sections were then mounted and coverslipped.
Aromatase and vimentin immunocytochemistry
Zebra finches (n = 4) were lesioned unilaterally through the cerebellum as previously described. Staining against both proteins was performed sequentially in the order listed below. Aromatase staining was performed as described above. Sections were washed for 60 min in 10% normal horse serum in 0.3% PBT (Vector Laboratories). Primary antibody incubation occurred with a 60 min wash with 1: 50 anti-vimentin (40E-C; University of Iowa Developmental Studies Hybridoma Bank) in 0.3% PBT and then storage for 24 h at 4°C. Sections were washed three times for 15 min in 0.1% PBT. Secondary antibody incubation was for 60 min with 1: 200 biotinylated horse anti-mouse IgG in 0.3% PBT (Vector Laboratories). Sections were washed three times for 15 min in 0.1% PBT. Sections were incubated for 60 min in avidin Texas red (20 μg/mL) (Vector Laboratories). Sections were washed five times for 15 min in 0.1 m PB. Sections were then mounted and coverslipped.
Microscopy
Single-labeled brain sections were viewed with the Axioskop 20 (Carl Zeiss) using objectives from 10× to 40× with bright-field or appropriate fluorescent filters. The aromatase and vimentin double-labeled slides were viewed with a confocal microscope as follows. Confocal fluorescence images were taken using Leica Confocal Software on a Leica TCS-SP MP Confocal and Multiphoton Inverted Microscope (Heidelberg, Germany) equipped with argon laser (488 nm blue excitation) and 561 nm diode laser (green excitation) and a two-photon laser setup consisting of a Spectra-Physics Millenia × 532 nm green diode pump laser and a Tsunami Ti-Sapphire picosecond pulsed infrared laser tuned at 770 nm for UV excitation. Images were taken at either 3 or 1 μm layers under 20× or 40× magnification.
Results
Lesion site confirmation
Figure 2 illustrates a typical lesion. The maximum lesion produced damage to the caudal area of folia I–VI and the minimal lesion damaged folia II–VI. All birds had damage to the medial deep cerebellar nucleus. Lesion volume was about 2.25 mm3 for each hemisphere.
Fig. 2.
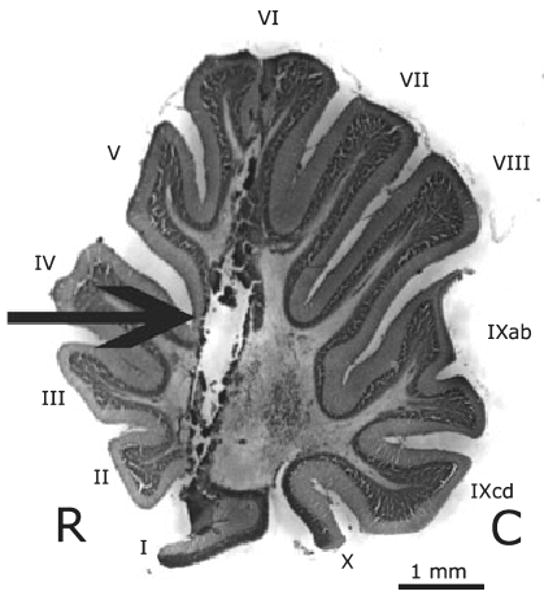
Sagittal section of an adult female zebra finch illustrating a typical lesion (arrow). R, rostral; C, caudal. The lesion tract penetrates folia I–VI and extends into the medial deep cerebellar nucleus. All lesions were bilateral; only a single lesion is visible in this sagittal section. Folia are labeled with roman numerals according to the scheme established by Larsell (1967).
Experiment 1
As expected, animals with bilateral lesions of the cerebellum performed more poorly than those with sham lesions on all three dependent measures, i.e. falls, errors and time to the correct feeder. We did not statistically compare the difference in the number of falls that occurred during the daily testing period (Fig. 3A) because only cerebellar-lesioned birds fell. Not all lesioned birds showed significant loss of balance, however, as only three of the seven lesioned birds were seen to fall during the testing.
Fig. 3.
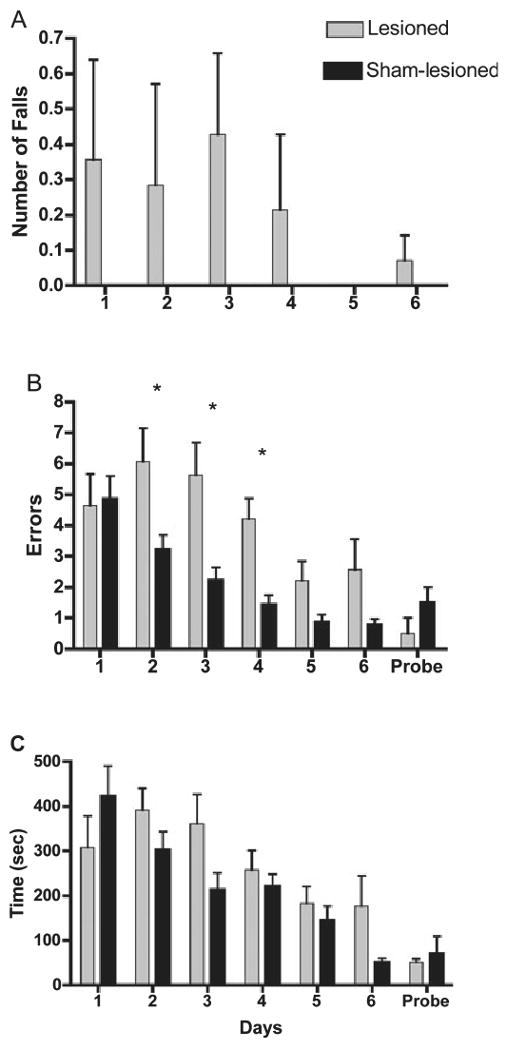
Performance of cerebellar-lesioned and sham-lesioned control birds on motor and cognitive components of a spatial working memory task (mean ± SEM). (A) Falls while traversing maze. (B) Errors into incorrect arms. (C) Time to reach the correct feeder. *Days on which lesioned birds differed from controls (Fisher's PLSD, Ps < 0.05). The probe trial involved removal of food from the goal cup and removal of parafilm from the non-goal cups to assess whether olfactory or visual cues differing between cups influenced performance (n = 2 for probe trail only).
