Abstract
Aspirin-intolerant chronic urticaria (AICU) is a common condition among the chronic urticaria population, but the genetic mechanism is not yet understood. In this study, the genotypes and haplotypes of three interleukin (IL)-13 polymorphisms, -1510 A>C, -1055C>T, and Arg110Gln (110G>A), as well as their respective clinical phenotypes were examined to determine whether genetic variants of IL-13 play a role in AICU. Single-nucleotide polymorphism (SNP) genotyping was used to compare IL-13 genotype and allele frequencies among 135 patients with AICU, 146 with aspirin-tolerant chronic urticaria (ATCU), and 430 normal controls (NC). Relationships among the AICU phenotype, atopy, and total IgE level were also investigated. The results failed to show a significant difference in the allele or genotype frequencies between the AICU group and either the ATCU or NC group (P>0.05, respectively). Haplotype analysis confirmed that there was no significant difference among the three study groups (P>0.05), nor was there a significant difference in atopy or total IgE level according to the three genetic polymorphisms (P>0.05, respectively). Our data lead to the conclusion that there is no evidence supporting genetic polymorphisms in IL-13 as a genetic risk factor for the development of AICU.
Keywords: aspirin, IL-13, urticaria
INTRODUCTION
Aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) cause symptoms in 20-30% of patients with chronic urticaria (CU).1,2 In the Korean population, 35.7% of CU patients are affected, as confirmed by oral provocation testing.3 Several genes are likely to be involved in the etiology and pathogenesis of aspirin-intolerant urticaria (AIU), including both the acute and chronic forms as well as intermediate phenotypes. The candidate genes include HLA-DRB1 *1302-DQB1*0609,4 ALOX5,5 FCER1A,6 HNMT,7 and FCER1G.8
The cytokine interleukin (IL)-13 is produced primarily by activated Th2 cells9 and is thought to play a role in the mechanisms of several atopic conditions. IL-13 promotes B cell differentiation and is capable of inducing isotype-switching in B cells, resulting in the production of IgG4 and IgE.10 Human lung mast cells express IL-13Rα1, and activation of these cells by the cytokine for 5 days increased FcεRI expression and proliferation.11 IL-13 maps to chromosome 5q31, which has been implicated in the development of asthma and atopy.12 It is a critical effector in the induction and maintenance of IgE production and IgE-mediated allergic airway responses.13
Analyses of IL-13 gene polymorphisms have produced various results depending on the specific study population. For example, genetic association studies in German14 and Chinese populations have suggested a role for the IL13 variant Arg110Gln in the increased IgE production and atopic sensitization that characterizes allergic rhinitis, whereas this allele was found to be associated with asthma in British and Japanese populations.15 Previous studies have reported that AICU patients had a significantly higher rate of atopy than ATCU patients and normal controls, suggesting that atopy is a risk factor for AICU.3,8 A study on FcER1α promoter polymorphisms found that the prolonged exposure of AICU patients to IgE induces the persistent accumulation and activation of mast cells and basophils via increased surface expression of high-affinity IgE receptors.6
To identify genetic factors in the development of AICU, we analyzed the genotypes and haplotypes of three polymorphisms of IL-13 to determine whether genetic variants of IL-13 play an important role in AICU.
MATERIALS AND METHODS
Study subjects and phenotyping
Two groups of CU patients, AICU (n=135) and ATCU (n=146), who were identified based on the results of n oral provocation test with up to 500 mg of aspirin (Rhonal; KunWha Pharmaceutical Co., Seoul, Korea), were enrolled in the study, as described previously.4 AICU patients who had aspirin-intolerant asthma (AIA) were excluded. Normal healthy controls (NC; n=430) with no personal or family history of allergic diseases, or aspirin or drug hypersensitivity were recruited from the general Korean population. Informed consent was obtained from all participants, and the institutional review board of Ajou University Hospital approved this study. Skin-prick tests were performed with 55 common aeroallergens (Bencard Co., West Sussex, UK), and atopy was defined as one or more positive reactions to common inhalant allergens. Total IgE concentrations were measured using a UniCAP system (Phadia, Uppsala, Sweden), according to the manufacturer's instructions. Anti-thyroglobulin levels in serum were measured using anti-Tg (B.R.A.H.M.S Aktiengesellschaft, Hennigsdorf, Germany) and serum antimicrosomal antibodies with a thyroid peroxidase IgG ELISA (Zeus Scientific, Zierikzee, Netherlands).
