Abstract
Autophagy is a process of self-degradation of cellular components in which double-membrane autophagosomes sequester organelles or portions of cytosol and fuse with lysosomes or vacuoles for breakdown by resident hydrolases. Autophagy is upregulated in response to extra- or intracellular stress and signals such as starvation, growth factor deprivation, ER stress, and pathogen infection. Defective autophagy plays a significant role in human pathologies, including cancer, neurodegeneration, and infectious diseases. We present our current knowledge on the key genes composing the autophagy machinery in eukaryotes from yeast to mammalian cells and the signaling pathways that sense the status of different types of stress and induce autophagy for cell survival and homeostasis. We also review the recent advances on the molecular mechanisms that regulate the autophagy machinery at various levels, from transcriptional activation to post-translational protein modification.
Keywords: lysosome, Atg proteins, target of rapamycin, stress, pathogen, transcription
Introduction
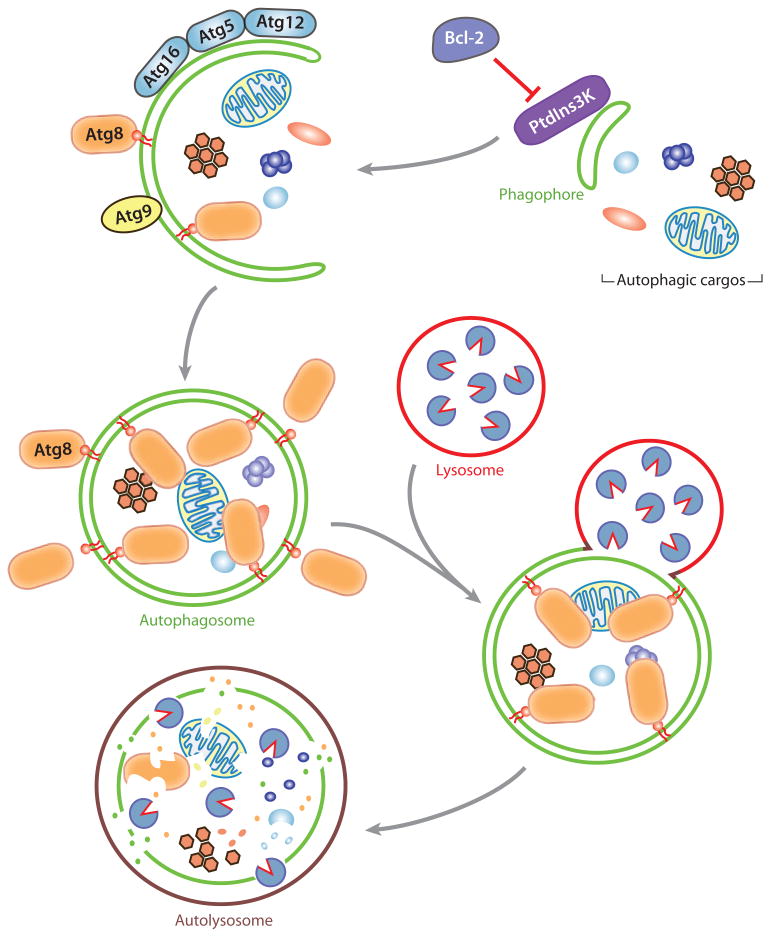
Cell homeostasis is achieved by balancing biosynthesis and turnover. In eukaryotic cells, the lysosome (or the analogous yeast and plant vacuole) is the primary organelle for degradation through its wide array of resident acid hydrolases. As an adaptive response in unfavorable conditions such as nutrient deprivation, autophagy mediates a highly regulated self-eating process via lysosomes. Double-membrane vesicles, termed autophagosomes, engulf long-lived proteins, damaged organelles, and even invasive pathogens, and transport these cargos to the lysosomes. There, the outer-membrane of the autophagosome fuses with the lysosomal membrane, and the inner vesicle, together with its cargo, is degraded (Figure 1). The resulting macromolecules can be recycled back to the cytosol for reuse during starvation (165). Malfunction of autophagy contributes to a variety of diseases, including cancer, neurodegeneration, cardiovascular disorders, and microbe infection, because efficient sequestration and clearance of unneeded or damaged cellular or nonself components is crucial for cell survival and function.
Figure 1.
Schematic model of autophagy. The class III PtdIns3K complex mediates nucleation of the phagophore membrane, enwrapping cytosolic proteins, protein aggregates, and organelles (such as mitochondria). Bcl-2 blocks this step by binding and inhibiting Beclin 1, a component in the PtdIns3K complex. Atg12–Atg5-Atg16 and Atg8–PE conjugates are recruited to the phagophore, together with the transmembrane protein Atg9, facilitating the phagophore expansion step. Upon vesicle completion, most of the Atg proteins are dissociated from the autophagosome, allowing autophagosome-lysosome fusion and cargo degradation by lysosomal proteases.
Autophagosomes have been observed by electron microscopy in mammalian cells since as early as the 1950s (68), whereas the molecular era of autophagy study began little more than a decade ago, starting primarily from genetic screens in the budding yeast Saccharomyces cerevisiae and the methylotrophic yeasts Pichia pastoris and Hansenula polymorpha, leading to the identification of 31 autophagy-related (ATG) genes. The Atg proteins function at several physiologically continuous steps in autophagy (for instance, induction, cargo recognition and packaging, and vesicle formation and breakdown) and orchestrate much of the process. Many ATG homologs have subsequently been identified and characterized in higher eukaryotes, suggesting that autophagy is a highly conserved pathway through evolution. In fungal cells, the site of autophagosome formation is termed the phagophore assembly site (PAS, also known as the preautophagosomal structure). This is the location where the phagophore, the initial sequestering structure, expands and the Atg proteins are recruited. Whereas in mammalian cells autophagosomes emerge from multiple sites, fungi appear to have one distinct PAS adjacent to the vacuole, which allows convenient studies of the mechanisms and dynamic stages of autophagosome biogenesis.
Although bulk cytosol can be randomly turned over through autophagy, in many cases autophagy displays substrate-specificity. For example, two yeast vacuolar proteases, α-mannosidase and the precursor form of aminopeptidase I [Ape1 (prApe1)], are synthesized in the cytoplasm and transported to the vacuole via a selective autophagy pathway, known as the cytoplasm-to-vacuole targeting (Cvt) pathway. The Cvt pathway is mechanistically and genetically similar to bulk autophagy, except that it occurs constitutively in normal growing conditions and in contrast to autophagy is biosynthetic (70). In addition, ubiquitinated protein aggregates, and damaged or superfluous organelles are selectively targeted for degradation by autophagy. Different terms have been used to describe the selectivity of each process according to the cargo, such as autophagic degradation of mitochondria (mitophagy) (63), ribosomes (ribophagy) (73), peroxisomes (pexophagy) (25), and endoplasmic reticulum (ER; reticulophagy) (5, 69). This review provides an overview of recent advances in our understanding of the regulation of different types of autophagy in response to various stimuli or inhibitors, involving signaling pathways, transcription factors, and protein modifiers, as well as functions and interactions of the Atg proteins.
Molecular Machinery of Autophagy
Autophagy-Related (Atg) Proteins: The Core Machinery
The autophagy process is divided into mechanistically distinct steps, including induction, cargo recognition and selection, vesicle formation, autophagsome-vacuole fusion, and breakdown of the cargo followed by release of the degradation products back into the cytosol. Different sets of Atg proteins are involved in these steps and consist of the core autophagic machinery.
Induction
Basal-level autophagy is very low under normal conditions; therefore, an efficient mechanism to induce autophagy is crucial for organisms to adapt to stress and extracellular cues. A central inhibitor of autophagy is the serine/threonine protein kinase TOR (target of rapamycin). In yeast and Drosophila, Tor/dTOR integrates input information from multiple upstream signal transduction pathways (discussed below) and negatively regulates another serine/threonine kinase, Atg1, in nutrient-rich conditions (15, 59, 133) (Figure 2b). In yeast, upon Tor inhibition by starvation or rapamycin treatment, the kinase activity of Atg1 is activated and Atg1 binding affinity to Atg13 and Atg17 may also increase (59), which promotes the formation of an Atg1-Atg13-Atg17 scaffold and the recruitment of multiple Atg proteins to the PAS to initiate autophagosome formation (20, 21, 57, 60, 144). Thus, a role of the Atg1 kinase complex in protein recruitment is indispensable for autophagy induction. Moreover, in Drosophila, Atg1 is able to inhibit the phosphorylation and activation of a downstream TOR effector, S6K, during nutrient starvation (77), yet it is not clear how S6K signaling modulates other autophagy proteins and/or autophagy activity.
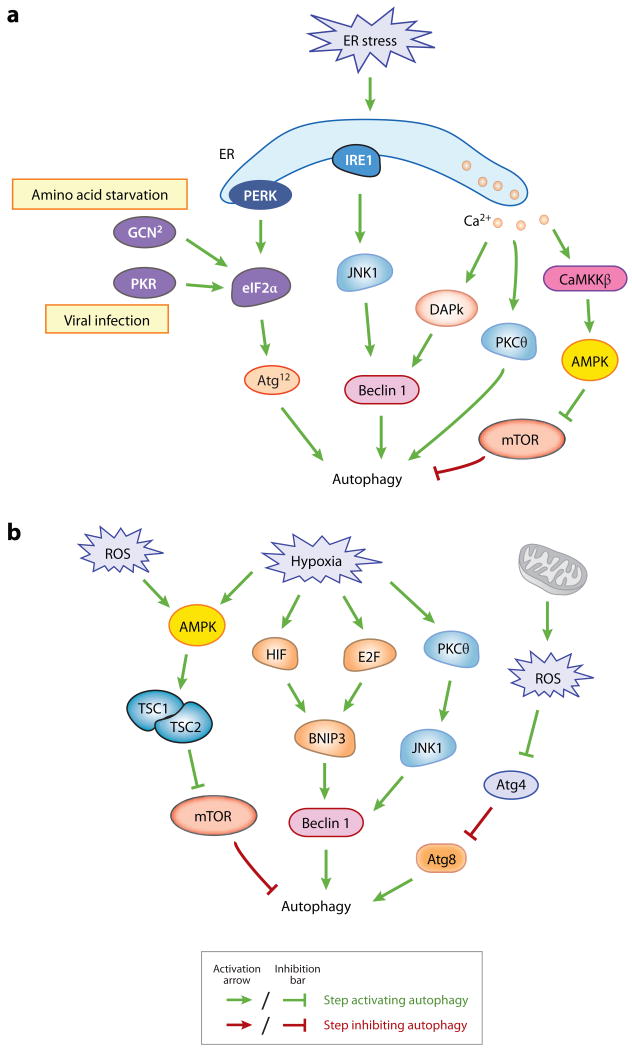
Figure 2.
Autophagy is induced by deprivation of nutrients, hormones, and energy. (a) Regulatory pathways of autophagy by amino acids, hormones, and energy in mammals. (b) Signaling of autophagy in yeast.
There are two mammalian homologs of Atg1 that appear to function in autophagy, the Unc-51-like kinase 1 (ULK1) and -2 (ULK2), and one homolog of yeast Atg17, FIP200 (the focal adhesion kinase family-interacting protein of 200 kD), which forms a complex with ULKs and mammalian Atg13 and localizes to the phagophore upon starvation (38, 56). Regarding the substrates of the Atg1 kinase during autophagy, it is suggested that mammalian Atg13 and FIP200 are phosphorylated by ULKs (56), and ULKs also undergo autophosphorylation, which is conducive to a conformational change and autophagy induction (13). Different from what has been reported in yeast, ULKs-Atg13-FIP200 seem to form a stable complex regardless of nutritional conditions in mammalian cells. mTOR interacts with, phosphorylates, and inactivates ULKs and Atg13 under nutrient-rich conditions. Upon mTOR inhibition by starvation or rapamycin, ULK1 and ULK2 are activated and phosphorylate Atg13 and FIP200, which are essential for autophagy activity (41, 56). These studies raise an interesting hypothesis that Atg13 is phosphorylated by TOR or Atg1/ULKs on different residues, which may exert opposite effects on autophagy induction dependent on nutrient status. Indeed, yeast Atg13 is rapidly dephosphorylated during starvation (59), whereas Atg13 phosphorylation is enhanced during autophagic conditions in Drosophila (15); it is likely that phosphorylation of Atg13 is dependent more on Tor in yeast, and to a greater extent on Atg1 in Drosophila. Atg101, a newly identified protein component in the ULKs-Atg13-FIP200 complex, binds and stabilizes Atg13, and is required for autophagy in mammals (91).
