Abstract
Extracellular superoxide dismutase (ecSOD) protects the extracellular matrix (ECM) from oxidative stress. We previously reported a new allele for ecSOD, expressed in 129P3/J mice (129), which differs from the wild-type (wt), expressed in C57BL/6J and other strains, by two amino acid substitutions and a 10 bp deletion in the 3' UTR of the mRNA [1]. The newly discovered allele is associated with a phenotype of significantly increased circulating and heparin-releasable enzyme activities and levels. In order to examine the properties of the two forms of ecSOD in an identical environment we generated, by extensive backcrossing of ecSOD heterozygous progeny to C57BL/6J females, a congenic C57 strain with the 129 (or wt) allele of ecSOD. These mice are homozygous for nearly 5,000 SNPs across all chromosomes, as determined by Affymetrix Parallele Mouse 5K SNP panel. The present study describes the generation of the congenic mice (genetically >99.8 % identical) and their ecSOD phenotype. The congenic mice plasma ecSOD activities before and after heparin administration recapitulate the differences reported in the founder mice. Tissue enzyme distribution is similar in both congenic groups, although the 129 allele is associated with higher levels of enzyme expression despite lower levels of enzyme mRNA. In these characteristics the phenotype is also allele driven, with little impact by the rest of the genome. The congenic mice carrying the 129 allele have mRNA levels that are in between those found in the founder 129P3/J and C57BL/6J strains. We conclude that the ecSOD phenotype in most aspects of enzyme expression is allele- driven, with the exception of tissue mRNA levels, where a significant contribution by the surrounding (host) genome is observed. These results also suggest potential allele-specific differences in the regulation of ecSOD synthesis and intracellular processing/secretion of ecSOD, independent of the genotype context. Most importantly, the congenic mice offer an excellent model to examine the regulatory mechanisms of ecSOD expression and the role of ecSOD in various diseases involving oxidative stress.
Keywords: ecSOD, polymorphism, C57BL/6J, 129P3/J, congenic mice, plasma, tissue, antioxidants
Introduction
Extracellular superoxide dismutase (ecSOD, also referred to as SOD3) is the only antioxidant enzyme in the extracellular compartment [including the extracellular matrix (ECM), endothelial cell surface and tissue fluids] that provides protection against superoxide and regulates the bioavailability of nitric oxide [2–4]. This is accomplished through the conversion of superoxide to hydrogen peroxide and diminished formation of peroxinitrite from nitric oxide. While hydrogen peroxide is also an oxidant, it also can serve as a signaling molecule and potentiate endothelium- dependent relaxation [5–7]. The physiological importance of this enzyme is illustrated by problems in mice lacking this enzyme: their increased sensitivity to lung injury, increased endothelial dysfunction and impaired neovascularization [8–10]. Over-expression of the human form of the enzyme on the other hand protects mice from global cerebral ischemia [11], preserves post-ischemic myocardial function [12], reduces lung injury during inflammation [13] and reduces aging-induced cognitive impairment [14]. More recent studies indicate that the protective effect of ecSOD on the oxidative fragmentation of the ECM components [15–17] is a key factor in controlling the inflammatory response in lung injury.
In mice, like humans, ecSOD is a glycosylated homotetramer localized mainly in the ECM and on cell surfaces, anchored to heparan sulfate proteoglycans and type I collagen through an interaction of a heparin binding domain (HBD) containing 6 positively charged amino acids in the C-terminal region of each monomer [18–20]. The C-terminal region can be proteolytically cleaved and, depending on the extent of monomer processing, ecSOD can be present in three types: type A, lacking heparin affinity (all monomers in the tetramer are devoid of the HBD); B, with intermediate affinity (some monomers are cleaved); and C, with strong affinity (all monomers in the tetramer are intact) [21]. Thus type A is normally found circulating in plasma, and types B and C are mostly tissue bound. Over 90% of the total body ecSOD is thus estimated to be associated with tissue extracellular matrix, including vascular tissue and, to a lesser extent, endothelial cell surface [22–24].
We previously observed that the 129 inbred strain of mice expresses a variant of the ecSOD mRNA, which includes the A61G and G556T point mutations and a 10bp deletion in the 3'UTR of the transcript [1]. Other strains tested thus far (C57BL/6J, C3H and Swiss-Webster) carry the “wild-type” (wt) allele [1]. The 129 allele is associated with a very different ecSOD phenotype; these mice have significantly higher activity and amount of circulating and heparin-releasable ecSOD, when compared to C57BL/6J mice. Co-incidentally, the 129 and C57 mice differ significantly in their response to proliferative lung disease, brain ischemia, vascular remodeling, susceptibility to atherosclerosis [25–28], as well as development of tolerance against global and focal cerebral ischemia [29] by hyperbaric oxygen treatment. None of these studies identified specific gene(s) that may account for the observed differences in the response of these strains.