Lesioned birds made more errors prior to finding the correct feeder compared with their sham-lesioned counterparts (Fig. 3B). Between-group repeated-measures anova identified significant effects of Treatment (F1,12 = 5.30, P = 0.04) and Day (F5,60 = 23.72, P = 0.0001) with a significant Day by Treatment interaction (F5,60 = 5.07, P = 0.0006). Fisher's PLSD post-hoc tests indicated significant differences between groups on Days 2, 3 and 4. The largest difference occurred on Day 4 when lesioned birds averaged 4.2 errors, whereas sham-lesioned birds averaged only 1.4 errors. Linear regression analysis showed that both groups had a linear reduction in the number of errors made over the 6 days of testing (lesioned group, F1,5 = 12.53, P = 0.001; sham-lesioned group, F1,5 = 52.58, P = 0.001). Paired t-tests comparing Days 1 and 6 confirmed that both groups had significantly fewer errors on the final day of testing than on the first day of testing (lesioned group, t6 = 3.33, P = 0.02; sham-lesioned group, t6 = 5.54, P = 0.02; Fig. 3B).
There was no significant difference in time to reach the correct feeder between treatment groups (F1,12 = 1.49, P = 0.246, Fig. 3C). However, there was a significant effect of Day (F5,60 = 11.60, P = 0.0001) and an interaction (F5,60 = 2.56, P = 0.04). Although both lesioned and sham-lesioned birds had a linear decrease in times across days as shown by linear regression analysis (lesioned birds, F1,5 = 8.46, P = 0.006; sham-lesioned birds, F1,5 = 49.54, P = 0.0001), sham-lesioned birds (t6 = 5.74, P = 0.01) showed significant improvements in time between the first and last day of training, whereas lesioned birds did not (t6 = 1.29, P = 0.25).
To determine whether the time that it took lesioned birds to reach the cup was influenced by impaired balance, we compared the time to reach the cup between birds that fell (n = 3) and birds that did not fall (n = 4) (F1,5 = 0.92, P = 0.81). These results suggest that falls had little impact on the overall time to reach the feeder. In addition, these results suggest that time to the correct feeder may directly reflect cognitive abilities rather than both motor and cognitive abilities as originally thought. However, we cannot discount the possibility that some motor deficit, less obvious than falls, slowed lesioned birds' time to the correct feeder.
Although a small sample size precluded statistical analyses of the probe trial, the mean errors and time to reach the previously correct cup were similar between the last day of training (Day 6) and the probe trial for both groups, suggesting that olfactory or visual cues were not used to find the goal during training (Fig. 3B and C). The lesioned group appeared to improve slightly on the probe trial just as one might expect for an added day of testing. The sham-lesioned group increased errors and time slightly but, with only two randomly selected birds used, not much can be made of this result.
Experiment 2
As predicted, replacement of estrogen by implantation improved the performance of lesioned birds given the aromatase blocker Fadrozole. However, estrogen appeared to mainly improve cognitive function without increasing recovery of motor function.
Overall, lesioned birds treated with Fadrozole/E2 were faster to reach the correct feeder than birds treated with Fadrozole only (F1,10 = 13.29, P = 0.005, Fig. 4C). The treatment groups differed specifically on Days 1 and 5 (Ps < 0.03, Fisher's PLSD post-hoc). There was no Day effect (F5,50 = 1.56, P = 0.189) or Treatment interaction effect (F5,50 = 0.58, P = 0.72). However, linear regression analysis showed that there was a trend for improved performance by birds treated with Fadrozole/E2 (F1,5 = 3.66, P = 0.06), whereas birds treated with Fadrozole alone showed no linear improvement (F1,5 = 0.77, P = 0.39).
Fig. 4.