Cargo recognition and selectivity
In selective autophagy, cargos are recognized through interactions with specific receptor proteins. The yeast Cvt pathway delivers prApe1 into the vacuole and generates the mature enzyme Ape1. The cargo prApe1 contains a vacuolar-targeting signal, which can be recognized and bound by the receptor protein Atg19. An adaptor protein, Atg11, binds Atg19 and recruits the Atg19-prApe1 complex to the PAS, where Atg19 interacts with one of the key components in the vesicle-forming machinery, Atg8, for packaging the receptor-cargo complex into Cvt vesicles (analogous to autophagosomes) (134, 137).
In multicellular organisms, an important function of autophagy is the clearance of cytosolic ubiquitinated substrates or aggregate-prone proteins. Recent studies suggest that this degradative process is also selective and mediated through the mammalian protein p62/sequestosome 1 (SQSTM1) (6, 114), or Ref(2)P, the Drosophila homolog of p62 (99). p62 directly binds both poly- or mono-ubiquitin via its ubiquitin-associated (UBA) domain and the mammalian Atg8 homolog, LC3 (microtubule-associated protein 1 light chain 3) (64, 110), and links the ubiquitinated cargos to the autophagy machinery for autophagic degradation. The structural and functional similarity between a C-terminal motif in mammalian p62 and yeast Atg19 (103) gives rise to an intriguing hypothesis that p62 is an Atg19 analog in higher eukaryotes and acts as a receptor for ubiquitinated proteins or organelles in selective autophagy. Recent work shows that the P granule proteins, the germ cell determinant in Caenorhabditis elegans, are also selectively degraded by autophagy in somatic cells (173). The cargo receptor, SEPA-1 (suppressor of ectopic P granule in autophagy mutants 1), directly interacts with the P granule components and LGG-1, the Atg8 homolog in C. elegans, and thus functions similarly to Atg19 and p62.
Another selective route of autophagy is pexophagy, and its mechanism has been best studied in the methylotrophic yeast Pichia pastoris. Peroxisome formation is greatly induced by growth on methanol as the sole carbon source; when the medium is replenished with glucose or ethanol, the peroxisomes are no longer needed and are selectively degraded in the vacuole through pexophagy. In P. pastoris, PpAtg30 functions as a peroxisome receptor by interacting both with peroxisomal membrane proteins PpPex14 and PpPex3 and with autophagy proteins PpAtg11 and PpAtg17, and therefore links peroxisomes destined for degradation to the PAS (28). A notable feature of the aforementioned receptor proteins is the high level of pathway-specificity. For example, Atg19 is not required for bulk autophagy or pexophagy, and PpAtg30 is not involved in the Cvt or bulk autophagy pathways.
Autophagosome formation
Unlike processes of vesicle formation in most endomembrane trafficking systems, double-membrane autophagosomes appear to be assembled at the PAS by addition of new membranes, rather than being generated by budding from the surface of a preexisting organelle or sealing of a single piece of continuous membrane. Thus, formation of the sequestering vesicles is likely the most complex step of autophagy. Multiple Atg proteins are recruited to the phagophore to participate in autophagosome formation, and this step requires the highly regulated coordination of all of these proteins (Figure 1).
The nucleation and assembly of the initial phagophore membrane requires the class III phosphatidylinositol 3-kinase (PtdIns3K) complex, which is composed of the PtdIns3K Vps34 (vacuolar protein sorting 34), a myristoylated serine/threonine kinase Vps15 (p150 in mammalian cells), Atg14 (Barkor or mAtg14 in mammalian cells) and Atg6/Vps30 (Beclin 1 in mammalian cells) (48, 61, 81, 142). The function of Beclin 1 in autophagy is regulated by Bcl-2 (B-cell lymphoma/leukemia-2), an antiapoptotic protein that inhibits autophagy by binding and sequestering Beclin 1 under nutrient-rich conditions; dissociation of Beclin 1 from Bcl-2 is required for autophagy induction. The PtdIns3K complex produces PtdIns3P (phosphatidylinositol 3-phosphate) and is involved in PAS targeting of a number of yeast Atg proteins that bind PtdIns3P, such as Atg18, Atg20, Atg21, and Atg24 (100, 105, 141). In yeast, Atg20 and Atg24 interact with the Atg1-Atg13-Atg17 complex, and the latter mediates autophagy induction (discussed above); however, the mammalian homologs of Atg20 and Atg24 are either not identified (Atg20) or not well characterized in autophagy (Atg24). The PtdIns3K complex, in part together with the above Atg proteins, further recruits two interrelated ubiquitin-like (Ubl) conjugation systems, Atg12–Atg5-Atg16 and Atg8–PE (phosphatidylethanolamine), to the phagophore (143, 144), which play an essential role in regulating the membrane elongation and expansion of the forming autophagosome.
The two Ubl proteins, Atg12 and Atg8, undergo conjugation in a similar manner as ubiquitin. Atg12 is activated by Atg7 (E1 activating enzyme), transferred to Atg10 (E2 conjugating enzyme) and attached to an internal lysine of the substrate protein Atg5 covalently. In contrast to ubiquitination, Atg12–Atg5 conjugation is constitutive and irreversible, and apparently no substrate-specific E3 ligase counterpart is required in this process (34). The Atg12–Atg5 conjugate further interacts with a coiled-coil protein Atg16, which links the Atg12–Atg5-Atg16 complex into a tetramer by self-oligomerization and attaches it to the phagophore (92, 93). In the Atg8 conjugation system, Atg8 is first processed by a cysteine protease, Atg4, exposing a C-terminal glycine residue. The same E1 enzyme Atg7 activates Atg8 and transfers it to Atg3 (E2). Atg8 is finally conjugated to the target lipid PE via an amide bond, facilitated by the E3-like Atg12–Atg5 conjugate (32, 37), although Atg12–Atg5 lacks the conserved HECT or RING domains possessed by typical E3 ligases, and is not essential for conjugation to occur. In nutrient-rich conditions, the majority of Atg8 is cytosolic; upon autophagy induction, Atg8 largely exists as the lipid-conjugated form and is localized to both sides of the phagophore (58, 65). Atg8 controls the size of the autophagosome (158), which may result from its ability to determine membrane curvature. The lipidation of Atg8 and its mammalian homolog LC3 are widely used to monitor autophagy induction.
Various sources, including mitochondria, the Golgi complex, and the ER, are proposed to be the origins of the autophagosomal membrane (54, 119). However, it is not completely clear by what mechanism the additional membranes are delivered to and fused with the growing phagophore. SNAREs (N-ethylmaleimide-sensitive factor attachment protein receptors) or small GTPases have not been shown to directly play a role in autophagosome formation. Although the Golgi-resident GTPase Rab33B binds Atg16L (the mammalian Atg16 homolog), the function of this interaction in autophagy is unclear (49). Recent studies demonstrate that self-multimerization of Atg9 may facilitate membrane tethering and/or fusion (39). Atg9 is the only identified integral membrane protein required for autophagosome formation. In yeast, Atg9 localizes to the PAS and peripheral structures and is suggested to cycle between the two sites. Atg11, Atg23, and Atg27 are essential in the anterograde transport of Atg9 to the PAS (14, 40, 78, 163), and the Atg1-Atg13 complex, Atg2, Atg18, and the PtdIns3K complex are involved in its retrograde transport (120). Thus, Atg9 may function as a carrier in supplying membrane and may also play a role during phagophore expansion through its dynamic self-interaction. Similarly, mammalian Atg9 (mAtg9) transports from the trans-Golgi network (TGN) to late endosomes, which are colabeled with LC3, when autophagy is induced. In mammalian cells, it is the redistribution of mAtg9 from the TGN to late endosomes that is dependent on ULK1 and human Atg13 (13, 168).
Vesicle fusion and autophagosome breakdown
When autophagosome formation is completed, Atg8 attached to the outer membrane is cleaved from PE by Atg4 and released back to the cytosol (66). However, the retrieval and uncoating mechanisms of other Atg proteins remain to be studied. Autophagosome-lysosome fusion is mediated by the same machinery that is involved in homotypic vacuole membrane fusion. In mammalian cells, the fusion event requires the lysosomal membrane protein LAMP-2 and the small GTPase Rab7 (51, 147), although the mechanism is less characterized. In yeast, the machinery consists of the Rab family GTPase Ypt7 (the homolog of Rab7), the NSF homolog Sec18, the SNARE proteins Vam3, Vam7, Vti1, and Ykt6, the class C Vps/HOPS complex proteins, and two other proteins, Ccz1 and Mon1 (67).
After fusion, degradation of the inner vesicle is dependent on a series of lysosomal/vacuolar acid hydrolases, including proteinases A and B (encoded by PEP4 and PRB1, respectively) and the lipase Atg15 in yeast (26, 149) and cathepsin B, D (a homolog of proteinase A), and L in mammalian cells (148). The resulting small molecules from the degradation, particularly amino acids, are transported back to the cytosol for protein synthesis and maintenance of cellular functions under starvation conditions. The identification of Atg22, together with other vacuolar permeases (such as Avt3 and Avt4) as vacuolar amino acid effluxers during yeast autophagy (160), has helped in the understanding of the mechanisms of nutrient recycling; these permeases represent the last step in the degradation and recycling process.
Non-Atg Components Required for Autophagy
Besides the aforementioned Atg proteins, certain subcellular systems, including the secretory pathway, the endocytic pathway, and the cytoskeletal network, may also carry out essential functions during autophagy, such as providing membrane, facilitating autophagosome transport, and enabling clearance of autophagic substrates.
The secretory and endocytic pathways
Autophagy involves dramatic subcellular membrane remodeling. The role of the secretory pathway in autophagy is largely illuminated by studies in yeast, where a functional ER and Golgi complex are required for autophagy and the Cvt pathway (47, 121). A subset of GTP exchange factors, including Sec12 and Sec16, and two coatomer subunits of the COPII coat, Sec23 and Sec24, are needed for the biogenesis of autophagosomes but not Cvt vesicles (47); although it is possible that a defect in the Cvt pathway is more subtle compared with nonselective autophagy because of the relatively reduced need for membrane. Nonetheless, there may be some aspects of vesicle formation that are unique to either autophagy or the Cvt pathway. For example, the SNARE-Sec complex containing the tSNARE Tlg2, the vSNARE Tlg1 and the Sec1 homolog Vps45, the Vps-fifty-three (VFT) complex, and the sorting nexins Atg20 and Atg24 are required solely for Cvt vesicle formation (1, 100, 122). In addition, Trs85, a component in the TRAPP (transport protein particle) complexes, which mediate ER-to-Golgi and intra-Golgi trafficking, is essential for both nonselective autophagy and selective autophagic processes such as the Cvt pathway and pexophagy (90, 98). Although the underlying mechanism is not well characterized, it is likely that the early secretory mutants affect the membrane flow or correct sorting of some Atg proteins, such as Atg9 transport to the PAS.
