Abstract
Background
The ε4 allele of the apolipoprotein E gene (APOE) is the chief known genetic risk factor for Alzheimer’s disease, the most common cause of dementia late in life. To determine the relation between brain responses to tasks requiring memory and the genetic risk of Alzheimer’s disease, we performed APOE genotyping and functional magnetic resonance imaging (MRI) of the brain in older persons with intact cognition.
Methods
We studied 30 subjects (age, 47 to 82 years) who were neurologically normal, of whom 16 were carriers of the APOE ε4 allele and 14 were homozygous for the APOE ε3 allele. The mean age and level of education were similar in the two groups. Patterns of brain activation during functional MRI scanning were determined while subjects memorized and recalled unrelated pairs of words and while subjects rested between such periods. Memory was reassessed in 14 subjects two years later.
Results
Both the magnitude and the extent of brain activation during memory-activation tasks in regions affected by Alzheimer’s disease, including the left hippocampal, parietal, and prefrontal regions, were greater among the carriers of the APOE ε4 allele than among the carriers of the APOE ε3 allele. During periods of recall, the carriers of the APOE ε4 allele had a greater average increase in signal intensity in the hippocampal region (1.03 percent vs. 0.62 percent, P<0.001) and a greater mean (±SD) number of activated regions throughout the brain (15.9±6.2 vs. 9.4±5.5, P=0.005) than did carriers of the APOE ε3 allele. Longitudinal assessment after two years indicated that the degree of base-line brain activation correlated with degree of decline in memory.
Conclusions
Patterns of brain activation during tasks requiring memory differ depending on the genetic risk of Alzheimer’s disease and may predict a subsequent decline in memory.
Alzheimer’s disease is the most common cause of dementia late in life, affecting approximately 8 percent of people who are 65 years of age or older.1 Clinically diagnosed Alzheimer’s disease is preceded by gradual, progressive memory loss. Neuritic plaques2 and neurofibrillary tangles,3 the neuropathological hallmarks of Alzheimer’s disease, have also been found in adults without dementia, suggesting that the neuronal deficits leading to Alzheimer’s disease begin years before any clinical changes occur. New and potential treatments for dementia focus on slowing the progression of the disease rather than regenerating neural cells, making it important to identify at an early stage markers of future cognitive decline.
Genetic studies have identified an association between the presence of the ε4 allele of the apolipoprotein E (APOE) gene on chromosome 19 and the common form of Alzheimer’s disease, which begins after the age of 60 years.4 APOE has three allelic variants (APOE ε2, APOE ε3, and APOE ε4) and five common genotypes (ε2/ε3, ε3/ε3, ε2/ε4, ε3/ε4, and ε4/ε4). The APOE ε4 allele has a dose-related effect on risk and the age at onset of late-onset familial Alzheimer’s disease and sporadic cases of the disease,4,5 whereas the APOE ε2 allele appears to confer protection against the disease.6 Although the presence of the APOE ε4 allele may be associated with cognitive decline in older persons, the APOE genotype alone is not considered useful in predicting whether the disease will develop in people without dementia.7
Structural magnetic resonance imaging (MRI) in older persons with normal cognition may show medial temporal atrophy and thus indicate the possibility of future cognitive decline8; cerebral atrophy, however, is seen only after a substantial proportion of neural cells have died. Positron-emission tomographic (PET) studies obtained during mental rest have identified parietal, temporal, and prefrontal deficits in glucose metabolism in middle-aged persons who have normal cognition and the APOE ε4 allele,9,10 in whom Alzheimer’s disease is not likely to develop for decades.
Activation imaging, which compares the level of brain activity while subjects perform a task with the level of activity in a control, or resting, state, may reveal more subtle alterations in brain function, perhaps before the emergence of mild memory impairment. Several activation PET studies that have used cognitive stimuli have revealed a greater extent and magnitude of brain activity among patients with Alzheimer’s disease than among age-matched subjects with normal cognition.11–13 Like PET, functional MRI provides measures of signal intensity associated with relative cerebral blood flow during tasks requiring memory or other types of cognitive skills,14,15 but it has the advantages of producing more detailed pictures in less time and does not involve exposure to radiation. The signal intensity associated with a particular task in comparison with that associated with the control condition reflects relative blood flow and, consequently, neural activity, though indirectly.16–18
Previous activation PET and functional MRI studies revealed that the degree of neural activity increases with the demands of the cognitive task,19 so that a greater cognitive effort or a more difficult task increases the magnitude and the spatial extent of brain activation.19–21 These observations led us to hypothesize that a challenging task requiring memory would result in increased MRI signal intensity in presymptomatic subjects at genetic risk for Alzheimer’s disease.