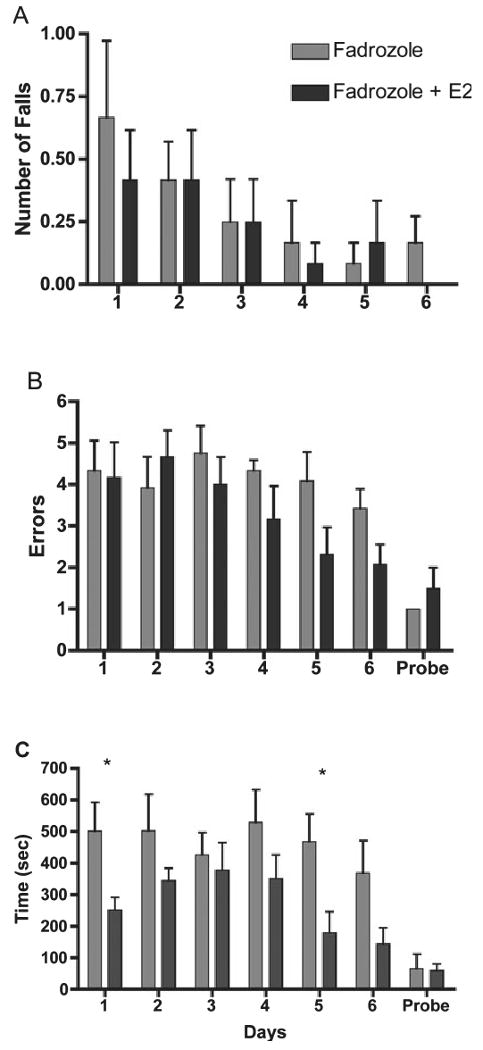
Performance of cerebellar-lesioned birds treated with either Fadrozole alone or Fadrozole plus E2 on motor and cognitive components of a spatial working memory task (mean ± SEM). (A) Falls while traversing maze. (B) Errors into incorrect arms. (C) Time to reach the correct feeder. *Days on which Fadrozole birds differed from Fadrozole + E2-treated birds (Fisher's PLSD, Ps < 0.05). The probe trial involved removal of food from the goal cup and removal of parafilm from the non-goal cups to assess whether olfactory or visual cues differing between cups influenced performance (n = 2 for probe trial only).
All lesioned birds had some reduction in errors to find the correct feeder (Fig. 4B) across days (F5,50 = 2.96, P = 0.02). There was no significant effect of Treatment (F1,10 = 1.54, P = 0.24) and no Treatment by Day interaction (F5,50 = 1.37, P = 0.25). However, linear regression analysis showed that the Fadrozole/E2 group had a significant linear improvement with an overall decrease in the number of errors per day (F1,5 = 11.859, P = 0.0015), whereas the group with Fadrozole alone did not (F1,5 = 0.25, P = 0.62).
As was seen in experiment 1, bilateral lesions produced motor deficits in some birds indicated by their number of falls during the daily testing period (Fig. 4A). However, there was no difference in the number of falls between birds treated with Fadrozole only and those treated with Fadrozole/E2 (F1,10 = 0.87, P = 0.93).
Similar to experiment 1, the mean errors and time to reach the previously correct cup were similar between the last day of training (Day 6) and the probe trial for both groups, suggesting that spatial cues rather than olfactory or visual cues emanating from the goal were used to find the goal during training.
Experiments 3–5: aromatase immunocytochemistry
Following injury to the folia, aromatase-positive cells were seen in the granule cell layer, white matter layer and molecular layer (Fig. 5). No aromatase-immunoreactive cells were seen in these layers in the absence of a lesion. A few Purkinje cells (as identified by double-labeling for aromatase and GAD67; not shown) were stained for aromatase in folia with or without lesions. Interestingly, the upregulation of aromatase immunoreactivity is usually restricted to neural tissue adjacent to a lesion (Peterson et al., 2001, 2004) as was seen in the molecular layer (Fig. 5A). However, when any part of the granule cell layer or white matter layer was injured, aromatase-positive cells were seen spread laterally throughout the entirety of both layers (Fig. 5A).
Fig. 5.
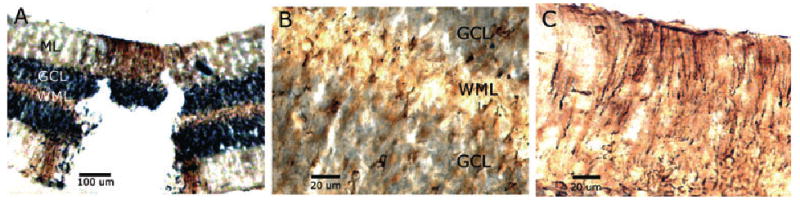
Coronal sections of the cerebellum of an adult female zebra finch showing aromatase immunoreactivity (brown staining) surrounding a cerebellar lesion. (A) Aromatase immunostaining extends laterally from the lesion in the white matter layer (WML) and granule cell layer (GCL) but in the molecular layer (ML) staining occurs only adjacent to the lesion site. (B) Both the GCL and WML show aromatase-positive cells. (C) Aromatase-positive cells in the ML appear to be Bergman glia based on morphology. The GCL stains black non-specifically with this 3′3-diaminobenzidine tetrahydrochloride treatment.
As has been shown previously, the majority of cells in which aromatase was upregulated by injury had the morphology of astrocytes (Fig. 5B). The double-label immunocytochemistry for aromatase and vimentin supports our morphological assessment. Many cells stained positive for both aromatase and vimentin, an intermediate filament of immature and reactive astrocytes (Garcia-Segura et al., 1999b; Fig. 6A–C). A morphologically distinct cell type was seen to express aromatase near the injury in the molecular layer (Fig. 5C). Based on position and morphology, these elongated cells appear to be Bergmann glia. This was also suggested by double-label immunocytochemistry for aromatase and vimentin (Fig. 6D–F) because vimentin is an intermediate filament of radial glia.
