Abstract
There are no data currently available on gender and racial variation in smallpox vaccine immune responses. We recruited 1076 healthy adults 18–40 years old who received one dose of the US-licensed smallpox vaccine (Dryvax®). Vaccinia neutralizing antibody titers in each subject’s serum were determined using a high throughput neutralization assay based on a recombinant, β-gal expressing vaccinia virus. Results are reported as the serum dilution inhibiting 50% of virus activity (ID50). The median ID50 for all subjects was 132.2 (inter-quartile range (IQR) = 78.8, 205.6). While no significant differences were observed with race and ethnicity, females had significantly higher neutralizing antibody titers than males (158.5 [93.2, 255.8] vs. 124.1 [75.2, 185.9]; p < 0.0001). As expected, time since vaccination was also associated with variations in neutralizing antibody titers in our subjects. These data indicate that neutralizing antibody titers following primary smallpox vaccination vary by gender.
Keywords: Smallpox vaccine, Vaccinia, Neutralizing antibody, Vaccine response
1. Introduction
Smallpox, caused by variola virus, is a disease which killed hundreds of millions of people before its eradication in 1980 [1]. The key component of the eradication effort was an effective live virus vaccine composed of vaccinia virus, an immunologically cross-reactive orthopox virus. Following the eradication of smallpox, routine vaccination was discontinued due to the small but definite risk of serious, life-threatening adverse events following immunization [2,3]. Vaccine production ceased and many basic questions regarding poxvirus immunity were left unresolved. Unfortunately, the possibility of the intentional use of variola virus as a biological weapon has engendered considerable interest in next generation vaccines, antiviral agents and basic research into poxvirus pathogenesis and correlates of protective immunity to diseases such as smallpox and monkeypox [4]. Recent research has highlighted the importance of both humoral and cellular immunity in protection against poxviruses [5,6]. One significant conclusion from this research is the critical role that vaccine-induced anti-body responses play in protection against subsequent exposure to poxviruses [7].
Ethnic and racial differences in immune responses to infection and vaccination have been described [8–11]. Individuals of African decent are reported to have higher levels of inflammatory cytokines such as IL-8 and granulocyte colony stimulating factor (G-CSF) as compared to Caucasians, resulting in a condition referred to as benign ethnic neutropenia [10]. Race has been identified as a risk factor for dengue hemorrhagic fever (DHF) for Caucasians as compared to African and Black Caribbean populations because of the differences in pathogenesis related to distinct allelic pools of immune response genes [12].
Gender has also been associated with the outcome of immune response to infection or vaccination. Higher levels of rubella and mumps antibodies are seen in the females [11,13,14], while a more robust cellular response is detected to rubella and varicellazoster in males [15,16]. In addition, the antibody levels to individual components of the measles–mumps–rubella (MMR) vaccine are reported to wane over time at different rates based on gender [17–20]. Furthermore, we have previously reported that the antibody titers to mumps decrease with an increasing time between immunization and the subsequent blood draw [14]. In this report we examined vaccinia-specific neutralizing antibody responses in a cohort of young, healthy individuals after receipt of a single dose of the Dryvax® vaccine.
2. Methods
2.1. Subject recruitment
Healthy individuals between 18 and 40 years of age who had previously received a single dose of Dryvax® were recruited into the study. All subjects had been immunized within 4 years prior to recruitment. Subjects were selected for our study based on the presence of a documented “take” or formation of the pustule at the vaccination site. Local participants of the Department of Health and Human Services civilian healthcare worker smallpox vaccination program were recruited at Mayo Clinic in Rochester, MN, while the majority of the subjects were recruited from among eligible armed forces personnel by the Naval Health Research Center in San Diego. Institutional Review Board approval was granted for all study procedures and written informed consent was obtained from each participant. A serum sample was collected from each study subject. Serum samples were separated from the clotted blood and aliquoted into sterile microcentrifuge tubes and stored at −70 °C until use.