METHODS
Study Subjects
From December 1996 to May 1999, we performed base-line studies in 30 subjects who were neurologically normal and for whom technically adequate MRI scans of the brain were available. These subjects were selected initially from a pool of 267 potential subjects (age, 40 to 85 years) recruited through advertisements. From this pool of subjects, we excluded left-handed subjects and anyone who took drugs that could influence cognition, those who had dementia, and those who had other medical, psychiatric, or neurologic conditions, including cerebrovascular disease or hypertension. The remaining 37 subjects underwent MRI scanning, and 5 with technically inadequate scans were excluded. The remaining 32 subjects underwent genotyping for APOE, according to previously described methods.4,5 We excluded two subjects with the APOE ε2 allele. Among the remaining 30 subjects, 16 had the APOE ε4 allele — 14 were heterozygous (ε3/ε4) and 2 were homozygous (ε4/ε4) — and 14 were homozygous for the APOE ε3 allele (ε3/ε3). The subjects had above-average intelligence, and had scores on tests assessing memory that were normal for their age. Memory was assessed with three standardized tests: the Consistent Long-Term Retrieval section of the Buschke–Fuld Selective Reminding test,22 in which subjects are asked to learn and then recite a list of 16 unrelated words over a total of 10 trials; the Logical Memory portion of the Wechsler Memory Scale,23 in which subjects hear and are then asked to recall two short stories immediately after hearing them and after a 20-minute delay; and the Benton Visual Retention examination,24 in which subjects are shown various designs and then asked to reproduce them from memory. Fourteen subjects underwent memory assessments again two years later. The study was approved by the UCLA human-subjects protection committee, and all subjects gave written informed consent.
Imaging Procedures
We performed MRI with a 3-T unit (General Electric, Waukesha, Wis.) with echo–planar imaging capability (Advanced NMR Systems, Wilmington, Mass.). Functional MRI scanning was conducted with a gradient echo, echo–planar acquisition sequence in which the repetition time was 2.5 msec, the echo time was 45 msec, the flip angle was 80 degrees, the matrix image was 128 by 64, the field of view was 40 by 20 cm, and the in-plane resolution was 3 mm. Sixteen slices that were each 4 mm thick, with a 1-mm gap between slices, were obtained every 2.5 seconds for 9 minutes while the subjects performed the memory-activation tasks and during control periods. High-resolution spin–echo scans (matrix, 128 by 256; in-plane resolution, 1.5 mm; repetition time, 4000 msec; echo time, 54 msec; and number of excitations, 4) acquired in the same plane as the functional scans were used to normalize spatial relations and help pinpoint regions of interest for the analysis of data within subjects.
Memory-Activation Task
During functional MRI scanning, subjects performed a learning task involving unrelated pairs of words that is particularly sensitive for the identification of damage to the medial temporal lobe25 and that was chosen to engage memory systems maximally. In this test, subjects listen to seven unrelated pairs of words (e.g., up and foot or table and flower) for six separate periods, or “learning” blocks, each of which is followed by 30-second periods of rest, or “rest” blocks. Finally, during six periods of recall, or “recall” blocks, the subjects hear the first word in each pair and try to recall the second silently (to avoid head motion). An alternative form of this test was also administered two hours before scanning in which each learning period was followed by a cued-recall period.
Statistical Analysis
Because individual differences in the degree of cortical atrophy can distort efforts to normalize the results of MRI with respect to spatial relations,26 group-averaged statistics may yield spurious results in direct comparisons of groups. Therefore, we used two approaches to analyze the patterns of activation on functional MRI: group-averaged statistical parametric mapping analysis and an analysis of the regions of interest within subjects. The latter approach reduces the potential effects of cortical atrophy on the results.
Statistical Parametric Mapping Analysis
Parametric maps, in which statistically significant differences in activity between learning and recall periods and periods of rest were averaged for the APOE ε3 and APOE ε4 groups, were devised and placed in a system with common coordinates. Each subject’s T2-weighted echo–planar structural scan was fitted to the standard Talairach and Tournoux template27 with use of an 11-parameter rigid-body transformation. After correcting for head motion,28 we applied the transformation parameters to the coplanar functional images. Statistical analyses were performed with SPM’96 software (Wellcome Foundation, London). The functional images were smoothed to a full width of 6 mm at half-maximal resolution with use of a gaussian filter. We used the general linear model to analyze fixed effects within groups, specifying a six-second delayed-response function.29 We used proportional scaling to remove individual differences in the changes in global activity, and we assessed the differences in activity between three pairs of periods (learning vs. rest, recall vs. rest, and learning and recall combined vs. rest). This analysis included 10 carriers of the APOE ε4 allele and 11 carriers of the APOE ε3 allele; the remaining subjects, whose images were acquired with a smaller field of view, were excluded from this analysis to accommodate limitations in the memory of the hardware.