Fig. 6.
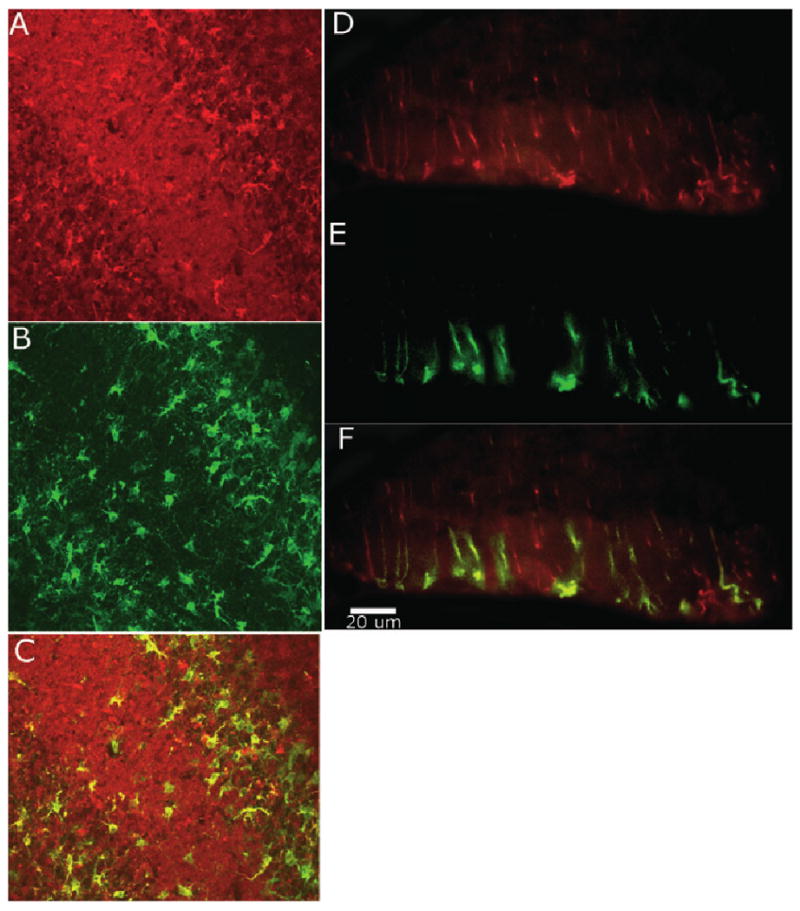
Coronal section of injured cerebellum stained for vimentin (Texas red) and aromatase (fluorescein) at 20× (A–C) and 40× (D–F). (A) Vimentin-stained reactive astrocytes in the molecular and white matter layer. (B) Aromatase-stained cells in the molecular and white matter layer. (C) Confocal image identifies considerable overlap between aromatase and vimentin indicating reactive astrocyte aromatase expression. (D) Vimentin-stained Bergmann glia cells in the molecular layer. (E) Aromatase-positive cells in the molecular layer. (F) Overlay of D and E shows Bergmann glia co-expressing vimentin and aromatase.
Discussion
Motor and cognitive function
Little is known about the role of the cerebellum in songbird behavior or the effects of steroids and injury on songbird cerebellar function. Consequently our research on the songbird cerebellum was guided by several objectives. Although it is well known that the cerebellum plays a prominent role in vertebrate motor control (Molinari et al., 2007), there is evidence implicating the cerebellum in cognitive performance (Gandhi et al., 2000; Rodriguez et al., 2005), in particular spatial memory (Willson et al., 2007).
Our results clearly show that destruction of several folia and portions of deep medial cerebellar nuclei had an impact on the motor and cognitive function of the zebra finch. Following cerebellar lesioning, some birds fell during behavioral testing. As expected, sham-lesioned birds never fell during these same trials. Clearly, the songbird cerebellum participates in normal movement in songbirds as previously found in other avian species (Necker & Neumann, 1997). Our experiments also provide evidence for a role of the cerebellum in cognition associated with a spatial working memory task. Cerebellar-lesioned birds made more errors than sham-lesioned birds and did not show decreased time to locate the correct cup between the first and last day of testing, whereas sham-lesioned birds did. However, both groups showed a general linear decline in errors and time to reach the correct cup over days. The probe trial suggests that, in locating the goal, birds were not relying on local olfactory or visual cues from the seed-filled cup. Therefore, songbirds with cerebellar lesions can learn a spatial task using distal cues to guide the search but improve more slowly and less consistently than sham-lesioned birds. These results provide support for the conclusion that the avian cerebellum is involved in spatial working memory in addition to its role in motor function consistent with previous studies showing cerebellar involvement in spatial memory in goldfish and rats (Gandhi et al., 2000; Rodriguez et al., 2005).