2.1.1. Neutralizing antibody assay
The vaccinia-specific neutralization assay developed at the Food and Drug Administration (FDA) was adapted for our use [21]. Briefly, heat inactivated serum samples were serially diluted and then mixed with a known concentration of a recombinant, β-galactosidase expressing vaccinia virus for 1 h and then added to Hela cells overnight. Vaccinia Immune Globulin (VIG), kindly provided by Christine Anderson (Center for Biologics Evaluation and Research/FDA) was used as a positive control in each assay, while negative controls consisted of medium only. After an overnight incubation, cells were lysed and β-gal activity levels were monitored using a colorimetric substrate and used as a surrogate marker for virus activity. Results are defined as the serum dilution which inhibits 50% of virus activity (ID50). ID50 values for each subject were estimated using the M estimation approach introduced by Huber [22]. We relied on the iteratively re-weighted least squares approach for performing M-estimation, with a bisquare weighting function that is implemented in the ROBUSTREG procedure of the SAS software package (Cary, NC). Each serum sample was tested at least three times.
2.1.2. Statistical analyses
The purpose of the efforts reported here was to assess associations between demographic and clinical variables with measures of serum neutralizing antibodies. Serum antibody titers were tested multiple times for each individual. For descriptive purposes, a single antibody response measure per individual was obtained using the median of these multiple measures. Data were descriptively summarized across individuals using frequencies and percentages for categorical variables, and medians and inter-quartile ranges for continuous variables.
Associations of clinical and demographic characteristics with antibody response were formally evaluated using linear regression models. Unlike the descriptive analyses, we included each of the multiple observations per subject for these formal analyses. Repeated measures approaches were implemented in order to account for the multiple observed values within an individual, using a compound symmetry variance–covariance matrix. The following demographic and clinical characteristics were examined: age at blood draw (categorized into approximate quartiles), gender, race, ethnicity, and time from immunization to blood draw (also categorized into approximate quartiles). We first ran a series of univariate analyses, examining in turn the associations of these characteristics with antibody response. We then fit a multivariate regression model simultaneously including all demographic and clinical variables. For this latter model, any observed association of antibody levels with a given characteristic was adjusted for the effects of all other characteristics. Data transformations were used to correct for data skewness in the antibody response measures. An inverse cumulative normal (probit) transformation was used in each model to ensure distributional assumptions were met. All statistical tests were two-sided, and all analyses were carried out using the SAS software system (SAS Institute, Inc., Cary, NC).
3. Results
The vast majority of our study subjects were military personnel recruited at the Naval Health Research Center (NHRC) in San Diego, and our study demographics reflect this fact (Table 1). Even though our study population was predominantly white males, we were able to recruit a significant number of females as well as a fairly diverse racial group; including 231 (21.5%) subjects self-reporting ethnicity as Hispanic. A difficulty in our analysis was that many of the Hispanic participants listed their race as “Other”, “Don’t Know” or “More Than One”. In total, 243 (22.6%) of participants did not indicate race.
Table 1.
Demographics of the study cohort.
| Attribute | Number (percent) |
|---|---|
| Study site | |
| Mayo Clinic | 14 (1.3) |
| Naval Health Research Center | 1062 (98.7) |
| Gender | |
| Males | 795 (73.9) |
| Females | 281 (26.1) |
| Race | |
| American Indian | 20 (1.9) |
| Asian, Pacific Islander | 54 (5.0) |
| African American | 187 (17.4) |
| Caucasian | 572 (53.2) |
| More than one race | 89 (8.3) |
| Other or don’t know | 154 (13.3) |
| Ethnicity | |
| Hispanic | 231 (21.5) |
| Non-Hispanic | 800 (74.3) |
| Don’t know | 45 (4.2) |
| Attribute | Median (IQR) |
| Age at enrollment (years) | 24 (18–40) |
| Time since vaccination (years) | 1.3 (0.1–4.1) |
IQR: inter-quartile range.