Although completed autophagosomes can directly fuse with lysosomes, when cells are overloaded with aggregate-prone proteins, fusion of autophagosomes with the endocytic compartments is essential to facilitate efficient autophagic removal of these proteins. In Drosophila and mammalian cells, the ESCRT (endosomal sorting complexes required for transport) machinery and multivesicular body (MVB)-localized Rab11 play an important role in the fusion of MVBs with completed autophagosomes, and the Drosophila endosomal PtdIns3P 5-kinase Fab1 is involved in fusion of the resulting amphisomes with lysosomes (27, 30, 125). Both steps are required for promoting degradation of aggregate-prone substrate proteins sequestered by autophagosomes.
Cytoskeleton
Efficient protein trafficking during autophagosome formation is presumably mediated by cytoskeletal networks. For example, a functional actin cytoskeleton and the Arp (actin-related protein) 2/3 complex, which nucleates branching of actin filaments, are required for Atg9 anterograde transport to the PAS and selective autophagy activity in yeast (94, 118). Microtubules are involved in autophagy in higher eukaryotes. In primary rat hepatocytes, the microtubule depolymerizing drug nocodazole inhibits autophagosome formation (71). In addition, microtubules, the tubulin deacetylase HDAC6, and the microtubule motor protein dynein are required for the autophagic clearance of various aggregate-prone proteins in flies and mammalian cells (50, 109, 117). In mammalian cells, it appears that autophagosomes are formed at random locations in the cell, but transported directionally toward the nucleus after completion (52). Several lines of evidence suggest that autophagosomes associate with microtubule tracks, move to the microtubule-organizing centre, and fuse with endosomes or lysosomes, and the dynamic process is driven by the dynein motor (29, 52, 71).
Signaling Pathways Regulating Autophagy
Nutrient Signaling
During nutrient deprivation, autophagosome formation is dramatically induced. In both yeast and mammalian cells, two well-characterized signaling cascades that sense nutrient status, activate cell division and growth, and negatively regulate autophagy are the TOR and Ras-cAMP-PKA pathways.
TOR complex 1
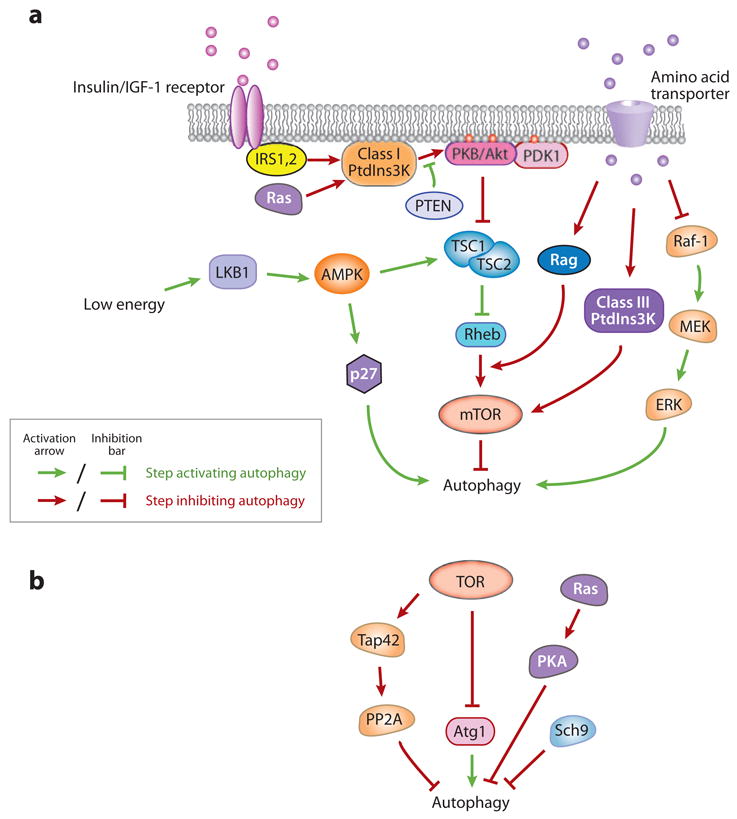
TOR complex 1 (TORC1) is sensitive to inhibition by rapamycin. Inactivation of TORC1 by rapamycin stimulates autophagy in the presence of nutrients, suggesting that TOR downregulates autophagy (104). Extracellular amino acids enter mammalian cells through transporters such as SLC1A5 (solute carrier family 1 member 5) and SLC7A5 (101), and it is proposed that mTORC1 directly senses and is phosphorylated in response to nutrient signals (84); however, recent observations in Drosophila and mammals suggest that Rag proteins, the Ras-related small GTPases, activate TORC1 in response to amino acids (62) through mediating translocation of TORC1 to a specific subcellular compartment that contains the TORC1 activator Rheb (Ras homolog enriched in brain) (127). Other studies indicate that amino acids also activate mTOR via class III PtdIns3K (hVps34) (10, 102) (Figure 2a). The presence of amino acids stimulates hVps34, which leads to mTOR activation and autophagy inhibition. However, this creates a discrepancy with the role of the class III Pt-dIns3K in promoting nucleation and assembly of Atg proteins at early steps of autophagosome formation (53). A possible explanation is that the PtdIns3K exists in distinct subpopulations or protein complexes in the cell, which carry out different functions, or function at different times.
Besides regulating the Atg1/ULK complex, in yeast TORC1 also suppresses autophagy via phosphorylation of Tap42, which activates the catalytic subunits of PP2A (the serine/threonine protein phosphatase 2A), a negative regulator of autophagy (164) (Figure 2b). Although downstream targets of PP2A have not been identified, it is possible that Atg proteins may be directly involved, such as the Atg1 kinase complex (as described above) and other phosphorylated Atg proteins.
The Ras/PKA pathway
The Ras/cAMP-dependent protein kinase A (PKA) signaling pathway plays an important role in glucose sensing from yeast to mammals. Yeast PKA contains a heterotetramer composed of the regulatory subunit Bcy1 and three apparently redundant catalytic subunits Tpk1, Tpk2, and Tpk3. In nutrient-rich conditions, the small GTPases Ras1 and Ras2 are active and enhance cAMP generation by the adenylyl cyclase. Elevated cAMP binds to Bcy1 and releases its inhibitory effect on PKA. Constitutive activation of the Ras/PKA pathway suppresses autophagy induced by TOR inhibition in yeast (9, 131), suggesting that the Ras-PKA pathway downregulates autophagy in parallel with the TOR-Tap42 pathway. Autophagy inhibition by Ras/PKA may be mediated through regulation of Atg1, which is identified as a phosphorylation substrate of PKA (8) (Figure 2b). In the presence of nutrients, PKA phosphorylation causes Atg1 to be largely cytosolic and dissociated from the PAS, whereas during starvation, Atg1 is dephosphorylated and localized to the PAS. It should be noted that Atg1 may not be the sole PKA target because a hyperactive Ras mutant, Ras2G19V, which constitutively activates PKA signaling, is still able to inhibit autophagy in cells expressing an Atg1 variant lacking the PKA phosphorylation sites.
In addition to PKA, the protein kinase Sch9, the closest yeast homolog to the mammalian protein kinase B (PKB)/Akt as well as to the TOR target S6 kinase (S6K), is involved in nutrient sensing (170). Simultaneous inactivation of PKA and Sch9 induces autophagy, which can be further increased by inactivation of TORC1 (167), suggesting that autophagy is negatively regulated by at least three parallel pathways in yeast, TORC1, Ras/PKA, and Sch9 (Figure 2b). Ras/PKA and Sch9 may regulate autophagy at the transcriptional level, as the transcription factors Msn2/4 and the Rim15 kinase are required for autophagic flux induced by inactivation of both PKA and Sch9, but not for that induced by inactivation of TOR (167).
Insulin/Growth Factor Pathways
When growth factors are withdrawn from the extracellular milieu, in spite of sufficient nutrients, autophagy is induced and is indispensable for maintaining cellular functions and energy production (85). In higher eukaryotes such as Drosophila and mammalian cells, the pathways through which hormones regulate autophagy are different from those of nutrients, but both converge on TOR (Figure 2a). Insulin and insulin-like growth factors regulate mTOR through the class I PtdIns3K. Upon insulin binding, autophosphorylation of the insulin receptor on tyrosine residues results in the recruitment and phosphorylation of IRS1 and IRS2 (insulin receptor substrate 1 and 2), which creates a docking scaffold that allows binding of adaptor proteins, including subunits of the class I PtdIns3K such as p85. Generation of PIP3 (phosphatidylinositol (3,4,5)-trisphosphate; Figure 2a, red circles in the membrane) by the class I PtdIns3K increases membrane recruitment of both protein kinase B (PKB)/Akt and its activator PDK1 (phosphoinositide-dependent protein kinase 1), leading to phosphorylation and activation of PKB/Akt by PDK1 (2, 140). The 3′-phosphoinositide phosphatase PTEN reverses PIP3 production, decreases the downstream PKB/Akt signaling and thus positively regulates autophagy (3). Activated PKB/Akt promotes phosphorylation of the protein encoded by the TSC2 tumor suppressor gene that is mutated in the tuberous sclerosis complex (TSC) tumor syndrome. The phosphorylation blocks TSC2 interaction with TSC1 and prevents formation of the TSC1/2 complex (88), which causes Rheb to exist in the active GTP-bound form (45, 172) and allows it to directly bind and activate mTORC1 (83). When hormones are absent, mTOR is inactivated, which releases the inhibitory effect on autophagy.
Besides TOR, Ras signaling also plays a role in autophagy regulation by growth factors (Figure 2a). Ras transduces signals from growth factor receptor tyrosine kinases to intracellular effectors, such as Raf-1/MAP (mitogen-activated protein) kinases and the class I PtdIns3K. In NIH3T3 mouse embryonic fibroblasts (MEFs), activated Ras suppresses autophagy through the class I PtdIns3K, but not through Raf-1 (33). In contrast to PtdIns3K, the Ras effector Raf-1 is an amino acid sensor and positively regulates autophagy in HT-29 human colon cancer cells (113). In this situation, amino acids target and inhibit the activity of the Raf-1 kinase, which downregulates the downstream MEK1/2 [MAPK(mitogen-activated protein kinase)/ERK kinase 1/2] and ERK1/2 (extracellular signal-regulated kinase 1/2) kinases and autophagy activity. Amino acid deprivation reverses this inhibition and induces ERK1/2 and autophagy. As a consequence, two downstream effector cascades of Ras, the Ras-PtdIns3K and Ras-Raf-1-ERK1/2 pathways, are likely to oppose each other in autophagy regulation through signaling in response to growth factors versus the absence of amino acids. It is also possible that the genetic differences between normal and cancer cell lines determine how Ras signaling controls autophagy.
Energy Sensing
During periods of intracellular metabolic stress, activation of autophagy is essential for cell viability, and the underlying pathways are understood in considerable detail. In mammalian cells, a reduced cellular energy (ATP) level is sensed by AMPK (5′-AMP-activated protein kinase) (Figure 2a). AMPK is activated by a decreased ATP/AMP ratio through the upstream LKB1 kinase (encoded by the Peutz-Jeghers syndrome gene). Active AMPK leads to phosphorylation and activation of the TSC1/2 complex, which inhibits mTOR activity through Rheb (46). Autophagy stimulated by mTOR downregulation results in elevated ATP production via recycling of nutrients. In addition, the LKB1-AMPK pathway phosphorylates and activates p27kip1, a cyclin-dependent kinase inhibitor leading to cell cycle arrest, which is essential to prevent cells from undergoing apoptotic death and to induce autophagy for survival in response to bioenergetic stress during growth factor withdrawal and nutrient deprivation (80). Similarly, Snf1, the yeast homolog of mammalian AMPK, also positively modulates autophagy, possibly through independent mechanisms involving regulation of Atg1 (154).
Stress Response
Various extra- and intracellular stresses potently induce autophagy, which is important for organisms to adapt to or overcome unfavorable conditions. Recent studies have provided insight into the molecular mechanisms that regulate autophagy in response to different stresses.
