Abstract
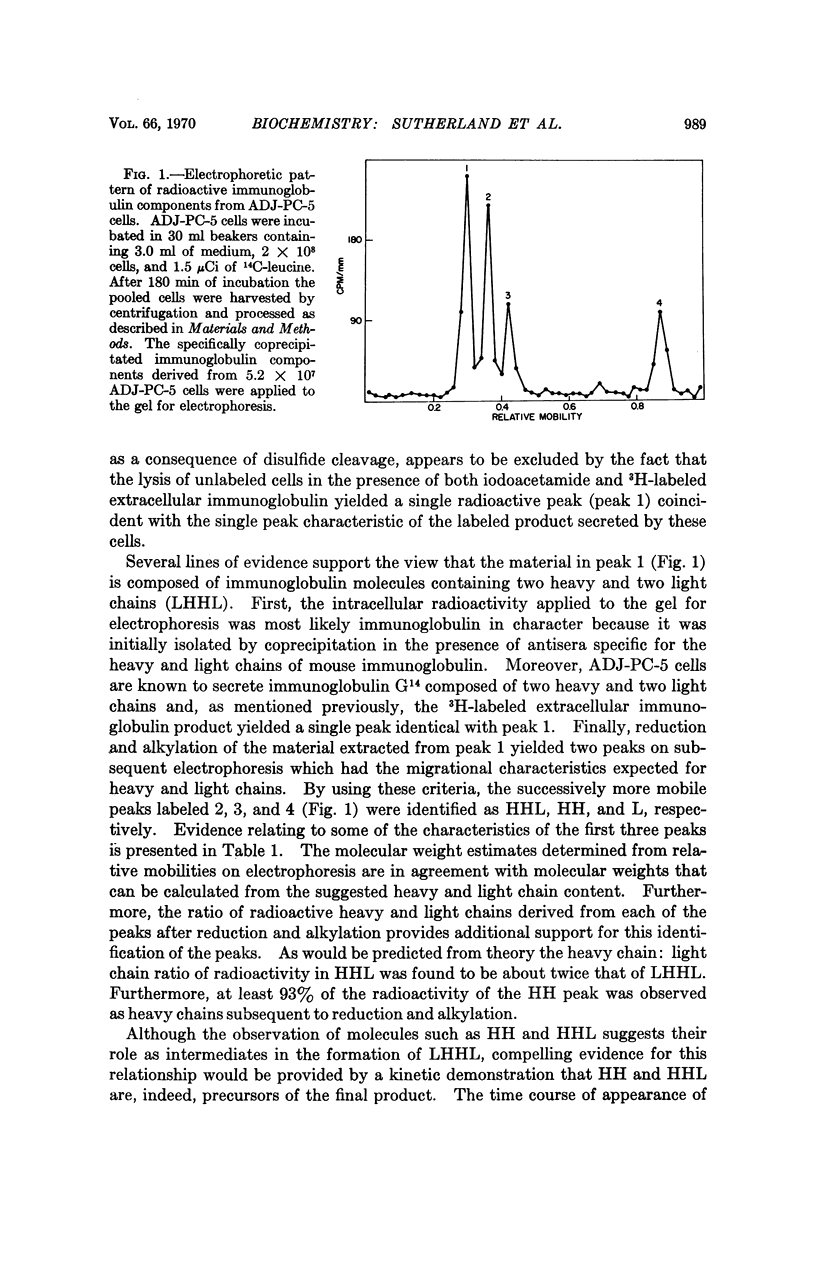
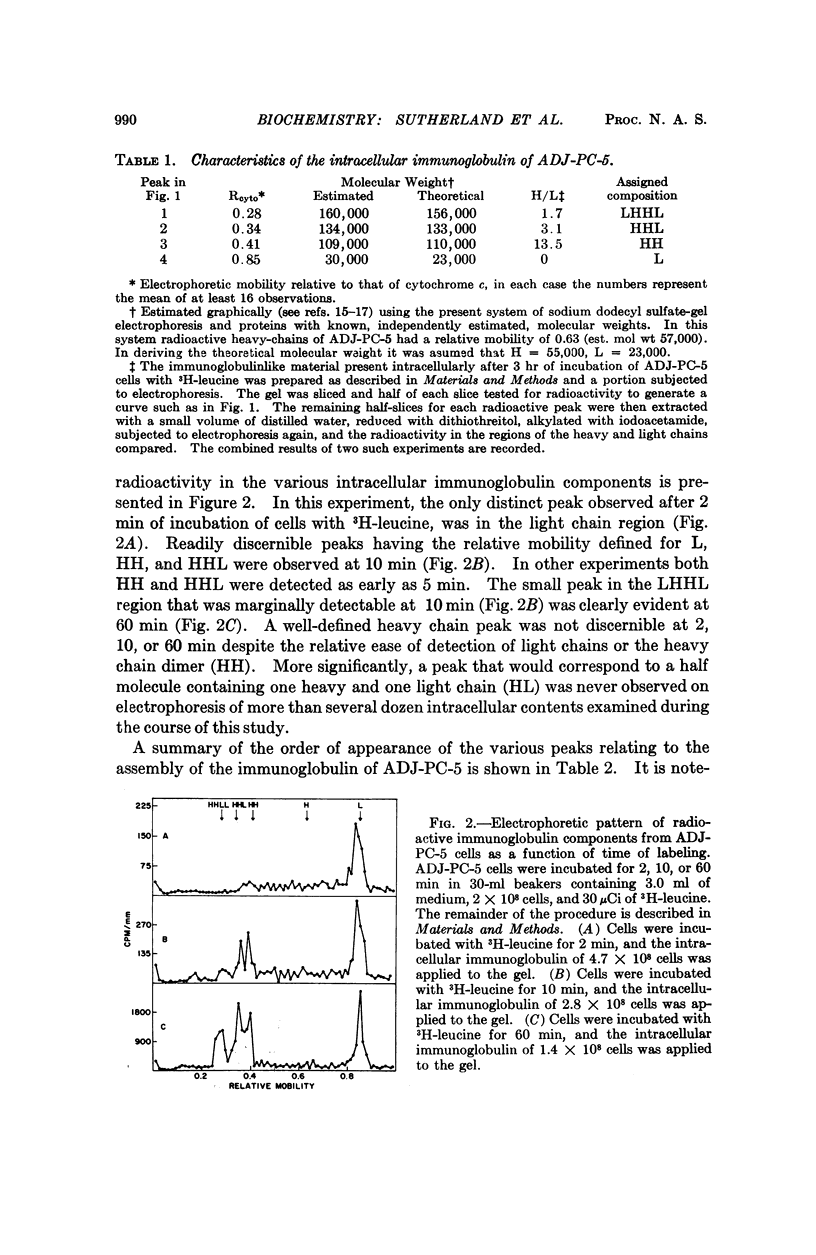
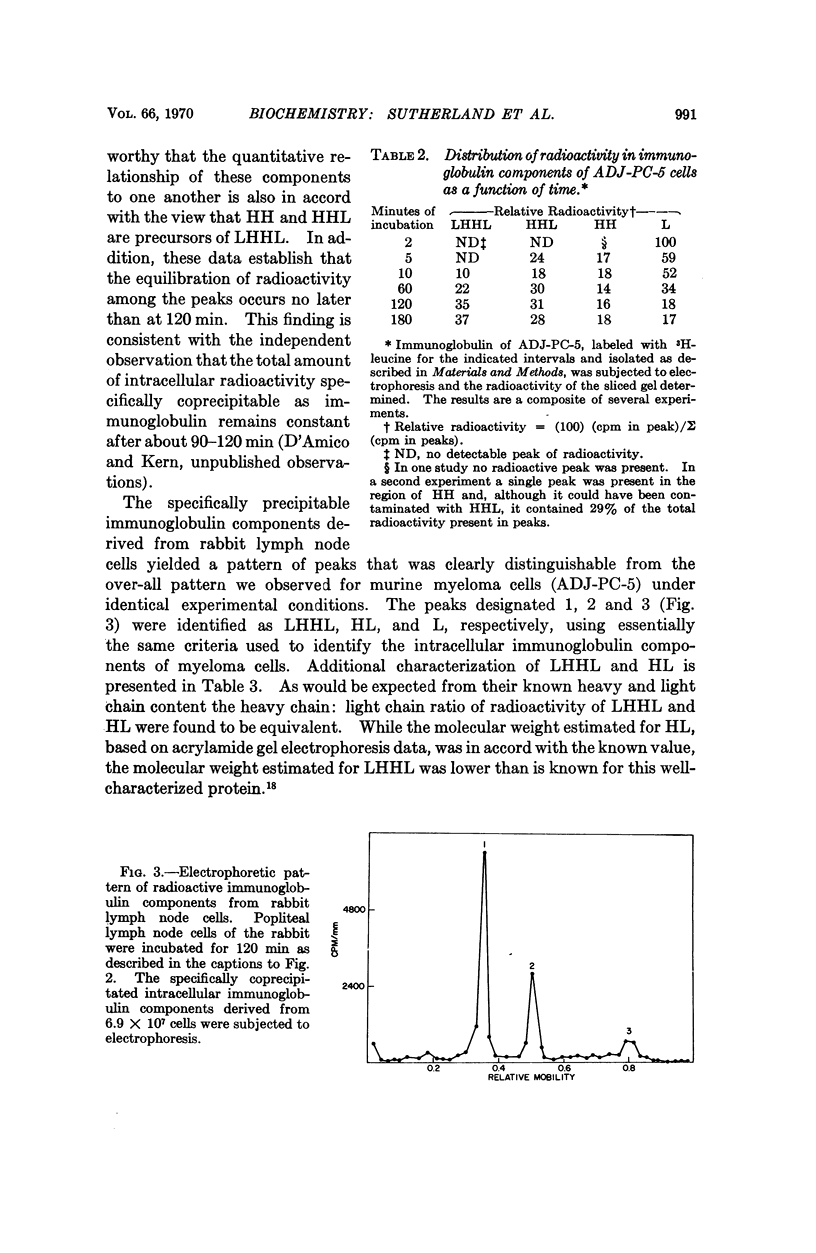
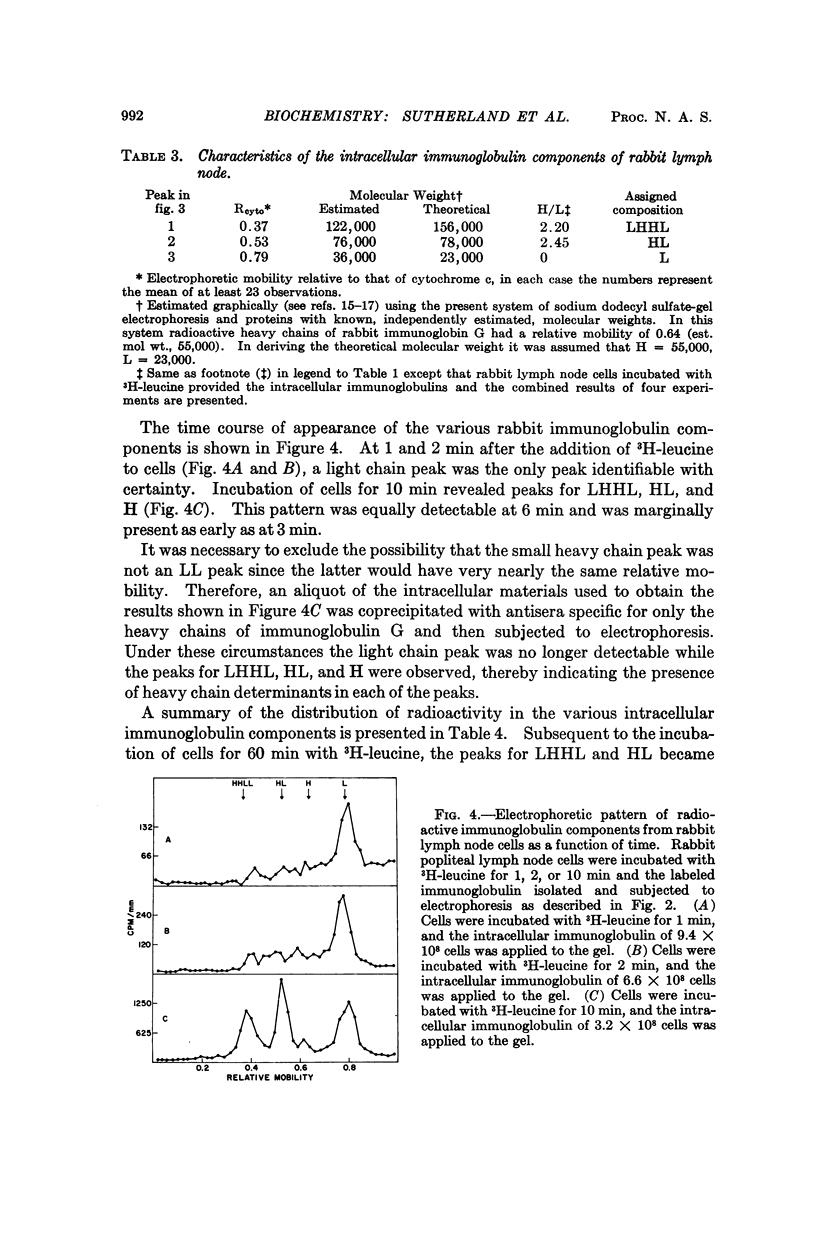
Murine myeloma cells (ADJ-PC-5), incubated in vitro with 3H-leucine, secrete 3H-immunoglobulin G as a single molecular species as judged by the migration characteristics of the labeled product on sodium dodecyl sulfateacrylamide gel electrophoresis. However, the fact that some of the interchain disulfide linkages of intracellular immunoglobulins had not been acquired permitted the identification of the following intracellular species: LHHL (identical to immunoglobulin G), HHL, HH, and L (H and L refer to heavy and light polypeptide chains, respectively). Although HH and HHL were readily observed, radioactivity was not detected in the region of the gel where HL would be expected. The time course for the appearance of the intermediates indicates that in these cells the first interchain disulfide bond to be formed occurs