Neutralizing antibody titers in our study population varied greatly, with ID50 values ranging from 15.71 to 1314.15. The median ID50 was 132.19 (IQR: 78.79–205.56). Serum samples from non-immune volunteers were used to establish the lower level of detection in our neutralizing antibody assay (defined as ID50 ≤ 10). We found that 100% of our participants had detectable levels of neutralizing antibody activity in their serum. This result is not surprising given that each of our study subjects had a documented vaccine take following immunization. In spite of the fact that we utilized a different assay to measure neutralizing antibody titers, our results are broadly similar to other, recent, large-scale studies reporting neutralizing antibody titers [23–25]. Historically, it has been estimated that neutralizing antibody titers of either 1:20 or 1:32 are protective [26,27]. Comparing our population to these estimates of protection we found that the vast majority of our subjects could be considered “immune”, i.e. 99.7% of our subjects had titers >1:20 and 98.2% had titers above 1:32.
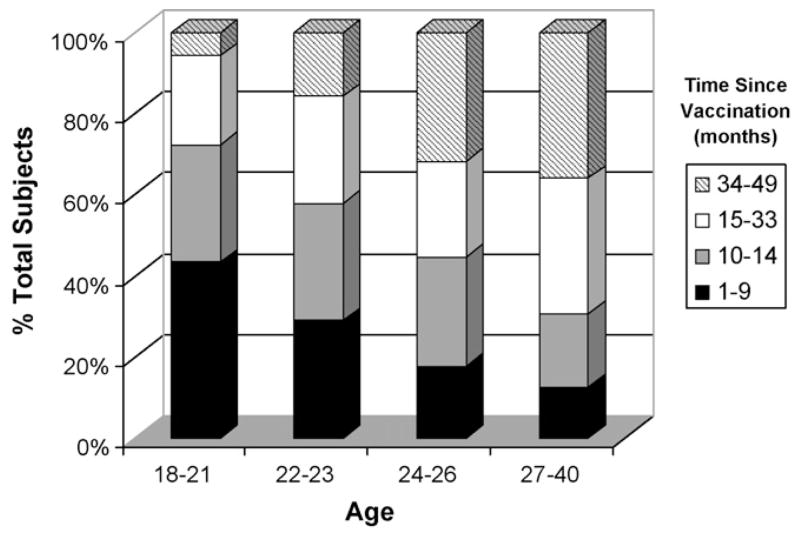
Our first analysis was to determine if age at vaccination was associated with different neutralizing antibody titer. Our population was stratified by age as shown in Table 2. While younger participants had higher neutralizing antibody levels, the differences were not statistically significant. In fact, the younger study subjects were more likely to have had a more recent vaccination (Fig. 1) which likely accounts for the difference in ID50 measurements between our age groups. Table 2 also shows the associations between race or ethnicity and neutralizing antibody titer. Neither of these two variables was significantly associated with differences in the measured humoral immune response.
Table 2.
Associations of neutralizing antibody titers with demographic and clinical variables.
| Attribute | No. of subjects | Median (IQR) | p-Value1 | p-Value2 |
|---|---|---|---|---|
| Age at enrollment | ||||
| 0.0736 | 0.2036 | |||
| 18–21 | 244 | 151.6 (81.21–237.54) | ||
| 22–23 | 261 | 134.83 (81.18–206.93) | ||
| 24–26 | 238 | 134.17 (83.14–207.11) | ||
| 27–40 | 333 | 120.14 (73.03–183.7) | ||
| Gender | ||||
| <0.0001 | <0.0001 | |||
| Female | 381 | 158.47 (93.15–255.77) | ||
| Male | 795 | 124.13 (75.21–185.89) | ||
| Race | ||||
| 0.2887 | 0.4362 | |||
| Asian, Pacific Islander | 187 | 132.5 (80.15–234.39) | ||
| African American | 74 | 148.28 (93.15–202.13) | ||
| Caucasian | 572 | 156.46 (76.65–196.54) | ||
| More than one race | 89 | 146.04 (77.0–243.49) | ||
| Other or don’t know | 154 | 133.46 (76.94–191.36) | ||
| Ethnicity | ||||
| 0.2101 | 0.3613 | |||
| Hispanic | 231 | 153.06 (91.04–527.36) | ||
| Non-Hispanic | 800 | 133.77 (78.39–190.91) | ||
| Don’t know | 45 | 129.42 (78.53–207.10) | ||
| Time since vaccination | ||||
| 0.0038 | 0.0313 | |||
| 1–9 months | 264 | 140.67 (87.87–220.45) | ||
| 10–14 months | 271 | 130.96 (84.99–205.63) | ||
| 15–33 months | 278 | 113.76 (66.53–184.74) | ||
| 34–49 months | 263 | 139.96 (82.2–210.31) | ||
IQR: inter-quartile range. p-Values from linear regression model. Repeated measures analyses were used to account for multiple measures of antibody titers per individual. Age at enrollment and time since vaccination categorized into approximate quartiles.