When we reported on the different ecSOD phenotype between these two strains [1], we were unable to ascertain the magnitude of the contribution to these differences by the ecSOD allele itself and the effects of the surrounding milieu. In order to examine the contribution of the genetic environment vs. the ecSOD allele to the observed ecSOD phenotype, we bred congenic mice (C57.129-sod3) that carry either of the ecSOD alleles in an otherwise identical C57BL/6J background. Our results clearly demonstrate that the ecSOD phenotype is largely due to the allele-specific effects independent of other strain-specific factors. Our results also suggest significant allele-specific differences in the regulation of ecSOD synthesis and intracellular processing/secretion, independent of the genotype/strain context.
Materials and methods
Animals
C57BL/6J and 129P3/J mice were obtained from Jackson Laboratories (Bar Harbor, ME) and were maintained on normal chow and water. All experimental protocols were approved by the UNT HSC Institutional Animal Care and Use Committee.
Selection of microsatellite markers
The microsatellite polymorphism markers between the C57BL/6J and 129P3/J strains were selected from the Jackson mouse informatics data base. Only markers with at least a 6 bp difference in the size of the amplicons were chosen and are listed in Table 1.
Table 1.
Selected microsatellite polymorphism markers between the C57BL/6J and 129P3/J strains that were used for the selection of male heterozygous ecSOD progeny for subsequent backcrossing into C57 females.
| Chromosome | Locus (cM) | Marker | Primer | Primer sequence | Strain | Fragment size (bp) | Size difference |
|---|---|---|---|---|---|---|---|
| Chr 1 | 106.3 | D1Mit362 | D1Mit362 | TGTGTGACTGCTTGGAAGATG CTGAGTCCCTAAAGTTGTCCTTG |
129P3/J C57BL/6J |
148 120 |
28 |
| Chr 5 | 18 | D5Mit388 | D5Mit388 | TTTCAGAGGGTGGGAGGTAA CCTGGACTCATGGAAGCATT |
129P3/J C57BL/6J |
183 192 |
11 |
| Chr 5 | 1 | D5Mit346 | D5Mit346 | TCAAACTCCTCTAATATGGAAGTGC CTGTCTCATTAATCCATGGATCC |
129P3/J C57BL/6J |
141 121 |
21 |
| Chr 6 | 0.9 | D6Rp2 | TB6F TB6R |
TCCTTATAAATCAGTTTCTTAG GCAATGGAGGTAGAAAACTG |
129P3/J C57BL/6J |
179 165 |
14 |
| Chr 7 | 3.9 | D7Msu1 | pA pB |
ATTCCAAAGTGTTCAATGCC ACTTCCCAGCTGATGTGACT |
129P3/J C57BL/6J |
175 185 |
10 |
| Chr 11 | 20 | D11Mit188 | D11Mit188 | CTATTTCCTCAGTGCTCGGC AGCATGTACCTTGAAAACCAGA |
129P3/J C57BL/6J |
122 128 |
6 |
DNA isolation and genotyping
DNA was isolated from small amount of tail tissue incubated with 200μl of lysis buffer (Viagen) with proteinase K for 18hr at 56 °C. EcSOD genotyping was carried out as previously described [1], using primers spanning bp −33 to +94 (forward: 5'- GGGGACATTCCACAGGTGCAG–3', reverse: 5' – TGTCTGCTAGGTCGAAGCTGGAC–3') of the ecSOD gene. The amplicons were then digested with MboI (Promega) for 3 hr. at 37 °C. Undigested and digested amplicons were fractionated using 12% PAGE with ethidium bromide staining. For microsatellite genotyping, the primer sets listed in Table 1 were used and the resulting amplicons were separated using 12% or 15% PAGE.
Sample collection
For free (circulating) plasma ecSOD measurements blood was collected from anesthetized 6–8 wk old female mice into heparinized capillary tubes from the retro-orbital plexus. Blood was also collected 10 min after a tail vein injection of small M.W. heparin (100 U) to measure heparin-releasable ecSOD. Upon sacrifice, tissues were perfused with ice-cold PBS via cardiac puncture to remove residual blood. Tissues/organs were removed and immediately snap-frozen in liquid nitrogen for subsequent analyses or were kept in RNAlater (Ambion) for RNA analyses.
Analyses
Frozen tissues were ground to a fine powder in liquid nitrogen, and homogenized with 50 mM potassium phosphate buffer containing 0.3 M KBr and 3 mM EDTA, pH 7.4 containing a 1:1000 dilution of a protease inhibitor cocktail (100 μM 4-(2-aminoethyl) benzensulfonyl fluoride, 10 μM leupeptin, 10 μM E-64, 1 μM bestatin, 15 nM aprotinin, 1.0 μM pepstatin-A). The protein concentration of tissue homogenate was determined by Lowry assay [30]. RNA isolation was carried out by TRIreagent® (Molecular Research Center) extraction of tissues stored in RNAlater® (Ambion) and concentrations measured by a ND-1000 spectrophotometer (NanoDrop).