SNP identification and genotyping
Peripheral blood samples from 40 healthy Korean volunteers were used to identify single nucleotide polymorphisms (SNPs). Genomic DNA was prepared from the blood samples using a Puregene DNA purification kit (Gentra, Minneapolis, MN, USA), according to the manufacturer's protocol.
The IL-13 gene was examined for SNPs using an ABI Prism 3100 DNA analyzer (Applied Biosystems, Foster City, CA, USA). Three polymorphisms of IL-13, -1510 A>C, -1055C>T, and Arg110Gln (110G>A), were genotyped using a primer-extension method and a SNAPshot ddNTP primer-extension kit (Applied Biosystems). The primers used were: for IL-13 -1510A>C, forward (F) 5'-TGTGGGAGATGCCGTGGG-3', reverse (R) 5'-TCTGACTCCCAGAAGTCTGC-3', and extension 5'-TACAGATTAGGAAACAGGCCCGTAG-3'; for IL-13-1055C>T, (F) 5'-AGAGAGGGTGGGAATGAC-3', (R) 5'-CCAGTCTCTGCAGGATCA-3', and extension 5'-TGTCGCCTTTTCCTGCTCTTCCCTC-3'; and for IL-13 110G>A, (F) 5'-AGTTTGTAAAGGACCTGCTCTTAC-3', (R) 5'-TCAGGTCCTGTCTCTGCAA-3', and extension 5'-TTAAAGAAACTTTTTCGCGAGGGAC-3'.
Statistical analysis
Chi-squared tests were used to detect significant departures in genotype frequency from the Hardy-Weinberg equilibrium at each SNP. Haplotypes of IL-13 were analyzed using Haploview v2.0, based on the expectation maximization algorithm. Differences in the mean values of phenotypic characteristics among AICU patients were compared using the chi-squared test, or Fisher's exact test and independent t-test. Continuous variables that did not have a normal distribution, such as the levels of serum total IgE, were log-transformed. Differences in genotype frequency between groups were examined with the chi-squared test, and three logical regression models (co-dominant, dominant, recessive) were used after accounting for age and gender covariates. The level of statistical significance was set at P<0.05. SPSS 12.0 for Windows software (SPSS Inc., Chicago, IL, USA) was used for the statistical analyses.
RESULTS
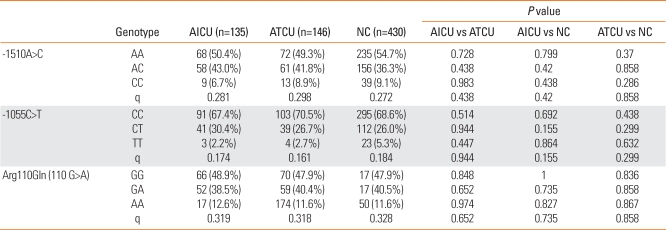
In our study, genetic associations among three genetic polymorphisms of IL-13, -1510A>C, -1055C>T, and Arg110Gln (110G>A), and the three study groups (AICU, ATCU, and NC) were examined. No significant association was detected between the IL-13 polymorphisms and susceptibility to AICU (P>0.05).
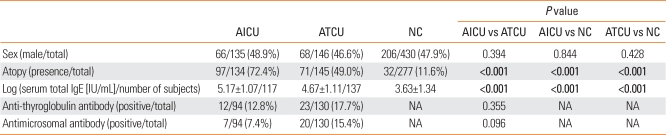
Significant differences in AICU phenotypes, based on the presence of atopy and log serum total IgE levels, were found between the AICU, ATCU, and NC groups (P<0.001, respectively) (Table 1). However, there were no significant differences between the two CU groups with respect to allele and genotype frequencies (P>0.05) (Table 2). Furthermore, the three polymorphisms of IL-13 were not in linkage disequilibrium.