Because differences between groups in the MRI signal intensity may result from differences present during the rest (control) period rather than during the memory-activation tasks, we compared the signal intensity between groups during the memory-activation tasks and ignored values obtained during the rest periods. These analyses were also assessed for random effects with SPM’96 software. Images from all 30 subjects were averaged into one summary image representing the combined results obtained during the learning and recall periods. We adjusted for base-line differences in signal intensity by scaling the average signal-intensity values for each subject to the group average. Statistical images were generated with use of the SPM’96 PET group-analysis module and were not corrected for multiple comparisons.
Region-of-Interest Analysis
We assessed the relation between the performance on the memory-activation task and the MRI signal intensity for each subject by correlating the actual signal intensity in each voxel over time with the predicted increase in signal intensity during learning or recall periods and the decrease during rest periods, taking into account the slow rise and fall of the blood-flow response.30 We then used a cutoff value of 0.30 for Pearson’s r statistic (corresponding to a P value of less than 0.01) in six or more contiguous voxels to define activated regions. For each subject, we then used a template to locate all the activated regions.31 A region was defined as important if it contained any contiguous cluster of six or more voxels. The mean number of activated regions above the threshold value was calculated for learning and recall periods and for periods of rest, and the results were compared.
For the 14 subjects studied two years later, we correlated the number of activated regions in the brain with the extent of memory decline at follow-up using Spearman’s rank-order correlation coefficient. All statistical tests were two-tailed.
RESULTS
The demographic and clinical characteristics of the subjects in each group were similar, except that the carriers of the APOE ε4 allele had lower scores on the delayed-recall test than did carriers of the APOE ε3 allele (Table 1). The scores for both groups, however, fell within the normal range for this age group.
TABLE 1.
Demographic and Clinical Characteristics of the Study Groups.*
| Characteristic | Range of Possible Scores† |
APOE ε3 Carriers (N=14) |
APOE ε4 Carriers (N=16) |
|---|---|---|---|
| Female sex — no. (%) | 7 (50) | 9 (56) | |
| Family history of dementia — no. (%) |
8 (57) | 10 (62) | |
| Age — yr | 62±8 | 63±8 | |
| Years of education | 15±2 | 15±2 | |
| Benton Visual Retention test, total errors‡ |
0–30 | 3.5±2.6 | 4.3±2.4 |
| Wechsler Memory Scale, Logical Memory Delayed Recall portion§ |
0–50 | 21.6±7.1 | 16.7±6.4 |
| Buschke–Fuld Selective Remind- ing test, Consistent Long-Term Retrieval section¶ |
0–144 | 48.8±28.1 | 49.4±28.3 |
| Unrelated-word-pair learning task‖ |
0–42 | 25.2±11.9 | 25.3±10.6 |
Plus–minus values are means ±SD.
For standardized rating scales and memory tests, lower scores reflect poorer performance, except in the case of the Benton test, in which higher scores indicate greater impairment.
A score of 4±3 is considered normal for subjects who are 60 to 64 years old, and a score of 5±5 is considered normal for subjects 65 to 74 years old.
P=0.06 for the difference between groups. A score of 18.1±6.0 is considered normal in this age group (55 to 64 years).
A score of 60.2±32.4 for men and 71.4±36.8 for women is considered normal in this age group (55 to 70 years).
This test was performed before functional MRI scanning; seven pairs of unrelated words were presented six times to the subjects, with the degree of cued recall assessed after each presentation. The total score reflects the total number of correct responses during retrieval for the six periods.
In the statistical parametric mapping analysis, we found significant increases in the MRI signal intensity during learning or recall periods as compared with resting periods for all subjects, regardless of the APOE allele status (Fig. 1). Specifically, in both groups learning or recall resulted in increases in signal intensity in the left inferior frontal region (Broca’s area), the right prefrontal cortex, the transverse temporal gyri bilaterally, and the left posterior temporal and inferior parietal regions (Wernicke’s area). However, the extent and the intensity of activation in these regions were greater in the carriers of the APOE ε4 allele than in the carriers of the APOE ε3 allele, and there were significant increases in additional regions in these subjects (Fig. 1). The magnitude of the increase was greater in the left hemisphere. For example, in the parietal lobe, the signal intensity increased by 888 voxels in the left hemisphere, as compared with 44 in the right.