Univariate analysis.
multivariate analysis, statistically adjusting for all other variables included in the table.
Fig. 1.
Breakdown of vaccination history by age group. The bar graph shows the temporal distribution of vaccination receipt by age group.
We also examined what effect increasing time since vaccination has on the immune response and found similar results to those seen in other studies examining the kinetics of smallpox vaccine responses. As expected, those with the shortest interval between smallpox vaccination and serum testing showed the highest immune response (Table 2).
Gender-based differences in immune responses are not uncommon, therefore we analyzed whether or not gender was associated with significant differences in neutralizing antibody titers. As shown in Table 2, we found that females (median ID50 = 158.47 and IQR = 93.15–255.77) had significantly higher antibody levels (p < 0.0001) than the male study participants (median ID50 = 124.13 and IQR = 75.21–185.89). Fig. 2 illustrates the spectrum of humoral responses for both male and female subjects.
Fig. 2.
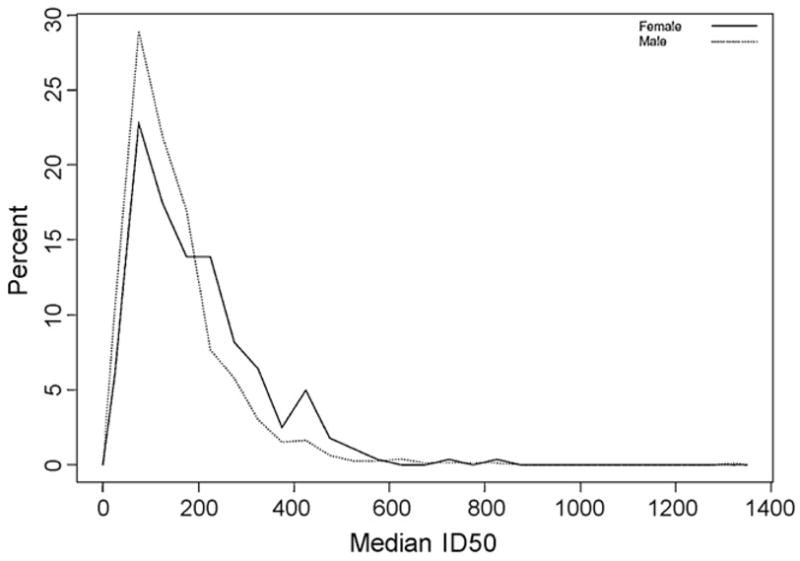
Neutralizing antibody responses by gender. The histogram plots the median ID50 measurement for each male (dotted line) and female (solid line) study subject. The y-axis represents the % of total subjects for each respective gender.
In our study 243 (22.6%) participants either did not indicate their race or claimed more than one race. We therefore re-ran all of the analyses without these individuals. As before, neither race nor ethnicity nor age correlated with differences in neutralizing antibody titer. However, both gender and time since vaccination remained significantly associated with divergent ID50 titers.
4. Discussion
Here we report the effect of various demographic characteristics on the humoral immune response (as measured by vaccinia-specific neutralizing antibody) elicited by primary smallpox vaccination in a large population. All of our subjects were selected for our study based on the presence of a documented “take” or formation of the pustule at the vaccination site. Historically, a take was used as evidence for vaccine-induced protection. This lesion is the result of local viral replication and was correlated with the development of vaccinia-specific immune responses and clinical protection against smallpox [1]. Given our inclusion criteria we found that the all of our subjects had detectable levels of neutralizing antibodies in their serum. The range of antibody titers was quite broad, ranging from the teens to over 1300. A variety of factors can contribute to the wide range of antibody levels seen in response to the vaccine including: host and pathogen genetic factors, age, race, gender, time since vaccination, nutritional and socio-economic status, and many others. In this study we sought to gain a greater understanding of the influence of demographic factors on immune response to primary smallpox vaccination.