between heavy chains. In contrast, the interchain disulfide bonds of immunoglobulins derived from rabbit lymph node cells were acquired in a different order. The principal intracellular species observed were LHHL and HL, whereas HHL and HH were not detectable. These findings indicate that in this species the first interchain disulfide bond to be formed is that between the heavy and light chains of immunoglobulin G.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askonas B. A., Williamson A. R. Interchain disulphide-bond formation in the assembly of immunoglobulin G. Heavy-chain dimer as an intermediate. Biochem J. 1968 Oct;109(4):637–643. doi: 10.1042/bj1090637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrambach A. Device for sectioning of polyacrylamide gel cylinders and its use in determining biological activity in the sections. Anal Biochem. 1966 Jun;15(3):544–548. doi: 10.1016/0003-2697(66)90121-7. [DOI] [PubMed] [Google Scholar]
- D'Amico R. P., Kern M. Synthesis and secretion of gamma-globulin by lymph node cells. VII. The cell-free incorporation of galactose and sialic acid into the carbohydrate component of endogenous and exogenous immunoglobulin. Biochim Biophys Acta. 1970 Jul 21;215(1):78–87. doi: 10.1016/0304-4165(70)90389-2. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- HELMREICH E., KERN M., EISEN H. N. The secretion of antibody by isolated lymph node cells. J Biol Chem. 1961 Feb;236:464–473. [PubMed] [Google Scholar]
- Kern M., Helmreich E., Eisen H. N. A DEMONSTRATION OF ANTIBODY ACTIVITY ON MICROSOMES. Proc Natl Acad Sci U S A. 1959 Jun;45(6):862–867. doi: 10.1073/pnas.45.6.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- Potter M., Lieberman R. Genetics of immunoglobulins in the mouse. Adv Immunol. 1967;7:91–145. doi: 10.1016/s0065-2776(08)60127-3. [DOI] [PubMed] [Google Scholar]
- SOBER H. A., PETERSON E. A. Protein chromatography on ion exchange cellulose. Fed Proc. 1958 Dec;17(4):1116–1126. [PubMed] [Google Scholar]
- Schubert D. Immunoglobulin assembly in a mouse myeloma. Proc Natl Acad Sci U S A. 1968 Jun;60(2):683–690. doi: 10.1073/pnas.60.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. M., Kern M. THE SYNTHESIS AND SECRETION OF gamma-GLOBULINS BY LYMPH NODE CELLS, I. THE MICROSOMAL COMPARTMENTALIZATION OF gamma-GLOBULINS. Proc Natl Acad Sci U S A. 1967 Feb;57(2):417–422. doi: 10.1073/pnas.57.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. M., Kern M. The synthesis and secretion of gamma-globulin by lymph node cells. 3. The slow acquisition of the carbohydrate moiety of gamma-globulin and its relationship to secretion. Proc Natl Acad Sci U S A. 1968 Feb;59(2):546–553. doi: 10.1073/pnas.59.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishler P. V., Epstein C. J. A convenient method of preparing polyacrylamide gels for liquid scintillation spectrometry. Anal Biochem. 1968 Jan;22(1):89–98. doi: 10.1016/0003-2697(68)90262-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