Table 1.
Clinical characteristics of study subjects
Values in bold indicate significant P value. The value was presented as means±S.D.
NA=not applicable.
Table 2.
Allele and genotype frequencies of AICU
Each P value was calculated with co-dominant, dominant, and recessive models. Logistic regression analysis was applied to control for age and sex as covariables. q; Minor allele frequency.
AICU, aspirin-intolerant chronic urticaria; NC, normal controls; ATCU, aspirin-tolerant chronic urticaria; N, number of patients.
Using Haploview 2.0 and the expectation maximization algorithm, five common haplotypes were constructed: ht1 (ACG), ht2 (CTA), ht3 (ACA), ht4 (CCG), and ht5 (CTG). Haplotype analysis further confirmed that there were no significant differences among the three study groups (P>0.05) (data not shown).
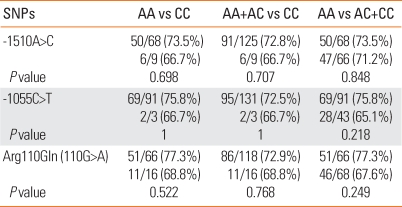
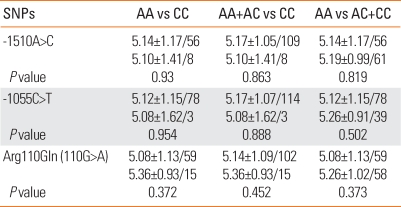
The relationship between the investigated genotypes of the three polymorphisms and AICU phenotype was not significantly different for atopy (Table 4) or total IgE (Table 3) according to the three different methods of analysis (P>0.05).
Table 4.
Comparison of the atopy (presence/total) according to the genotype of IL 13 in AICU
Table 3.
Comparison of the log total IgE according to the genotype of IL 13 in AICU
The value was presented as means±S.D.
DISCUSSION
This study evaluated three AICU-susceptibility loci in the IL-13 gene: two SNPs in the promoter region (-1510 A>C and -1055C>T) and one nonsynonymous SNP (Arg110Gln, 110G>A). In a case control study, we evaluated the three polymorphisms for evidence of association with AICU and related phenotypes. Based on the results, no evidence was found to support a significant association between these three SNPs and the diagnosis of AICU.
The cytokines IL-13 and IL-4 share biological properties such as isotype switching to IgE and expression of endothelial cell adhesion molecules.16 Accordingly, it was reasonable to propose that in chronic urticaria, IL-13 increases mast cell FcεR1 expression and mediator release,17 leading to the development of hives and the enhancement of cutaneous inflammation. In a previous report, patients with more frequent itch were found to have higher cytokine levels, suggesting that IL-13 may serve as a serological marker of disease activity.18
Owing to the lack of previously published data on IL-13 SNPs in patients with AICU, our results cannot be compared directly with others. However, several studies showed a linkage between IL-13 and a high serum total IgE level,19,20 including an association of IL-13 Arg110Gln with serum IgE levels in patients with asthma21,15 and atopic dermatitis.22 IL-13 variants have been associated with airway responsiveness23 and with eosinophil count.24 Recently, IL-13 Arg110Gln was reported to be significantly associated with reduced lung function in Korean children and adolescents.25 Nevertheless, we found no significant association of either of the promoter SNPs or the coding SNP with AICU traits, including serum total IgE and atopy. Our findings indicate that a genetic variation in the form of any of the three SNPs at the IL-13 locus is not likely to be involved in the development of AICU.
