Abstract
Objective:
Aging is associated with elevated levels of glucose, insulin, and triglycerides. Our objective was to assess the effect of a nutritional program designed to reduce these correlates of aging.
Design:
This is a retrospective chart review of patients attending an outpatient metabolic management program including a high-fat, adequate-protein, low-carbohydrate diet, nutritional supplementation and periodic individual visits. Outcomes measured at baseline and follow-up included body weight, fasting serum glucose, insulin, leptin, lipids, and thyroid hormone.
Results:
Thirty-one patients were identified with complete information. The mean age of patients was 57.6 ± 2.4 consisting of 53% female and 47% male patients. The average duration between follow up visits was 91.5 ± 8.5 days. Of the parameters measured at the follow-up visit, body weight, serum leptin, insulin, fasting glucose, triglyceride, and free T3 significantly decreased by 8.1 ± 0.8%, 48.2 ± 3.8%, 40.1 ± 4.7%, 7.6 ± 2.1%, 28.3 ± 5.7%, and 10.8 ± 1.8%, respectively. Furthermore, the triglyceride/high density lipoprotein ratio decreased from 5.1 ± 1.7 to 2.6 ± 0.5.
Conclusions:
In the context of an outpatient medical clinic, a high-fat, adequate-protein, low-carbohydrate diet with nutritional supplementation led to improvements in serum factors related to the aging process. Further research regarding this dietary approach and its relationship to aging is in order.
BACKGROUND
Since the discovery that caloric restriction extended the lifespan of rats 70 years ago (1), many model organisms such as yeast (2, 3), nematodes (4), fruit flies (5), and mice (6, 7) have been subjected to some form of food restriction that resulted in extended lifespan. These and many other studies regarding the impact of caloric restriction on lifespan has generated such compelling evidence that the National Institute on Aging has initiated studies in non-human primates (rhesus macaques) examining caloric restriction on a number of metabolic and biological parameters including lifespan (8).
Nevertheless, the mechanisms driving the increased lifespan by calorie restriction or weight loss are unclear. Data from C. elegans (9, 10), Drosophila, and yeast (11) and, more recently, rodents (12) implicate the insulin/Insulin-like growth factor signaling pathways as a potential regulator of this process. Moreover, the ongoing data collection on the physiologic effects of caloric restriction in rhesus macaques parallels rodent studies demonstrating reduced body and fat mass, improved glucoregulatory function, decreased blood pressure and blood lipids, and decreased body temperature (13). Interestingly, centenarians have lower blood glucose, insulin, leptin, free T3 and serum triglycerides than those who do not live to be over one hundred years old (14). Therefore, the fundamental mechanism by which calorie restriction improves lifespan appears to alter these metabolic parameters.
In this paper, we examine the impact of a diet specifically designed to improve metabolic parameters independent of caloric intake. The diet is based on the premise that by reducing glucose and protein as substrates for oxidative metabolism and enhancing fatty acid oxidation, many of the same physiologic changes that are seen in calorie-restricted animals will also be seen in individuals following this type of diet.
Methods
Design
This is a retrospective analysis of clinical information from patients attending a private practice. Patients were referred for the treatment of diabetes, cardiovascular disease, excessive weight, fatigue, and other chronic diseases of aging. All patients with sufficient baseline and follow-up parameters were included in this series.
Clinic procedures
On the first visit, a comprehensive history and basic physical examination was performed. Clinical information was obtained and dietary instruction was provided by a clinical team consisting of a naturopath, nutritionist, and physician. After the first visit, the patients were typically contacted by telephone after 2-4 weeks, and a return visit was scheduled for 2-3 months later for follow-up evaluation and labs.