Importantly, we found that gender was highly correlated with differences in neutralizing antibody titers, with females having significantly higher responses than males. At the population level this difference is unlikely to have major clinical consequences, i.e. an individual with an ID50 of 124.13 (the median male ID50 value) is likely to be as “immune” to smallpox as the individual with an ID50 of 158.47 (the median female ID50 value). At the individual level however, these differences may have significant consequences, especially among persons predisposed to lower vaccine responses. Protective efficacy may wane more quickly with the male segment of this population or the initial priming may not induce sufficient “immunity”. These individuals may benefit from additional vaccine doses or adjuvanted versions of vaccines. A greater understanding of the genetic basis for this insufficient immune response could allow us to tailor vaccination strategies to suit the individual’s needs.
The results shown here parallel our previous findings that females have higher antibody titers to mumps and rubella after immunization [11,14,28]. Gender-specific differences in humoral responses have been found for a large number of viral and bacterial vaccines including: influenza, hepatitis A and B, rubella, measles, rabies, yellow fever, meningococcus, pneumococcus, diphtheria, tetanus and brucella [29]. In accordance with our results, many of these other studies found significantly higher antibody responses in adult females compared to males. While these gender-based differences may be a result of sex hormones or functional disparities in B cells or T helper lymphocytes, these alone are unlikely to fully account for differences [29,30].
While we saw slight differences in the range of neutralizing antibody titers among the different racial groups in our study, these differences were not statistically significant. Historically there was scant evidence to indicate that different racial or ethnic groups responded differently to either the vaccine or smallpox itself. The consensus seems to have been that any racial differences seen were related to the relative newness of the disease among that racial group and not to any racial susceptibility or predilection towards more severe disease [31]. Prime examples of this are the devastating early epidemics among Native Americans after the arrival of European settlers in the Americas.
Age has also been shown to affect both smallpox vaccine response and disease mortality, with young children being especially susceptible to lethal disease [1,31]. Likewise, immunity wanes with the passing of time since vaccination; historically it was felt that full protection against smallpox lasted only a few years, with revaccination recommended every 5–10 years [1]. Current CDC recommendations are for revaccination every 10 years, and every 3 years for laboratory workers utilizing pathogenic orthopox viruses and the first responders who are part of the smallpox response teams. In fact, in the 1960s it was shown that clinical take rates upon revaccination correlated with the age of vaccinees and consequently the time since last smallpox vaccination [32]. Current studies have shown that, following immunization, vaccinia-specific neutralizing antibody responses peak several weeks post-immunization, gradually decline over a period of 3–5 years and then remain steady for decades [23,33]. We saw a similar trend in our population; those individuals with more recent vaccinations had higher levels of neutralizing antibody titers (Table 2). Current vaccination guidelines recommend against vaccinating young children and none of our participants were under 18. In Table 2 we did see a gradual decrease in ID50 as our population got older, however as shown in Fig. 2 younger individuals in our study were more likely to have had a more recent immunization and once this confounding effect was taken into account we saw no significant age-based differences in neutralizing antibody titer.
Further research into the underlying mechanisms for these gender-based differences may provide valuable insight into immune reactivity and allow for the development of improved vaccines and/or adjuvants to overcome inherent characteristics which predispose to suboptimal vaccine responses.
Acknowledgments
We would like to thank Kevin L. Russell and the Naval Health Research Center team as well as the nurses and study coordinators of the Mayo Vaccine Research Group for their tremendous efforts in recruiting the large number of subjects needed for the study. We gratefully acknowledge the subjects who participated in our studies. We thank Dr. Hana Golding for sharing the β-gal vaccinia virus neutralization assay and for helpful discussions. We thank David A. Watson for statistical analysis and Cheri A. Hart for her editorial assistance. We acknowledge support from National Institutes of Health grants AI 40065 and 1 UL1 RR024150-01* for this work.