ER stress
The ER is the key compartment in the cell to facilitate folding of newly synthesized proteins and initiate the pathway of vesicular movement of membrane and proteins to various organelles and the cell surface. In mammalian cells, the ER also serves as the major intracellular Ca2+ reservoir. A number of ER stress stimuli, for example, expression of aggregate-prone proteins, glucose deprivation (resulting in reduced glycosylation and decreased energy for chaperone activity), hypoxia and oxidative stress (causing decreased disulfide bond formation), and Ca2+ efflux from the ER, lead to the accumulation of unfolded proteins in the ER, which exceeds its folding capacity. An increasing number of studies indicate that autophagy is induced by ER stress in organisms from yeast to mammals. However, the signaling mechanisms linking ER stress to autophagy vary, dependent on the specific stress conditions and the organisms being studied (Figure 2a).
In yeast, ER chemical stressors blocking formation of disulfide bonds or protein glycosylation, such as DTT and tunicamycin, effectively trigger autophagy, which requires Atg1 kinase activity (166). ER stress-induced autophagy is required for cell survival in the presence of tunicamycin, likely through compensatory removal of expanded and disorganized ER (which results from the unfolded protein response; UPR), along with the misfolded proteins within (5). The UPR signaling pathway in yeast is mediated by Ire1 (inositol-requiring kinase 1), an ER transmembrane protein with a lumenal stress-sensing domain and a cytosolic endoribonuclease domain. In response to the accumulation of unfolded proteins, an ER-specific member of the heat shock protein 70 family, Grp78/BiP, dissociates from its ER-sensing domain and activates the cytosolic endonuclease domain of Ire1, which triggers the splicing of the Ire1 substrate Hac1. The latter encodes a transcription factor (the yeast homolog of mammalian XBP1) that activates transcription of target genes involved in protein modification/folding, vesicle transport, phospholipid biosynthesis, and ERAD (ER-associated degradation) (86). Although the Ire1-Hac1 pathway is required for autophagy induction by ER stress, it seems dispensable for the transcriptional upregulation of ATG genes (5). How Ire1 and Hac1 exert their function to stimulate autophagy in yeast remains to be understood.
In mammalian cells, knockdown of the upstream UPR regulator Grp78/BiP by siRNA inhibits autophagosome formation induced by both ER stress and nutrient deprivation, but does not affect the conversion of LC3-I to LC3-II, suggesting that Grp78/BiP is an obligatory factor for autophagy and may function at the phagophore expansion rather than induction step (79). It should be noted that this conclusion is mainly based on knockdown of Grp78/BiP, an artificial condition that spontaneously activates UPR pathways and induces LC3 conversion, which makes it difficult to differentiate between the roles of Grp78/BiP and UPR signaling in autophagy induction.
Additional studies focusing on the downstream UPR targets have provided useful information on the mechanisms of ER stress-induced autophagy in mammals. Mammalian UPR signaling is more complex than in yeast and involves three distinct downstream pathways, IRE1 (similar to yeast Ire1), ATF6 (activating transcription factor 6), and PERK (RNA-dependent protein kinase-like ER kinase). These factors signal misfolded protein levels in the ER and activate transcription of different target genes. One downstream target of IRE1 is c-Jun N-terminal kinase (JNK), which is essential for lipid conjugation of LC3 induced by tunicamycin or by accumulation of cytosolic misfolded proteins due to proteasome inhibition in MEFs and cancer cells (24, 106). Recent data using murine cells suggest that in response to ER stress induced by expression of misfolded polyQ72 or mutant dysferlin proteins, phosphorylation of eIF2α (eukaryotic initiation factor 2 α) by the eIF2α kinase PERK is required for mediating LC3 conversion and autophagic degradation of the mutant proteins in the ER (31, 72). Thus, both the IRE1-JNK and the PERK-eIF2α pathways seem to play a pivotal role in UPR-induced autophagy.
In addition to UPR signaling, ER stress also induces release of lumenal Ca2+ to the cytosol. Calcium-activated calmodulin-dependent kinase kinase-β (CaMKKβ) is stimulated by the increase in the intracellular Ca2+ level and further activates AMPK, the latter potently inducing autophagy (42). Elevated Ca2+ levels also trigger phosphorylation of protein kinase Cθ (PKCθ), which induces LC3 conversion and autophagy in immortalized hepatocytes in response to the ER stressors thapsigargin and tunicamycin (126).
Although the above studies show that ER stress-induced autophagy has a prosurvival role in mammalian cells, others suggest that ER stressors may cause autophagic cell death. Injection of tunicamycin into renal tubules in mice induces kidney tubular cell death via both apoptosis and autophagy, which are mediated by the catalytic activity of the tumor suppressor DAPk (calmodulin-regulated serine/threonine kinase death-associated protein kinase). DAPk is activated by PP2A-like phosphatase-dependent dephosphorylation of an inhibitory serine that is derived from autophosphorylation (35), and activated DAPk induces autophagy likely through its ability to phosphorylate Beclin 1 and promote Beclin 1 dissociation from Bcl-2 (169). It is possible that autophagy plays dual roles in determining cell fate, depending on specific cell types and stimuli (23), a theme that appears repeatedly.
Hypoxia
Low levels of oxygen at or below 1% (hypoxic stress) versus 2–9% (normoxia for most mammalian cell types), exist in physiologically developing embryos as well as many pathological conditions, such as solid tumors, cardiovascular ischemia, and brain injuries. Accumulating data show that hypoxia induces autophagy in mammalian cells, yet the area of study is at a beginning stage, and the signaling pathways responsible for autophagy induction and its cellular consequences seem to be different, contingent on the types of cells and autophagic pathways (Figure 3b). For example, enhanced mitochondrial autophagy (mitophagy) during hypoxia is suggested to be an adaptive response, reducing the levels of reactive oxygen species (ROS) and protecting cell integrity, although in several glioma and breast cancer cell lines, prolonged hypoxia mediates autophagic cell death (4).
Figure 3.
Autophagy regulation in response to stress. (a) ER stress stimulates autophagy through the PERK-eIF2α pathway, the IRE1-JNK1 pathway and Ca2+ release. Activation of eIF2α by PERK may upregulate transcription of certain autophagy genes, such as ATG12. Phosphorylation and activation of eIF2α by two other eIF2α kinases, GCN2 and PKR, upon amino acid starvation and viral infection, respectively, are also depicted. (b) Autophagy induction by mechanisms sensing hypoxia or oxidative stress. JNK1, DAPk, and BNIP3 induce autophagy by disrupting the Bcl-2-Beclin 1 interaction and activating Beclin 1.
Hypoxia-inducible factor-1 (HIF-1) is the primary transcription factor acutely induced by hypoxic conditions, and it drives transcription of hundreds of genes that promote erythropoiesis and angiogenesis and decrease mitochondrial biogenesis and respiration, counterbalancing deleterious effects caused by O2 deficiency. In MEFs, mitochondria are removed by mitophagy in response to hypoxia, which is dependent on HIF-1 and the induction of its downstream target BNIP3 (Bcl-2 adenovirus E1a nineteen kDa interacting protein 3; a prodeath Bcl-2 family member) (171). In addition, during reticulocyte maturation in mice, BNIP3L (BNIP3-like protein, also known as NIX), another HIF-1-induced target, is also required for programmed mitochondrial clearance by autophagy (128, 132). BNIP3 competes with Beclin 1 for binding Bcl-2 and thus releases Beclin 1 for participating in mitophagy. It should be noted that, besides being under the control of HIF-1, BNIP3 is also a target gene of the E2F transcription factors, which are inhibited by the RB tumor suppressor during RB-induced cell cycle arrest (150). Therefore, hypoxia induces BNIP3 transcription via binding of HIF-1 and/or E2F to the BNIP3 promoter, and the RB-E2F-BNIP3 signaling is also involved in hypoxia-induced autophagy.
Indeed, upregulation of bulk autophagy by hypoxia in tumor cells seems independent of the HIF-1 pathway; instead, the AMPK-mTOR (111, 152) and PKCδ (protein kinase Cδ)-JNK1 (16) cascades are responsible for signaling autophagy induction. In addition, hypoxia inhibits TOR and blocks eIF4F (eukaryotic initiation factor 4F) complex formation and mRNA translation (7, 123). Thus, autophagy induced by hypoxia can be at least partially TOR-dependent, and hypoxia-stimulated ER stress may also play a role in autophagy induction.
Oxidative stress
A common intracellular stress that effectively leads to induction of autophagy is the formation of ROS. Mitochondria are the major source generating ROS, which will in turn damage these organelles. ROS-generating agents (such as hydrogen peroxide and 2-methoxyestradiol), or chemicals inhibiting the mitochondrial electron transport chain, induce ROS production and autophagic cell death in transformed and cancer cell lines (17, 18). Intriguingly, these drugs induce a much lower level of ROS in nontransformed primary mouse astrocytes compared with cancer cells and fail to stimulate autophagy, suggesting that normal cells effectively maintain ROS at a tolerable level and are able to protect themselves from mitochondrial damage, likely through antioxidant mechanisms such as superoxide dismutase (SOD), catalase, and the redox system, because applying chemical ROS scavengers or overexpressing SOD2 reduces autophagy (17, 18). In addition, impaired mitochondria may be selectively degraded through mitophagy in yeast and mammalian cells (96, 115, 124, 151), which may constitute another mechanism to reduce ROS levels and maintain cell survival. Therefore, ROS selectively targets malignant but not normal cells for autophagy induction, which has important implications for anticancer therapy.
The link between ROS and autophagy induction may be the cysteine protease Atg4 (Figure 3b), which cleaves Atg8/LC3 from the autophagosome outer-membrane before, or soon after, autophagosome-lysosome fusion. ROS targets a conserved Cys81 on Atg4, which is in the vicinity of the catalytic Cys77 residue; oxidation of cysteines inhibits Atg4 protease activity and promotes lipidation of Atg8/LC3, an essential step for autophagy (130). It is not known, however, how ROS levels might be temporally and spatially controlled inside the cell, so that Atg4 can be locally activated to allow delipidation and recycling of Atg8/LC3, or whether another mechanism is involved.
In addition, Atg4 seems not the sole molecule that underlies the oxidative regulation of autophagy. A recent study suggests that hydrogen peroxide activates poly(ADP-ribose) polymerase-1 (PARP-1), which stimulates the LKB1-AMPK pathway and leads to autophagy induction (44). It is likely that DNA damage induced by oxidative stress is involved in the activation of PARP-1 and autophagy (95).
Pathogen Infection
Autophagy has an important role in eliminating invading pathogens, and pathogen-induced autophagy appears to be TOR-independent (153). Although many studies report autophagy induction in host cells in response to various bacteria and viruses, and subsequent autophagic sequestration and degradation of the pathogens, information about the signaling pathways activating autophagy in innate and adaptive immunity has only been revealed recently (Figure 4).
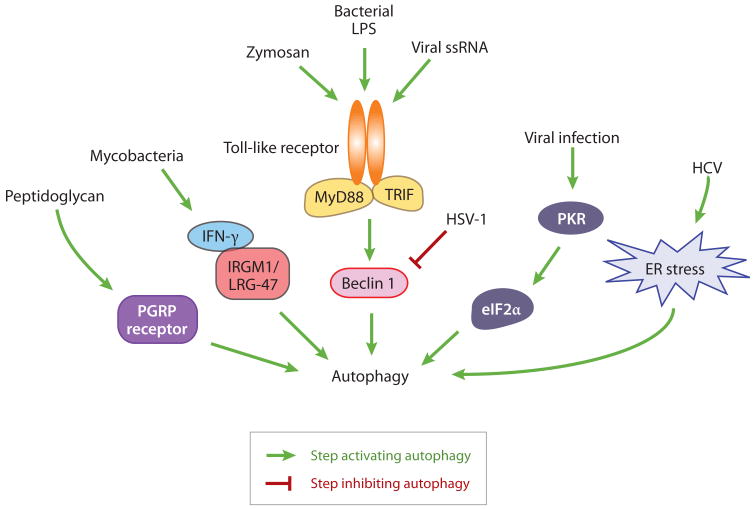
Figure 4.