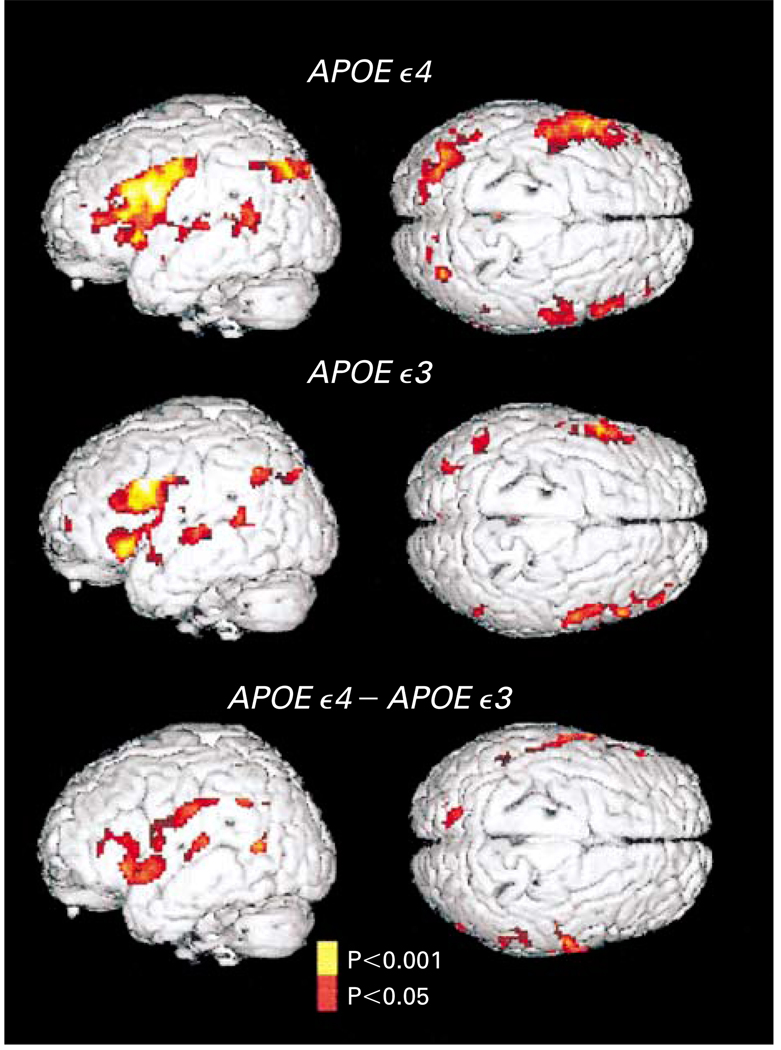
Figure 1. Statistical Parametric Maps of the Brain Used to Assess Subjects’ Performance on Memory-Activation Tests in Carriers of the APOE ε4 Allele and Carriers of the APOE ε3 Allele.
Three-dimensional renditions of the surface of the brain are shown in gray, and colored areas indicate regions of significantly increased MRI signal intensity during learning or recall periods as compared with resting periods. The signal intensity increased significantly in the left inferior frontal region, the right prefrontal cortex, the transverse temporal gyri bilaterally, and the left posterior temporal and inferior parietal regions in both groups. However, both the extent and the intensity of activation were greater among the carriers of the APOE ε4 allele. The carriers of the APOE ε4 allele also had significant increases in the left parahippocampal region (Talairach and Tournoux atlas co-ordinates, −12, −38, and −10), the left dorsal prefrontal cortex (−56, 0, and 34; −50, −5, and 44), and in the inferior and superior parietal lobes (−48, −52, and 44 and −20, −80, and 26, respectively) and the anterior cingulate gyrus (12, 20, and 32). Direct comparisons of the carriers of the APOE ε4 allele and the carriers of the APOE ε3 allele (bottom panel, which shows the difference between the carriers) further demonstrated the greater extent and magnitude of activity in the left prefrontal region (atlas coordinates −60, 2, and 14 and −54, −18, and 32) and bilateral orbitofrontal, superior temporal, and inferior and superior parietal regions in the carriers of the APOE ε4 allele.
Direct comparisons of the signal intensity during the periods of learning or recall alone (ignoring rest periods) showed that the signal was more intense among carriers of the APOE ε4 allele in the left prefrontal and bilateral orbitofrontal, superior temporal, and inferior and superior parietal regions, indicating that the differences between groups resulted from differences in the way the brain functioned during the memory-activation task and not during the resting state.
Visual inspection of the activation maps of individual subjects also indicated a pattern of greater signal intensity during the periods of learning or recall among the carriers of the APOE ε4 allele (Fig. 2). The mean (±SD) number of regions of interest in which activation increased above the threshold value during periods of learning or recall as compared with periods of rest was significantly greater among the carriers of the APOE ε4 allele than among the carriers of the APOE ε3 allele (15.9±6.2 vs. 9.4±5.5, P=0.005) — but only for comparisons in the left hemisphere (Table 2).