Dietary recommendation
The diet included unlimited amounts of certain fats and oils, a restricted amount of protein, and a very limited amount of carbohydrate. Patients were told to eat when they were hungry. Calories were not explicitly restricted; calorie intake was determined only by levels of hunger. Recommended sources of fat included raw nuts and seeds, avocados, olives and olive oil, flax oil and cod liver oil. The intake of protein was told to be limited to approximately 1.0 grams/kg lean body mass per day (increased for exercise to 1.25 grams/day). As a result, most patients were instructed to eat from 50-80 grams of protein per day. Recommended sources of protein included sardines, fish, eggs, tofu, chicken, turkey, wild meats, low-fat cheeses (cottage, ricotta, swiss), seafood, and veggie burgers. Only non-starchy, fibrous vegetables were acceptable: lettuce, greens, broccoli, cauliflower, cucumbers, mushrooms, onions, peppers, sprouts, asparagus, and seaweed. Though not explicitly stated, the general dietary intake as percent daily caloric intake from macronutrients for most people ended up by history to be approximately 20% carbohydrate, 20% protein, and 60% fat. For drinking, 6-8 eight ounce glasses of water and/or herbal tea were recommended. A written handout with a list of acceptable and unacceptable foods was provided.
Nutritional supplementation
Nutritional supplements to support fat metabolism and enhance insulin sensitivity were recommended to all patients to be taken on a daily basis: L-carnitine 2000mg, alpha-lipoic acid 400mg, coenzyme Q10 100 mg, 1 tbsp cod liver oil, magnesium 300mg, potassium 300mg, vitamin C 1000mg, vitamin E 800mg daily, and a multivitamin consisting of all essential B vitamins and minerals. (15, 16).
Medication adjustment
If an individual was taking lipid-lowering or sulfonylurea medications, these medications were discontinued at the first visit before starting the diet. In those patients taking blood pressure medication, medication was adjusted or, altogether discontinued, if low normal blood pressures were observed during the course of the intervention. Because these patients were seen in clinical practice, patients were responsible for purchasing their own supplements.
Outcome measures
At baseline and return clinic visits, body weight was measured on the same scale (Tanita Model TBF-300A, Tanita Corporation of America, Inc. Arlington Heights, Illinois). Baseline and follow-up laboratory measurements included body weight and sitting blood pressure. Laboratory parameters included serum glucose, insulin, leptin, total cholesterol, LDL, HDL, triglycerides (TG), free T3 and thyroid stimulating hormone (TSH) following a 12 hour fast. The clinical data were abstracted from the medical charts and entered into a computer database without identifying information.
Statistical Analysis
The primary analysis was a “pre-post” analysis comparing baseline to follow-up values using a paired t-test. Individual percent changes for each laboratory parameter were determined and used to calculate the mean percent change. This de-identified analysis of existing clinical data was approved by the Duke University Medical Center Institutional Review Board.
Results
Patient demographics of those who had complete baseline and follow-up laboratory tests are displayed in Table 1a. The mean age was 57.6 ± 2.4 years consisting of 53% female and 47% male patients. The average duration between baseline and follow up measurements was 91.5 ± 8.5 days.
Table 1a.
Baseline patient characteristicsSummary of patient demographics. This population of patients consisted of highly motivated, primarily Caucasian individuals. (Data is presented as ± S.E.M.)
| n: | 31 |
| Age (in years): | 57.6 ± 2.4 |
| Sex: | 53% female, 47% male |
| Follow up duration (in days): | 91.5 ± 8.5 |
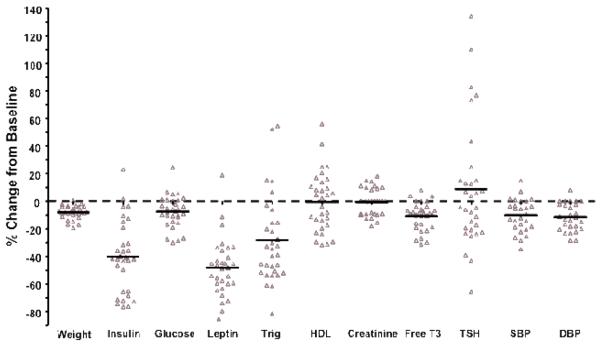
The recommendation of a high fat, adequate protein, low carbohydrate diet resulted in a significant loss of body weight by 7.1 ± 0.8 lbs in this patient population (Table 1b; Figure 1). Accompanied by the weight was a significant reduction in both systolic and diastolic blood pressure by 10.2 ± 2.1% and 11.4 ± 1.8% mmHg, respectively. Serum levels of leptin, insulin, fasting glucose, and free T3 significantly decreased from baseline levels by 48.2 ± 3.8%, 40.1 ± 4.7%, 7.6 ± 2.1%, and 10.8 ± 1.8%, respectively (Table 1b; Figure 1). In addition, despite the intake of predominantly fat, there was a significant decrease in triglyceride (28.3 ± 5.7%) in this patient group. The triglyceride/HDL ratio decreased from 5.1 ± 1.7 to 2.6 ± 0.5. Serum creatinine and TSH did not change significantly.