We wondered whether differences between groups could be attributed to errors in spatial reference memory vs. working memory as there has been some debate about the role of the cerebellum in working memory (Caston et al., 1997; Nixon & Passingham, 1999; Mandolesi et al., 2001). We attempted to segregate these error types by independently analysing the number of initial errors (arms looked into prior to entering the correct arm) and repeat errors into these same incorrect arms during the same trial. When these effects are analysed independently, there are no significant treatment, day or interaction effects for either the repeated or non-repeated errors (data not shown). However, the combined errors (non-repeated and repeated) taken together do show a treatment effect as shown in results. These results suggest that birds used both reference and working memory to complete the task and that lesioned birds showed deficits in both. Alternatively, these measures may not accurately segregate these two types of learning in our task. We suspect that there is some validity to both of these interpretations. The behavior of zebra finches in our task suggests that repeat errors may not accurately reflect poor working memory. Rather they may be related to persistent efforts to gain access to seed cups. Birds frequently pecked at the parafilm covers on seed cups when they reached the end of an incorrect arm. Repeat errors could also reflect an inability to recognize the spatial cues indicating that the arm entered was a previously entered arm. In this case repeat errors would be spatial reference errors. Given these possible confounds and our behavioral observations during repeat entries, we did not attempt to analyse this variable in experiment 2 and do not discuss it further.
Rate of recovery
The ability of the brain to undergo repair post-injury remains a widely researched topic. Our data clearly show that bilateral cerebellar-lesioned birds are able to recover both cognitive and motor function. Of the birds that fell in the first few days after a lesion, there was no evidence for deficits in motor coordination or postural control at the end of behavioral testing at 8 days post-lesion. Although we do not yet know the basis for this recovery, motor deficits of balance and postural sway and their gradual recovery are classic responses to cerebellar injury (Konczak et al., 2005; Schalow, 2006; Williams et al.,2006; Willson et al., 2007) and we now have an avian model to explore the mechanisms involved.
Birds also appeared to recover from deficits in cognitive function created by a cerebellar lesion. Rather than showing no improvement over days, lesioned birds made fewer errors at the end of behavioral testing than at the beginning. It is difficult to be certain if improvements by lesioned birds in these cognitive aspects indicate recovery of cognition or merely demonstrate that deficits were not severe enough to prevent gradual learning over days. We plan future experiments to examine the recovery of cognitive function more specifically by using a task that requires learning novel elements daily. Comparisons of the two lesioned groups in experiment 2 suggest that estrogen aids in recovery of function and the aromatase immunocytochemistry suggests a cellular mechanism for recovery.
Aromatase immunocytochemistry
Aromatase activity is upregulated in the zebra finch cerebellum after injury (this experiment and Peterson et al., 2007) and our present results show that this upregulation involves cerebellar glial cells adjacent to the injury site, similar to what is seen in other avian brain regions (Peterson et al., 2001, 2004). Given the possible neuroprotective effects of estrogen, we assume that the upregulation of aromatase provides estrogens that may participate in cerebellar repair (Azcoitia et al., 2001, 2003; Sierra et al., 2003; Lavaque et al., 2006), possibly associated with the recovery of behavioral function that we have observed.
Of particular interest is the upregulation of aromatase in Bergman glia. During mammalian development Bergmann glia provide a tract along which Purkinje neurons and granule cells migrate (Kawamura et al., 1988). In adults, they can continue to guide new neurons. Granule and Purkinje neurons grafted into the cerebellum in adults migrate along Bergman glia into their appropriate positions (Kawamura et al., 1988). It is thought that Bergmann glia express estrogen receptor α and neurotrophin receptor to influence adult cell migration (Kawamura et al., 1988). Because they express aromatase along the full length of their fibers near the site of injury, estrogens produced by these cells may assist with neural survival, differentiation or migration. Aromatase is also expressed in a subset of Purkinje cells but unrelated to the lesion. E2 may normally act on estrogen receptor β on Purkinje neurons to play a role in motor learning (Andreescu et al., 2007). It is unknown why only some Purkinje cells express aromatase.
Fadrozole and Fadrozole/E2
Estradiol has a wide range of effects on neuroplasticity and can enhance recovery of function (Wise, 2002, 2003). Interestingly, we saw improvements in cognitive recovery without improvements in motor recovery in the Fadrozole/E2 group compared with the Fadrozole-treated group.
Cerebellar-lesioned birds with excess estrogen showed just as little recovery of loss of balance across days as birds with depleted estrogen. However, birds treated with estrogen showed improved cognitive recovery. Although errors did not differ between groups, linear regression analysis showed that E2-treated birds reduced the number of errors over the 6 days of testing, whereas the Fadrozole-alone group did not. This suggests some improved recovery of this cognitive element in the E2-treated birds compared with the estrogen-depleted group. In addition, estrogen-treated birds found the correct feeder more quickly than estrogen-depleted birds. Given that the number of falls was similar between groups and that birds that fall generally take no less time to reach the feeder than birds that do not fall (shown in experiment 1), the decrease in time to locate the correct feeder across days probably reflects improved recovery of cognitive capabilities in the estrogen group compared with the estrogen-depleted group after injury. Time to reach the correct cup was superior in the E2-treated group even on the first trial. Many factors could contribute to this result. These trials span several minutes. On the first trial birds have to learn that, unlike pre-training, only one cup is now accessible and start to acquire spatial knowledge of the goal location. Birds that are faster learners may decrease latency on this first trial by reducing the time spent trying to gain access to covered cups and moving on to a new cup as well as by reducing re-entries into incorrect arms. The number of errors can contribute to overall time. Although errors did not differ significantly between groups, even the slight numerical decrease in errors observed in the estrogen-treated group on Day 1 could have saved them time (Fig. 4B). It is also possible that the numerically higher number of falls in the estrogen-depleted group compared with the estrogen-treated group may have slowed the estrogen-treated group's time to reach the cup on Day 1. It is important to remember that falls did not generally contribute to the time to reach the cup, did not differ between groups and did not show a pattern suggesting improved motor recovery in the estrogen-treated group. However, we cannot rule out a slight influence of falls on differences between groups in time to reach the correct cup on Day 1 only.