Plasma and tissue ecSOD activities
ecSOD was partially purified using ConA Sepharose (Sigma) as described [31], using 100 μl of plasma or 2–6 mg of tissue homogenate protein. The columns (0.5×10 mm) were washed with 50mM Na-HEPES (pH 7.0) and 0.25 M NaCl, and ecSOD was eluted with a washing buffer containing 0.5 M α-methyl mannoside (Sigma), pH 6.0. ConA-Sepaharose efficiency and binding capacity were monitored by Western blotting of the wash.
The activity of ecSOD (released from ConA) was determined using a system based on the oxidation of NAD(P)H [32]. Briefly, 23.5μl of sample was combined with 187μl of 100 mM triethanolamine/diethanolamine-HCl buffer pH 7.4, 6μl of 100mM EDTA/50mM MnCl2 solution, and 10μl 7.5 mM NAD(P)H. The reaction was started with the addition of 23.5μl of 10 mM ß-mercaptoethanol. Samples were tested for NAD(P)H oxidase activity before the addition of ß-mercaptoethanol. Dismutase activity was estimated from a standard curve constructed by measuring the activity of increasing and known amounts of Cu/Zn SOD (Sigma, cat. #S2515). Activities are therefore expressed as ng of Cu/Zn SOD equivalents.
Western blots
Rabbit anti-mouse ecSOD antiserum was produced against a synthetic mouse-specific 21-amino acid peptide corresponding to the N-terminus of the mature protein, as previously described [1]. Aliquots equivalent to 0.25μL of whole plasma or 10–100μg of tissue protein were dissolved and boiled in Laemmli buffer containing 5% β-mercaptoethanol [33] for 5 min, separated by 12% SDS-PAGE at 100 V for 3.5 hours and transferred to polyvinylidine fluoride membranes (Bio-Rad) for 2 hours. After blocking for 1 hour the membranes were incubated with the primary antibodies for ecSOD (1:20,000) and/or β-actin (1:5,000) rabbit anti-mouse antiserum (Sigma) overnight at 4 °C. Secondary antibodies (goat anti-rabbit IgG), conjugated to horseradish peroxidase (Jackson Immunoresearch) were added for 2 hours at RT (1:5,000 dilution). EcSOD and ß-actin bands were visualized by the ECL system (Amersham) using FluorChem® FC2 Imaging System (Alpha Innotech) and densitometric analysis was done using the software supplied (AlphaEaseFC) for the instrument. Transfer efficiency between runs was checked and corrected for by using aliquots of pooled mouse plasma in each of the outside lanes. Moreover, scans were also corrected for loading by detecting ß-actin in all tissue samples except blood.
Quantitative Real-Time PCR (qRT-PCR)
cDNA was synthesized from 2mg of total RNA (Retro-Script kit, Ambion) using oligo-dT primers. One eighth (0.25μg) of this reaction mixture was used in the subsequent qRT-PCR reactions, using the following primers for ecSOD: forward, 5' –AGGACGACCTGGGTAAAGGT–3'; reverse: 5' –AGTGGTCTTGCACTCGCTCT–3' and S15, (a ribosomal protein, as an internal standard): forward: 5' –CGGGCCGGCCGTGCTTCACG–3', reverse: 5' –TTCCGCAAGTTCACCTACC–3' on the Mastercycler realplex2 (Eppendorf) using Taq ReadyMix™ JumpStart™ mix containing SYBR® Green (Sigma) to visualize the amplified product. For all reactions, the cycle threshold (Ct) values of ecSOD were normalized by the Ct of S15 (a ribosomal protein) as an internal standard by the following formula: ΔCt = CtecSOD − CtS15. The ΔCt value of the sod3wt liver was considered as the calibrator of ecSOD mRNA expression. Thus the abundance of each tissue ecSOD mRNA was expressed a fold change relative to sod3wt liver by 2−ΔΔCt, where ΔΔCt = ΔCttissue − ΔCtsod3wt liver.
Statistical Analyses
Results from the experiments are reported as means ± SEM. Statistical significance was determined using one- or two- way ANOVA (SPSS 14.0 for Windows and Prism). A p-value of <0.05 was considered statistically significant.
Results
Generation of congenic (C57.129-sod3) mice on C57BL/6J background
In order to examine the phenotypic effects of the allelic differences in an otherwise essentially identical environment, we generated C57.129-sod3 mice, expressing the wild-type (C57.129-sod3wt) or the 129 allele for ecSOD (C57.129-sod3129). Briefly, an F1 generation male of the C57BL/6J × 129P3/J cross (heterozygous for the ecSOD gene) was backcrossed to C57BL/6J female (N2). Heterozygous male progeny were backcrossed to C57BL/6J females for 10 generations. Each time the male litter was genotyped for ecSOD as well as for six additional microsatellite polymorphism markers (as shown in Table 1). Two of the markers are on chromosome 5, including one 13cM away from the ecSOD locus at 31cM. Heterozygous males with the most C57BL/6J - matching microsatellite markers were chosen for breeding with the C57 females. The extensive (10 generations) backcrossing and the use of strain-specific markers assures theoretically at least a 99.8% homozygosity of the congenic mice with the C57BL/6J strain (% homozygosity = [1−(1/2)n−1]). By generation N5 all of the chosen markers in Table 1, including ones on Ch5, were homozygous with the C57BL/6J background. The homozygosity of the congenic mice with the C57BL/6J strain was also confirmed by analyzing nearly 5,000 SNPs across all chromosomes using the Affymetrics Parallele Mouse 5K SNP panel (courtesy of Dr. Aldons Lusis, UCLA).