Different mechanisms that regulate autophagy by pathogen invasion. Toll-like receptor adaptor proteins, MyD88 and TRIF, dissociate Beclin 1 from its inhibitor Bcl-2 and induce autophagy, whereas the HSV-1 protein ICP34.5 blocks autophagy by binding and sequestering Beclin 1. HSV-1, herpes simplex virus type 1; HCV, hepatitis C virus.
The innate immune system is an evolution-arily ancient and conserved defense mechanism and is found in almost all types of multicellular organisms, including insects, plants, and mammals. In Drosophila, which depend nearly entirely on innate immunity to fight against infection, the peptidoglycan-recognition protein (PGRP) family members play crucial roles in surveillance and microbe sensing. The PGRP receptors are present in immune cells, recognize bacterial-derived peptidoglycans, and activate the production of antimicrobial peptides. A PGRP family member, PGRP-LE, is the first identified cytoplasmic sensor in insects that recognizes the presence of intracellular bacteria and triggers autophagy in host cells, which is required for suppressing the growth of Listeria monocytogenes in hemocytes and enhancing host viability (161). The pathway that transduces signals from the PGRP-LE receptor to the autophagy machinery, and whether it crosstalks to the classical signaling pathways downstream of PGRP receptors that activate genes encoding antimicrobial peptides, remain to be investigated.
Signaling of Toll-like receptors (TLRs) triggers autophagy during mammalian innate and adaptive immunity. TLRs are membrane receptors localized at the cell surface and endosomes, and TLR signaling activates transcription of genes responsible for T cell stimulation, inflammation, and antiviral immune responses. Different TLRs are involved in autophagy induction upon binding their specific pathogen-derived ligands. For example, viral ssRNA (single-stranded RNA) induces autophagy via TLR7 (22), zymosan stimulates LC3 translocation to phagosomes by activating TLR2 (129), and lipopolysaccharide (LPS), which is derived from cell walls of Gram-negative bacteria, triggers autophagy through TLR4 (136, 159). TLRs recruit adaptor proteins to transduce signals to downstream effectors. MyD88 (myeloid differentiation factor 88) is the major adaptor protein used by almost all activated TLRs and mediates autophagy induction downstream of TLR7 (22). In human and murine macrophages, the TLR4 adaptor protein TRIF (Toll-interleukin-1 receptor domain-containing adaptor-inducing interferon-β) is suggested to mediate LPS-induced autophagy, and the same downstream signaling pathway is shared by both innate immunity and autophagy upregulation (159). In addition, a unique mechanism is proposed for induction of macrophage autophagy; in murine macrophages, MyD88, together with TRIF, interacts with Beclin 1 more strongly in the presence of LPS or poly(I·C) (ligands for TLR4 and TLR3, respectively), which reduces the binding of Beclin 1 and Bcl-2, and thus triggers autophagy activation (136). Moreover, interferon (IFN)-γ and its effector Irgm1 (the IFN-inducible immunity-related GTPase family M member 1; also known as LRG-47) are also required to stimulate autophagy in macrophages and inhibit the survival of intracellular mycobacteria (36, 138).
In response to viral infection, the antiviral eIF2α kinase signaling pathway, including eIF2α and the IFN-inducible double-stranded RNA-dependent protein kinase R (PKR), is activated and upregulates autophagy (145, 146). Some viral proteins may antagonize autophagy induction by directly modulating Atg proteins. An example is the HSV-1 (herpes simplex virus type 1)-encoded neurovirulence protein ICP34.5, which inhibits autophagy via interaction and sequestration of Beclin 1 (108). It is also reported that viral infection, such as infection with hepatitis C virus, induces ER stress, and autophagy is triggered through the unfolded protein response, including the downstream IRE1, ATF6, and PERK signaling pathways (139).
Transcriptional and Epigenetic Regulation of Autophagy
Transcription
Autophagy genes are regulated at a transcriptional level in response to stress. For example, under starvation conditions, transcription of the autophagosome marker Atg8/LC3 is rapidly upregulated in yeast and certain mammalian cells. However, not much is known about the underlying transcriptional machinery. FoxO (Forkhead box transcription factor class O) is the first transcription factor that is shown to be necessary and sufficient to induce autophagy in the Drosophila larval fat body (55). For mammalian cells, discovery of a transcription-dependent mechanism via FoxO3 comes from studies on protein degradation during muscle atrophy (87, 174). FoxO3 induces the transcription of multiple autophagy genes, including LC3B, Gabarapl1, atg12, atg4B, vps34, ulk2, beclin 1, Bnip3, and Bnip3l. FoxO3 directly binds to the promoters of LC3B, Gabarapl1, atg12, Bnip3l, and Bnip3 to activate gene transcription. Constitutively active FoxO3 is sufficient to induce autophagosome formation in adult mouse skeletal muscle, which promotes lysosomal proteolysis and leads to muscle wasting. Importantly, FoxO3 functions in parallel to the mTOR pathway, whilst both pathways are downstream of IGF-1/insulin-PtdIns3K-PKB/Akt signaling. Thus, autophagy is regulated by two different mechanisms: nontranscriptional inhibition by mTOR and transcription-dependent upregulation through FoxO3. Nevertheless, transcriptional mechanisms that physiologically regulate expression of autophagy genes in tissues other than myotubes have not been characterized.
Autophagy induction inversely correlates with active protein synthesis. In the presence of abundant nutrients, proteins are synthesized and autophagy suppressed, whereas in response to stress stimuli, cells trigger translational arrest and autophagy induction. Recent studies suggest that translational initiation factors, for example, eIF4GI, that specifically activate translation of genes controlling cell growth and proliferation, block the induction of autophagy downstream of TOR (116), whereas signaling pathways that induce translational arrest, such as the eEF-2 (eukaryotic elongation factor-2) kinase and the eIF2α kinase signaling pathway, positively regulate autophagy (145, 157). Phosphorylation of eIF2α at Ser51 by the conserved eIF2α protein kinase family is essential for the global translational arrest and selective upregulation in the translation of transcriptional activators (such as GCN4) that stimulate transcription of starvation-induced genes. Yeast has one eIF2α kinase, Gcn2 (general control nonderepressible-2), which is negatively regulated by Tor (74), whereas mammals have four known eIF2α kinases, GCN2, PKR, PERK, and HRI (heme-regulated inhibitor), activated by amino acid starvation, viral infection, ER stress, and heme deprivation, respectively (Figure 3a). In both yeast and MEFs, eIF2α phosphorylation by GCN2 is required for starvation-induced autophagy, and in MEFs, PKR is required for autophagy induced by viral infection (145). The downstream effect of eIF2α phosphorylation on autophagy is likely to be transcriptional, as transcription of Atg12 is upregulated by phosphorylated eIF2α in polyQ72-loaded mammalian cells (72). In yeast, selective translation of Gcn4 (rather than the global translational arrest) by eIF2α phosphorylation is essential for autophagy, consistent with a report identifying several autophagy genes, including ATG1, ATG13, and APE1, as downstream transcription activation targets of Gcn4 (97). However, the study is largely based on microarray analysis, and independent assays are needed to test the functions of transcriptional activators in yeast autophagy.
Chromosome Modification
Accumulating data implicate an important role of epigenetic factors in regulating autophagy in various pathological conditions. Alteration of the acetylation status of histones through histone deacetylases (HDACs) is a key mechanism that controls chromatin structural remodeling and gene transcription. Chemical or genetic inhibition of HDACs leads to autophagy induction (107). Various HDAC chemical inhibitors, including SAHA (suberoylanilide hydroxamic acid) and butyrate, promote hyperacetylation of histones and preferentially target cancer cells for both apoptotic and caspase-independent autophagic cell death in cell lines and animal models (89,135). Although there are debates about the anticancer role of autophagy induced by HDAC suppression (12), a number of mechanisms have been proposed for autophagy induction by HDAC inhibitors based on studies with different diseases. For example, it is suggested that autophagy is stimulated by SAHA via a decreased level of mTOR expression and activity in solid tumor endometrial stromal sarcoma cells, and via PKB/Akt inhibition and Beclin 1 induction in HeLa cells (11, 43), whereas in malignant rhabdoid tumor cells, the HDAC inhibitor FK228 induces autophagy by triggering translocation of AIF (apoptosis inducing factor) into the nucleus, which mediates caspase-independent tumor cell death (155).
Specific transcriptional effects on ATG genes by HDAC suppression have also been reported. In lung tissues obtained from patients with chronic obstructive pulmonary disease caused by cigarette smoking, inhibition of HDAC activity by cigarette smoke extract increases binding of Egr-1 (early growth response-1) and E2F transcription factors to the LC3B promoter region, and activates LC3B expression. In addition, expression of ATG4B also depends on Egr-1 (19). Although one cannot rule out the possibility that enhanced autophagy by HDAC inhibition may be due to global cell cycle arrest (82) or nonspecific effects on the overall chromosome structure, it opens up opportunities for regulating autophagy by chromosome modifiers as a therapeutic target for clinical intervention. Future studies to define the specific functions of different histone deacetylases on autophagy genes are much awaited.
Post-Translational Regulation of the Autophagy Machinery
In addition to the aforementioned ubiquitin-like modification of Atg12 and Atg8/LC3, and Tor/PKA-dependent phosphorylation of the Atg1/ULK kinase complex, a number of other post-translational modifications on various Atg proteins have been recently studied and suggested to be crucial in regulating autophagy activity.
Phosphorylation
One major function of protein phosphorylation is to form a docking scaffold that serves to recruit other proteins. An example is seen during pexophagy in the yeast Pichia pastoris, where the receptor protein for peroxisomes, PpAtg30, is phosphorylated. Through phosphorylated residues, PpAtg30 interacts with other autophagy machinery components, such as PpAtg11, targeting peroxisomes for autophagic breakdown (28).
On the other hand, phosphorylation can also serve a role to sterically prevent protein-protein interaction. In mammalian cells, in response to ceramide or starvation, the Beclin 1 binding partner and inhibitor Bcl-2 is phosphorylated on three residues, Thr69, Ser70, and Ser87, by JNK1 (112, 156). Hyperphosphorylated Bcl-2 dissociates from Beclin 1 and releases it for autophagy induction, whereas viral Bcl-2 (derived from Kaposi's sarcoma-associated herpes virus), which lacks the phosphorylation residues, cannot dissociate from Beclin 1 and thus inhibits autophagy. In addition, phosphorylation of Beclin 1 on Thr119 in its BH3 domain by DAPk also reduces Bcl-2-Beclin 1 interaction and activates autophagy (169). Thus, phosphorylation of autophagy-related proteins provides additional aspects for autophagy regulation.
Acetylation
Deacetylation modification of the autophagy machinery proteins is also required for autophagy (75, 76). A number of Atg proteins, including Atg5, Atg7, Atg8, and Atg12, are acetylated in nutrient-rich conditions and deacetylated during starvation, both in HeLa cells and in vivo. Acetylation of Atg proteins is dependent specifically on the p300 acetyltransferase but not on two other acetyltransferases, CBP and PCAF, and the deacetylation process is dependent on the NAD-dependent deacetylase Sirt1 (a sirtuin family member). Components of the autophagy machinery physically interact with p300 or Sirt1, which is regulated by nutrient availability and contributes to changes in the acetylation status.
Sirt1 expression is induced by caloric restriction, which correlates with increased longevity (162). Therefore, direct deacetylation of the autophagy machinery by Sirt1 provides an important mechanistic link between autophagy induction and life span extension. However, the molecular function of deacetylation of Atg proteins in autophagy is essentially unknown, although deacetylation may help remove spatial blockades for protein interaction or recruitment, or modify protein activity. In addition, the mechanisms by which Sirt1 and p300 sense upstream input signals have not been demonstrated. It is possible that the insulin/growth factor pathway is involved in the control of the reversible acetylation process.