Table 1b.
Effect of Diet Program on Body Weight and Fasting Serum LevelsEffect of dietary and nutritional supplement therapy on gravimetric measurements and fasting serum levels. Patient physicals were performed at the initial and final, follow up clinic visits. At each time, blood was drawn for the determination of fasting serum levels of the measured parameters.
| Parameter | Baseline | Follow-up | % Change |
|---|---|---|---|
| Body weight (kg) | 86.3 ± 3.2 | 79.2 ± 3.0* | −8.1 ± 0.8* |
| Insulin (mg/dl) | 22.2 ±2.9 | 11.1 ± 0.9* | −40.1 ± 4.7* |
| Glucose (mg/dl) | 108.3 ± 5.0 | 99.3 ± 4.5* | −7.6 ± 2.1* |
| Leptin (mg/dl) | 16.5 ± 2.5 | 8.2 ± 1.4* | −48.2 ± 3.7* |
| Triglyceride (mg/dl) | 229.3 ± 75.1 | 110.7 ± 14.2* | −28.3 ± 5.7* |
| High density lipoprotein, HDL (mg/dl) |
55.4 ± 3.5 | 53.5 ± 3.0 | −0.3 ± 3.9 |
| Creatinine (mg/dl) | 1.02 ± 0.03 | 1.01± 0.03 | 0.5± 1.7 |
| Free T3 (pg/ml) | 2.97 ± 0.05 | 2.64 ± 0.06* | −10.8 ± 1.8* |
| Thyroid Stimulating Hormone (pg/ml) |
2.53 ± 0.25 | 2.57 ± 0.22 | 8.9 ± 8.1 |
| Systolic blood pressure (mmHg) | 136.6 ± 3.7 | 121.7 ± 2.3* | −10.2 ± 2.1* |
| Diastolic blood pressure (mmHg) | 86.5 ± 1.7 | 76.4 ± 1.7* | −11.4 ± 1.8* |
p<0.001; data presented as ± S.E.M.
Figure 1.
Directional impact of dietary intervention on clinical parameters of aging. The percent change from baseline is indicated for the averaged data taken from each patient. (*p<0.001; data presented as ± S.E.M.) Abbreviations: Gluc, glucose; Crea, Creatinine; Trig, triglyceride; HDL, high-density lipoprotein; TSH, thyroid stimulating hormone; FT3, free T3; Wt, weight; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Discussion
This retrospective analysis of patients from a private clinic adhering to a high-fat, low carbohydrate, adequate protein diet demonstrated reductions in critical metabolic mediators including insulin, leptin, glucose, triglycerides, and free T3. Moreover, the patient group, on average, lost significant weight even though they were not instructed to reduce calorie intake. These findings are consistent with other studies showing weight loss and reductions in fasting serum insulin with a low carbohydrate, ketogenic diet (17, 18). The ability of the diet to lower these parameters, especially TGs and body weight, seems counter to standard thinking considering the increased ingestion of fats. A possible explanation is that the resultant lower leptin and insulin was indicative of increased leptin and insulin sensitivity, resulting in increased β-oxidation, hypothalamic mediated increased satiety, and possibly subconsciously lowered caloric intake (19, 20).
