Abstract
More than 15 million people in the United States consume herbal remedies or high-dose vitamins. The number of visits to providers of complementary and alternative medicine exceeds those to primary care physicians, for annual out-of-pocket costs of $30 billion. Use of herbal products forms the bulk of treatments, particularly by elderly persons who also consume multiple prescription medications for comorbid conditions, which increases the risk of adverse herb-drug-disease interactions. Despite the paucity of scientific evidence supporting the safety or efficacy of herbal products, their widespread promotion in the popular media and the unsubstantiated health care claims about their efficacy drive consumer demand. In this review, we highlight commonly used herbs and their interactions with cardiovascular drugs. We also discuss health-related issues of herbal products and suggest ways to improve their safety to better protect the public from untoward effects.
Keywords: cardiovascular agents, complementary therapies, drug approval, herbal medicine, herb-drug interaction
Introduction
Herbal supplements have been used for thousands of years in the East and have had a recent resurgence in popularity among consumers in the West. More than 15 million people in the United States consume herbal remedies or high-dose vitamins, and the total number of visits to complementary and alternative medicine (CAM) providers far exceeds those to primary physicians (Figure 1A), amounting to more than $34 billion out-of-pocket costs for CAM annually (1) (Figure 1B). Of the $37.1 billion spent for weight-loss products in 2001, $17.7 billion was for dietary and herbal weight-loss supplements, a number projected to increase by 6% to 7% per year (2). Multiple factors contribute to the increased use of CAM, including the obesity epidemic, the prevalence of chronic disorders and pain syndromes, anxiety, depression (Figure 1C), the general desire for good health and wellness, disease prevention, the increasing cost of conventional medicines, and the traditional belief that CAM is safer and more effective than prescription drugs that commonly have adverse effects.
Figure 1.
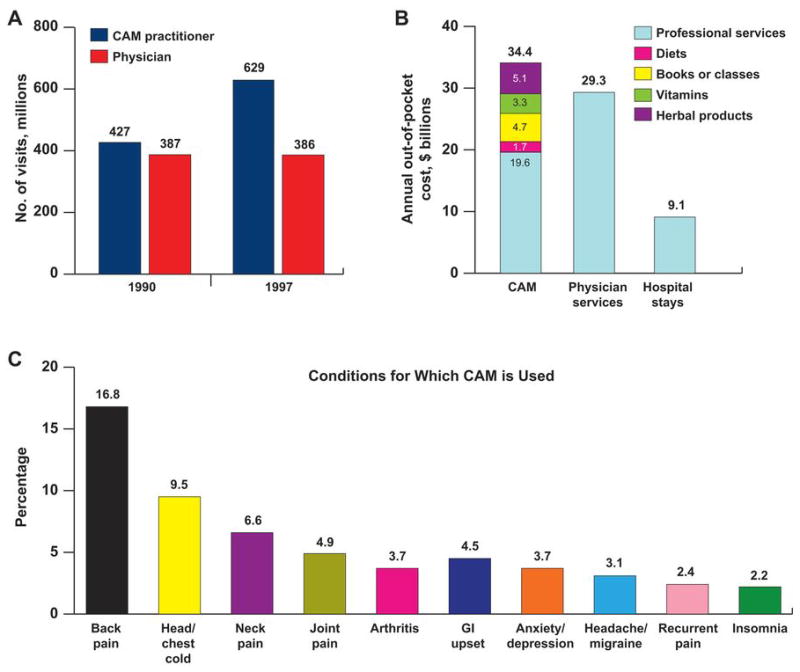
Comparison of Number of Visits, Costs, and Conditions Treated Medically or by Complementary and Alternative Medicine. A, Annual visits to physicians vs those to complementary and alternative medicine (CAM) practitioners; B, the costs of CAM services by type compared with the costs of physician services and hospitalizations; and, C, the most common conditions for which CAM therapies are used in the United States. GI indicates gastrointestinal. (A and B, Adapted from Eisenberg et al [1]. Used with permission.) (C, Data from Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Advance data from vital and health statistics; no 343. Hyattsville [MD]: National Center for Health Statistics; c2004.)
Herbs, generally defined as any form of plant or plant product, constitute the largest proportion of CAM use in the United States (Figure 2). Because herbs are regarded as food products, they are not subject to the same scrutiny and regulation as traditional medications. As a result, manufacturers are exempt from premarket safety and efficacy testing before the release of an herbal product and from any postmarketing surveillance. Although herbal remedies are perceived as being natural and therefore safe, many have adverse effects that can sometimes produce life-threatening consequences.
Figure 2.
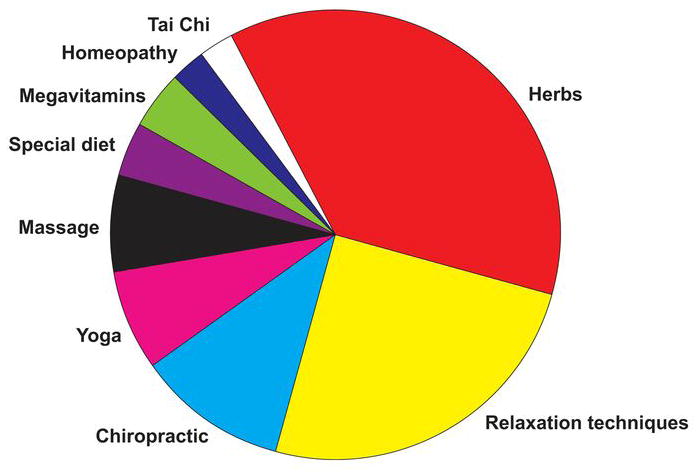
Types of Complementary and Alternative Medicine Used by U.S. Consumers. (Data from Tindle HA, Davis RB, Phillips RS, Eisenberg DM. Trends in use of complementary and alternative medicine by US adults: 1997–2002. Altern Ther Health Med. 2005 Jan-Feb;11[1]:42–9.)
Despite the paucity of scientific evidence about the safety or efficacy of herbal products, widespread promotion of CAM products in the popular media and unsubstantiated health care claims seem to be driving their demand and forcing even conventional medical practitioners to incorporate CAM therapies into their practice. In 2 nationwide surveys conducted in 1990 and 1997, Eisenberg et al (1,3) found an increased number of visits to CAM providers from 427 million to 629 million, whereas the number of visits to primary physicians remained, in essence, unchanged (Figure 1A). Millions of people are therefore exposed to the risk of these potential adverse interactions, especially with products that contain several herbs.
The use of herbal supplements is prevalent among patients who are taking prescription medications, particularly senior citizens (4). Yet few clinical studies have systematically assessed potential interactions between herbs and medications (5). Most patients do not readily disclose their use of CAM to their health care providers (1), and physicians may not routinely ask about such use. As a result, dangerous herb-drug interactions may be missed. However, potentially serious consequences might be avoided by obtaining a more careful history about CAM use. In this review, we highlight some common herbal remedies used, their adverse cardiovascular effects, and their potential interactions with cardiovascular drugs. We also discuss issues about the use of herbal products and suggest ways to improve their safety.
Search Strategy and Selection Criteria
A search of the PubMed and Medline databases was performed for the years 1966 to 2008 using the search terms “cardiovascular agents,” “complementary therapies,” “herb-drug interaction,” and “cardiovascular disease interactions” to identify citations, abstracts, and articles on herbs and cardiovascular disease.
Cardiovascular Adverse Effects of Herbal Remedies
Patients are increasingly using herbal products for purportedly preventive and therapeutic purposes (6). Some products have direct effects on the cardiovascular or hemostatic system, whereas others have indirect effects through interactions with medications that could lead to serious consequences (4). Common herbal remedies that produce adverse effects on the cardiovascular system include St. John’s wort, motherwort, ginseng, gingko biloba, garlic, grapefruit juice, hawthorn, saw palmetto, danshen, echinacea, tetrandrine, aconite, yohimbine, gynura, licorice, and black cohosh (Table 1).
Table 1.