Plasma ecSOD activities and protein levels in congenic mice
We previously reported large differences in the plasma pre- and post-heparin ecSOD activities between the two founder strains. The 129P3/J strain had significantly higher plasma ecSOD activity (as well as amounts), both before as well as after heparin administration [1], when compared to the C57BL/6J mice. As shown in Figure 1, these differences are recapitulated in the congenic mice as well. The sod3129 mice have higher activity and amount of ecSOD in the circulation, both before and after heparin administration, than the sod3wt congenic mice. Moreover, these mice also have a significantly larger heparin-accessible pool of ecSOD. Based on these findings we conclude that the phenotypic differences in ecSOD expression in plasma are largely strain- independent and allele-specific.
Figure 1. Plasma ecSOD expression in congenic mice before and after heparin administration.
Plasma ecSOD activities (A) and protein levels (B) were measured in aliquots of plasma, as described in Materials and Methods. A typical Western blot is shown in C. C57.129-sod3wt (black bars) mice are compared to the C57.129-sod3129 (grey bars) congenic mice before (−) and after (+) heparin administration. (A) Activities were measured following partial purification by ConA-Sepharose affinity chromatography and are expressed as ng of Cu/Zn SOD equivalents/mL of plasma; (B) ecSOD protein levels were assessed by Western blotting of a 20μL of diluted plasma (equivalent to 0.25 μL of whole plasma), resolved by 12% SDS-PAGE; (C) a typical blot of plasma ecSOD from 2 mice in each of the experimental groups. The single band in the plasma sample represents the HBD-cleaved form of ecSOD; an additional band of higher molecular weight, representing the uncleaved monomers, appears after heparin administration. The efficiency of transfer was monitored by standard pooled plasma aliquots applied to both outside lanes. Results in (A) and (B) represent means ± SEM of 5 animals per group. All comparisons were done with two-way ANOVA. The heparin releasable pool of ecSOD activity or amount are significantly greater (p<0.044 and p<0.001, respectively) in the C57.129-sod3129 mice.
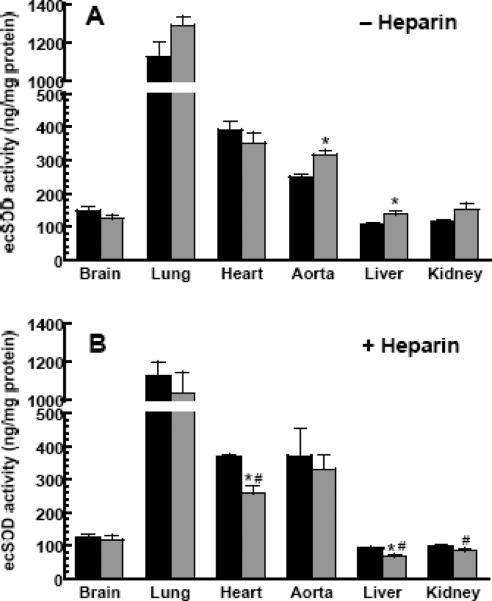
Tissue distribution of ecSOD in congenic mice
Tissue distribution of ecSOD activity is shown in Figure 2, both before (A) and after (B) heparin administration. The highest enzyme activity was measured in the lung, followed by the heart and aorta. The liver, kidney and brain enzyme have the lowest activity. This observation can be misleading; although the activity of the enzyme in liver homogenates is low, because of its high protein content the total pool of the enzyme is equivalent to the lung pool (data not shown). With the exception of the brain and heart, in all tissues examined, the sod3129 mice have higher, or in the case of aorta and liver, significantly higher enzyme activity than the sod3wt mice. Heparin administration does not significantly change the activity of ecSOD of the brain or lung in either group; a significant fraction of enzyme was lost from the liver, kidney (>50%) and heart (30%) of sod3129 mice, but not the sod3wt mice, as shown in (B).
Figure 2. ecSOD activity in selected tissues of congenic mice before and after heparin administration.
Enzyme activities were determined before (A) and after (B) heparin administration in C57.129-sod3wt (black bars) and C57.129-sod3129 (grey bars) mice. For activity assays, equal amounts of homogenate protein were loaded to ConA columns and eluted, as described in Materials and Methods. Results represent a mean ± SEM of 5 mice/group.
* indicates a significant difference (at p<0.05) from C57.129-sod3wt mice (black bars).