It has been known for some time that different dietary fats appear to have varying effects on insulin-sensitive tissues and this may differentially impact the metabolic parameters measured in this study (21, 22). Numerous studies have demonstrated that reduced glucose (carbohydrate) substrates in the diet suppress hepatic de novo fatty acid biosynthesis, triglyceride production, and triglyceride secretion, while enhancing hepatic, adipose, and skeletal muscle fatty acid oxidation (22-24).
Nonetheless, the impact of this dietary approach on aging mechanisms can only be implied from comparisons with longevity studies that have examined the same metabolic parameters. Many aging studies have used calorie restriction as the means to impact aging. These types of studies become difficult in humans for the obvious reasons of dietary compliance, human lifespan duration, and the multiple and complex confounding factors. For this reason, investigators have examined the effectiveness of weight loss as a surrogate for caloric restriction on human mortality rates (25) but have found an increased mortality rate and reduced lifespan (26). The reason is that many of these studies did not distinguish between intentional and unintentional weight loss with the latter usually resulting from disease and, thus, increased mortality. It is now speculated that fat loss as opposed to weight loss decreases all-cause mortality in humans (25).
Patients in this study demonstrated a similar directional impact on the measured parameters when compared to studies using more established models of longevity such as caloric restriction. The patients in this study demonstrated significant weight loss along with a reduction in glucoregulatory mediators including insulin and leptin similar to those found in calorie-restricted primates (27, 28). Moreover, calorie restricted primates demonstrated a positive relationship between food intake and leptin levels (29). Thus, although patients were not told to restrict calories, there may have been a reduction in caloric intake secondary to reduced hungar,with the decrease in circulating leptin reflecting an increase in leptin sensitivity (19, 30). Interestingly, this study cohort exhibited a reduction in circulating free T3, the secreted form of thyroid hormone thought to mediate most of thyroid actions. Paralleling this reduction in circulating free T3, 9 patients of this study cohort that had basal body temperatures measured before and after intervention showed a significant decrease (p=0.004) in basal body temperature of 0.182 degrees C. Similar findings were reported in caloric restricted rodents, monkeys, humans, and centenarians (31-34). It has been suggested that the reduction in T3 and body temperature could alter the aging process by reflecting a reducing metabolic rate, oxidative stress, and systemic inflammation (35, 36).
It is important to note that the dietary recommendation in this study is unique from other ketogenic diets in that this dietary intervention limits protein intake as well as carbohydrates (though not total fat intake). It has been demonstrated that the longevity effects of calorie restriction can be partially attributed to the reduction in protein intake (37). It has been shown that limiting dietary amino acids, specifically methionine, inhibits signaling through mammalian target of rapamycin (mTOR) thereby decreasing mitochondrial damage and protein translation (38, 39). Future studies will be aimed at investigating the impact of a high fat, low carbohydrate with limited but adequate protein on mTOR signaling.
Because this was a retrospective analysis of a clinical practice, there may be bias introduced in the patient sampling procedure. This study reflects the effect of recommending this diet in a clinical practice, so food intake was not directly measured. In addition, this sample population may reflect the results in highly motivated individuals. Though the metabolic improvements occurred in patients who had both high and low weight loss, the improvements in metabolic parameters may be all or partially due to the weight loss. It should be noted, however, that the percent reduction in leptin particularly far exceeded the percentage of fat loss and may not be explained solely as a result of this fat loss. Also, it remains unclear as to the contributions of the suggested nutritional supplementation to the observed outcomes.
In conclusion, a nutritional program recommendation originally designed to treat chronic diseases of aging led to weight loss and metabolic changes currently thought to be beneficial in reducing the aging process. Further research regarding this dietary approach and its relationship to aging is in order.
ACKNOWLEDGEMENTS
This work was supported by a National Research Service Award and a Mentored Research Scientist Development Award from the NIH awarded to J.P. Konhilas (F32 HL 70509 and K01 AR052840).