Herbal Products to Avoid in Patients With Cardiovascular Diseasesa
| Herb | Purported Use | Cardiac Adverse Effect of Interaction |
|---|---|---|
| Alfalfa | Arthritis, asthma, dyspepsia, hyperlipidemia, diabetes | Increases bleeding risk with warfarin |
| Aloe vera | Wounds (topical), diabetes (oral) | Hypokalemia causing digitalis toxicity and arrhythmia |
| Angelica (dong quai) | Appetite loss, dyspepsia, infection | Increases bleeding risk with warfarin |
| Bilberry | Circulatory disorders, local inflammation, skin conditions, diarrhea, arthritis | Increases bleeding risk with warfarin |
| Butcher’s broom | Circulatory disorders, inflammation, leg cramps | Decreases effects of α-blockers |
| Capsicum | Shingles, trigeminal and diabetic neuralgia | Increases blood pressure (with MAOI) |
| Fenugreek | High cholesterol | Increases bleeding risk with warfarin; hypoglycemia |
| Fumitory | Infection, edema, hypertension, constipation | Increases effects of β-blockers, calcium channel blockers, cardiac glycosides |
| Garlic | High cholesterol, hypertension, heart disease | Increases bleeding risk with warfarin |
| Ginger | High cholesterol, motion sickness, indigestion, antioxidant | Increases bleeding risk with warfarin |
| Ginkgo | Poor circulation, cognitive disorder | Increases bleeding risk with warfarin, aspirin, or COX-2 inhibitors Potential risk of seizures |
| Ginseng | Aging, diminished immunity, improves mental and physical capacity and stress tolerance | Increases blood pressure; Decreases effects of warfarin; Hypoglycemia |
| Gossypol | Male contraceptive | Increases effects of diuretics; Hypokalemia |
| Grapefruit juice | Weight loss, to promote cardiovascular health | Increases effects of statins, calcium channel blockers, or cyclosporines |
| Green tea | Improve cognitive performance, mental alertness, weight loss, diuretic | Decreases effects of warfarin (contains vitamin K) |
| Hawthorn | CHF, hypertension | Potentiates action of cardiac glycosides and nitrates |
| Irish moss | Ulcers, gastritis | Increases effects of antihypertensives |
| Kelp | Cancer, obesity | Increases effects of antihypertensives and anticoagulants |
| Khella | Muscle spasms | Increases effects of anticoagulants and calcium channel blockers |
| Licorice | Ulcer, cirrhosis, cough, sore throat, infections | Increases blood pressure; Hypokalemia; May potentiate digoxin toxicity |
| Lily of the valley | CHF | Increases effects of β-blockers, calcium channel blockers, digitalis, quinidine, steroids |
| Ma-huang (ephedra) | Obesity, cough | Increases heart rate and blood pressure |
| Night-blooming cereus | CHF | Increases effects of angiotension-converting enzyme inhibitors, antiarrhythmics, β-blockers, calcium channel blockers, cardiac glycosides |
| Oleander | Muscle cramps, asthma, cancer, CHF, hepatitis, psoriasis, arthritis | Heart block; Hyperkalemia; Arrhythmia; Death |
| St. John’s wort | Depression | Increases heart rate and blood pressure (with MAOI); Decreases digoxin concentration |
| Storphanthus | CHF | Increases effects of cardiac glycosides |
| Yohimbine | Impotence | Increases heart rate; Increases or decreases blood pressure |
Abbreviations: CHF, congestive heart failure; COX, cyclooxygenase; MAOI, monoamine oxidase inhibitor.
Only major indications, adverse effects, and interactions are listed; thus, the list is not all-inclusive.
St. John’s Wort
St. John’s wort is one of the top 10 best-selling herbs in the United States (7). It is typically used to treat depression, anxiety, sleep disorders, the common cold, herpes, and the human immunodeficiency virus. It is used as a topical analgesic, and even as an enema for ulcerative colitis.
Use of St. John’s wort could potentially result in serious adverse reactions because of its effect on drug metabolism; it induces the hepatic cytochrome P450 system (8), particularly CYP3A4, an enzyme involved in oxidative metabolism of more than 50% of all prescription medications (Table 2) (9). Therefore, coadministration of this herb and drugs metabolized by CYP3A4 (Table 2) should be avoided, as it may result in reduced bioavailability and effectiveness with subsequent recurrence of arrhythmia, hypertension, or other undesirable effects (9).
Table 2.
Common Drugs Metabolized by the CYP3A4 Systema
| Medication Class | Drug Names |
|---|---|
| Antiarrhythmic | amiodarone, disopyramide, flecainide,b lidocaine, mexiletine, quinidinec |
| Angiotensin receptor antagonist | irbesartan |
| Antihistamine | loratidine, fexofenadine |
| Antibiotic or antimicrobial | macrolides (erythromycins), floxacins (ciprofloxacin), azoles (itraconazole, ketoconazole), protease inhibitors (indinavir sulfate, ritonavir, saquinavir, nelfinavir mesylate) |
| β-Adrenergic blocker | metoprolol,b carvedilol |
| Calcium channel blocker | amlodipine besylate, felodipine, nifedipine, diltiazem, verapamil |
| Chemotherapeutic | cyclophosphamide, docetaxel, doxorubicin, etoposide, ifosfamide, paclitaxel, tamoxifen citrate, teniposide, vinblastine sulfate, vindesine sulfate, gefitinib |
| Immunosuppressant | cyclosporine, tacrolimus, sirolimus |
| Psychotropic | benzodiazepines (alprazolam, clonazepam, flunitrazepam, midazolam, triazolam; tricyclic antidepressants (amitriptyline, clomipramine, imipramine); SSRIs (citalopram, fluoxetine, norfluoxetine, sertraline) |
| HMG-CoA reductase inhibitor (statins) | atorvastatin, cerivastatin, fluvastatin, lovastatin, simvastatin |
| Other | cisapride, cortisol, ethinyl estradiol, progesterone, sildenafil, terazocin, warfarind |
Abbreviations: SSRI, selective serotonin reuptake inhibitor.
List is not all inclusive.
Also by CYP2D6.
Also by CYP2D1.
Also by CYP2D9.
Reduced drug levels of ethinyl estradiol (10), indinavir (11), and cyclosporine (12,13) have been reported in patients using St. John’s wort. In 1 study of organ transplant patients, St. John’s wort caused a decrease of almost 50% in the concentration of cyclosporine (14). A similar experience was reported in renal and cardiac transplant patients who had a reduction in the efficacy of immunosuppressants taken with St. John’s wort, with consequent transplant rejection (9,14).
Concomitant use of warfarin with St. John’s wort decreases prothrombin time, which may result in subtherapeutic anticoagulation and increased risk of thromboembolism (9,10). Persons taking warfarin who have a history of stroke, thrombosis, atrial fibrillation, or prosthetic cardiac valves should avoid the use of St. John’s wort (Table 3). Reduced concentrations of statins may increase the risk of cardiovascular events (15). St. John’s wort can induce the multidrug resistance gene product P-glycoprotein, which may reduce the blood levels and efficacy of drugs such as digoxin, which is normally excreted by this glycoprotein (16). Hypoglycemia may occur with concomitant use of antidiabetic agents (17). Serotonin syndrome, a potentially life-threatening adverse drug reaction caused by excess serotonergic activity in the central and peripheral nervous system, has also been reported with concomitant use of antidepressants (18–20).
Table 3.
Commonly Used Herbs That Can Potentiate the Risk of Bleeding or Arrhythmogenesis
| Bleeding | |
| Alfalfa | Garlic |
| Bilberry | Ginkgo biloba |
| Danshen | Ginseng |
| Dong quai | Motherwort |
| Fenugreek | Saw palmetto |
| Arrhythmogenesis (QT Prolongation) | |
| Aloe vera | Lily of the valley |
| Bitter orange | Night-blooming cereus |
| Echinacea | Oleander |
| Ginkgo biloba | Rhodiola |
| Ginseng | St. John’s wort |
| Guarana | |
| Hawthorn | |
| Horny goat weed | |
| Licorice | |
Motherwort
Motherwort has a long history of use in both European and Asian traditional medicine because of its purported sedative and antispasmodic properties. Traditionally, it has been used for “cardiac debility,” tachycardia, anxiety, insomnia, and amenorrhea. It is also used as a hypotensive and a diuretic. When administered intravenously, motherwort reduces platelet aggregation and fibrinogen levels (21). It potentiates antithrombotic and antiplatelet effects and increases the risk of bleeding (Table 3). Taken with benzodiazepines, motherwort can have a synergistic sedative effect and may result in coma.
Ginseng
The origin of the ginseng root and its manner of extraction can produce wide variations in ginseng products. Ginseng is advertised as an immune system stimulant that increases vigor, sexual potency, and longevity, and for use as an antidiabetic agent (9). Ginseng has both hypertensive and hypotensive effects, the latter caused by the enhanced synthesis of nitric oxide (22). In Chinese medicine, ginseng is used for myocardial infarction, congestive heart failure (CHF), and angina pectoris; however, current evidence does not support its use for cardiovascular conditions.