# indicates a significant difference (at p<0.05) from pre-heparin values.
Tissue ecSOD levels were assessed by Western blotting and are shown in Figure 3, both before (A) and after (B) heparin administration with typical Western blots (C). The levels of enzyme, as determined by Western blotting closely parallel the activity data shown in Figure 2; with the exception of the brain, the sod3129 mice have higher, or in the case of liver, significantly higher enzyme levels than the sod3wt mice. The effects of heparin on enzyme release are also similar to those shown in Figure 2, except that the liver and kidney of both congenic groups loose a similar fraction of the ecSOD pool.
Figure 3. ecSOD protein levels in selected tissues of congenic mice before and after heparin administration.

Enzyme levels were determined by Western blotting before (A) and after (B) heparin administration in C57.129-sod3wt (black bars) and C57.129-sod3129 (grey bars) mice. Also shown are typical blots of tissue ecSOD in each of the experimental groups (C). Tissue homogenate proteins (10 – 100μg) were resolved by 12% SDS-PAGE and transferred to PVDF membranes and scans quantified, as described in the Materials and Methods. Protein loading within each tissue/organ was corrected by ß-actin loading. Transfer efficiency was also monitored by using a sample of pooled standard plasma on both sides of each gel. Results represent a mean ± SEM of 5 mice/group.
* indicates a significant difference (at p<0.05) from C57.129-sod3wt mice (black bars).
# indicates a significant difference (at p<0.05) from pre-heparin values.
Data in Figures 2 and 3 suggest that heparin administration has little effect on the brain and lung enzyme content (both, activity and level). Overall, only tissues from the sod3129 mice (heart, liver and kidney) release statistically significant amounts of enzyme. The greatest phenotypic difference in this respect is seen in the liver; heparin releases well over 50% of the enzyme activity and mass in the sod3129 mice, while the release from the livers of sod3wt is much smaller, and statistically not significant. These data lead us to conclude that a significantly greater proportion of the liver, kidney and heart enzyme in the sod3129 mice resides on the endothelial interface, where it is accessible to heparin action. Surprisingly, heparin administration consistently leads to small increases in enzyme content (both activity and amount) of the aorta (the total of ascending, arch, descending and abdominal aorta).
ecSOD mRNA levels in tissues of congenic mice
We next examined the abundance of ecSOD mRNA in the selected tissues. In Figure 4 we compare ecSOD mRNA abundance in sod3wt (equivalent to the founder C57BL/6J mice) to that of sod3129 mice as well as the founder 129P3/J mice.
Figure 4. Relative abundance of ecSOD mRNA in selected tissues of mice.
mRNA levels were measured as described in the Materials and Methods from C57.129-sod3wt (black bars), C57.129-sod3129 (grey bars) and the founder 129P3/J mice (clear bars). Ribosomal protein S15 was used as a control for each tissue. Bars represent means ± SEM of four to five animals in each group.
* indicates a significant difference (at p<0.05) from C57.129-sod3wt mice (black bars)
# indicates a significant difference (at p<0.05) from C57.129-sod3129 mice (grey bars).
In agreement with published data [34], ecSOD mRNA abundance is highest in the kidney, lung and aorta, and lower in the brain, heart and liver, regardless of the strain. With the exception of the liver, tissues of the sod3129 mice, much like the founder 129P3/J mice, have consistently lower levels of ecSOD mRNA, statistically significantly so in the brain, lung and kidney, even though enzyme expression levels are similar or higher. The highest discrepancy is observed in the brain: the levels of ecSOD mRNA driven by the 129 allele in this organ range from 45–50% of those driven by the wt allele, and yet ecSOD expression is similar. Although the mRNA levels of the sod3129 mice trend with those of the 129P3/J founder strain, they are generally higher. Overall, our mRNA data therefore suggest that the ecSOD mRNA abundance phenotype may be determined partially by the strain (the C57BL/6J genome) and partially be allele-specific.
Discussion
C57BL/6J and 129P3/J are some of the most commonly used mouse strains for disease studies and for generating genetically engineered mice. Interestingly, these strains differ significantly in their response to proliferative lung disease, brain ischemia and vascular remodeling and susceptibility to atherosclerosis [25–28]. Even though it is widely accepted that oxidative stress is an important component of the etiology of these conditions, studies have yet to identify the gene(s) responsible for these differences. We recently reported that the 129P3/J strain expresses a novel ecSOD allele which differs in several aspects from the wt allele expressed by the C57BL/6J and C3H strains: it contains an N21D (or N-4D of the mature protein) substitution in the signal sequence of ecSOD, an A186S substitution in the catalytic domain and a 10bp deletion in the 3' untranslated region of the mRNA [1]. These mice have higher activity and amount of circulating and heparin-releasable ecSOD, when compared to C57BL/6J mice. Because of its function, ecSOD may be an important component of the anti-oxidant defense repertoire. To be able to compare the effect of two different ecSOD isoforms in an identical environment, we generated congenic mice that express either of the ecSOD alleles on a C57BL/6J genomic background. Based on the extent backcrossing (10 generations) and the additional use of 6 microsatellite polymorphism markers and an analysis by the Affimetrix Parallele Mouse 5K SNP panel, the congenic mice we generated are well over 99.8% identical to the C57BL/6J mice. The extensive backcrossing also eliminates any potential epigenetic factors that may have existed in the founder mice.