REFERENCES
- 1.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. Journal of Nutrition. 1935;10:63–79. [PubMed] [Google Scholar]
- 2.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Guarente L. Calorie restriction and SIR2 genes--towards a mechanism. Mech Ageing Dev. 2005;126:923–928. doi: 10.1016/j.mad.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren JR. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp Gerontol. 2003;38:947–954. doi: 10.1016/s0531-5565(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 5.Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miskin R, Tirosh O, Pardo M, Zusman I, Schwartz B, Yahav S, Dubnov G, Kohen R. AlphaMUPA mice: a transgenic model for longevity induced by caloric restriction. Mech Ageing Dev. 2005;126:255–261. doi: 10.1016/j.mad.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- 8.Ingram DK, Cutler RG, Weindruch R, Renquist DM, Knapka JJ, April M, Belcher CT, Clark MA, Hatcherson CD, Marriott BM, et al. Dietary restriction and aging: the initiation of a primate study. J Gerontol. 1990;45:B148–163. doi: 10.1093/geronj/45.5.b148. [DOI] [PubMed] [Google Scholar]
- 9.Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- 10.Johnson TE. Caenorhabditis elegans 2007: the premier model for the study of aging. Exp Gerontol. 2008;43:1–4. doi: 10.1016/j.exger.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaza H, Chiba T, Higami Y, Shimokawa I. Lifespan extension by caloric restriction: an aspect of energy metabolism. Microsc Res Tech. 2002;59:325–330. doi: 10.1002/jemt.10212. [DOI] [PubMed] [Google Scholar]
- 12.Bluher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 13.Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 14.Paolisso G, Gambardella A, Ammendola S, D'Amore A, Balbi V, Varricchio M, D'Onofrio F. Glucose tolerance and insulin action in healty centenarians. Am J Physiol. 1996;270:E890–894. doi: 10.1152/ajpendo.1996.270.5.E890. [DOI] [PubMed] [Google Scholar]
- 15.Rebouche CJ, Paulson DJ. Carnitine metabolism and function in humans. Annu Rev Nutr. 1986;6:41–66. doi: 10.1146/annurev.nu.06.070186.000353. [DOI] [PubMed] [Google Scholar]
- 16.Genova ML, Pich MM, Biondi A, Bernacchia A, Falasca A, Bovina C, Formiggini G, Parenti Castelli G, Lenaz G. Mitochondrial production of oxygen radical species and the role of Coenzyme Q as an antioxidant. Exp Biol Med (Maywood) 2003;228:506–513. doi: 10.1177/15353702-0322805-14. [DOI] [PubMed] [Google Scholar]
- 17.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 18.Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348:2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 19.Dyck DJ. Leptin sensitivity in skeletal muscle is modulated by diet and exercise. Exerc Sport Sci Rev. 2005;33:189–194. doi: 10.1097/00003677-200510000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002;967:379–388. doi: 10.1111/j.1749-6632.2002.tb04293.x. [DOI] [PubMed] [Google Scholar]
- 21.Storlien LH, Kriketos AD, Jenkins AB, Baur LA, Pan DA, Tapsell LC, Calvert GD. Does dietary fat influence insulin action? Ann N Y Acad Sci. 1997;827:287–301. doi: 10.1111/j.1749-6632.1997.tb51842.x. [DOI] [PubMed] [Google Scholar]
- 22.Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science. 1987;237:885–888. doi: 10.1126/science.3303333. [DOI] [PubMed] [Google Scholar]
- 23.Clarke SD, Gasperikova D, Nelson C, Lapillonne A, Heird WC. Fatty acid regulation of gene expression: a genomic explanation for the benefits of the mediterranean diet. Ann N Y Acad Sci. 2002;967:283–298. [PubMed] [Google Scholar]