Ginseng abuse syndrome causes hypertension, behavioral changes, and diarrhea (23). A nephrotoxic contaminant, germanium may damage cells of the thick ascending limb of the loop of Henle, thereby diminishing responsiveness to loop diuretics (24). When administered with warfarin, ginseng resulted in reduced prothrombin time (25). The inconsistency of ginseng in lowering blood glucose may be related to the type of preparation. Ginseng can produce effects similar to those of estrogen because its active component, ginsenosides, has a chemical structure similar to that of testosterone, estrogen, and glucocorticoids (26). Ginseng should not be used by women who are pregnant or receiving hormone replacement therapy. Neonatal death has been related to maternal use (27). Increased levels of digoxin are associated with Siberian ginseng, which interferes with the digoxin assay (Table 4).
Table 4.
Herbal Products That May Interfere With Digoxin Level and Assays
| Herb | Extent of Interference | Comments |
|---|---|---|
| Chan su | High | Active components (eg, bufalin) cross-react with digoxin assay; Monitoring free digoxin eliminates interference |
| Danshen | Moderate | Falsely increases (FPIA) or falsely decreases low levels (MEIA) of digoxin; Monitoring free digoxin eliminates interference |
| Asian ginseng | Moderate | Falsely increases elevated (FPIA) or falsely decreases low (MEIA) digoxin level; Monitoring free digoxin does not eliminate interference |
| Siberian ginseng | Moderate | Falsely increases elevated (FPIA) or falsely decreases low (MEIA) digoxin; Monitoring free digoxin does not eliminate interference |
| Uzara root (diuretic) | NA | Increases effect with digoxin; Interferes with digoxin assay |
Abbreviations: FPIA, fluorescence polarization immunoassay; MEIA, microparticle enzyme immunoassay; NA, not available.
Adapted from Dasgupta A. Review of abnormal laboratory test results and toxic effects due to use of herbal medicines. Am J Clin Pathol. 2003 Jul;120(1):127–37. Used with permission.
Ginkgo
Ginkgo biloba is one of the world’s oldest living tree species, dating back to the Permian period. Purported indications include conditions such as cardiovascular disease, cerebrovascular or peripheral vascular insufficiency, impotence, inner ear dysfunction, retinopathy, premenstrual syndrome, stress, depression, and dementia. Ginkgo is one of the best-selling herbal remedies in the United States for cognitive impairment (7). However, early trials that suggested it had beneficial effects on cognition were limited by inadequate methods, low numbers of patients, absence of hard end points, and publication bias (28). Recent randomized trials demonstrated no difference between ginkgo and placebo (28,29). A large clinical trial of ginkgo for prevention of dementia, supported by the National Center for Complementary and Alternative Medicine (NCCAM) is under way.
The concurrent use of ginkgo with antiplatelet agents, anticoagulants, or antithrombotics increases the risk of bleeding. Hyphema, subphrenic hematoma, and intracranial hemorrhage have been reported (9). In clinical trials, ginkgo has also been shown to reduce the effectiveness of nicardipine by interacting with the cytochrome P450 system (9).
Garlic
Garlic has been mentioned in medicinal texts since the Ebers papyrus (circa 1550 BCE). It has been used for treatment of infectious conditions because of its presumed antimicrobial and immune-enhancing properties. Garlic is thought to have cholesterol-lowering and other antiatherosclerotic and antihypertensive effects and is used for prevention of cardiovascular disease (30). Despite such claims, a recent study concluded that raw, powdered, or aged garlic extract vs placebo for 6 months had no significant effect on low-density lipoprotein cholesterol or other plasma lipids in adults with moderate hypercholesterolemia (31). The active component ajoene in garlic inhibits collagen-induced platelet aggregation (32), and garlic is used for its antiplatelet and fibrinolytic effects in patients with cardiovascular disease. However, the risk of bleeding in persons using anticoagulant or antiplatelet agents increases, so its concomitant use should be avoided (33,34). Garlic supplements should be discontinued about 10 days before elective surgical procedures, especially by patients taking aspirin or warfarin (33).
Grapefruit Juice
Grapefruit is used as a dietary intervention to lose weight and improve cardiovascular health. Its constituents of naringenin and bergamottin inhibit the CYP3A4 enzyme in small-intestine enterocytes, which increases blood levels of CYP3A4 substrate drugs, including calcium channel blockers, cyclosporine, statins, midazolam, estrogen, and terazosin (Table 2). Thus, the action of these medications is potentiated by their increased bioavailability (35–38) that can potentially result in dangerous hypotension, myopathy, or liver toxicity. In postmenopausal women taking estrogen, grapefruit juice may increase the risk of breast cancer by inhibiting estrogen metabolism by CYP3A4 (39). These potential interactions should be discussed with patients taking medications metabolized by the CYP3A4 system and they should be advised to avoid grapefruit consumption.
Hawthorn
Hawthorn extract is commonly used by herbalists for treatment of angina, CHF, bradyarrhythmia, and cerebral insufficiency. Hawthorn has positive inotropic and vasodilatory effects and is thought to increase myocardial perfusion and reduce afterload. As an adjunct treatment for CHF, hawthorn has been reported to have beneficial effects on symptom control and physiologic outcomes (40,41), but the efficacy and safety of its supposed inotropic activity and effect on morbidity and mortality have not been systematically assessed (40). Hawthorn enhances the activity of digitalis (42), and its concomitant use should be monitored carefully for potential toxic effects. Hawthorn also and it could potentially increase the risk of inhibits the biosynthesis of thromboxane A2 bleeding in patients taking antiplatelets or anticoagulants (9,43). Without additional data on safety and efficacy, clinicians should discourage unsupervised use of hawthorn in patients with CHF who are taking heart failure medications.
Saw Palmetto
Saw palmetto is used by more than 2 million men for treatment of benign prostatic hypertrophy (BPH) (7). It is also used as a diuretic and urinary antiseptic. The exact biologic mechanism of action of saw palmetto is not clear. In vitro, it potently inhibits α1-adrenergic receptors (44). Despite claims that saw palmetto helps relieve BPH symptoms, recent clinical trials did not demonstrate any beneficial effects on BPH symptoms or postvoid residual bladder volume (45). Additional prospective studies are needed to establish the role of herbal extracts in alleviating BPH symptoms.
Saw palmetto inhibits cyclooxygenase and increases bleeding with warfarin (46). In addition, its unsupervised use can result in cholestatic hepatitis, acute pancreatitis (47), and intraoperative floppy iris syndrome during cataract removal because of loss of iris tone (48). Ophthalmologists should be aware of this important association so that they can take the necessary steps to prevent surgical complications.
Danshen
Danshen is used in traditional Chinese medicine for treatment of coronary artery disease and menstrual abnormalities. Danshen reduces elimination of warfarin and inhibits cyclic adenosine monophosphate phosphodiesterase, which results in additive antiplatelet effects and risk of bleeding (Table 5). Concomitant use with warfarin increases prothrombin time (49). Danshen may also interfere with digoxin assay (Table 4); in the absence of signs or symptoms of digoxin toxicity, the possibility of a false elevation of digoxin concentration should be explored.
Table 5.