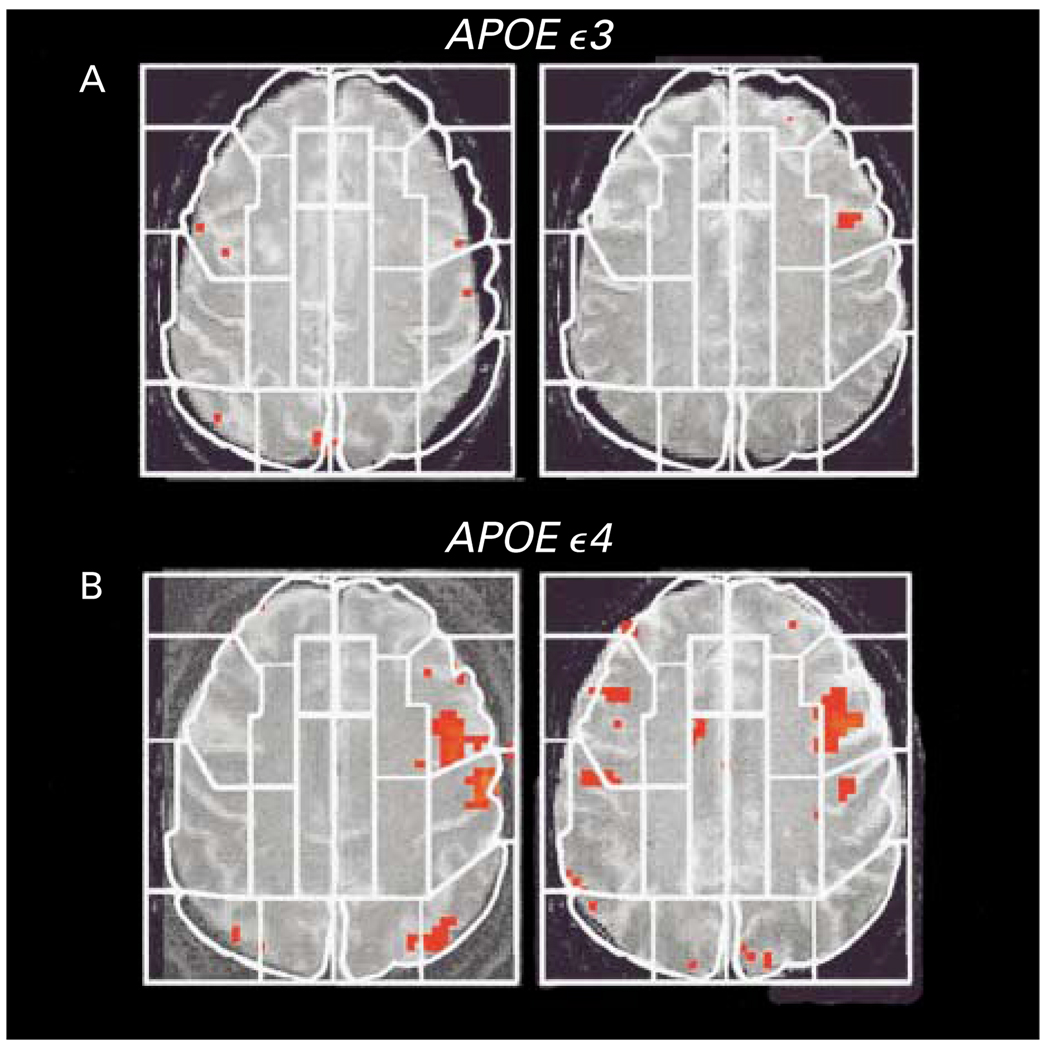
Figure 2. Examples of Activation Maps on Single MRI Planes.
Two carriers of the APOE ε3 allele (Panel A) had fewer and less extensive areas of statistically significant activation (indicated in red) than did two carriers of the APOE ε4 allele (Panel B). The white lines indicate examples of regions of interest used for the analyses of data within subjects.
TABLE 2.
Difference between the Mean Number of Regions of Interest Activated during Learning or Recall Periods and the Number Activated during Rest Periods.*
| Region of Interest and Hemisphere |
APOE ε3 Carriers (N=14) |
APOE ε4 Carriers (N=16) |
|---|---|---|
| Language cortex | ||
| Left | 2.8±1.2 | 3.6±0.8† |
| Right | 1.9±1.1 | 2.5±0.8 |
| Dorsolateral prefrontal cortex | ||
| Left | 1.2±0.9 | 2.8±1.1‡ |
| Right | 1.3±1.2 | 1.3±1.2 |
| Medial temporal lobe | ||
| Left | 0.1±0.4 | 1.0±1.0§ |
| Right | 0.1±0.4 | 0.6±0.7 |
| Parietal lobe | ||
| Left | 0.7±0.7 | 1.3±0.8¶ |
| Right | 0.4±0.6 | 0.5±0.7 |
Images obtained during the learning or recall periods were compared with those obtained during periods of rest. Regions with statistically significant increases in signal intensity during memory-activation tasks (those with a Pearson’s r statistic of more than 0.3; P<0.01) were identified according to the template of Damasio and Damasio.31 The regions of interest were grouped into the language cortex (inferior frontal gyrus [Brodmann’s area 44], anterior insula, superior temporal gyrus, and middle temporal gyrus), the dorsal prefrontal cortex (Brodmann’s areas 9, 46, 10, 6, and 8 in the cortex), the medial temporal lobe (hippocampal formation, parahippocampal gyrus, and the amygdala), and the parietal lobe (superior and inferior parietal lobules). Plus–minus values are means ±SD.