- 24.Taouis M, Dagou C, Ster C, Durand G, Pinault M, Delarue J. N-3 polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. Am J Physiol Endocrinol Metab. 2002;282:E664–671. doi: 10.1152/ajpendo.00320.2001. [DOI] [PubMed] [Google Scholar]
- 25.Coffey CS, Gadbury GL, Fontaine KR, Wang C, Weindruch R, Allison DB. The effects of intentional weight loss as a latent variable problem. Stat Med. 2005;24:941–954. doi: 10.1002/sim.1964. [DOI] [PubMed] [Google Scholar]
- 26.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. Jama. 2003;289:187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 27.Tigno XT, Gerzanich G, Hansen BC. Age-related changes in metabolic parameters of nonhuman primates. J Gerontol A Biol Sci Med Sci. 2004;59:1081–1088. doi: 10.1093/gerona/59.11.1081. [DOI] [PubMed] [Google Scholar]
- 28.Colman RJ, Ramsey JJ, Roecker EB, Havighurst T, Hudson JC, Kemnitz JW. Body fat distribution with long-term dietary restriction in adult male rhesus macaques. J Gerontol A Biol Sci Med Sci. 1999;54:B283–290. doi: 10.1093/gerona/54.7.b283. [DOI] [PubMed] [Google Scholar]
- 29.Mattison JA, Black A, Huck J, Moscrip T, Handy A, Tilmont E, Roth GS, Lane MA, Ingram DK. Age-related decline in caloric intake and motivation for food in rhesus monkeys. Neurobiol Aging. 2005;26:1117–1127. doi: 10.1016/j.neurobiolaging.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Brehm BJ, Seeley RJ, Daniels SR, D'Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88:1617–1623. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- 31.Mariotti S, Barbesino G, Caturegli P, Bartalena L, Sansoni P, Fagnoni F, Monti D, Fagiolo U, Franceschi C, Pinchera A. Complex alteration of thyroid function in healthy centenarians. J Clin Endocrinol Metab. 1993;77:1130–1134. doi: 10.1210/jcem.77.5.8077303. [DOI] [PubMed] [Google Scholar]
- 32.Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab. 2006;91:3232–3235. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- 33.Herlihy JT, Stacy C, Bertrand HA. Long-term food restriction depresses serum thyroid hormone concentrations in the rat. Mech Ageing Dev. 1990;53:9–16. doi: 10.1016/0047-6374(90)90030-j. [DOI] [PubMed] [Google Scholar]
- 34.Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- 35.Chung HY, Kim HJ, Kim JW, Yu BP. The inflammation hypothesis of aging: molecular modulation by calorie restriction. Ann N Y Acad Sci. 2001;928:327–335. [PubMed] [Google Scholar]
- 36.Tapia G, Fernandez V, Varela P, Cornejo P, Guerrero J, Videla LA. Thyroid hormone-induced oxidative stress triggers nuclear factor-kappaB activation and cytokine gene expression in rat liver. Free Radic Biol Med. 2003;35:257–265. doi: 10.1016/s0891-5849(03)00209-0. [DOI] [PubMed] [Google Scholar]
- 37.Ayala V, Naudi A, Sanz A, Caro P, Portero-Otin M, Barja G, Pamplona R. Dietary protein restriction decreases oxidative protein damage, peroxidizability index, and mitochondrial complex I content in rat liver. J Gerontol A Biol Sci Med Sci. 2007;62:352–360. doi: 10.1093/gerona/62.4.352. [DOI] [PubMed] [Google Scholar]
- 38.Sanz A, Caro P, Ayala V, Portero-Otin M, Pamplona R, Barja G. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. Faseb J. 2006;20:1064–1073. doi: 10.1096/fj.05-5568com. [DOI] [PubMed] [Google Scholar]
- 39.Tesseraud S, Bigot K, Taouis M. Amino acid availability regulates S6K1 and protein synthesis in avian insulin-insensitive QM7 myoblasts. FEBS Lett. 2003;540:176–180. doi: 10.1016/s0014-5793(03)00260-6. [DOI] [PubMed] [Google Scholar]