Common Herb-Drug Interactions
| Herb | Drug or Drug Class | Interaction or Other Comments |
|---|---|---|
| Comfrey | Phenobarbital | Increases metabolism of comfrey, producing a lethal metabolite from pyrrolizidine that results in severe hepatotoxicity |
| Danshen | Anticoagulants or antiplatelets | Increases bleeding due to additive effects |
| Digoxin | Increases side effects of digoxin | |
| Echinacea | Amiodarone or ibutilide | Increases QT interval |
| Statins, fibrates, niacin | Increases risk of hepatotoxic effects | |
| Ephedra | Antidiabetes drugs | Increases blood glucose; Decreases effectiveness of oral hypoglycemic agents |
| Class IA and class III antiarrhythmics | Increases QT interval | |
| β-Blockers | Decreases effects of β-blockers, leading to hypertension and tachycardia | |
| Monoamine oxidase inhibitors | Hypertension | |
| Evening primrose oil | Phenobarbital | Decreases seizure threshold |
| Garlic | Aspirin, clopidogrel, warfarin, or heparinoids | Increases bleeding risk |
| Ginkgo biloba | Antidiabetes drugs | Increases hypoglycemia |
| Aspirin | Increases bleeding; inhibits PAF hemorrhage | |
| Warfarin | ||
| Ginseng | Antidiabetes drugs | Increases hypoglycemia |
| Digoxin | Interferes with digoxin assay, leading to falsely increased levels | |
| Warfarin | Decreases effectiveness of warfarin | |
| Phenelzine sulfate | Headache; Irritability; Insomnia |
|
| Hawthorn | Digoxin | Increases effects of digoxin |
| Calcium channel blockers or nitrates | Increases coronary vasodilatory effects | |
| Kava | Alprazolam | Increases CNS depression; Increases effects of alcohol |
| Licorice | Spironolactone | Increases effects of spironolactone |
| Saw palmetto | Anticoagulants or antiplatelets | Increases bleeding |
| Soy milk | Warfarin | Decreases effectiveness of warfarin |
| St. John’s wort | Digoxin | Decreases serum digoxin concentration |
| Clopidogrel | Increases activity of clopidogrel; Increases bleeding |
|
| Warfarin | Decreases warfarin bioavailability and effectiveness | |
| Simvastatin | Decreases effectiveness of simvastatin | |
| Paroxetine | Nausea; Lethargy; Incoherence |
|
| Class IA and III antiarrhythmic agents | Decreases effectiveness (precipitating arrhythmias) | |
| Cyclosporine | Decreases cyclosporine concentration due to increased clearance (transplant rejection) | |
| Theophylline | Decreases serum concentration | |
| Indinavir | Decreases serum concentration (treatment failure in HIV patients) | |
| Yohimbine | Clonidine | Decreases blood pressure reduction effect of centrally active agents |
| Guanabenz | ||
| CNS stimulants (eg, amphetamines) | Risk of hypertensive crisis due to its monoamine oxidase inhibitor activity | |
| ACE inhibitors | Decreases effectiveness of ACE inhibitors; Hypertension |
|
| β-Blockers | Decreases effectiveness of β-blockers; Hypertension; Increases heart rate |
Abbreviations: ACE, angiotensin-converting enzyme; CNS, central nervous system; HIV, human immunodeficiency virus; PAF, platelet-activating factor.
Echinacea
Echinacea is popularly believed to stimulate the immune system and prevent infections. The evidence is still insufficient to support clear therapeutic recommendations. The results of a controlled double-blind study indicated that echinacea had no clinically significant effects on rhinovirus infection (50). Persistent use may result in or potentiate the hepatotoxic effects of other medications (eg, statins, fibrates, niacins, or amiodarone). Side effects include nausea, dizziness, dyspnea, rash, and dermatitis. Flavonoids from echinacea may inhibit or induce cytochrome P450 enzymes, depending on their structure and assay conditions (9).
Tetrandrine
Tetrandrine is a vasoactive alkaloid used in Chinese medicine to treat hypertension and angina. Its vasodilative effect is due to inhibition of the L-type calcium channels (51) and possible competition with other calcium channel blockers (52). Tetrandrine lowers plasma glucose and causes hepatotoxicity and renal toxicity (53).
Aconite
Traditional Chinese practitioners use aconite for pain relief caused by trigeminal and intercostal neuralgia, rheumatism, migraine, and general debilitation. Aconite initially stimulates and then paralyzes nerves that communicate pain, touch, and temperature, producing anesthesia mediated by numerous different alkaloids blocking sodium current (54). It is also used as a mild diaphoretic and to slow pulse rate by its effect on brainstem centers. Atrial or ventricular fibrillation, however, may result from the direct effect of aconite on the myocardium. Side effects occur even after contact with leaves or sap from Aconitum plants (or monkshood) and can range from bradycardia and hypotension to fatal ventricular arrhythmia induced by triggered activity (55,56).
Yohimbine
Yohimbine is marketed for treatment of sexual disorders and exhaustion. Many of its effects are attributed to its α2-adrenergic receptor antagonist activity. Yohimbine increases the release of norepinephrine, resulting in inadequate blood pressure control in persons also using antihypertensives and diuretics (57) (Table 5). Use of yohimbine is contraindicated in patients with hypertension, angina, and renal impairment.
Gynura
Widely used in Chinese folk medicine, gynura purportedly improves microcirculation and relieves pain; however, it has been associated with hepatic toxicity (58–61). Resultant conditions include hepatic venoocclusive disease, which is characterized by painful hepatomegaly, fluid avidity, weight gain, and jaundice (58–60). In animals, gynura has been shown to inhibit angiotensin-converting enzyme activity, resulting in hypotension (62).
Licorice
Licorice is used as an expectorant. Modern cough syrups often include licorice extract. It can result in pseudoaldosteronism with concomitant hypokalemia, hypertension, and edema that may reduce the effectiveness of antihypertensive drugs (63). Licorice-induced hypokalemia can lead to increased risk for ventricular arrhythmia, particularly torsades de pointes (64,65) (Table 3). It can also potentiate effects of spironolactone and digoxin (Tables 1 and 5), and can cause hyperglycemia, rendering antidiabetic agents less effective. Its ability to inhibit thrombin and platelet aggregation enhances the risk of bleeding with antiplatelets and anticoagulants.
Black Cohosh
Black cohosh contains triterpene glycosides and has been used in remedies for relief of symptoms of menopause, premenstrual tension, and other gynecologic problems. The mechanism of action is unclear. It may bind to estrogen and serotonin receptors (66). After estrogen replacement therapy was shown to increase the risk of thromboembolic and cardiovascular events and breast cancer, sales of black cohosh supplements soared ($79 million in 2003) (67,68). In 2006 a clinical trial supported by NCCAM failed to show that treatments containing black cohosh relieved menopause-associated symptoms (67). Commercially available dietary supplements made from black cohosh inhibit CYP3A4 and potentially increase the risk of adverse effects from some drugs (Table 2). Hepatotoxicity has been reported (66,69), and black cohosh should not be used during pregnancy or lactation (9).
Problems Related to the Use of Herbal Products
The use of herbal products is complicated by numerous problems as summarized below.
Lack of Scientific Evidence of Safety and Efficacy
Most herbal products have not been scientifically evaluated; thus, information is limited about their pharmacokinetics, pharmacodynamics, efficacy, and safety. Randomized controlled trials are the best way to determine efficacy and safety; however, controlled trials for most herbal products are not available, not demanded by consumers or health care practitioners, and not required by regulatory agencies. Belief in the safety of CAM products remains unsubstantiated and, even when data are available, findings are often questionable because of a lack of consistency in research methods, the small number of subjects, the absence of placebo groups, the inclusion of healthy volunteers or low-risk populations without comorbid conditions, lack of standardization of supplements being investigated, and absence of data about drug-herb interactions. Thus, findings for specific herbal products are often of limited usefulness for making decisions about efficacy or safety.
Lack of Regulatory Oversight
Despite the lack of scientific evidence about the efficacy and safety of herbal products, more than 30,000 dietary supplements are sold in the United States, with an additional 1,000 new supplements introduced annually. Most patients believe that the government oversees the safety of CAM; but the fact is that the only requirement is for the manufacturer to send a copy of the product label to the U.S. Food and Drug Administration (FDA). A new dietary supplement or new formulation can be introduced and marketed overnight, without significant restriction, despite containing new, experimental, and unregulated herbal ingredients. The Dietary Supplement Health and Education Act of 1994 provides some oversight but nonetheless allows untested herbal products on the market without safety testing (70). Unfortunately, many supplements contain ingredients or contaminants with adverse effects or interactions. If these supplements were subject to premarket safety testing, many would no doubt be banned.
The FDA has identified several supplements, including ephedra, chaparral, comfrey, lobelia, and yohimbe, that have caused serious harm, yet it took 9 years to get ephedra alone removed from the market (71). The other products are still available for sale in stores and over the Internet. Restricting a specific substance or group of substances is not particularly helpful because manufacturers can simply introduce new formulations. From 1995 to 1997, hundreds of cases of ephedra toxicity were reported to the FDA as causing stroke, myocardial infarction, seizure, and death from cardiac arrhythmia (72). Side effects occurred in otherwise healthy young adults who used ephedra for energy boost and weight loss (72). As a result, ephedra was eventually banned by the FDA, but other structurally related sympathomimetic amines emerged to take its place in weight-loss and energy-enhancement products, such as synephrine, which is now the most popular ephedra replacement. It has a similar pharmacology and structure. Internet resources describe synephrine as an herbal supplement that stimulates fat metabolism, suppresses appetite, and boosts caloric expenditure without side effects. Synephrine inhibits intestinal cytochrome P450 and increases blood levels of many drugs (Table 2). Like ephedra and caffeine, the combination of synephrine and caffeine produces arrhythmogenesis, stroke, CHF, and seizures (73).