Our data show that the congenic mice recapitulate the ecSOD phenotype in an allele-specific manner, in terms of free and heparin-releasable plasma ecSOD levels and activities, tissue enzyme distribution and relative tissue mRNA abundance. These results suggest that a substantial portion of the observed ecSOD phenotype is allele-dependent. It should be noted that the analysis of total tissue enzyme activity and levels does not discriminate between enzymes localized extracellularly or still within the secretory pathway. Heparin administration, on the other hand, can only clear enzyme that is accessible: bound to the vascular cell surface or the endothelial lining of various organs [35]. Our data suggest that the sod129 mice have a larger pool size of the enzyme in most, but especially highly vascular tissues, including the liver and kidney and thus a higher heparin-accessible pool size. The physiological consequence of this is yet to be determined. The mature enzyme differs in a single amino acid substitution (A186S) which is unlikely to have an effect on its ability to form tetramers and thus influence the amounts of heparin-releasable enzyme. Both isoforms of the enzyme elute, upon FPLC chromatography at an identical point corresponding to ~130 kDa (data not shown). Heparin administration is not informative in tissues like lung or brain. The physiologically relevant location of ecSOD in the alveolae may make the enzyme inaccessible to heparin, while the extent of heparin penetration of the blood-brain barrier is uncertain. The apparent and consistent increase in aortic ecSOD content and activity after heparin administration may be due to a large influx of uncleaved ecSOD (released from peripheral tissues) that is able to re-bind to the endothelial lining of the vessel, while the concentration of heparin may be falling, due to “trapping” in the peripheral tissues.
The significant discrepancy in the relative enzyme content in the tissues of congenic mice vs. the amounts of corresponding mRNA is also of interest. At this point we can only speculate about potential differences in the mRNA translational efficiency and protein processing during the synthetic/secretory pathway. Our data using a stably transfected CHO cell line expressing either of the ecSOD alleles are consistent with this observation. Given equal copy numbers of the ecSOD allele, CHO cells transfected with the 129 allele consistently secrete larger amounts of ecSOD [36].
We suggest that the N21D (N-4D in the mature protein) mutation may play an important role. The effect of this change may result in significant changes in the signal peptide processing. While the precise processing site of the wt signal peptide is not known, the well-accepted −3, −1 rule [34, 37] and computer analysis (SignalP 3.0 http://www.cbs.dtu.dk/services/SignalP/), points to the −4 aa (Asn in wt, Asp in 129 alleles). The relative cleavage site probability at this point rises from 0.611 in the wt product to 0.771 in the 129 isoform. The change at this position from a polar, uncharged Asn to a negatively charged Asp may thus lead to increased efficiency of processing and secretion. Different susceptibilities of the two transcripts to translational regulation or degradation may also play a role. Clearly more work is required in the future to elucidate this discrepancy.
A similar transcript (N21D and a 10 bp deletion) has been identified in apoE/LDLR KO mice and its appearance was erroneously reported to occur due post-transcriptional modification induced by atherosclerosis [38]. A more likely explanation is the significant extent of heterozygosity, with respect to ecSOD, in the apoE/LDLR KO mice as described previously [1]. A substantial portion of these mice carry over the 129 allele for ecSOD from the embryonic stem cells derived from the 129 strain and used in the generation of the knock-out mice.
Recently, Ganguly et al. [39] identified an ecSOD transcript variant in the JF1/Ms (JF1) mouse strain, which has several mutations, including two (A61G and G556T) point mutations that are shared with the 129 strain and associated with lower lung function [40]. The JF1 mouse strain has 2–3 fold lower mRNA as well as protein levels in the lung compared to C3H strain which express wt ecSOD. It does not share the 10bp deletion in the 3' UTR of its transcript (L. Dory, unpublished observation). It should be emphasized however that the comparison of different variants of ecSOD transcripts and their products is not informative, when compared in a context of large differences in the rest of the genome. This is precisely the reason we generated the congenic mice.