Footnotes
Disclosures
None.
References
- 1.Fenner F. Smallpox and its Eradication. xvi. Geneva: World Health Organization; 1988. p. 1460. [Google Scholar]
- 2.Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: a review. Part II Adverse events. Clin Infect Dis. 2003;37(2):251–71. doi: 10.1086/375825. [DOI] [PubMed] [Google Scholar]
- 3.Arness MK, Eckart RE, Love SS, Atwood JE, Wells TS, Engler RJ, et al. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA. 2003;289(24):3283–9. doi: 10.1001/jama.289.24.3283. [DOI] [PubMed] [Google Scholar]
- 4.Henderson DA, Inglesby TV, Bartlett JG, Ascher MS, Eitzen E, Jahrling PB, et al. Smallpox as a biological weapon: medical and public health management. JAMA. 1999;281(22):2127–37. doi: 10.1001/jama.281.22.2127. [DOI] [PubMed] [Google Scholar]
- 5.Belyakov IM, Earl P, Dzutsev A, Kuznetsov VA, Lemon M, Wyatt LS, et al. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc Natl Acad Sci USA. 2003;100(16):9458–63. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172(10):6265–71. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 7.Panchanathan V, Chaudhri G, Karupiah G. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. J Virol. 2006;80(13):6333–8. doi: 10.1128/JVI.00115-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thio CL, Thomas DL, Goedert JJ, Vlahov D, Nelson KE, Hilgartner MW, et al. Racial differences in HLA class II associations with hepatitis C virus outcomes. J Infect Dis. 2001;184(1):16–21. doi: 10.1086/321005. [DOI] [PubMed] [Google Scholar]
- 9.Zabaleta J, Schneider BG, Ryckman K, Hooper PF, Camargo MC, Piazuelo MB, et al. Ethnic differences in cytokine gene polymorphisms: potential implications for cancer development. Cancer Immunol Immunother. 2008;57(1):107–14. doi: 10.1007/s00262-007-0358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayr FB, Spiel AO, Leitner JM, Firbas C, Kliegel T, Jilma B. Ethnic differences in plasma levels of interleukin-8 (IL-8) and granulocyte colony stimulating factor (G-CSF) Transl Res. 2007;149(1):10–4. doi: 10.1016/j.trsl.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Dhiman N, Ovsyannikova IG, Vierkant RA, Pankratz VS, Jacobson RM, Poland GA. Associations between cytokine/cytokine receptor single nucleotide polymorphisms and humoral immunity to measles, mumps and rubella in a Somali population. Tissue Antigens. 2008;72(3):211–20. doi: 10.1111/j.1399-0039.2008.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la CSB, Kouri G, Guzman MG. Race: a risk factor for dengue hemorrhagic fever. Arch Virol. 2007;152(3):533–42. doi: 10.1007/s00705-006-0869-x. [DOI] [PubMed] [Google Scholar]
- 13.Domínguez A, Plans P, Costa J, Torner N, Cardenosa N, Batalla J, et al. Seroprevalence of measles, rubella, and mumps antibodies in Catalonia Spain: results of a cross-sectional study. Eur J Clin Microbiol Infect Dis. 2006;25(5):310–7. doi: 10.1007/s10096-006-0133-z. [DOI] [PubMed] [Google Scholar]
- 14.Ovsyannikova IG, Jacobson RM, Dhiman N, Vierkant RA, Pankratz VS, Poland GA. Human leukocyte antigen and cytokine receptor gene polymorphisms associated with heterogeneous immune responses to mumps viral vaccine. Pediatrics. 2008;121(5):e1091–1099. doi: 10.1542/peds.2007-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell LA. Sex differences in antibody- and cell-mediated immune response to rubella re-immunisation. J Med Microbiol. 1999;48(12):1075–80. doi: 10.1099/00222615-48-12-1075. [DOI] [PubMed] [Google Scholar]