This result is consistent with an earlier report showing that there was no relationship between the coding SNP and allergic rhinitis induced by artemisia pollen and/or DerpI in a Chinese population. Marginal significance was found for atopy-related phenotypes associated with the Arg110Gln variant and in vitro specific IgE responses to common inhalant allergens.26 In our previous study, two SNPs, at FcεR1β E237G and FcεR1γ, were associated with atopy in AICU patients, but not in ATCU patients, and were proposed to explain the increased release of histamine from basophils and subsequent development of the AICU phenotype.8 Furthermore, other studies have noted a significant association of the FcεR1β-109T>C and FcεR1β E237G polymorphisms with serum IgE levels in asthmatic patients,27,28 and the FcεR1α -344C>T polymorphism in AICU.6 However, in our study, IL-13 genetic polymorphisms were not related to either atopy or total IgE levels in AICU patients, implying that neither the two SNPs in the functional promoter nor the SNP in the coding sequence contribute significantly to AICU susceptibility. This is the first detailed investigation showing the absence of a genetic association between IL-13 and AICU and its phenotypes, i.e., serum total IgE and atopy. There is no evidence that these three SNPs are associated with AICU; instead, SNPs of IL-13 may confer a greater susceptibility to asthma or other inflammatory diseases.
ACKNOWLEDGMENTS
This study was supported by a grant of the Korean Health 21 R&D Project, Ministry For Health, Welfare and Family Affairs, Republic of Korea (A050571 and A030001).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Grattan CE. Aspirin sensitivity and urticaria. Clin Exp Dermatol. 2003;28:123–127. doi: 10.1046/j.1365-2230.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 2.Mastalerz L, Setkowicz M, Sanak M, Szczeklik A. Hypersensitivity to aspirin: common eicosanoid alterations in urticaria and asthma. J Allergy Clin Immunol. 2004;113:771–775. doi: 10.1016/j.jaci.2003.12.323. [DOI] [PubMed] [Google Scholar]
- 3.Ye YM, Kim JE, Nahm DI, Kim SH, Suh CH, Nahm DH, Park HS. Comparison of clinical characteristics and prognosis of chronic urticaria according to the aspirin sensitivity. J Asthma Allergy Clin Immunol. 2005;25:194–199. [Google Scholar]
- 4.Kim SH, Choi JH, Lee KW, Kim SH, Shin ES, Oh HB, Suh CH, Nahm DH, Park HS. The human leucocyte antigen-DRB1*1302-DQB1*0609-DPB1*0201 haplotype may be a strong genetic marker for aspirin-induced urticaria. Clin Exp Allergy. 2005;35:339–344. doi: 10.1111/j.1365-2222.2004.02197.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim SH, Choi JH, Holloway JW, Suh CH, Nahm DH, Ha EH, Park CS, Park HS. Leukotriene-related gene polymorphisms in patients with aspirin-intolerant urticaria and aspirin-intolerant asthma: differing contributions of ALOX5 polymorphism in Korean population. J Korean Med Sci. 2005;20:926–931. doi: 10.3346/jkms.2005.20.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae JS, Kim SH, Ye YM, Yoon HJ, Suh CH, Nahm DH, Park HS. Significant association of FcepsilonRIalpha promoter polymorphisms with aspirin-intolerant chronic urticaria. J Allergy Clin Immunol. 2007;119:449–456. doi: 10.1016/j.jaci.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Kang YM, Kim SH, Cho BY, Ye YM, Hur GY, Park HS. Histamine N-methyltransferase 939A>G polymorphism affects mRNA stability in patients with acetylsalicylic acid-intolerant chronic urticaria. Allergy. 2009;64:213–221. doi: 10.1111/j.1398-9995.2008.01795.x. [DOI] [PubMed] [Google Scholar]