Regardless of the specific behaviors that contribute to Day 1 differences, it should not be surprising to see such behavioral differences in these two groups on the first day of testing, as was seen with time to reach the correct cup. The estrogen-treated birds had estrogen treatment for 2 days prior to starting behavioral testing. Thus, differences between groups at the onset of testing, whether purely cognitive or cognitive and some motor, could be due to the neuroprotective and neurogenerative effects of estrogen on the cognitive aspects of cerebellar function.
It is unclear why E2 would improve cognitive recovery of function but not motor recovery of function over days. One possibility is that the improvements seen in motor function across days involve different neural pathways than those used for improvements in cognitive function. We suspect that improvements in gross motor function, which are typical following cerebellar lesions in animals (Konczak et al., 2005; Schalow, 2006; Williams et al.,2006; Willson et al., 2007), may occur via the taking over of functions by pathways outside the lesioned area, whereas cognitive improvements may require repair at the lesion site via estrogen-dependent pathways. Many more experiments will be required to support this view, suggested by the cognitive recovery accompanied by persistent motor deficits in the Fadrozole/E2 group compared with the Fadrozole-alone group.
In particular, we need to perform experiments that would show that inhibition of aromatase directly affects cells in the cerebellum. Given that we did not include a group in experiment 2 that had no Fadrozole, we cannot say for certain how Fadrozole affected the performance of lesioned birds compared with lesion alone. A comparison of experiments 1 (Fig. 3) and 2 (Fig. 4) suggests that Fadrozole impairs performance on time to reach the correct feeder and may reduce the extent of improvement in errors over trials. We have recently completed experiments using a different spatial maze that directly compared cerebellar-lesioned birds given another aromatase inhibitor, Letrozole, with cerebellar-lesioned birds not treated with Letrozole. These results support the view that aromatase inhibitors increase cognitive impairments in cerebellar-lesioned birds (L.B. Day, J. Hamer and G. Stinson, unpublished results). The experiments presented in this study, the first to show that E2 might improve cognitive deficits after cerebellar lesions in birds, are necessarily only a first step in determining how estrogen influences recovery of function after cerebellar lesion in birds.
Conclusion
Our data offer insights into the role of the cerebellum in motor and cognitive performance in a songbird. There is no doubt that lesions of the cerebellum produce profound motor deficits and it appears that the same lesions induce deficits in performance on a spatial memory task. There is also clear evidence that exogenous estrogens improve recovery of cognitive elements of this task. It is likely that some estrogens are produced naturally within the cerebellum following neural injury and these estrogens may also assist with the repair and recovery. Overall, more work is needed to understand what role the cerebellum plays in cognition. Although the size and shape of the cerebellum differs greatly among vertebrates, the circuitry is highly conserved between birds and mammals (Paula-Barbosa & Sobrinho-Simões, 1976; Tohyama, 1976; Paula-Barbosa et al., 1980; Smith et al., 1993). Therefore, the songbird may be an excellent model to explore a role for the cerebellum in cognition and a role for estrogens in recovery of cerebellar function.
Acknowledgments
Thanks to Niranjala Tillakaratne for supplying antibodies to GAD67. Supported by MH061994 and funds from the University of Mississippi Biology Department and Office of Sponsored Research Projects.
Abbreviations
- E2
estradiol
- GAD
glutamic acid decarboxylase
- PB
phosphate buffer
- PBT
Triton X-100 in phosphate buffer
References
- Adkins-Regan E, Abdelnabi M, Mobarak M, Ottinger MA. Sex steroid levels in developing and adult male and female zebra finches (Poephila guttata) Gen Comp Endocrinol. 1990;78:93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- Andreescu CE, Milojkovic BA, Haasdijk ED, Kramer P, De Jong FH, Krust A, De Zeeuw CI, De Jeu MT. Estradiol improves cerebellar memory formation by activating estrogen receptor. J Neurosci. 2007;27:10832–10839. doi: 10.1523/JNEUROSCI.2588-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM. Brain aromatase is neuroprotective. J Neurobiol. 2001;47:318–329. doi: 10.1002/neu.1038. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. Aromatase expression by reactive astroglia is neuroprotective. Steroid Nerv Syst. 2003;1007:298–305. doi: 10.1196/annals.1286.028. [DOI] [PubMed] [Google Scholar]
- Baulieu EE, Robel P. Neurosteroids – a new brain-function. J Steroid Biochem Mol Biol. 1990;37:395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Bentley GE, Balthazart J, Turek FW, Ball GF. Androgen receptor, estrogen receptor alpha, and estrogen receptor beta show distinct patterns of expression in forebrain song control nuclei of European starlings. Endocrinology. 1999;140:4633–4643. doi: 10.1210/endo.140.10.7024. [DOI] [PubMed] [Google Scholar]