The general public regards herbal products as natural. Thus, there is a widespread yet false perception that herbal products are safe. According to a 2002 nationwide Harris interactive poll of more than 1,000 adults, 60% of respondents believed that supplements were approved by a governmental agency before they could be sold, that warning labels were required to list potential side effects, and that supplement manufacturers could not make safety claims without solid scientific evidence (74).
Lack of Quality Control
The wide variations in herbal supplement manufacturing techniques, storage methods, and lot-to-lot variability require urgent attention from the FDA and other regulatory agencies. U.S. pharmacopeia has been developing quality control standards but supplement manufacturers are not required to follow these guidelines. Such latitude allows cheaply manufactured and even expired herbal products to be sold without restriction. The variation in herbal sources and the inconsistent labeling of ingredients continue to be of major concern. More than 40% of all herbal products fail to contain as much of their active ingredients as claimed on their labels (75) or substitute cheaper ingredients for more expensive ones (23).
Contamination with heavy metals, adulteration with pharmaceuticals, and prohibited animal and plant ingredients are repeatedly found in herbal products (76,77). Intentional and unintentional adulteration with antibiotics, nonsteroidal anti-inflammatory drugs, hormones, and heavy metals is common. Untested and unregulated steroids are sold to the public, including minors (78). In 2007, the FDA issued 9 safety alerts warning consumers to stop using 13 brands marketed as dietary supplements because testing identified undocumented prescription medications in them (78). Nine contained erectile dysfunction drugs such as sildenafil or tadalafil, 3 contained lovastatin, and 1 contained the weight-loss drug sibutramine.
The ramifications of using herbal supplements are sometimes discerned through subsequent medical tests or treatment. For example, colchicine has been found in the placenta of women taking ginkgo (79). Aristolochic acid nephropathy has been reported in patients who took herbal supplements without knowing that the manufacturer had substituted Aristolochia for another herb (80,81). In addition to being a renal toxin, Aristolochic acid is on the World Health Organization’s list of human carcinogens. It is banned from use in several European countries but is still readily available in the U.S. (78,81).
FDA guidelines for manufacturers mandate that they avoid contaminating products with other herbs, pesticides, heavy metals, or prescription drugs (82). Nonetheless, products have been repeatedly reported to contain adulterant compounds and to put unknowing consumers at risk of adverse side effects and drug interactions.
Public Misinformation
Unethical marketing techniques have led to false advertisements about the safety and efficacy of the herbs reaching the public today more so than at any other time. Herbal products are promoted in magazines, newspapers, and books, on radio and television, and through the Internet. Herbs are advocated for treatment on the basis of unproven, word-of-mouth traditions and beliefs. Some promotional materials even claim that the featured product is “doctor recommended,” “the world’s most powerful,” “patented,” or “now presented without a prescription” (even if it had never been prescribed). In a study of Internet marketing of herbal products, Morris and Avorn (30) found that at least 81% of Web sites made 1 or more health claims; with more than 50% claiming to treat, prevent, diagnose, or cure specific diseases despite regulations barring such statements (30). The sale of herbal supplements can be halted only if the product has been proven dangerous and documentary proof has been submitted to the Secretary of the Department of Health and Human Services. However, the FDA cannot routinely test food supplements and “considerable risk or injury to health” must be established before it can ban a product. The burden of proof for demonstrating that a supplement is safe must be placed on manufacturers rather than the government or consumers and consumer protection laws must be instituted.
Lack of Knowledge About Herb-Drug Interactions by Patients and Health Care Providers
Devastating effects due to a lack of understanding about adverse herb-drug interactions can occur in vulnerable populations, including the elderly, children, pregnant women, immunocompromised persons (eg, with human immunodeficiency virus or transplant recipients), and those with hepatic or renal dysfunction. Most (64%) of the patients with a diagnosis of atrial fibrillation, CHF, or ischemic heart disease who were attending a cardiovascular clinic reported concomitant use of alternative therapies and prescription drugs (83). More than half (58%) took supplements that had potential interactions with warfarin, amiodarone, sotalol, or digoxin. In a geriatric clinic, 46% of the patients were taking supplements with anticoagulation properties. Most (73%) were also taking a prescription anticoagulant and were unaware of potential interactions. Findings from another study indicated that 1 in 6 adults taking conventional prescription medications reported concomitant use of at least 1 CAM product during the preceding week (37). More than 40% of the respondents said that taking herbal medicine along with prescription medication was more useful than taking either alone (84).
Underreporting of Adverse Drug Reactions
Most consumers are unlikely to attribute health problems to a supplement that they assume is safe and natural, and are reluctant to report an adverse effect from a self-medicated substance. Supplement manufacturers also rarely report serious adverse effects to the FDA despite federal requirements. Between 1990 and 1994, fewer than 10 of more than 2,500 reports of adverse effects made to the FDA came from a herbal product manufacturer. A report from the inspector general of the Department of Health and Human Services revealed that <1% of adverse reactions caused by dietary supplements are reported to the FDA (85) (Figure 3). Recent FDA postmarketing guidance for the supplement industry mandates the reporting of all serious adverse events to the FDA within 15 days (86).
Figure 3.
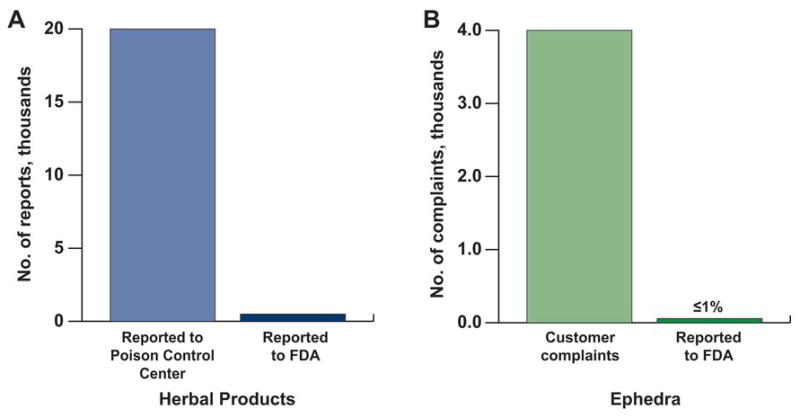
Adverse Effects of Herbal Products. Adverse effects of, A, Herbal products and, B, ephedra are underreported to the U.S. Food and Drug Administration. (Data from Litovitz TL, Klein-Schwartz W, Rodgers GC Jr, Cobaugh DJ, Youniss J, Omslaer JC, et al. 2001 Annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2002 Sep;20[5]:391–452.)
Conclusion
The use of herbal remedies in the United States is widespread and increasing dramatically, yet current laws allow them to be marketed as dietary supplements not subject to the same regulations required for prescription drugs. Thus, the purity, efficacy, and safety of herbal products are often unknown, and individual products may not even contain the amount of active ingredients listed on the label. In addition, manufacturers seldom report adverse events to the FDA. Manufacturers should be required to register with the FDA and to provide evidence of good manufacturing practices (ie, standardization, storage, preparation techniques, and manufacturing sites). Evidence of the safety and efficacy of the herbal product should be obtained by well-designed clinical trials, premarketing approval regarding safety, and strict postmarketing surveillance.
Herb-drug interactions are especially relevant when cardiovascular medications with a narrow therapeutic index, such as digoxin and warfarin, are coadministered with herbs that can potentiate or reduce pharmacologic effects (4,6). Thus, health care professionals should carefully question patients about their use of herbal products. Treating physicians are often unaware of patients’ use of such products because patients are not routinely asked about it. Collecting such information is important, particularly in elderly patients at higher risk of adverse interactions. Physicians should therefore have a good knowledge base about herbal remedies and should inquire about their use, discuss adverse effects, and monitor and identify possible herb-drug interactions. In addition, properly designed clinical trials are needed to assess the safety and efficacy of herbal remedies, including potential interactions with concurrently used medications.