- 8.Palikhe N, Kim SH, Yang EM, Kang YM, Ye YM, Hur GY, Park HS. Analysis of high-affinity IgE receptor (FcepsilonR1) polymorphisms in patients with aspirin-intolerant chronic urticaria. Allergy Asthma Proc. 2008;29:250–257. doi: 10.2500/aap.2008.29.3116. [DOI] [PubMed] [Google Scholar]
- 9.Nagai S, Hashimoto S, Yamashita T, Toyoda N, Satoh T, Suzuki T, Matsushima K. Comprehensive gene expression profile of human activated T(h)1- and T(h)2-polarized cells. Int Immunol. 2001;13:367–376. doi: 10.1093/intimm/13.3.367. [DOI] [PubMed] [Google Scholar]
- 10.Cocks BG, de Waal Malefyt R, Galizzi JP, de Vries JE, Aversa G. IL-13 induces proliferation and differentiation of human B cells activated by the CD40 ligand. Int Immunol. 1993;5:657–663. doi: 10.1093/intimm/5.6.657. [DOI] [PubMed] [Google Scholar]
- 11.Kaur D, Hollins F, Woodman L, Yang W, Monk P, May R, Bradding P, Brightling CE. Mast cells express IL-13R alpha 1: IL-13 promotes human lung mast cell proliferation and Fc epsilon RI expression. Allergy. 2006;61:1047–1053. doi: 10.1111/j.1398-9995.2006.01139.x. [DOI] [PubMed] [Google Scholar]
- 12.Shirakawa I, Deichmann KA, Izuhara I, Mao I, Adra CN, Hopkin JM. Atopy and asthma: genetic variants of IL-4 and IL-13 signalling. Immunol Today. 2000;21:60–64. doi: 10.1016/s0167-5699(99)01492-9. [DOI] [PubMed] [Google Scholar]
- 13.van der Pouw Kraan TC, van Veen A, Boeije LC, van Tuyl SA, de Groot ER, Stapel SO, Bakker A, Verweij CL, Aarden LA, van der Zee JS. An IL-13 promoter polymorphism associated with increased risk of allergic asthma. Genes Immun. 1999;1:61–65. doi: 10.1038/sj.gene.6363630. [DOI] [PubMed] [Google Scholar]
- 14.Wills-Karp M, Chiaramonte M. Interleukin-13 in asthma. Curr Opin Pulm Med. 2003;9:21–27. doi: 10.1097/00063198-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Graves PE, Kabesch M, Halonen M, Holberg CJ, Baldini M, Fritzsch C, Weiland SK, Erickson RP, von Mutius E, Martinez FD. A cluster of seven tightly linked polymorphisms in the IL-13 gene is associated with total serum IgE levels in three populations of white children. J Allergy Clin Immunol. 2000;105:506–513. doi: 10.1067/mai.2000.104940. [DOI] [PubMed] [Google Scholar]
- 16.Lee KH, Kim JY, Kang DS, Choi YJ, Lee WJ, Ro JY. Increased expression of endothelial cell adhesion molecules due to mediator release from human foreskin mast cells stimulated by autoantibodies in chronic urticaria sera. J Invest Dermatol. 2002;118:658–663. doi: 10.1046/j.1523-1747.2002.01733.x. [DOI] [PubMed] [Google Scholar]
- 17.Ryzhov S, Goldstein AE, Matafonov A, Zeng D, Biaggioni I, Feoktistov I. Adenosine-activated mast cells induce IgE synthesis by B lymphocytes: an A2B-mediated process involving Th2 cytokines IL-4 and IL-13 with implications for asthma. J Immunol. 2004;172:7726–7733. doi: 10.4049/jimmunol.172.12.7726. [DOI] [PubMed] [Google Scholar]