- Caston J, Vasseur F, Delhaye-Bouchaud N, Mariani J. Delayed spontaneous alternation in intact and cerebellectomized control and lurcher mutant mice: differential role of cerebellar cortex and deep cerebellar nuclei. Behav Neurosci. 1997;11:214–218. doi: 10.1037//0735-7044.111.1.214. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;6699:272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Gandhi CC, Kelly RM, Wiley RG, Walsh TJ. Impaired acquisition of a Morris water maze task following selective destruction of cerebellar purkinje cells with OX7-saporin. Behav Brain Res. 2000;109:37–47. doi: 10.1016/s0166-4328(99)00160-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Naftolin F, Hutchison JB, Azcoitia I, Chowen JA. Role of astroglia in estrogen regulation of synaptic plasticity and brain repair. J Neurobiol. 1999a;40:574–584. [PubMed] [Google Scholar]
- Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: Implications for local estrogen formation in brain repair. Neuroscience. 1999b;89:567–578. doi: 10.1016/s0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Azcoitia I. Aromatase: a neuroprotective enzyme. Prog Neurobiol. 2003;71:31–41. doi: 10.1016/j.pneurobio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Gaytán-Tocavén L, Olvera-Cortés ME. Bilateral lesion of the cerebellar-dentate nucleus impairs egocentric sequential learning but not egocentric navigation in the rat. Neurobiol Learn Mem. 2004;2:120–127. doi: 10.1016/j.nlm.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Ghidoni R, Boccardi M, Benussi L, Testa C, Villa A, Pievani M, Gigola L, Sabattoli F, Barbiero L, Frisoni GB, Binetti G. Effects of estrogens on cognition and brain morphology: involvement of the cerebellum. Maturitas. 2006;54:222–228. doi: 10.1016/j.maturitas.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Gordon N. The cerebellum and cognition. Eur J Paediatr Neurol. 2007;4:232–234. doi: 10.1016/j.ejpn.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Henderson VW. Cognition and cognitive aging. Climacteric. 2007;10:88–91. doi: 10.1080/13697130701537363. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Nanami T, Kikuchi Y, Kitakami A. Grafted granule and Purkinje-cells can migrate into the mature cerebellum of normal adult-rats. Exp Brain Res. 1988;70:477–484. doi: 10.1007/BF00247596. [DOI] [PubMed] [Google Scholar]
- Konczak J, Schoch B, Dimitrova A, Gizewski E, Timmann D. Functional recovery of children and adolescents after cerebellar tumour resection. Brain. 2005;128:1428–1441. doi: 10.1093/brain/awh385. [DOI] [PubMed] [Google Scholar]
- Larsell O. The Comparative Anatomy and Histology of the Cerebellum from Myxinoids through Birds. University of Minnesota Press; Minneapolis, MN: 1967. [Google Scholar]
- Lavaque E, Mayen A, Azcoitia I, Tena-Sempere M, Garcia-Segura LM. Sex differences, developmental changes, response to injury and cAMP regulation of the mRNA levels of steroidogenic acute regulatory protein, cytochrome P450scc, and aromatase in the olivocerebellar system. J Neurobiol. 2006;66:308–318. doi: 10.1002/neu.20221. [DOI] [PubMed] [Google Scholar]
- Lee DW, Fernando G, Peterson RS, Allen TA, Schlinger BA. Estrogen mediation of injury-induced cell birth in neuroproliferative regions of the adult zebra finch brain. Dev Neurobiol. 2007;11:1546–1557. doi: 10.1002/dneu.20399. [DOI] [PubMed] [Google Scholar]
- Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123:1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- London SE, Boulter J, Schlinger BA. Cloning of the zebra finch androgen synthetic enzyme CYP17: a study of its neural expression throughout posthatch development. J Comp Neurol. 2003;467:496–508. doi: 10.1002/cne.10936. [DOI] [PubMed] [Google Scholar]
- London SE, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology. 2006;147:5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandolesi L, Leggio MG, Graziano A, Neri P, Petrosini L. Cerebellar contribution to spatial event processing: involvement in procedural and working memory components. Eur J Neurosci. 2001;14:2011–2022. doi: 10.1046/j.0953-816x.2001.01819.x. [DOI] [PubMed] [Google Scholar]
- Molinari M, Leggio MG, Thaut MH. The cerebellum and neural networks for rhythmic sensorimotor synchronization in the human brain. Cerebellum. 2007;6:18–23. doi: 10.1080/14734220601142886. [DOI] [PubMed] [Google Scholar]
- Necker R, Neumann V. Response characteristics of cerebellar nuclear cells in the pigeon. NeuroReport. 1997;8:1485–1489. doi: 10.1097/00001756-199704140-00032. [DOI] [PubMed] [Google Scholar]
- Nixdorf-Bergweiler B, Bischof HJ. A Stereotaxic Atlas of the Brain of the Zebra Finch Taeniopygia guttata with Special Emphasis on Telencephalic Visual and Song System Nuclei in Tansverse and Sagittal Sections. National Library of Medicine, NCBI Bookshelf; Bethesda: 2007. [Google Scholar]