There is a clear need for better public and physician understanding of herbal products through health education, early detection and management of herbal toxicities, scientific scrutiny of their use, and research on their safety and effectiveness. Regulatory policies are also needed to protect persons from untoward effects on their health and finances. The principles and standards of evidence for safety and efficacy of drugs used in conventional medicine should also apply to herbal and other CAM products, with decisions about their use based on the results of scientific inquiry rather than on long-held but untested belief systems or traditions (87).
Acknowledgments
Dr. Jahangir is supported in part by grants from the National Institute on Aging (AG21201), the National Heart, Lung, and Blood Institute (HL089542), the Mayo Clinic Marriott Mitochondrial Medicine Award, and the Angel and Paul Harvey Cardiovascular Research Endowment.
Abbreviations
- BPH
benign prostate hypertrophy
- CAM
complementary and alternative medicine
- cAMP
cyclic adenosine monophosphate
- CHF
congestive heart failure
- FDA
U.S. Food and Drug Administration
- NCCAM
National Center for Complementary and Alternative Medicine
Footnotes
Conflict of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ara Tachjian, Visiting physician at the Division of Cardiovascular Diseases, Mayo Clinic, Rochester, Minnesota.
Viqar Maria, Division of Cardiovascular Diseases, Mayo Clinic, Rochester, Minnesota.
Arshad Jahangir, Division of Cardiovascular Diseases, Mayo Clinic, Scottsdale, Arizona.
References
- 1.Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA. 1998 Nov 11;280(18):1569–75. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 2.The U.S. weight loss & diet control market. 9. Marketdata Enterprises Inc; c2007. [Google Scholar]
- 3.Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States: prevalence, costs, and patterns of use. N Engl J Med. 1993 Jan 28;328(4):246–52. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 4.Vogel JH, Bolling SF, Costello RB, Guarneri EM, Krucoff MW, Longhurst JC, et al. American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (Writing Committee to Develop an Expert Consensus Document on Complementary and Integrative Medicine) Integrating complementary medicine into cardiovascular medicine: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (Writing Committee to Develop an Expert Consensus Document on Complementary and Integrative Medicine) J Am Coll Cardiol. 2005 Jul 5;46(1):184–221. doi: 10.1016/j.jacc.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 5.Cherniack EP, Senzel RS, Pan CX. Correlates of use of alternative medicine by the elderly in an urban population. J Altern Complement Med. 2001 Jun;7(3):277–80. doi: 10.1089/107555301300328160. [DOI] [PubMed] [Google Scholar]
- 6.Valli G, Giardina EG. Benefits, adverse effects and drug interactions of herbal therapies with cardiovascular effects. J Am Coll Cardiol. 2002 Apr 3;39(7):1083–95. doi: 10.1016/s0735-1097(02)01749-7. [DOI] [PubMed] [Google Scholar]
- 7.Barnes PM, Powell-Griner E, McFann K, Nahin RL, editors. Complementary and alternative medicine use among adults, United States, 2002. Hyattsville (MD): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; c2004. [Google Scholar]
- 8.Ernst E. Second thoughts about safety of St. John’s wort. Lancet. 1999 Dec 11;354(9195):2014–6. doi: 10.1016/S0140-6736(99)00418-3. Erratum in: Lancet. 2000 Feb 12;355(9203):580. [DOI] [PubMed] [Google Scholar]
- 9.AltCareDex® System [intranet database]. Version 5.1. Greenwood Village (CO): Thomson Healthcare; [Google Scholar]
- 10.Yue QY, Bergquist C, Gerden B. Safety of St John’s wort (Hypericum perforatum) Lancet. 2000 Feb 12;355(9203):576–7. doi: 10.1016/S0140-6736(05)73227-X. [DOI] [PubMed] [Google Scholar]
- 11.Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. Indinavir concentrations and St John’s wort. Lancet. 2000 Feb 12;355(9203):547–8. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- 12.Ernst E. St John’s Wort supplements endanger the success of organ transplantation. Arch Surg. 2002 Mar;137(3):316–9. doi: 10.1001/archsurg.137.3.316. [DOI] [PubMed] [Google Scholar]
- 13.Mai I, Stormer E, Bauer S, Kruger H, Budde K, Roots I. Impact of St John’s wort treatment on the pharmacokinetics of tacrolimus and mycophenolic acid in renal transplant patients. Nephrol Dial Transplant. 2003 Apr;18(4):819–22. doi: 10.1093/ndt/gfg002. [DOI] [PubMed] [Google Scholar]
- 14.Breidenbach T, Hoffmann MW, Becker T, Schlitt H, Klempnauer J. Drug interaction of St John’s wort with cyclosporin. Lancet. 2000 May 27;355(9218):1912. doi: 10.1016/s0140-6736(05)73359-6. [DOI] [PubMed] [Google Scholar]
- 15.Sugimoto K, Ohmori M, Tsuruoka S, Nishiki K, Kawaguchi A, Harada K, et al. Different effects of St John’s wort on the pharmacokinetics of simvastatin and pravastatin. Clin Pharmacol Ther. 2001 Dec;70(6):518–24. doi: 10.1067/mcp.2001.120025. [DOI] [PubMed] [Google Scholar]
- 16.Johne A, Brockmoller J, Bauer S, Maurer A, Langheinrich M, Roots I. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum) Clin Pharmacol Ther. 1999 Oct;66(4):338–45. doi: 10.1053/cp.1999.v66.a101944. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Gorski JC, Hamman MA, Huang SM, Lesko LJ, Hall SD. The effects of St John’s wort (Hypericum perforatum) on human cytochrome P450 activity. Clin Pharmacol Ther. 2001 Oct;70(4):317–26. [PubMed] [Google Scholar]
- 18.Beckman SE, Sommi RW, Switzer J. Consumer use of St. John’s wort: a survey on effectiveness, safety, and tolerability. Pharmacotherapy. 2000 May;20(5):568–74. doi: 10.1592/phco.20.6.568.35152. [DOI] [PubMed] [Google Scholar]
- 19.Brown TM. Acute St. John’s wort toxicity. Am J Emerg Med. 2000 Mar;18(2):231–2. doi: 10.1016/s0735-6757(00)90030-5. [DOI] [PubMed] [Google Scholar]
- 20.Lantz MS, Buchalter E, Giambanco V. St. John’s wort and antidepressant drug interactions in the elderly. J Geriatr Psychiatry Neurol. 1999 Spring;12(1):7–10. doi: 10.1177/089198879901200103. [DOI] [PubMed] [Google Scholar]
- 21.Zou QZ, Bi RG, Li JM, Feng JB, Yu AM, Chan HP, et al. Effect of motherwort on blood hyperviscosity. Am J Chin Med. 1989;17(1–2):65–70. doi: 10.1142/S0192415X89000115. [DOI] [PubMed] [Google Scholar]
- 22.Sung J, Han KH, Zo JH, Park HJ, Kim CH, Oh BH. Effects of red ginseng upon vascular endothelial function in patients with essential hypertension. Am J Chin Med. 2000;28(2):205–16. doi: 10.1142/S0192415X00000258. [DOI] [PubMed] [Google Scholar]
- 23.Siegel RK. Ginseng abuse syndrome: problems with the panacea. JAMA. 1979 Apr 13;241(15):1614–5. [PubMed] [Google Scholar]
- 24.Becker BN, Greene J, Evanson J, Chidsey G, Stone WJ. Ginseng-induced diuretic resistance. JAMA. 1996 Aug 28;276(8):606–7. doi: 10.1001/jama.1996.03540080028021. [DOI] [PubMed] [Google Scholar]
- 25.Yuan CS, Wei G, Dey L, Karrison T, Nahlik L, Maleckar S, et al. Brief communication: American ginseng reduces warfarin’s effect in healthy patients: a randomized, controlled trial. Ann Intern Med. 2004 Jul 6;141(1):23–7. doi: 10.7326/0003-4819-141-1-200407060-00011. [DOI] [PubMed] [Google Scholar]