- 16.Klein NP, Holmes TH, Sharp MA, Heineman TC, Schleiss MR, Bernstein DI, et al. Variability and gender differences in memory T cell immunity to varicellazoster virus in healthy adults. Vaccine. 2006;24(33–34):5913–8. doi: 10.1016/j.vaccine.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 17.Johnson CE, Kumar ML, Whitwell JK, Staehle BO, Rome LP, Dinakar C, et al. Antibody persistence after primary measles–mumps–rubella vaccine and response to a second dose given at four to six vs. eleven to thirteen years. Pediatr Infect Dis J. 1996;15(8):687–92. doi: 10.1097/00006454-199608000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Christenson B, Bottiger M. Measles antibody: comparison of long-term vaccination titres, early vaccination titres and naturally acquired immunity to and booster effects on the measles virus. Vaccine. 1994;12(2):129–33. doi: 10.1016/0264-410x(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 19.Mossong J, O’Callaghan CJ, Ratnam S. Modelling antibody response to measles vaccine and subsequent waning of immunity in a low exposure population. Vaccine. 2000;19(4–5):523–9. doi: 10.1016/s0264-410x(00)00175-4. [DOI] [PubMed] [Google Scholar]
- 20.Pebody RG, Gay NJ, Hesketh LM, Vyse A, Morgan-Capner P, Brown DW, et al. Immunogenicity of second dose measles–mumps–rubella (MMR) vaccine and implications for serosurveillance. Vaccine. 2002;20(7–8):1134–40. doi: 10.1016/s0264-410x(01)00435-2. [DOI] [PubMed] [Google Scholar]
- 21.Manischewitz J, King LR, Bleckwenn NA, Shiloach J, Taffs R, Merchlinsky M, et al. Development of a novel vaccinia-neutralization assay based on reporter-gene expression. J Infect Dis. 2003;188(3):440–8. doi: 10.1086/376557. [DOI] [PubMed] [Google Scholar]
- 22.Huber PJ. Robust regression: asymptotics, conjectures and Monte Carlo. Ann Stat. 1973;1(5):799–821. [Google Scholar]
- 23.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171(10):4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 24.Belshe RB, Newman FK, Frey SE, Couch RB, Treanor JJ, Tacket CO, et al. Dose-dependent neutralizing-antibody responses to vaccinia. J Infect Dis. 2004;189(3):493–7. doi: 10.1086/380906. [DOI] [PubMed] [Google Scholar]
- 25.Combadiere B, Boissonnas A, Carcelain G, Lefranc E, Samri A, Bricaire F, et al. Distinct time effects of vaccination on long-term proliferative and IFN-gamma-producing T cell memory to smallpox in humans. J Exp Med. 2004;199(11):1585–93. doi: 10.1084/jem.20032083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mack TM, Noble J, Jr, Thomas DB. A prospective study of serum antibody and protection against smallpox. Am J Trop Med Hyg. 1972;21(2):214–8. doi: 10.4269/ajtmh.1972.21.214. [DOI] [PubMed] [Google Scholar]
- 27.Sarkar JK, Mitra AC, Mukherjee MK. The minimum protective level of antibodies in smallpox. Bull World Health Organ. 1975;52(3):307–11. [PMC free article] [PubMed] [Google Scholar]
- 28.Ovsyannikova IG, Jacobson RM, Vierkant RA, Jacobsen SJ, Pankratz VS, Poland GA. The contribution of HLA class I antigens in immune status following two doses of rubella vaccination. Hum Immunol. 2004;65(12):1506–15. doi: 10.1016/j.humimm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26(29–30):3551–5. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 30.Girón-González JA, Moral FJ, Elvira J, García-Gil D, Guerrero F, Gavilán I, et al. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur J Endocrinol. 2000;143(1):31–6. doi: 10.1530/eje.0.1430031. [DOI] [PubMed] [Google Scholar]
- 31.Dixon CW. Smallpox. London: J. & A. Churchill; 1962. p. 512. [Google Scholar]
- 32.Nyerges G, Hollos I, Barsy G. The significance of serological tests in controlling the success of smallpox revaccination. Acta Microbiol Acad Sci Hung. 1966;13(2):97–112. [PubMed] [Google Scholar]
- 33.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9(9):1131–7. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]