- Nixon PD, Passingham RE. The cerebellum and cognition: cerebellar lesions do not impair spatial working memory or visual associative learning in monkeys. Eur J Neurosci. 1999;11:4070–4080. doi: 10.1046/j.1460-9568.1999.00825.x. [DOI] [PubMed] [Google Scholar]
- Patel SN, Clayton NS, Krebs JR. Hippocampal tissue transplants reverse lesion-induced spatial memory deficits in zebra finches (Taeniopygia gutta) J Neurosci. 1997;17:3861–3869. doi: 10.1523/JNEUROSCI.17-10-03861.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula-Barbosa MM, Sobrinho-Simões MA. An ultrastructural morphometric study of mossy fiber endings in pigeon, rat and man. J Comp Neurol. 1976;170:365–379. doi: 10.1002/cne.901700307. [DOI] [PubMed] [Google Scholar]
- Paula-Barbosa MM, Sobrinho-Simões MA, Ruela C. Comparative morphometric study of cerebellar neurons. I. Granule cells. Acta Anat (Basel) 1980;106:262–269. doi: 10.1159/000145189. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Saldanha CJ, Schlinger BA. Rapid upregulation of aromatase mRNA and protein following neural injury in the zebra finch (Taeniopygia guttata) J Neuroendocrinol. 2001;13:317–323. doi: 10.1046/j.1365-2826.2001.00647.x. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Lee DW, Fernando G, Schlinger BA. Radial glia express aromatase in the injured zebra finch brain. J Comp Neurol. 2004;475:261–269. doi: 10.1002/cne.20157. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Fernando G, Day LB, Allen TA, Chapleau JD, Menjivar J, Schlinger BA, Lee DW. Aromatase expression and cell proliferation following injury of the adult zebra finch hippocampus. Dev Neurobiol. 2007;67:1867–1878. doi: 10.1002/dneu.20548. [DOI] [PubMed] [Google Scholar]
- Price RH, Jr, Handa RJ. Expression of estrogen receptor-beta protein and mRNA in the cerebellum of the rat. Neurosci Lett. 2000;288:115–118. doi: 10.1016/s0304-3940(00)01221-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez F, Duran E, Gomez A, Ocana FM, Alvarez E, Jimenez-Moya F, Broglio C, Salas C. Cognitive and emotional functions of the teleost fish cerebellum. Brain Res Bull. 2005;66:365–370. doi: 10.1016/j.brainresbull.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Mezaki Y, Shikimi H, Ukena K, Tsutsui K. Dendritic growth and spine formation in response to estrogen in the developing Purkinje cell. Endocrinology. 2003;144:4466–4477. doi: 10.1210/en.2003-0307. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Schalow G. Surface EMG- and coordination dynamics measurements-assisted cerebellar diagnosis in a patient with cerebellar injury. Electromyogr Clin Neurophysiol. 2006;46:371–384. [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Sierra A, Azcoitia I, Garcia-Segura LM. Endogenous estrogen formation is neuroprotective in model of cerebellar ataxia. Endocriniology. 2003;21:43–51. doi: 10.1385/endo:21:1:43. [DOI] [PubMed] [Google Scholar]
- Smith TG, Brauer K, Reichenbach A. Quantitative phylogenetic constancy of cerebellar Purkinje cell morphological complexity. J Comp Neurol. 1993;331:402–406. doi: 10.1002/cne.903310309. [DOI] [PubMed] [Google Scholar]
- Tohyama M. Comparative anatomy of cerebellar catecholamine innervation from teleosts to mammals. J für Hirnforsch. 1976;17:43–60. [PubMed] [Google Scholar]
- Tsutsui K. Biosynthesis, mode of action and functional significance of neurosteroids in the developing Purkinje cell. J Steroid Biochem Mol Biol. 2006;102:187–194. doi: 10.1016/j.jsbmb.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Bischof HJ. Effects of hippocampal lesions on acquisition and retention of spatial learning in zebra finches. Behav Brain Res. 2004;155:147–152. doi: 10.1016/j.bbr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Williams H, Cynx J, Nottebohm F. Timbre control in zebra finch (Taeniopygia guttata) song syllables. J Comp Psychol. 1989;103:366–380. doi: 10.1037/0735-7036.103.4.366. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Ling GSF, Tortella FC. Severity level and injury track determine outcome following a penetrating ballistic-like brain injury in the rat. Neurosci Lett. 2006;408:183–188. doi: 10.1016/j.neulet.2006.08.086. [DOI] [PubMed] [Google Scholar]
- Willson ML, Bower AJ, Sherrard RM. Developmental neural plasticity and its cognitive benefits: olivocerebellar reinnervation compensates for spatial function in the cerebellum. Eur J Neurosci. 2007;25:1475–1483. doi: 10.1111/j.1460-9568.2007.05410.x. [DOI] [PubMed] [Google Scholar]
- Wise PM. Estrogens and neuroprotection. Trends Endocrinol Metab. 2002;13:229–230. doi: 10.1016/s1043-2760(02)00611-2. [DOI] [PubMed] [Google Scholar]
- Wise PM. Estradiol exerts neuroprotective actions against ischemic brain injury: insights derived from animal models. Endocrinology. 2003;21:11–15. doi: 10.1385/endo:21:1:11. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Funabiki K, Hirano T. Increased occurrence of climbing fiber inputs to the cerebellar flocculus in a mutant mouse is correlated with the timing delay of optokinetic response. Eur J Neurosci. 2007;25:1467–1474. doi: 10.1111/j.1460-9568.2007.05394.x. [DOI] [PubMed] [Google Scholar]