- 26.Sierpina VS. Integrative health care: complementary and alternative therapies for the whole person. Philadelphia: FA Davis; c2001. pp. 134–5. [Google Scholar]
- 27.Awang DV. Maternal use of ginseng and neonatal androgenization. JAMA. 1991 Jul 17;266(3):363. [PubMed] [Google Scholar]
- 28.Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. 2007;(2):CD003120. doi: 10.1002/14651858.CD003120.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Solomon PR, Adams F, Silver A, Zimmer J, DeVeaux R. Ginkgo for memory enhancement: a randomized controlled trial. JAMA. 2002 Aug 21;288(7):835–40. doi: 10.1001/jama.288.7.835. [DOI] [PubMed] [Google Scholar]
- 30.Morris CA, Avorn J. Internet marketing of herbal products. JAMA. 2003 Sep 17;290(11):1505–9. doi: 10.1001/jama.290.11.1505. [DOI] [PubMed] [Google Scholar]
- 31.Gardner CD, Lawson LD, Block E, Chatterjee LM, Kiazand A, Balise RR, et al. Effect of raw garlic vs commercial garlic supplements on plasma lipid concentrations in adults with moderate hypercholesterolemia: a randomized clinical trial. Arch Intern Med. 2007 Feb 26;167(4):346–53. doi: 10.1001/archinte.167.4.346. [DOI] [PubMed] [Google Scholar]
- 32.Apitz-Castro R, Cabrera S, Cruz MR, Ledezma E, Jain MK. Effects of garlic extract and of three pure components isolated from it on human platelet aggregation, arachidonate metabolism, release reaction and platelet ultrastructure. Thromb Res. 1983 Oct 15;32(2):155–69. doi: 10.1016/0049-3848(83)90027-0. [DOI] [PubMed] [Google Scholar]
- 33.German K, Kumar U, Blackford HN. Garlic and the risk of TURP bleeding. Br J Urol. 1995 Oct;76(4):518. doi: 10.1111/j.1464-410x.1995.tb07766.x. [DOI] [PubMed] [Google Scholar]
- 34.Rose KD, Croissant PD, Parliament CF, Levin MB. Spontaneous spinal epidural hematoma with associated platelet dysfunction from excessive garlic ingestion: a case report. Neurosurgery. 1990 May;26(5):880–2. doi: 10.1097/00006123-199005000-00026. [DOI] [PubMed] [Google Scholar]
- 35.Bailey DG, Kreeft JH, Munoz C, Freeman DJ, Bend JR. Grapefruit juice-felodipine interaction: effect of naringin and 6′,7′-dihydroxybergamottin in humans. Clin Pharmacol Ther. 1998 Sep;64(3):248–56. doi: 10.1016/S0009-9236(98)90173-4. [DOI] [PubMed] [Google Scholar]
- 36.Bailey DG, Malcolm J, Arnold O, Spence JD. Grapefruit juice-drug interactions. Br J Clin Pharmacol. 1998 Aug;46(2):101–10. doi: 10.1046/j.1365-2125.1998.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gross AS, Goh YD, Addison RS, Shenfield GM. Influence of grapefruit juice on cisapride pharmacokinetics. Clin Pharmacol Ther. 1999 Apr;65(4):395–401. doi: 10.1016/S0009-9236(99)70133-5. [DOI] [PubMed] [Google Scholar]
- 38.Kivisto KT, Lilja JJ, Backman JT, Neuvonen PJ. Repeated consumption of grapefruit juice considerably increases plasma concentrations of cisapride. Clin Pharmacol Ther. 1999 Nov;66(5):448–53. doi: 10.1016/S0009-9236(99)70007-X. [DOI] [PubMed] [Google Scholar]
- 39.Monroe KR, Murphy SP, Kolonel LN, Pike MC. Prospective study of grapefruit intake and risk of breast cancer in postmenopausal women: the Multiethnic Cohort Study. Br J Cancer. 2007 Aug 6;97(3):440–5. doi: 10.1038/sj.bjc.6603880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pittler MH, Guo R, Ernst E. Hawthorn extract for treating chronic heart failure. Cochrane Database Syst Rev. 2008;(1):CD005312. doi: 10.1002/14651858.CD005312.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tauchert M. Efficacy and safety of crataegus extract WS 1442 in comparison with placebo in patients with chronic stable New York Heart Association class-III heart failure. Am Heart J. 2002 May;143(5):910–5. doi: 10.1067/mhj.2002.121463. [DOI] [PubMed] [Google Scholar]
- 42.Mashour NH, Lin GI, Frishman WH. Herbal medicine for the treatment of cardiovascular disease: clinical considerations. Arch Intern Med. 1998 Nov 9;158(20):2225–34. doi: 10.1001/archinte.158.20.2225. [DOI] [PubMed] [Google Scholar]
- 43.Vibes J, Lasserre B, Gleye J, Declume C. Inhibition of thromboxane A2 biosynthesis in vitro by the main components of Crataegus oxyacantha (Hawthorn) flower heads. Prostaglandins Leukot Essent Fatty Acids. 1994 Apr;50(4):173–5. doi: 10.1016/0952-3278(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 44.Goepel M, Hecker U, Krege S, Rubben H, Michel MC. Saw palmetto extracts potently and noncompetitively inhibit human α1-adrenoceptors in vitro. Prostate. 1999 Feb 15;38(3):208–15. doi: 10.1002/(sici)1097-0045(19990215)38:3<208::aid-pros5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 45.Bent S, Kane C, Shinohara K, Neuhaus J, Hudes ES, Goldberg H, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006 Feb 9;354(6):557–66. doi: 10.1056/NEJMoa053085. [DOI] [PubMed] [Google Scholar]
- 46.Bressler R. Herb-drug interactions: interactions between saw palmetto and prescription medications. Geriatrics. 2005 Nov;60(11):32, 4. [PubMed] [Google Scholar]
- 47.Hamid S, Rojter S, Vierling J. Protracted cholestatic hepatitis after the use of prostata. Ann Intern Med. 1997 Jul 15;127(2):169–70. doi: 10.7326/0003-4819-127-2-199707150-00033. [DOI] [PubMed] [Google Scholar]
- 48.Yeu E, Grostern R. Saw palmetto and intraoperative floppy-iris syndrome. J Cataract Refract Surg. 2007 May;33(5):927–8. doi: 10.1016/j.jcrs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 49.Izzat MB, Yim AP, El-Zufari MH. A taste of Chinese medicine! Ann Thorac Surg. 1998 Sep;66(3):941–2. doi: 10.1016/s0003-4975(98)00624-9. [DOI] [PubMed] [Google Scholar]
- 50.Turner RB, Bauer R, Woelkart K, Hulsey TC, Gangemi JD. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N Engl J Med. 2005 Jul 28;353(4):341–8. doi: 10.1056/NEJMoa044441. [DOI] [PubMed] [Google Scholar]
- 51.Kwan CY, Leung YM, Kwan TK, Daniel EE. Tetrandrine inhibits Ca2+ release-activated Ca2+ channels in vascular endothelial cells. Life Sci. 2001 Jan 5;68(7):841–7. doi: 10.1016/s0024-3205(00)00988-7. [DOI] [PubMed] [Google Scholar]
- 52.Felix JP, King VF, Shevell JL, Garcia ML, Kaczorowski GJ, Bick IR, et al. Bis (benzylisoquinoline) analogs of tetrandrine block L-type calcium channels: evidence for interaction at the diltiazem-binding site. Biochemistry. 1992 Dec 1;31(47):11793–800. doi: 10.1021/bi00162a017. [DOI] [PubMed] [Google Scholar]
- 53.Seeff LB. Herbal hepatotoxicity. Clin Liver Dis. 2007 Aug;11(3):577–96. doi: 10.1016/j.cld.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Bello-Ramirez AM, Nava-Ocampo AA. The local anesthetic activity of Aconitum alkaloids can be explained by their structural properties: a QSAR analysis. Fundam Clin Pharmacol. 2004 Apr;18(2):157–61. doi: 10.1111/j.1472-8206.2004.00222.x. [DOI] [PubMed] [Google Scholar]
- 55.Lowe L, Matteucci MJ, Schneir AB. Herbal aconite tea and refractory ventricular tachycardia. N Engl J Med. 2005 Oct 6;353(14):1532. doi: 10.1056/NEJMc051568. [DOI] [PubMed] [Google Scholar]
- 56.Smith SW, Shah RR, Hunt JL, Herzog CA. Bidirectional ventricular tachycardia resulting from herbal aconite poisoning. Ann Emerg Med. 2005 Jan;45(1):100–1. doi: 10.1016/j.annemergmed.2004.07.454. [DOI] [PubMed] [Google Scholar]
- 57.Tam SW, Worcel M, Wyllie M. Yohimbine: a clinical review. Pharmacol Ther. 2001 Sep;91(3):215–43. doi: 10.1016/s0163-7258(01)00156-5. [DOI] [PubMed] [Google Scholar]