Concluding Remarks
Substantial progress has been made in the past few years in understanding the molecular mechanism and regulatory network of autophagy from yeast to mammals. As a major cellular catabolic system targeting a variety of substrates, the autophagic process needs to be tightly controlled. The coordination of Atg proteins with other subcellular components, including the cytoskeleton, the secretory pathway, oncogenes and tumor suppressors, and immunoproteins, is crucial for correct autophagy induction. Both canonical (such as the insulin and growth factor pathway) and novel signaling cascades have been implicated in regulating autophagy, in response to different intra- or extracellular stimuli. Current knowledge and further investigation on the genetic regulation of autophagy will provide a broad spectrum of potential pharmacological targets for modulating autophagy in various disease conditions.
Summary Points
Autophagy mediates the lysosomal degradation of cytosolic proteins, damaged or excess organelles, protein aggregates, and invasive microbes.
Autophagy can nonselectively degrade bulk cytosol or selectively target specific cargos through receptor and adaptor proteins.
Autophagy-related (ATG) gene products cooperatively function at multiple steps to facilitate the formation of a double-membrane autophagosome.
Various stress conditions stimulate induction of autophagy, and the signal transduction mechanisms of autophagy are being identified.
Not much is known about transcriptional regulation of autophagy genes, yet several transcription factors have been suggested to activate or suppress expression of certain ATG genes in yeast, Drosophila, and mammalian tissues.
Autophagy proteins undergo phosphorylation and acetylation modifications that modulate autophagy activity.
Future Issues
Crosstalk between signaling pathways that act in controlling autophagy needs to be further explored, and detailed studies on a potential convergent molecule of several upstream pathways, such as the link between TOR downregulation and autophagy induction, should be carried out.
Characterization of Atg1 kinase substrates and new components in the Atg1 complex in both yeast and higher eukaryotes will provide useful information on the exact functions this important kinase carries out during autophagy.
How substrate specificity is achieved by selective autophagic degradation in various circumstances, for example, cell cycle progression, cell division, development, differentiation, and diseases, deserves extensive investigation. It should be noted that homologs of many ATG genes required for selective autophagy in yeast, such as Atg11, have not been identified in higher eukaryotes. Thus, it is likely that they may share functional and structural similarities but not sequence homology.
Future studies on the mechanistic aspects of autophagosome formation can be performed either in vivo or in vitro. For instance, it will be important to determine how small GTPases and SNARE proteins may collaborate with the autophagy machinery during phagophore assembly, if any of them are involved, and what regulates dissociation of various Atg proteins from a completed autophagosome.
Transcriptional and translational regulation of autophagy genes, such as mRNA expression and stability, and whether there is tissue specificity, need to be better understood in both physiological and pathological stress conditions.
Discovery of new posttranslational modifications on Atg proteins and their regulating mechanisms will add to our current understanding of the genetic regulation of autophagy.
Acknowledgments
We thank Drs. Usha Nair and Clinton Bartholomew for helpful discussions and comments. This work was supported by a Rackham Predoctoral Fellowship from the University of Michigan to C.H., and National Institutes of Health Public Health Service grant GM53396 to D.J.K.
- Lysosome
A degradative organelle in higher eukaryotes that compartmentalizes a range of hydrolytic enzymes and maintains a highly acidic pH
- Vacuole
The yeast and plant equivalent of the lysosome; this organelle also carries out storage and osmoregulatory functions
- Autophagosome
A cytosolic double-membrane vesicle that sequesters intracellular components for degradation in lysosomes/vacuoles
- Atg
autophagy-related
- Phagophore assembly site (PAS)
A perivacuolar compartment or location where autophagosomes and similar types of sequestering vesicles are formed in yeast
- Cytoplasm-to-vacuole targeting (Cvt)
A biosynthetic pathway in yeast that transports resident hydrolases to the vacuole through a selective autophagy-like process
- Pexophagy
A selective type of autophagy involving the sequestration and degradation of peroxisomes; can occur by a micro- or macroautophagic process
- TOR
Target of rapamycin
- Phagophore
The initial sequestering compartment that expands into an autophagosome
- LC3
microtubule-associated protein 1 light chain 3
- Phosphatidylinositol 3-kinase (PtdIns3K)
Enzyme phosphorylating the 3′ hydroxyl on the phosphoinositide inositol ring. Class III PtdIns3K stimulates autophagy whereas class I is inhibitory
- PE
phosphatidylethanolamine
- Amphisome
An intermediate vesicle derived from the fusion of an autophagosome with an endosome or multivesicular body
- PKA
cAMP-dependent protein kinase A
- UPR
unfolded protein response
- JNK
c-Jun N-terminal kinase
- eIF2α
eukaryotic initiation factor 2α
- DAPk
calmodulin-regulated serine/threonine kinase death-associated protein kinase
- ROS
reactive oxygen species
- TLR
Toll-like receptor
Footnotes
Disclosure Statement: The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Literature Cited
- 1.Abeliovich H, Darsow T, Emr SD. Cytoplasm to vacuole trafficking of aminopeptidase I requires a t-SNARE-Sec1p complex composed of Tlg2p and Vps45p. EMBO J. 1999;18:6005–16. doi: 10.1093/emboj/18.21.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Ba. Curr Biol. 1997;7:261–69. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 3.Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, et al. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2001;276:35243–46. doi: 10.1074/jbc.C100319200. [DOI] [PubMed] [Google Scholar]
- 4.Azad MB, Chen Y, Henson ES, Cizeau J, McMillan-Ward E, et al. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy. 2008;4:195–204. doi: 10.4161/auto.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4:e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–14. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]; First demonstration that p62/SQSTM1 is required for autophagic degradation of ubiquitinated aggregate-prone proteins in mammals.
- 7.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budovskaya YV, Stephan JS, Deminoff SJ, Herman PK. An evolutionary proteomics approach identifies substrates of the cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 2005;102:13933–38. doi: 10.1073/pnas.0501046102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budovskaya YV, Stephan JS, Reggiori F, Klionsky DJ, Herman PK. The Ras/cAMP-dependent protein kinase signaling pathway regulates an early step of the autophagy process in Saccharomyces cerevisiae. J Biol Chem. 2004;279:20663–71. doi: 10.1074/jbc.M400272200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem. 2005;280:33076–82. doi: 10.1074/jbc.M507201200. [DOI] [PubMed] [Google Scholar]
- 11.Cao Q, Yu C, Xue R, Hsueh W, Pan P, et al. Autophagy induced by suberoylanilide hydroxamic acid in Hela S3 cells involves inhibition of protein kinase B and up-regulation of Beclin 1. Int J Biochem Cell Biol. 2008;40:272–83. doi: 10.1016/j.biocel.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Carew JS, Nawrocki ST, Kahue CN, Zhang H, Yang C, et al. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood. 2007;110:313–22. doi: 10.1182/blood-2006-10-050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan EYW, Longatti A, McKnight NC, Tooze SA. Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism. Mol Cell Biol. 2009;29:157–71. doi: 10.1128/MCB.01082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CY, Huang WP. Atg19 mediates a dual interaction cargo sorting mechanism in selective autophagy. Mol Biol Cell. 2007;18:919–29. doi: 10.1091/mbc.E06-08-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang YY, Neufeld TP. An Atg1/Atg13 complex with multiple roles in TOR-mediated autophagy regulation. Mol Biol Cell. 2009;20:2004–14. doi: 10.1091/mbc.E08-12-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JL, Lin HH, Kim KJ, Lin A, Forman HJ, Ann DK. Novel roles for protein kinase Cd-dependent signaling pathways in acute hypoxic stress-induced autophagy. J Biol Chem. 2008;283:34432–44. doi: 10.1074/jbc.M804239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J Cell Sci. 2007;120:4155–66. doi: 10.1242/jcs.011163. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15:171–82. doi: 10.1038/sj.cdd.4402233. [DOI] [PubMed] [Google Scholar]
- 19.Chen ZH, Kim HP, Sciurba FC, Lee SJ, Feghali-Bostwick C, et al. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS ONE. 2008;3:e3316. doi: 10.1371/journal.pone.0003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheong H, Nair U, Geng J, Klionsky DJ. The Atg1 kinase complex is involved in the regulation of protein recruitment to initiate sequestering vesicle formation for nonspecific autophagy in Saccharomyces cerevisiae. Mol Biol Cell. 2008;19:668–81. doi: 10.1091/mbc.E07-08-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheong H, Yorimitsu T, Reggiori F, Legakis JE, Wang CW, Klionsky DJ. Atg17 regulates the magnitude of the autophagic response. Mol Biol Cell. 2005;16:3438–53. doi: 10.1091/mbc.E04-10-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J. 2008;27:1110–21. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding WX, Ni HM, Gao W, Hou YF, Melan MA, et al. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem. 2007;282:4702–10. doi: 10.1074/jbc.M609267200. [DOI] [PubMed] [Google Scholar]
- 24.Ding WX, Ni HM, Gao W, Yoshimori T, Stolz DB, et al. Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am J Pathol. 2007;171:513–24. doi: 10.2353/ajpath.2007.070188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunn WA, Jr, Cregg JM, Kiel JAKW, van der Klei IJ, Oku M, et al. Pexophagy: the selective autophagy of peroxisomes. Autophagy. 2005;1:75–83. doi: 10.4161/auto.1.2.1737. [DOI] [PubMed] [Google Scholar]
- 26.Epple UD, Suriapranata I, Eskelinen EL, Thumm M. Aut5/Cvt17p, a putative lipase essential for disintegration of autophagic bodies inside the vacuole. J Bacteriol. 2001;183:5942–55. doi: 10.1128/JB.183.20.5942-5955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fader CM, Sanchez D, Furlan M, Colombo MI. Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in k562 cells. Traffic. 2008;9:230–50. doi: 10.1111/j.1600-0854.2007.00677.x. [DOI] [PubMed] [Google Scholar]
- 28.Farre JC, Manjithaya R, Mathewson RD, Subramani S. PpAtg30 tags peroxisomes for turnover by selective autophagy. Dev Cell. 2008;14:365–76. doi: 10.1016/j.devcel.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fass E, Shvets E, Degani I, Hirschberg K, Elazar Z. Microtubules support production of starvation-induced autophagosomes but not their targeting and fusion with lysosomes. J Biol Chem. 2006;281:36303–16. doi: 10.1074/jbc.M607031200. [DOI] [PubMed] [Google Scholar]
- 30.Filimonenko M, Stuffers S, Raiborg C, Yamamoto A, Malerod L, et al. Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J Cell Biol. 2007;179:485–500. doi: 10.1083/jcb.200702115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita E, Kouroku Y, Isoai A, Kumagai H, Misutani A, et al. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II) Hum Mol Genet. 2007;16:618–29. doi: 10.1093/hmg/ddm002. [DOI] [PubMed] [Google Scholar]
- 32.Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell. 2008;19:2092–100. doi: 10.1091/mbc.E07-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furuta S, Hidaka E, Ogata A, Yokota S, Kamata T. Ras is involved in the negative control of autophagy through the class I PI3-kinase. Oncogene. 2004;23:3898–904. doi: 10.1038/sj.onc.1207539. [DOI] [PubMed] [Google Scholar]
- 34.Geng J, Klionsky DJ. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. EMBO Rep. 2008;9:859–64. doi: 10.1038/embor.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gozuacik D, Bialik S, Raveh T, Mitou G, Shohat G, et al. DAP-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ. 2008;15:1875–86. doi: 10.1038/cdd.2008.121. [DOI] [PubMed] [Google Scholar]
- 36.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–66. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 37.Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, et al. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J Biol Chem. 2007;282:37298–302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- 38.Hara T, Takamura A, Kishi C, Iemura S, Natsume T, et al. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol. 2008;181:497–510. doi: 10.1083/jcb.200712064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He C, Baba M, Cao Y, Klionsky DJ. Self-interaction is critical for Atg9 transport and function at the phagophore assembly site during autophagy. Mol Biol Cell. 2008;19:5506–16. doi: 10.1091/mbc.E08-05-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He C, Song H, Yorimitsu T, Monastyrska I, Yen WL, et al. Recruitment of Atg9 to the preautophagosomal structure by Atg11 is essential for selective autophagy in budding yeast. J Cell Biol. 2006;175:925–35. doi: 10.1083/jcb.200606084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–91. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provides direct evidence on the nutrient-dependent phosphorylation of autophagy proteins by mTOR.