- 18.Caproni M, Cardinali C, Giomi B, Antiga E, D'Agata A, Walter S, Fabbri P. Serological detection of eotaxin, IL-4, IL-13, IFN-gamma, MIP-1alpha, TARC and IP-10 in chronic autoimmune urticaria and chronic idiopathic urticaria. J Dermatol Sci. 2004;36:57–59. doi: 10.1016/j.jdermsci.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Noguchi E, Shibasaki M, Arinami T, Takeda K, Maki T, Miyamoto T, Kawashima T, Kobayashi K, Hamaguchi H. Evidence for linkage between asthma/atopy in childhood and chromosome 5q31-q33 in a Japanese population. Am J Respir Crit Care Med. 1997;156:1390–1393. doi: 10.1164/ajrccm.156.5.9702084. [DOI] [PubMed] [Google Scholar]
- 20.Palmer LJ, Daniels SE, Rye PJ, Gibson NA, Tay GK, Cookson WO, Goldblatt J, Burton PR, LeSouef PN. Linkage of chromosome 5q and 11q gene markers to asthma-associated quantitative traits in Australian children. Am J Respir Crit Care Med. 1998;158:1825–1830. doi: 10.1164/ajrccm.158.6.9804037. [DOI] [PubMed] [Google Scholar]
- 21.DeMeo DL, Lange C, Silverman EK, Senter JM, Drazen JM, Barth MJ, Laird N, Weiss ST. Univariate and multivariate family-based association analysis of the IL-13 ARG130GLN polymorphism in the Childhood Asthma Management Program. Genet Epidemiol. 2002;23:335–348. doi: 10.1002/gepi.10182. [DOI] [PubMed] [Google Scholar]
- 22.Heinzmann A, Mao XQ, Akaiwa M, Kreomer RT, Gao PS, Ohshima K, Umeshita R, Abe Y, Braun S, Yamashita T, Roberts MH, Sugimoto R, Arima K, Arinobu Y, Yu B, Kruse S, Enomoto T, Dake Y, Kawai M, Shimazu S, Sasaki S, Adra CN, Kitaichi M, Inoue H, Yamauchi K, Tomichi N, Kurimoto F, Hamasaki N, Hopkin JM, Izuhara K, Shirakawa T, Deichmann KA. Genetic variants of IL-13 signalling and human asthma and atopy. Hum Mol Genet. 2000;9:549–559. doi: 10.1093/hmg/9.4.549. [DOI] [PubMed] [Google Scholar]
- 23.Howard TD, Whittaker PA, Zaiman AL, Koppelman GH, Xu J, Hanley MT, Meyers DA, Postma DS, Bleecker ER. Identification and association of polymorphisms in the interleukin-13 gene with asthma and atopy in a Dutch population. Am J Respir Cell Mol Biol. 2001;25:377–384. doi: 10.1165/ajrcmb.25.3.4483. [DOI] [PubMed] [Google Scholar]
- 24.Hunninghake GM, Soto-Quiros ME, Avila L, Su J, Murphy A, Demeo DL, Ly NP, Liang C, Sylvia JS, Klanderman BJ, Lange C, Raby BA, Silverman EK, Celedon JC. Polymorphisms in IL13, total IgE, eosinophilia, and asthma exacerbations in childhood. J Allergy Clin Immunol. 2007;120:84–90. doi: 10.1016/j.jaci.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 25.Park HW, Lee JE, Kim SH, Kim YK, Min KU, Kim YY, Cho SH. Genetic variation of IL13 as a risk factor of reduced lung function in children and adolescents: a cross-sectional population-based study in Korea. Respir Med. 2009;103:284–288. doi: 10.1016/j.rmed.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Nieters A, Linseisen J, Becker N. Association of polymorphisms in Th1, Th2 cytokine genes with hay-fever and atopy in a subsample of EPIC-Heidelberg. Clin Exp Allergy. 2004;34:346–353. doi: 10.1111/j.1365-2222.2004.01889.x. [DOI] [PubMed] [Google Scholar]
- 27.Young RP, Sharp PA, Lynch JR, Faux JA, Lathrop GM, Cookson WO, Hopkin JM. Confirmation of genetic linkage between atopic IgE responses and chromosome 11q13. J Med Genet. 1992;29:236–238. doi: 10.1136/jmg.29.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hizawa N, Yamaguchi E, Jinushi E, Kawakami Y. A common FCER1B gene promoter polymorphism influences total serum IgE levels in a Japanese population. Am J Respir Crit Care Med. 2000;161:906–909. doi: 10.1164/ajrccm.161.3.9903128. [DOI] [PubMed] [Google Scholar]