P=0.03 for the comparison with the APOE ε3 carriers.
P<0.001 for the comparison with the APOE ε3 carriers.
P=0.004 for the comparison with the APOE ε3 carriers.
P=0.04 for the comparison with the APOE ε3 carriers.
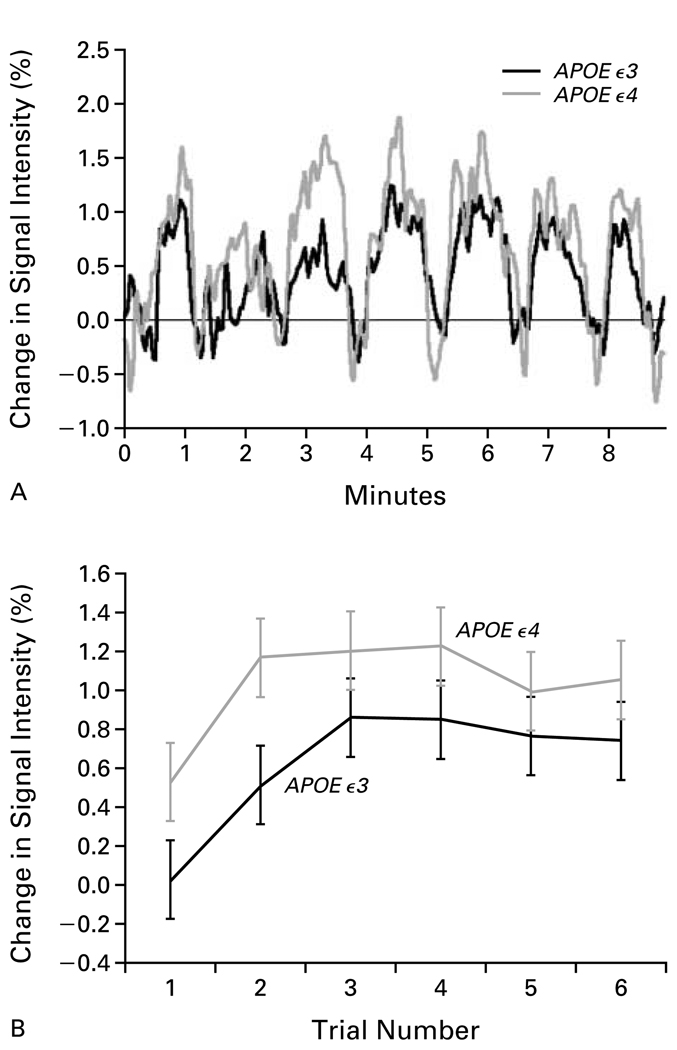
In both groups, the average signal intensity in the hippocampal regions clearly increased during periods of learning or recall as compared with periods of rest, but these increases were greater among the carriers of the APOE ε4 allele (Fig. 3A). The average percent change in signal intensity was consistently greater during recall periods than during learning periods. During periods of recall, the average increase in signal intensity was nearly twice as great among the carriers of the APOE ε4 allele as among carriers of the APOE ε3 allele (1.03 percent vs. 0.62 percent, P<0.001) (Fig. 3B). By contrast, the average increase in the hippocampal signal during learning periods was 0.90 percent among the carriers of the APOE ε4 allele and 0.61 percent among carriers of the APOE ε3 allele. The magnitude of these increases is similar to those in other functional MRI studies of memory activation. Such studies have typically reported increases of 2 to 3 percent in the primary sensory area and motor cortex and of less than 1 percent in the hippocampus.14,15
Figure 3. MRI Signal Intensity in the Hippocampus.
Panel A shows the percent increases in signal intensity during learning or recall periods as compared with periods of rest, for the hippocampus and parahippocampal gyrus, averaged among subjects in each group. These increases are plotted for the nine minutes of the experiment; the peaks indicate periods of learning or recalling the word pairs, whereas the valleys indicate periods when the subjects were at rest. In both groups the signal intensity increased during the learning or recall periods as compared with the interspersed periods of rest, though these increases were larger in the carriers of the APOE ε4 allele. Panel B shows the mean (±SD) percent change in the MRI signal intensity in the hippocampus and parahippocampal gyrus during each of the six periods of recall. The response among the carriers of the APOE ε4 allele was consistently larger than the response among the carriers of the APOE ε3 allele.
Fourteen subjects were studied again 2 years later, including eight carriers of the APOE ε4 allele (mean follow-up, 27±2 months) and six carriers of the APOE ε3 allele (mean follow-up, 28±2 months). The extent of the change in memory was defined as the follow-up score minus the base-line score. The number of regions of interest with significant activation in the left hemisphere at base line was significantly correlated with the degree of decline in verbal recall after two years, as measured by scores on the Consistent Long-Term Retrieval section of the Buschke–Fuld Selective Reminding test (r= − 0.65, P=0.02). Similar, but nonsignificant, correlations were found with respect to scores on the Logical Memory Delayed Recall section of the Wechsler Memory Scale (r= − 0.27) and the Benton Visual Retention test (r=0.24).