- 42.Høyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-b, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Hrzenjak A, Kremser ML, Strohmeier B, Moinfar F, Zatloukal K, Denk H. SAHA induces caspase-independent, autophagic cell death of endometrial stromal sarcoma cells by influencing the mTOR pathway. J Pathol. 2008;216:495–504. doi: 10.1002/path.2434. [DOI] [PubMed] [Google Scholar]
- 44.Huang Q, Wu YT, Tan HL, Ong CN, Shen HM. A novel function of poly(ADP-ribose) polymerase-1 in modulation of autophagy and necrosis under oxidative stress. Cell Death Differ. 2009;16:264–77. doi: 10.1038/cdd.2008.151. [DOI] [PubMed] [Google Scholar]
- 45.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–34. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 47.Ishihara N, Hamasaki M, Yokota S, Suzuki K, Kamada Y, et al. Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion. Mol Biol Cell. 2001;12:3690–702. doi: 10.1091/mbc.12.11.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell. 2008;19:5360–72. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itoh T, Fujita N, Kanno E, Yamamoto A, Yoshimori T, Fukuda M. Golgi-resident small GTPase Rab33B interacts with Atg16L and modulates autophagosome formation. Mol Biol Cell. 2008;19:2916–25. doi: 10.1091/mbc.E07-12-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwata A, Riley BE, Johnston JA, Kopito RR. HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. J Biol Chem. 2005;280:40282–92. doi: 10.1074/jbc.M508786200. [DOI] [PubMed] [Google Scholar]
- 51.Jager S, Bucci C, Tanida I, Ueno T, Kominami E, et al. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci. 2004;117:4837–48. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 52.Jahreiss L, Menzies FM, Rubinsztein DC. The itinerary of autophagosomes: from peripheral formation to kiss-and-run fusion with lysosomes. Traffic. 2008;9:574–87. doi: 10.1111/j.1600-0854.2008.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Juhasz G, Hill JH, Yan Y, Sass M, Baehrecke EH, et al. The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J Cell Biol. 2008;181:655–66. doi: 10.1083/jcb.200712051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Juhasz G, Neufeld TP. Autophagy: a forty-year search for a missing membrane source. PLoS Biol. 2006;4:e36. doi: 10.1371/journal.pbio.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Juhasz G, Puskas LG, Komonyi O, Erdi B, Maroy P, et al. Gene expression profiling identifies FKBP39 as an inhibitor of autophagy in larval Drosophila fat body. Cell Death Differ. 2007;14:1181–90. doi: 10.1038/sj.cdd.4402123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provides direct evidence on the nutrient-dependent phosphorylation of autophagy proteins by mTOR.
- 57.Kabeya Y, Kamada Y, Baba M, Takikawa H, Sasaki M, Ohsumi Y. Atg17 functions in cooperation with Atg1 and Atg13 in yeast autophagy. Mol Biol Cell. 2005;16:2544–53. doi: 10.1091/mbc.E04-08-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–28. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamada Y, Funakoshi T, Shintani T, Nagano K, Ohsumi M, Ohsumi Y. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol. 2000;150:1507–13. doi: 10.1083/jcb.150.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawamata T, Kamada Y, Kabeya Y, Sekito T, Ohsumi Y. Organization of the pre-autophagosomal structure responsible for autophagosome formation. Mol Biol Cell. 2008;19:2039–50. doi: 10.1091/mbc.E07-10-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kihara A, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol. 2001;152:519–30. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–45. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–53. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim PK, Hailey DW, Mullen RT, Lippincott-Schwartz J. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc Natl Acad Sci USA. 2008;105:20567–74. doi: 10.1073/pnas.0810611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, et al. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J Cell Biol. 1999;147:435–46. doi: 10.1083/jcb.147.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, et al. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J Cell Biol. 2000;151:263–76. doi: 10.1083/jcb.151.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klionsky DJ. The molecular machinery of autophagy: unanswered questions. J Cell Sci. 2005;118:7–18. doi: 10.1242/jcs.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–37. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 69.Klionsky DJ, Cuervo AM, Dunn WA, Jr, Levine B, van der Klei I, Seglen PO. How shall I eat thee? Autophagy. 2007;3:413–16. doi: 10.4161/auto.4377. [DOI] [PubMed] [Google Scholar]
- 70.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–21. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Köchl R, Hu XW, Chan EYW, Tooze SA. Microtubules facilitate autophagosome formation and fusion of autophagosomes with endosomes. Traffic. 2006;7:129–45. doi: 10.1111/j.1600-0854.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- 72.Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, et al. ER stress (PERK/eIF2a phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–39. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 73.Kraft C, Deplazes A, Sohrmann M, Peter M. Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat Cell Biol. 2008;10:602–10. doi: 10.1038/ncb1723. [DOI] [PubMed] [Google Scholar]
- 74.Kubota H, Obata T, Ota K, Sasaki T, Ito T. Rapamycin-induced translational derepression of GCN4 mRNA involves a novel mechanism for activation of the eIF2α kinase GCN2. J Biol Chem. 2003;278:20457–60. doi: 10.1074/jbc.C300133200. [DOI] [PubMed] [Google Scholar]
- 75.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA. 2008;105:3374–79. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee IH, Finkel T. Regulation of autophagy by the p300 acetyltransferase. J Biol Chem. 2009;284:6322–28. doi: 10.1074/jbc.M807135200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee SB, Kim S, Lee J, Park J, Lee G, et al. ATG1, an autophagy regulator, inhibits cell growth by negatively regulating S6 kinase. EMBO Rep. 2007;8:360–65. doi: 10.1038/sj.embor.7400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Legakis JE, Yen WL, Klionsky DJ. A cycling protein complex required for selective autophagy. Autophagy. 2007;3:422–32. doi: 10.4161/auto.4129. [DOI] [PubMed] [Google Scholar]
- 79.Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460–71. doi: 10.1038/cdd.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, et al. The energy sensing LKB1-AMPK pathway regulates p27kip1 phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–24. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]; First evidence that links cell-cycle arrest to autophagy induction during energy deprivation.
- 81.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–76. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 82.Long J, Zhao J, Yan Z, Liu Z, Wang N. Antitumor effects of a novel sulfur-containing hydroxamate histone deacetylase inhibitor H40. Int J Cancer. 2009;124:1235–44. doi: 10.1002/ijc.24074. [DOI] [PubMed] [Google Scholar]
- 83.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–13. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 84.Long X, Ortiz-Vega S, Lin Y, Avruch J. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J Biol Chem. 2005;280:23433–36. doi: 10.1074/jbc.C500169200. [DOI] [PubMed] [Google Scholar]
- 85.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–48. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]; Demonstrates that autophagy is essential for cell survival when cells are deprived of growth factors.
- 86.Ma Y, Hendershot LM. The unfolding tale of the unfolded protein response. Cell. 2001;107:827–30. doi: 10.1016/s0092-8674(01)00623-7. [DOI] [PubMed] [Google Scholar]
- 87.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–71. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]; Demonstrates the transcriptional regulation of autophagy genes by the transcription factor FoxO3 in mammals.
- 88.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/Akt pathway. Mol Cell. 2002;10:151–62. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 89.Marks PA, Richon VM, Miller T, Kelly WK. Histone deacetylase inhibitors. Adv Cancer Res. 2004;91:137–68. doi: 10.1016/S0065-230X(04)91004-4. [DOI] [PubMed] [Google Scholar]
- 90.Meiling-Wesse K, Epple UD, Krick R, Barth H, Appelles A, et al. Trs85 (Gsg1), a component of the TRAPP complexes, is required for the organization of the preautophagosomal structure during selective autophagy via the Cvt pathway. J Biol Chem. 2005;280:33669–78. doi: 10.1074/jbc.M501701200. [DOI] [PubMed] [Google Scholar]
- 91.Mercer CA, Kaliappan A, Dennis PB. A novel, human Atg13 binding protein, Atg101, interacts with ULK1 and is essential for macroautophagy. Autophagy. 2009 doi: 10.4161/auto.5.5.8249. In Press. [DOI] [PubMed] [Google Scholar]
- 92.Mizushima N, Kuma A, Kobayashi Y, Yamamoto A, Matsubae M, et al. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J Cell Sci. 2003;116:1679–88. doi: 10.1242/jcs.00381. [DOI] [PubMed] [Google Scholar]
- 93.Mizushima N, Noda T, Ohsumi Y. Apg16p is required for the function of the Apg12p-Apg5p conjugate in the yeast autophagy pathway. EMBO J. 1999;18:3888–96. doi: 10.1093/emboj/18.14.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Monastyrska I, He C, Geng J, Hoppe AD, Li Z, Klionsky DJ. Arp2 links autophagic machinery with the actin cytoskeleton. Mol Biol Cell. 2008;19:1962–75. doi: 10.1091/mbc.E07-09-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Munoz-Gamez JA, Rodriguez-Vargas JM, Quiles-Perez R, Aguilar-Quesada R, Martin-Oliva D, et al. PARP-1 is involved in autophagy induced by DNA damage. Autophagy. 2009;5:61–74. doi: 10.4161/auto.5.1.7272. [DOI] [PubMed] [Google Scholar]
- 96.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Natarajan K, Meyer MR, Jackson BM, Slade D, Roberts C, et al. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol Cell Biol. 2001;21:4347–68. doi: 10.1128/MCB.21.13.4347-4368.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nazarko TY, Huang J, Nicaud JM, Klionsky DJ, Sibirny AA. Trs85 is required for macroautophagy, pexophagy and cytoplasm to vacuole targeting in Yarrowia lipolytica and Saccharomyces cerevisiae. Autophagy. 2005;1:37–45. doi: 10.4161/auto.1.1.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nezis IP, Simonsen A, Sagona AP, Finley K, Gaumer S, et al. Ref(2)P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. J Cell Biol. 2008;180:1065–71. doi: 10.1083/jcb.200711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nice DC, Sato TK, Stromhaug PE, Emr SD, Klionsky DJ. Cooperative binding of the cytoplasm to vacuole targeting pathway proteins, Cvt13 and Cvt20, to phosphatidylinositol 3-phosphate at the pre-autophagosomal structure is required for selective autophagy. J Biol Chem. 2002;277:30198–207. doi: 10.1074/jbc.M204736200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–34. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA. 2005;102:14238–43. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Noda NN, Kumeta H, Nakatogawa H, Satoo K, Adachi W, et al. Structural basis of target recognition by Atg8/LC3 during selective autophagy. Genes Cells. 2008;13:1211–18. doi: 10.1111/j.1365-2443.2008.01238.x. [DOI] [PubMed] [Google Scholar]
- 104.Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273:3963–66. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- 105.Obara K, Sekito T, Niimi K, Ohsumi Y. The Atg18-Atg2 complex is recruited to autophagic membranes via phosphatidylinositol 3-phosphate and exerts an essential function. J Biol Chem. 2008;283:23972–80. doi: 10.1074/jbc.M803180200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–31. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates that ER stress triggers autophagy for cell survival in mammalian cells.
