Abstract
Background
Macaca nemestrina is a nonhuman primate used as a model in preclinical studies of hematopoietic stem cell transplantation and adoptive transfer of T cells. Adoptive T cell transfer studies typically require ex vivo expansion of substantial numbers of T cells prior to their reinfusion into the subject.
Results
We report an efficient method for the ex vivo expansion of CD4+ T cells from Macaca nemestrina peripheral blood. With this protocol, primary CD4+ T cells can be expanded between 300- to 6000-fold during 24-day period and can be efficiently transduced with lentiviral vectors. Furthermore, these T cells can be transformed by Herpesvirus saimiri and maintained in culture for several months. The transformed T cell lines can be productively infected with the simian immunodeficiency virus (SIV) strain SIVmac239.
Conclusions
We have established methods for the expansion and transformation of primary M. nemestrina CD4+ T cells and demonstrated the utility of these methods for several applications.
Keywords: CD4+ T cell expansion, H. saimiri transformation, and lentiviral transduction
INTRODUCTION
Adoptive T cell immunotherapy has been shown to be an efficient strategy for the treatment of infectious diseases such as cytomegalovirus (CMV) disease [1], Epstein-Barr virus-associated post-transplant lymphoproliferative disorders [2], AIDS [3], as well as for the treatment of malignant diseases like metastatic melanoma [4]. For this therapeutic approach, large numbers of genetically modified and/or antigen-specific T cells are expanded ex vivo and reinfused into the affected individual. If the reinfused T cells persist, they can help reconstitute the immune function of the immunodeficient patient.
The development of primate pre-clinical models has been critical for the study of several human diseases and also to develop therapeutic treatments for such conditions. The Macaca nemestrina (commonly known as pigtailed macaque) model is frequently used to study hematopoietic stem cell transplantation, human immunodeficiency virus (HIV) infection and T cell immunotherapy [5–7]. Previous reports have described methods for the ex vivo activation and expansion of rhesus macaque (Macaca mulatta) CD4+ T cells from peripheral blood, some of which have been successfully used for studies of autologous T cell infusion in this primate species [8–10]. Here we have established a protocol where we efficiently isolated and expanded CD4+ T cells from pigtailed macaques using paramagnetic beads coated with anti-CD3 and anti-CD28 antibodies and demonstrated the utility of these expanded cells for several applications. Additionally, we generated transformed cell lines from these primary cells that are susceptible to SIV infection and that can be used for long-term studies.
METHODS
Animals
This study used blood samples of four adult pigtailed macaques housed at the University of Washington Regional Primate Research Center under conditions approved by the American Association for Accreditation of Laboratory Animal Care. The Institutional Review Board and Animal Care and Use committee approved the protocols that were followed.
M. nemestrina CD4+ T cell isolation and ex vivo expansion
Peripheral blood CD4+ cells were isolated using the ‘Dynal CD4 Positive Isolation Kit’ (Invitrogen, Carlsbad CA) following the manufacturer’s instructions. The recovered cells were cultured in Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 10% Fetal Bovine Serum (FBS) and were activated by the addition of paramagnetic beads (Dynabeads M-450 Tosylactivated, Invitrogen, Carlsbad CA) coated with mouse monoclonal antibodies anti-human CD3 (clone SP34-2 BD Biosciences, San Jose CA) and anti-human CD28 (CD28.2 obtained from Dr. Daniel Olive, INSERM, France, through the NIH Nonhuman Primate Reagent Resource). The beads were prepared according to the maker’s indications and 1×107 beads were coated with 0.5μg of anti-CD3 and 4.5μg of anti-CD28. The CD4+ purified cells were stimulated with 3 beads per cell and 100U/ml recombinant human IL-2 (rhIL-2) (Chiron, Emeryville CA). The cultures were maintained at a density of 1–2×106 cells/ml and received more beads as required to maintain the 3:1 bead-to-cell ratio.