The availability of congenic mice with different ecSOD phenotypes within an otherwise identical genome provides an important tool to investigate the role of this enzyme in various diseases and a tool to study the transcription and translational regulation of ecSOD expression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pierce A, Whitlark J, Dory L. Extracellular superoxide dismutase polymorphism in mice. Arterioscler. Thromb. Vasc. Biol. 2003;23:1820–1825. doi: 10.1161/01.ATV.0000089011.51491.A6. [DOI] [PubMed] [Google Scholar]
- [2].Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U. S. A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismutase: A regulator of nitric oxide bioavailability. Lab. Invest. 1996;75:617–636. [PubMed] [Google Scholar]
- [4].Brahmajothi MV, Campbell DL. Heterogeneous basal expression of nitric oxide synthase and superoxide dismutase isoforms in mammalian heart : Implications for mechanisms governing indirect and direct nitric oxide-related effects. Circ. Res. 1999;85:575–587. doi: 10.1161/01.res.85.7.575. [DOI] [PubMed] [Google Scholar]
- [5].Ushio-Fukai M. Redox signaling in angiogenesis: Role of NADPH oxidase. Cardiovasc. Res. 2006;71:226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- [6].Capettini LS, Cortes SF, Gomes MA, Silva GA, Pesquero JL, Lopes MJ, Teixeira MM, Lemos VS. Neuronal nitric oxide synthase-derived hydrogen peroxide is a major endothelium-dependent relaxing factor. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2503–11. doi: 10.1152/ajpheart.00731.2008. [DOI] [PubMed] [Google Scholar]
- [7].Faraci FM, Didion SP. Vascular protection: Superoxide dismutase isoforms in the vessel wall. Arterioscler. Thromb. Vasc. Biol. 2004;24:1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- [8].Fattman CL, Tan RJ, Tobolewski JM, Oury TD. Increased sensitivity to asbestos-induced lung injury in mice lacking extracellular superoxide dismutase. Free Radic. Biol. Med. 2006;40:601–607. doi: 10.1016/j.freeradbiomed.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: In vivo and ex vivo evidence from ecSOD-deficient mice. Circ. Res. 2003;93:622–629. doi: 10.1161/01.RES.0000092140.81594.A8. [DOI] [PubMed] [Google Scholar]
- [10].Kim HW, Lin A, Guldberg RE, Ushio-Fukai M, Fukai T. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circ. Res. 2007;101:409–419. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- [11].Sheng H, Kudo M, Mackensen GB, Pearlstein RD, Crapo JD, Warner DS. Mice overexpressing extracellular superoxide dismutase have increased resistance to global cerebral ischemia. Exp. Neurol. 2000;163:392–398. doi: 10.1006/exnr.2000.7363. [DOI] [PubMed] [Google Scholar]
- [12].Chen EP, Bittner HB, Davis RD, Folz RJ, Van Trigt P. Extracellular superoxide dismutase transgene overexpression preserves postischemic myocardial function in isolated murine hearts. Circulation. 1996;94:II412–7. [PubMed] [Google Scholar]
- [13].Ghio AJ, Suliman HB, Carter JD, Abushamaa AM, Folz RJ. Overexpression of extracellular superoxide dismutase decreases lung injury after exposure to oil fly ash. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L211–8. doi: 10.1152/ajplung.00409.2001. [DOI] [PubMed] [Google Scholar]
- [14].Levin ED. Extracellular superoxide dismutase (EC-SOD) quenches free radicals and attenuates age-related cognitive decline: Opportunities for novel drug development in aging. Curr. Alzheimer Res. 2005;2:191–196. doi: 10.2174/1567205053585710. [DOI] [PubMed] [Google Scholar]
- [15].Gao F, Koenitzer JR, Tobolewski JM, Jiang D, Liang J, Noble PW, Oury TD. Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan. J. Biol. Chem. 2008;283:6058–6066. doi: 10.1074/jbc.M709273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kliment CR, Tobolewski JM, Manni ML, Tan RJ, Enghild J, Oury TD. Extracellular superoxide dismutase protects against matrix degradation of heparan sulfate in the lung. Antioxid. Redox Signal. 2008;10:261–268. doi: 10.1089/ars.2007.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kliment CR, Englert JM, Gochuico BR, Yu G, Kaminski N, Rosas I, Oury TD. Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J. Biol. Chem. 2009;284:3537–3545. doi: 10.1074/jbc.M807001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sandstrom J, Carlsson L, Marklund SL, Edlund T. The heparin-binding domain of extracellular superoxide dismutase C and formation of variants with reduced heparin affinity. J. Biol. Chem. 1992;267:18205–18209. [PubMed] [Google Scholar]
- [19].Karlsson K, Edlund A, Sandstrom J, Marklund SL. Proteolytic modification of the heparin-binding affinity of extracellular superoxide dismutase. Biochem. J. 1993;290(Pt 2):623–626. doi: 10.1042/bj2900623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Petersen SV, Oury TD, Ostergaard L, Valnickova Z, Wegrzyn J, Thogersen IB, Jacobsen C, Bowler RP, Fattman CL, Crapo JD, Enghild JJ. Extracellular superoxide dismutase (EC-SOD) binds to type i collagen and protects against oxidative fragmentation. J. Biol. Chem. 2004;279:13705–13710. doi: 10.1074/jbc.M310217200. [DOI] [PubMed] [Google Scholar]