- 107.Oh M, Choi IK, Kwon HJ. Inhibition of histone deacetylase1 induces autophagy. Biochem Biophys Res Commun. 2008;369:1179–83. doi: 10.1016/j.bbrc.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 108.Orvedahl A, Alexander D, Tallóczy Z, Sun Q, Wei Y, et al. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 109.Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–63. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 110.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–45. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 111.Papandreou I, Lim AL, Laderoute K, Denko NC. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death Differ. 2008;15:1572–81. doi: 10.1038/cdd.2008.84. [DOI] [PubMed] [Google Scholar]
- 112.Pattingre S, Bauvy C, Carpentier S, Levade T, Levine B, Codogno P. Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. J Biol Chem. 2009;284:2719–28. doi: 10.1074/jbc.M805920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pattingre S, Bauvy C, Codogno P. Amino acids interfere with the ERK1/2-dependent control of macroautophagy by controlling the activation of Raf-1 in human colon cancer HT-29 cells. J Biol Chem. 2003;278:16667–74. doi: 10.1074/jbc.M210998200. [DOI] [PubMed] [Google Scholar]
- 114.Pohl C, Jentsch S. Midbody ring disposal by autophagy is a post-abscission event of cytokinesis. Nat Cell Biol. 2009;11:65–70. doi: 10.1038/ncb1813. [DOI] [PubMed] [Google Scholar]
- 115.Priault M, Salin B, Schaeffer J, Vallette FM, di Rago JP, Martinou JC. Impairing the bioenergetic status and the biogenesis of mitochondria triggers mitophagy in yeast. Cell Death Differ. 2005;12:1613–21. doi: 10.1038/sj.cdd.4401697. [DOI] [PubMed] [Google Scholar]
- 116.Ramirez-Valle F, Braunstein S, Zavadil J, Formenti SC, Schneider RJ. eIF4GI links nutrient sensing by mTOR to cell proliferation and inhibition of autophagy. J Cell Biol. 2008;181:293–307. doi: 10.1083/jcb.200710215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ravikumar B, Acevedo-Arozena A, Imarisio S, Berger Z, Vacher C, et al. Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nat Genet. 2005;37:771–76. doi: 10.1038/ng1591. [DOI] [PubMed] [Google Scholar]
- 118.Reggiori F, Monastyrska I, Shintani T, Klionsky DJ. The actin cytoskeleton is required for selective types of autophagy, but not nonspecific autophagy, in the yeast Saccharomyces cerevisiae. Mol Biol Cell. 2005;16:5843–56. doi: 10.1091/mbc.E05-07-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reggiori F, Shintani T, Nair U, Klionsky DJ. Atg9 cycles between mitochondria and the pre-autophagosomal structure in yeasts. Autophagy. 2005;1:101–9. doi: 10.4161/auto.1.2.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reggiori F, Tucker KA, Stromhaug PE, Klionsky DJ. The Atg1-Atg13 complex regulates Atg9 and Atg23 retrieval transport from the pre-autophagosomal structure. Dev Cell. 2004;6:79–90. doi: 10.1016/s1534-5807(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 121.Reggiori F, Wang CW, Nair U, Shintani T, Abeliovich H, Klionsky DJ. Early stages of the secretory pathway, but not endosomes, are required for Cvt vesicle and autophagosome assembly in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:2189–204. doi: 10.1091/mbc.E03-07-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reggiori F, Wang CW, Stromhaug PE, Shintani T, Klionsky DJ. Vps51 is part of the yeast Vps fifty-three tethering complex essential for retrograde traffic from the early endosome and Cvt vesicle completion. J Biol Chem. 2003;278:5009–20. doi: 10.1074/jbc.M210436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reiling JH, Hafen E. The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes Dev. 2004;18:2879–92. doi: 10.1101/gad.322704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rodriguez-Hernandez A, Cordero MD, Salviati L, Artuch R, Pineda M, et al. Coenzyme Q deficiency triggers mitochondria degradation by mitophagy. Autophagy. 2009;5:19–32. doi: 10.4161/auto.5.1.7174. [DOI] [PubMed] [Google Scholar]
- 125.Rusten TE, Vaccari T, Lindmo K, Rodahl LM, Nezis IP, et al. ESCRTs and Fab1 regulate distinct steps of autophagy. Curr Biol. 2007;17:1817–25. doi: 10.1016/j.cub.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 126.Sakaki K, Wu J, Kaufman RJ. Protein kinase Cθ is required for autophagy in response to stress in the endoplasmic reticulum. J Biol Chem. 2008;283:15370–80. doi: 10.1074/jbc.M710209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–35. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–57. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 130.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–60. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schmelzle T, Beck T, Martin DE, Hall MN. Activation of the RAS/cyclic AMP pathway suppresses a TOR deficiency in yeast. Mol Cell Biol. 2004;24:338–51. doi: 10.1128/MCB.24.1.338-351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci USA. 2007;104:19500–5. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Scott RC, Juhasz G, Neufeld TP. Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death. Curr Biol. 2007;17:1–11. doi: 10.1016/j.cub.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Scott SV, Guan J, Hutchins MU, Kim J, Klionsky DJ. Cvt19 is a receptor for the cytoplasm-to-vacuole targeting pathway. Mol Cell. 2001;7:1131–41. doi: 10.1016/s1097-2765(01)00263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shao Y, Gao Z, Marks PA, Jiang X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci USA. 2004;101:18030–35. doi: 10.1073/pnas.0408345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shi CS, Kehrl JH. MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J Biol Chem. 2008;283:33175–82. doi: 10.1074/jbc.M804478200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shintani T, Huang WP, Stromhaug PE, Klionsky DJ. Mechanism of cargo selection in the cytoplasm to vacuole targeting pathway. Dev Cell. 2002;3:825–37. doi: 10.1016/s1534-5807(02)00373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Singh SB, Davis AS, Taylor GA, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006;313:1438–41. doi: 10.1126/science.1129577. [DOI] [PubMed] [Google Scholar]
- 139.Sir D, Chen WL, Choi J, Wakita T, Yen TS, Ou JH. Induction of incomplete autophagic response by hepatitis C virus via the unfolded protein response. Hepatology. 2008;48:1054–61. doi: 10.1002/hep.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, et al. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–70. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 141.Stromhaug PE, Reggiori F, Guan J, Wang CW, Klionsky DJ. Atg21 is a phosphoinositide binding protein required for efficient lipidation and localization of Atg8 during uptake of aminopeptidase I by selective autophagy. Mol Biol Cell. 2004;15:3553–66. doi: 10.1091/mbc.E04-02-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun Q, Fan W, Chen K, Ding X, Chen S, Zhong Q. Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 2008;105:19211–16. doi: 10.1073/pnas.0810452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Suzuki K, Kirisako T, Kamada Y, Mizushima N, Noda T, Ohsumi Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20:5971–81. doi: 10.1093/emboj/20.21.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Suzuki K, Kubota Y, Sekito T, Ohsumi Y. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells. 2007;12:209–18. doi: 10.1111/j.1365-2443.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- 145.Tallóczy Z, Jiang W, Virgin HW, IV, Leib DA, Scheuner D, et al. Regulation of starvation- and virus-induced autophagy by the eIF2a kinase signaling pathway. Proc Natl Acad Sci USA. 2002;99:190–95. doi: 10.1073/pnas.012485299. [DOI] [PMC free article] [PubMed] [Google Scholar]; First suggestion that autophagy induction by starvation or viral infection requires the eIF2α kinase pathway.
- 146.Tallóczy Z, Virgin HW, IV, Levine B. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy. 2006;2:24–29. doi: 10.4161/auto.2176. [DOI] [PubMed] [Google Scholar]
- 147.Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, et al. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406:902–6. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- 148.Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- 149.Teter SA, Eggerton KP, Scott SV, Kim J, Fischer AM, Klionsky DJ. Degradation of lipid vesicles in the yeast vacuole requires function of Cvt17, a putative lipase. J Biol Chem. 2001;276:2083–87. doi: 10.1074/jbc.C000739200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol. 2007;27:6229–42. doi: 10.1128/MCB.02246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–46. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vasseur S, Afzal S, Tardivel-Lacombe J, Park DS, Iovanna JL, Mak TW. DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc Natl Acad Sci USA. 2009;106:1111–16. doi: 10.1073/pnas.0812745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang Y, Weiss LM, Orlofsky A. Host cell autophagy is induced by Toxoplasma gondii and contributes to parasite growth. J Biol Chem. 2009;284:1694–701. doi: 10.1074/jbc.M807890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang Z, Wilson WA, Fujino MA, Roach PJ. Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol Cell Biol. 2001;21:5742–52. doi: 10.1128/MCB.21.17.5742-5752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Watanabe M, Adachi S, Matsubara H, Imai T, Yui Y, et al. Induction of autophagy in malignant rhabdoid tumor cells by the histone deacetylase inhibitor FK228 through AIF translocation. Int J Cancer. 2009;124:55–67. doi: 10.1002/ijc.23897. [DOI] [PubMed] [Google Scholar]
- 156.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–88. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu H, Yang JM, Jin S, Zhang H, Hait WN. Elongation factor-2 kinase regulates autophagy in human glioblastoma cells. Cancer Res. 2006;66:3015–23. doi: 10.1158/0008-5472.CAN-05-1554. [DOI] [PubMed] [Google Scholar]
- 158.Xie Z, Nair U, Klionsky DJ. Atg8 controls phagophore expansion during autophagosome formation. Mol Biol Cell. 2008;19:3290–98. doi: 10.1091/mbc.E07-12-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27:135–44. doi: 10.1016/j.immuni.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates that Toll-like receptor signaling stimulates autophagy in macrophages.
- 160.Yang Z, Huang J, Geng J, Nair U, Klionsky DJ. Atg22 recycles amino acids to link the degradative and recycling functions of autophagy. Mol Biol Cell. 2006;17:5094–104. doi: 10.1091/mbc.E06-06-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yano T, Mita S, Ohmori H, Oshima Y, Fujimoto Y, et al. Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nat Immunol. 2008;9:908–16. doi: 10.1038/ni.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yen WL, Klionsky DJ. How to live long and prosper: autophagy, mitochondria, and aging. Physiology. 2008;23:248–62. doi: 10.1152/physiol.00013.2008. [DOI] [PubMed] [Google Scholar]
- 163.Yen WL, Legakis JE, Nair U, Klionsky DJ. Atg27 is required for autophagy-dependent cycling of Atg9. Mol Biol Cell. 2007;18:581–93. doi: 10.1091/mbc.E06-07-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yorimitsu T, He C, Wang K, Klionsky DJ. Tap42-associated protein phosphatase type2Anegatively regulates induction of autophagy. Autophagy. 2009 doi: 10.4161/auto.5.5.8091. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12:1542–52. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281:30299–304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yorimitsu T, Zaman S, Broach JR, Klionsky DJ. Protein kinase A and Sch9 cooperatively regulate induction of autophagy in Saccharomyces cerevisiae. Mol Biol Cell. 2007;18:4180–9. doi: 10.1091/mbc.E07-05-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Young ARJ, Chan EYW, Hu XW, Köchl R, Crawshaw SG, et al. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci. 2006;119:3888–900. doi: 10.1242/jcs.03172. [DOI] [PubMed] [Google Scholar]
- 169.Zalckvar E, Berissi H, Mizrachy L, Idelchuk Y, Koren I, et al. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 2009;10:285–92. doi: 10.1038/embor.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zaman S, Lippman SI, Zhao X, Broach JR. How Saccharomyces responds to nutrients. Annu Rev Genet. 2008;42:27–81. doi: 10.1146/annurev.genet.41.110306.130206. [DOI] [PubMed] [Google Scholar]
- 171.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 172.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–81. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 173.Zhang Y, Yan L, Zhou Z, Yang P, Tian E, et al. SEPA-1 mediates the specific recognition and degradation of P granule components by autophagy in C. elegans. Cell. 2009;136:308–21. doi: 10.1016/j.cell.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 174.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–83. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]; Demonstrates the transcriptional regulation of autophagy genes by the transcription factor FoxO3 in mammals.