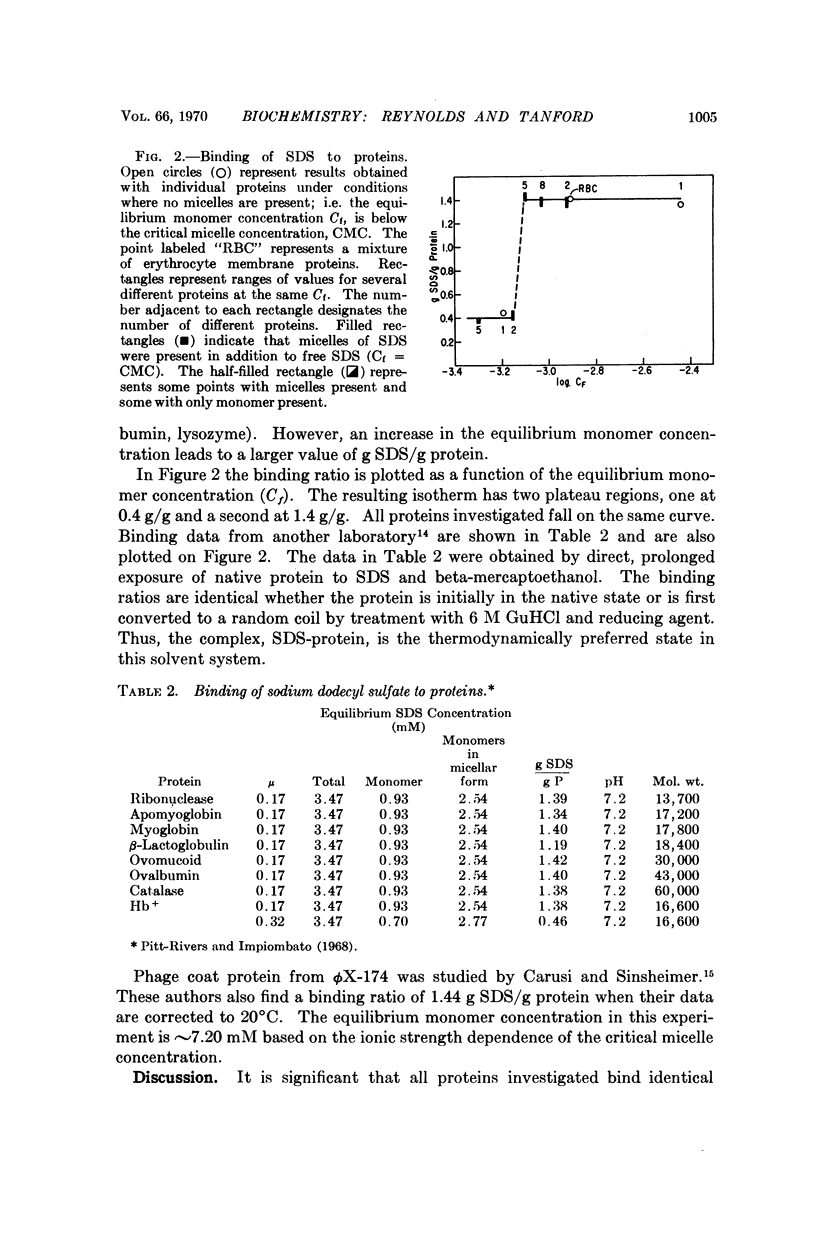
Abstract
A wide variety of proteins have been shown to bind identical amounts of an amphiphile, sodium dodecyl sulfate, on a gram per gram basis at monomer equilibrium concentrations above 0.5 mM. The binding is independent of ionic strength and primarily hydrophobic in nature. Only the monomeric form of the amphiphile binds to proteins, not the micellar form. The application of these results to models for biological membranes and to gel electrophoresis in sodium dodecyl sulfate is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun P. E., Radin N. S. Interactions of lipids with a membrane structural protein from myelin. Biochemistry. 1969 Nov;8(11):4310–4318. doi: 10.1021/bi00839a014. [DOI] [PubMed] [Google Scholar]
- CARUSI E. A., SINSHEIMER R. L. THE PHYSICAL PROPERTIES OF A PROTEIN ISOLATED FROM BACTERIOPHAGE PHI-X174. J Mol Biol. 1963 Oct;7:388–400. doi: 10.1016/s0022-2836(63)80032-7. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Emerson M. F., Holtzer A. On the ionic strength dependence of micelle number. II. J Phys Chem. 1967 May;71(6):1898–1907. doi: 10.1021/j100865a057. [DOI] [PubMed] [Google Scholar]
- Green D. E., Allmann D. W., Bachmann E., Baum H., Kopaczyk K., Korman E. F., Lipton S., MacLennan D. H., McConnell D. G., Perdue J. F. Formation of membranes by repeating units. Arch Biochem Biophys. 1967 Mar;119(1):312–335. doi: 10.1016/0003-9861(67)90461-4. [DOI] [PubMed] [Google Scholar]
- Ji T. H., Benson A. A. Association of lipids and proteins in chloroplast lamellar membrane. Biochim Biophys Acta. 1968 Jun 11;150(4):686–693. doi: 10.1016/0005-2736(68)90058-8. [DOI] [PubMed] [Google Scholar]
- Pitt-Rivers R., Impiombato F. S. The binding of sodium dodecyl sulphate to various proteins. Biochem J. 1968 Oct;109(5):825–830. doi: 10.1042/bj1090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Reynolds J. A., Polet H., Steinhardt J. Binding of large organic anions and neutral molecules by native bovine serum albumin. Biochemistry. 1966 Aug;5(8):2606–2616. doi: 10.1021/bi00872a019. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Herbert S., Polet H., Steinhardt J. The binding of divers detergent anions to bovine serum albumin. Biochemistry. 1967 Mar;6(3):937–947. doi: 10.1021/bi00855a038. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Engelman D. M. Current models for the structure of biological membranes. J Cell Biol. 1969 Sep;42(3):613–646. doi: 10.1083/jcb.42.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