- 58.Chen MY, Cai JT, Du Q. Hepatic veno-occlusive disease associated with the use of Gynura segetum. Eur J Intern Med. 2007 Dec;18(8):609. doi: 10.1016/j.ejim.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 59.Dai N, Yu YC, Ren TH, Wu JG, Jiang Y, Shen LG, et al. Gynura root induces hepatic veno-occlusive disease: a case report and review of the literature. World J Gastroenterol. 2007 Mar 14;13(10):1628–31. doi: 10.3748/wjg.v13.i10.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu XJ, Zhang K, Yu MY, Lv HP, Zhang GD. Clinical analysis of four cases of hepatic veno-occlusive disease caused by Gynura segetum (Lour.) Merr [Chinese] Zhonghua Gan Zang Bing Za Zhi. 2007 Feb;15(2):151–3. [PubMed] [Google Scholar]
- 61.Chitturi S, Farrell GC. Hepatotoxic slimming aids and other herbal hepatotoxins. J Gastroenterol Hepatol. 2008 Mar;23(3):366–73. doi: 10.1111/j.1440-1746.2008.05310.x. [DOI] [PubMed] [Google Scholar]
- 62.Hoe SZ, Kamaruddin MY, Lam SK. Inhibition of angiotensin-converting enzyme activity by a partially purified fraction of Gynura procumbens in spontaneously hypertensive rats. Med Princ Pract. 2007;16(3):203–8. doi: 10.1159/000100391. [DOI] [PubMed] [Google Scholar]
- 63.Mansoor GA. Herbs and alternative therapies in the hypertension clinic. Am J Hypertens. 2001 Sep;14(9 Pt 1):971–5. doi: 10.1016/s0895-7061(01)02172-0. [DOI] [PubMed] [Google Scholar]
- 64.Bryer-Ash M, Zehnder J, Angelchik P, Maisel A. Torsades de pointes precipitated by a Chinese herbal remedy. Am J Cardiol. 1987 Nov 15;60(14):1186–7. doi: 10.1016/0002-9149(87)90421-8. [DOI] [PubMed] [Google Scholar]
- 65.Eriksson JW, Carlberg B, Hillorn V. Life-threatening ventricular tachycardia due to liquorice-induced hypokalaemia. J Intern Med. 1999 Mar;245(3):307–10. doi: 10.1046/j.1365-2796.1999.00476.x. [DOI] [PubMed] [Google Scholar]
- 66.Mahady GB, Low Dog T, Barrett ML, Chavez ML, Gardiner P, Ko R, et al. United States Pharmacopeia review of the black cohosh case reports of hepatotoxicity. Menopause. 2008 Jul–Aug;15(4 Pt 1):628–38. doi: 10.1097/gme.0b013e31816054bf. [DOI] [PubMed] [Google Scholar]
- 67.Pockaj BA, Gallagher JG, Loprinzi CL, Stella PJ, Barton DL, Sloan JA, et al. Phase III double-blind, randomized, placebo-controlled crossover trial of black cohosh in the management of hot flashes: NCCTG Trial N01CC1. J Clin Oncol. 2006 Jun 20;24(18):2836–41. doi: 10.1200/JCO.2005.05.4296. [DOI] [PubMed] [Google Scholar]
- 68.The world almanac and book of facts, 2005. New York (NY): World Almanac Books: St. Martin’s Press; c2005. Top-selling medicinal herbs in the U.S., 1999–2003; p. 99. [Google Scholar]
- 69.Chow EC, Teo M, Ring JA, Chen JW. Liver failure associated with the use of black cohosh for menopausal symptoms. Med J Aust. 2008 Apr 7;188(7):420–2. doi: 10.5694/j.1326-5377.2008.tb01691.x. [DOI] [PubMed] [Google Scholar]
- 70.McNamara SH. FDA regulation of ingredients in dietary supplements after passage of the Dietary Supplement Health and Education Act of 1994: an update. Food Drug Law J. 1996;51(2):313–8. [PubMed] [Google Scholar]
- 71.U.S. Food and Drug Administration. Center for Food Safety and Applied Nutrition; Illnesses and injuries associated with the use of selected dietary supplements [Internet] [cited 2009 Apr] Available from: http://www.foodsafety.gov/~dms/ds-ill.html. [Google Scholar]
- 72.Haller CA, Benowitz NL. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N Engl J Med. 2000 Dec 21;343(25):1833–8. doi: 10.1056/NEJM200012213432502. [DOI] [PubMed] [Google Scholar]
- 73.Bouchard NC, Howland MA, Greller HA, Hoffman RS, Nelson LS. Ischemic stroke associated with use of an ephedra-free dietary supplement containing synephrine. Mayo Clin Proc. 2005 Apr;80(4):541–5. doi: 10.4065/80.4.541. [DOI] [PubMed] [Google Scholar]
- 74.Harris poll shows widespread public ignorance of supplement regulation [Internet] [cited 2008 Nov 10] Available from: http://www.supplementquality.com/news/Harris_survey.html.
- 75.Brody JE. Americans gamble on herbs as medicine. The New York Times; [Internet] [cited 2009 Jan 6]. Available from: http://www.http://query.nytimes.com/gst/fullpage.html?res=9B0DEFDA123BF93AA35751C0A96F958260. [Google Scholar]
- 76.Saper RB, Phillips RS, Sehgal A, Khouri N, Davis RB, Paquin J, et al. Lead, mercury, and arsenic in US- and Indian-manufactured Ayurvedic medicines sold via the Internet. JAMA. 2008 Aug 27;300(8):915–23. doi: 10.1001/jama.300.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Corns CM. Herbal remedies and clinical biochemistry. Ann Clin Biochem. 2003 Sep;40(Pt 5):489–507. doi: 10.1258/000456303322326407. [DOI] [PubMed] [Google Scholar]
- 78.Green GA, Catlin DH, Starcevic B. Analysis of over-the-counter dietary supplements. Clin J Sport Med. 2001 Oct;11(4):254–9. doi: 10.1097/00042752-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 79.Petty HR, Fernando M, Kindzelskii AL, Zarewych BN, Ksebati MB, Hryhorczuk LM, et al. Identification of colchicine in placental blood from patients using herbal medicines. Chem Res Toxicol. 2001 Sep;14(9):1254–8. doi: 10.1021/tx0155101. [DOI] [PubMed] [Google Scholar]
- 80.Debelle FD, Vanherweghem JL, Nortier JL. Aristolochic acid nephropathy: a worldwide problem. Kidney Int. 2008 Jul;74(2):158–69. doi: 10.1038/ki.2008.129. [DOI] [PubMed] [Google Scholar]
- 81.Nortier JL, Martinez MC, Schmeiser HH, Arlt VM, Bieler CA, Petein M, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi) N Engl J Med. 2000 Jun 8;342(23):1686–92. doi: 10.1056/NEJM200006083422301. [DOI] [PubMed] [Google Scholar]
- 82.U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER); Guidance for industry: botanical drug products [Internet] [cited 2009 Apr] Available from: http://www.fda.gov/cder/guidance/4592fnl.htm. [Google Scholar]
- 83.Cohen RJ, Ek K, Pan CX. Complementary and alternative medicine (CAM) use by older adults: a comparison of self-report and physician chart documentation. J Gerontol A Biol Sci Med Sci. 2002 Apr;57(4):M223–7. doi: 10.1093/gerona/57.4.m223. [DOI] [PubMed] [Google Scholar]
- 84.Kuo GM, Hawley ST, Weiss LT, Balkrishnan R, Volk RJ. Factors associated with herbal use among urban multiethnic primary care patients: a cross-sectional survey. BMC Complement Altern Med. 2004 Dec 2;4:18. doi: 10.1186/1472-6882-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Department of Health and Human Services. Office of Inspector General [Internet] [cited 2008 Jan 6] Available from: http://www.oig.hhs.gov/oei/reports/oei-01-00-00180.pdf.
- 86.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) [Internet] Guidance for industry: postmarketing adverse event reporting for nonprescription human drug products marketed without an approved application [cited 2008 Jan 6] Available from: http://www.fda.gov/cder/guidance/7950dft.htm.
- 87.Institute of Medicine (U.S.) Complementary and alternative medicine in the United States, Committee on the Use of Complementary and Alternative Medicine by the American Public, Board on Health Promotion and Disease Prevention. Washington (DC): National Academies Press; c2005. [Google Scholar]