DISCUSSION
We found that among older people who had the APOE ε4 allele and a normal memory for their age, both the magnitude and the extent of brain activation during verbal memory challenge were greater than those among similar subjects who had the APOE ε3 allele. These differences in patterns of brain activation in the left hemisphere correlated with the degree of decline in memory among subjects who were retested two years later. These functional MRI results extend established findings in PET studies of Alzheimer’s disease and aging to persons at genetic risk for Alzheimer’s disease.
Our findings are consistent with those of other neuroimaging studies that reported increased brain activity during cognitive challenge in subjects with normal cognitive function. More complex stimuli or more demanding cognitive processing results in a greater magnitude and area of signal intensity in regions critical to the task; likewise, as performance improves, through either innate ability or practice, the increase in signal intensity becomes smaller and more focal.19,20
The greater increase in signal intensity in brain regions necessary for tasks requiring memory among the carriers of the APOE ε4 allele suggests that they performed additional cognitive work to accomplish the task. Expanding the territory of neural tissue dedicated to such tasks, as well as increasing the number of neurons recruited or the firing rate within a given functional area, may augment the brain’s processing capacity, operating dynamically in response to cognitive demands. In persons at risk for Alzheimer’s disease, such increased brain activity may effectively serve a compensatory role, wherein subjects use additional cognitive resources to bring memory-related performance to a normal level.
In support of this compensatory hypothesis were the greater differences between groups during periods of recall, when subjects had to apply cognitive effort to retrieve the correct response. By contrast, differences between the groups were less pronounced during periods of learning, when subjects listened to but did not actively try to recall experimental stimuli. Furthermore, among the carriers of the APOE ε4 allele the MRI signal intensity was increased in the anterior cingulate gyrus and dorsal prefrontal cortex, regions that show greater activation as cognitive effort increases.32 The most plausible explanation for this pattern of response is that subjects at genetic risk for Alzheimer’s disease use greater cognitive effort to achieve the same level of performance as subjects who are not at genetic risk.
Although both groups of subjects had normal results on the logical-memory measure of the Wechsler Memory Scale, the results among carriers of the APOE ε4 allele were poorer.23 Such measures of delayed recall are particularly sensitive to the decline in memory associated with this allele.33 The scores themselves were within the normal range and, when interpreted concomitantly with the results of a battery of memory tests, were not low enough to arouse clinical concern. Nonetheless, slight declines in the results of such tests may have greater importance when they are interpreted in combination with functional imaging and genetic data.
For signal intensity to be increased in association with compensatory processing, there must be enough healthy neural tissue to accommodate such a change. A substantial neural loss, by contrast, would most likely be associated with attenuated brain activity. Indeed, activation-imaging studies of patients with Alzheimer’s disease revealed decreased brain activity in the parietal and hippocampal regions and relatively higher activity in regions of the cortex that were not affected by the disease.13,21 In those studies, tasks requiring memory made fewer demands on the subjects than in our study and apparently resulted in a passive approach to the task. By contrast, in our study, subjects closely attended to and actively retrieved stimuli. Such demanding paradigms may present a challenging behavioral probe that causes the observed increase in the patterns of signal intensity. Hence, we refer to our approach as a cognitive stress test.12
In the subgroup of subjects whom we studied two years later, the level of brain activation at base line correlated with the degree of longitudinal memory decline. The pattern of these results suggests the potential usefulness of combining studies of brain activation and assessments of genetic risk in predicting future cognitive decline.
Several methodologic issues deserve comment. Changes in magnetic susceptibility arising from increased cerebrospinal fluid as a result of atrophy may affect medial temporal structures. Atrophy alone, however, could not explain the different results in the two groups of subjects, since the effects were largely unilateral and were present primarily during the recall periods. Because the functional MRI measure is a relative one, reduced base-line blood flow could provide an alternative explanation for the results.11 Many factors influence the functional MRI signal, including the sensitivity of the scanner, the homogeneity of the field, the subject’s head motion, and the dependent signal measure.30,34
Our results indicate that, as a group, older persons with a genetic risk for Alzheimer’s disease have alterations in brain function without obvious morphologic or behavioral indications of impending disease. Initial longitudinal follow-up indicates that the baseline level of brain activation can be used to predict subsequent decline in memory.
Acknowledgments
Supported by grants (MH52453, AG13308, AG10123, MO1 RR00856-21, RG2-96-051, NS31153, NS26630, AG05128, and AG11268) from the National Institutes of Health, a grant (IIRG94101) from the Alzheimer’s Association, a grant (9523330) from the California Department of Health and Human Services, the Montgomery Street Foundation, the Fran and Ray Stark Foundation Fund for Alzheimer’s Disease Research, the Ahmanson Foundation, the Lovelace Foundation, and the Tamkin Foundation.