- [21].Sandstrom J, Karlsson L, Edlund T, Marklund SL. Heparin-affinity patterns and composition of extracellular superoxide dismutase in human plasma and tissues. Biochem J. 1993;294(Pt 3):853–7. doi: 10.1042/bj2940853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stralin P, Karlsson K, Johansson BO, Marklund SL. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler. Thromb. Vasc. Biol. 1995;15:2032–2036. doi: 10.1161/01.atv.15.11.2032. [DOI] [PubMed] [Google Scholar]
- [23].Karlsson K, Sandstrom J, Edlund A, Marklund SL. Turnover of extracellular-superoxide dismutase in tissues. Lab. Invest. 1994;70:705–710. [PubMed] [Google Scholar]
- [24].Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismutase in vessels and airways of humans and baboons. Free Radic. Biol. Med. 1996;20:957–965. doi: 10.1016/0891-5849(95)02222-8. [DOI] [PubMed] [Google Scholar]
- [25].Warshamana GS, Pociask DA, Sime P, Schwartz DA, Brody AR. Susceptibility to asbestos-induced and transforming growth factor-beta1-induced fibroproliferative lung disease in two strains of mice. Am. J. Respir. Cell Mol. Biol. 2002;27:705–713. doi: 10.1165/rcmb.2002-0096OC. [DOI] [PubMed] [Google Scholar]
- [26].Fujii M, Hara H, Meng W, Vonsattel JP, Huang Z, Moskowitz MA. Strain-related differences in susceptibility to transient forebrain ischemia in SV-129 and C57black/6 mice. Stroke. 1997;28:1805–10. doi: 10.1161/01.str.28.9.1805. discussion 1811. [DOI] [PubMed] [Google Scholar]
- [27].Ward NL, Moore E, Noon K, Spassil N, Keenan E, Ivanco TL, LaManna JC. Cerebral angiogenic factors, angiogenesis, and physiological response to chronic hypoxia differ among four commonly used mouse strains. J. Appl. Physiol. 2007;102:1927–1935. doi: 10.1152/japplphysiol.00909.2006. [DOI] [PubMed] [Google Scholar]
- [28].Paigen B, Ishida BY, Verstuyft J, Winters RB, Albee D. Atherosclerosis susceptibility differences among progenitors of recombinant inbred strains of mice. Arteriosclerosis. 1990;10:316–323. doi: 10.1161/01.atv.10.2.316. [DOI] [PubMed] [Google Scholar]
- [29].Prass K, Wiegand F, Schumann P, Ahrens M, Kapinya K, Harms C, Liao W, Trendelenburg G, Gertz K, Moskowitz MA, Knapp F, Victorov IV, Megow D, Dirnagl U. Hyperbaric oxygenation induced tolerance against focal cerebral ischemia in mice is strain dependent. Brain Res. 2000;871:146–150. doi: 10.1016/s0006-8993(00)02264-2. [DOI] [PubMed] [Google Scholar]
- [30].Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- [31].Marklund SL. Analysis of extracellular superoxide dismutase in tissue homogenates and extracellular fluids. Methods Enzymol. 1990;186:260–265. doi: 10.1016/0076-6879(90)86117-e. [DOI] [PubMed] [Google Scholar]
- [32].Paoletti F, Mocali A. Determination of superoxide dismutase activity by purely chemical system based on NAD(P)H oxidation. Methods Enzymol. 1990;186:209–220. doi: 10.1016/0076-6879(90)86110-h. [DOI] [PubMed] [Google Scholar]
- [33].Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- [34].Folz RJ, Guan J, Seldin MF, Oury TD, Enghild JJ, Crapo JD. Mouse extracellular superoxide dismutase: Primary structure, tissue-specific gene expression, chromosomal localization, and lung in situ hybridization. Am. J. Respir. Cell Mol. Biol. 1997;17:393–403. doi: 10.1165/ajrcmb.17.4.2826. [DOI] [PubMed] [Google Scholar]
- [35].Karlsson K, Marklund SL. Heparin-induced release of extracellular superoxide dismutase to human blood plasma. Biochem. J. 1987;242:55–59. doi: 10.1042/bj2420055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mirossay A, Jun S, Dory L. Cloning and characterization of two alleles of the murine extracellular superoxide dismutase gene. Biochem. Biophys. Res. Commun. 2007;352:739–743. doi: 10.1016/j.bbrc.2006.11.081. [DOI] [PubMed] [Google Scholar]
- [37].Folz RJ, Gordon JI. Computer-assisted predictions of signal peptidase processing sites. Biochem. Biophys. Res. Commun. 1987;146:870–877. doi: 10.1016/0006-291x(87)90611-5. [DOI] [PubMed] [Google Scholar]
- [38].Fukai T, Galis ZS, Meng XP, Parthasarathy S, Harrison DG. Vascular expression of extracellular superoxide dismutase in atherosclerosis. J. Clin. Invest. 1998;101:2101–2111. doi: 10.1172/JCI2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ganguly K, Stoeger T, Wesselkamper SC, Reinhard C, Sartor MA, Medvedovic M, Tomlinson CR, Bolle I, Mason JM, Leikauf GD, Schulz H. Candidate genes controlling pulmonary function in mice: Transcript profiling and predicted protein structure. Physiol. Genomics. 2007;31:410–421. doi: 10.1152/physiolgenomics.00260.2006. [DOI] [PubMed] [Google Scholar]
- [40].Ganguly K, Schulz H. Association studies of lung function in mice. Dtsch. Tierarztl. Wochenschr. 2008;115:276–284. [PubMed] [Google Scholar]