We are indebted to Ms. Andrea Kaplan and Ms. Debbie Dorsey for help in recruiting the subjects and coordinating the study.
REFERENCES
- 1.Small GW, Rabins PV, Barry PP, et al. Diagnosis and treatment of Alzheimer disease and related disorders: consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA. 1997;278:1363–1371. [PubMed] [Google Scholar]
- 2.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 4.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 5.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 6.Corder EH, Saunders AM, Risch NJ, et al. Protective effect of apolipoprotein E type 2 allele for late onset alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 7.National Institute on Aging/Alzheimer’s Association Working Group. Apolipoprotein E genotyping in Alzheimer’s disease. Lancet. 1996;347:1091–1095. [PubMed] [Google Scholar]
- 8.Golomb J, Kluger A, de Leon MJ, et al. Hippocampal formation size predicts declining memory performance in normal aging. Neurology. 1996;47:810–813. doi: 10.1212/wnl.47.3.810. [DOI] [PubMed] [Google Scholar]
- 9.Small GW, Mazziotta JC, Collins MT, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA. 1995;273:942–947. [PubMed] [Google Scholar]
- 10.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the ε4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 11.Grady CL, Haxby JV, Horwitz B, et al. Activation of cerebral blood flow during a visuoperceptual task in patients with Alzheimer-type dementia. Neurobiol Aging. 1993;14:35–44. doi: 10.1016/0197-4580(93)90018-7. [DOI] [PubMed] [Google Scholar]
- 12.Mentis MJ, Horwitz B, Grady CL, et al. Visual cortical dysfunction in Alzheimer’s disease evaluated with a temporally graded “stress test” during PET. Am J Psychiatry. 1996;153:32–40. doi: 10.1176/ajp.153.1.32. [DOI] [PubMed] [Google Scholar]
- 13.Backman L, Andersson JLR, Nyberg L, Winblad B, Nordberg A, Almkvist O. Brain regions associated with episodic retrieval in normal aging and Alzheimer’s disease. Neurology. 1999;52:1861–1870. doi: 10.1212/wnl.52.9.1861. [DOI] [PubMed] [Google Scholar]
- 14.Cohen MS, Bookheimer SY. Localization of brain function using magnetic resonance imaging. Trends Neurosci. 1994;17:268–277. doi: 10.1016/0166-2236(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 15.Gabrieli JDE, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- 16.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa S, Tank DW, Menon R, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwong KK, Belliveau JW, Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- 20.Raichle ME, Fiez JA, Videen TO, et al. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 21.Grady CL, Maisog JM, Horwitz B, et al. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 23.Wechsler D. Standardized memory scale for clinical use. J Psychol. 1945;19:87–95. [Google Scholar]
- 24.Benton AL, Hamsher K. Multilingual aphasia examination. Rev. ed. Iowa City: University of Iowa Press; 1978. [Google Scholar]
- 25.Rausch R, Babb TL. Hippocampal neuron loss and memory scores before and after temporal lobe surgery for epilepsy. Arch Neurol. 1993;50:812–817. doi: 10.1001/archneur.1993.00540080023008. [DOI] [PubMed] [Google Scholar]
- 26.Mega MS, Thompson PM, Cummings JL, et al. Sulcal variability in the Alzheimer’s brain: correlations with cognition. Neurology. 1998;50:145–151. doi: 10.1212/wnl.50.1.145. [DOI] [PubMed] [Google Scholar]
- 27.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. New York: Thieme Medical; 1988. [Google Scholar]
- 28.Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration. II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 29.Cohen MS. Parametric analysis of fMRI data using a linear systems method. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 30.Cohen MS, DuBois RM. Stability, repeatability, and the expression of signal magnitude in functional magnetic resonance imaging. J Magn Reson Imaging. 1999;10:33–40. doi: 10.1002/(sici)1522-2586(199907)10:1<33::aid-jmri5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 31.Damasio H, Damasio AR. Lesion analysis in neuropsychology. New York: Oxford University Press; 1989. [Google Scholar]
- 32.Cohen JD, Forman SD, Braver TS, Casey BJ, Servan-Schreiber D, Noll DC. Activation of the prefrontal cortex in a nonspatial working memory task with functional MRI. Hum Brain Mapping. 1994;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- 33.O’Hara R, Yesavage JA, Kraemer HC, Mauricio M, Friedman LF, Murphy GM., Jr The APOE epsilon4 allele is associated with decline on delayed recall performance in community-dwelling older adults. J Am Geriatr Soc. 1998;46:1493–1498. doi: 10.1111/j.1532-5415.1998.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 34.Song AW, Wong EC, Tan SG, Hyde JS. Diffusion weighted fMRI at 1.5 T. Magn Reson Med. 1996;35:155–158. doi: 10.1002/mrm.1910350204. [DOI] [PubMed] [Google Scholar]