H. saimiri transformation of M. nemestrina CD4+ T cells
H. saimiri strain C488 (obtained from the NIH Nonhuman Primate Reagent Resource) was propagated on Owl Monkey Kidney (OMK) cells and used to infect purified CD4+ T cells that had been stimulated with immunobeads and rhIL-2 for 3 days after isolation. The T cells were infected with H. saimiri at a multiplicity of infection (MOI) of 3. The cells were maintained in IMDM supplemented with 10% FBS and 20U/ml rhIL-2 until rapidly growing cells were visible (typically 25–40 days after infection). At that point, the cells were stimulated with 100U/ml rhIL-2.
Lentiviral infection of M. nemestrina CD4+ T cells
Primary CD4+ T cells stimulated with immunobeads and 100U/mL rhIL-2 for 1 day after isolation were infected with a VSV-pseudotyped lentiviral vector encoding GFP (pRRL.SIN.cPPT.PGK.GFP.WPRE) (obtained through Addgene, Cambridge MA, plasmid 12252) at MOI=0.25, 0.5 or 1.0 in the presence of 8μg/ml of protamine sulfate. Cell growth and GFP expression was monitored for four weeks after isolation.
Plasmids encoding the 5′ and 3′ halves of SIVmac239 [11,12] (obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: p239SpSp5′ and p239SpE3′ from Dr. Ronald Desrosiers) were linearized by Sph-I digestion, ligated, and transiently transfected into HEK-293T cells as described elsewhere [13]. The virus produced was propagated in 174xCEM cells [14] (obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: 174xCEM from Dr. Peter Cresswell) and titrated using the indicator cell line Magi-CCR5 [15] (obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID: MAGI-CCR5 from Dr. Julie Overbaugh). SIVmac239 containing medium was used to infect H. saimiri transformed cells at MOI=0.001. The replication of the cells as well as that of the SIVmac239 virus was monitored for several weeks after the infection.
RESULTS
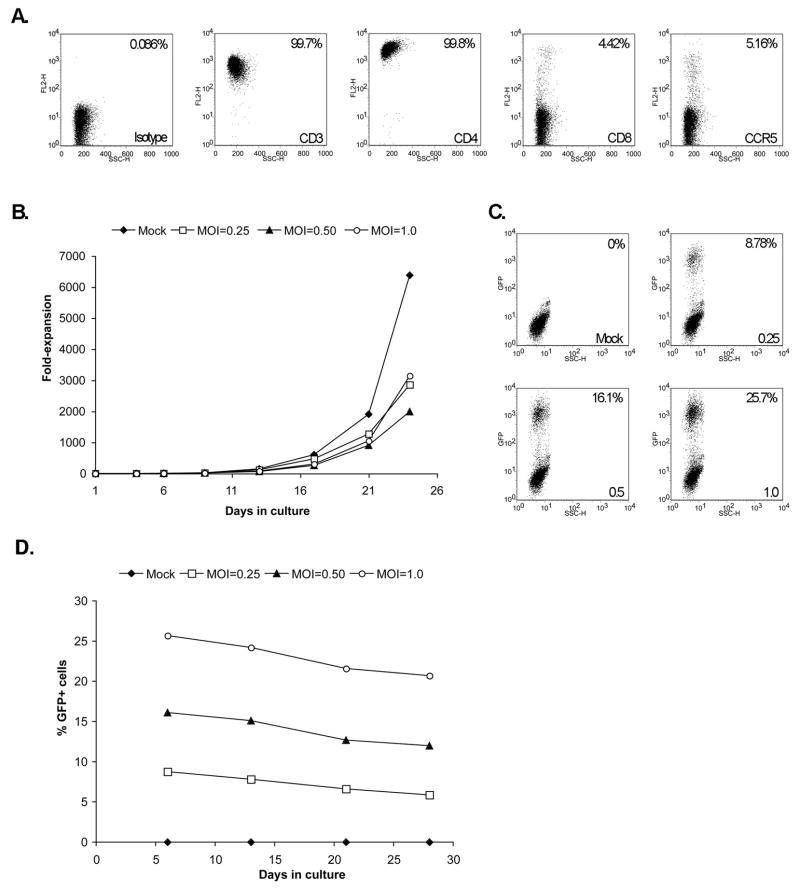
To maximize the expansion of M. nemestrina CD4+ lymphocytes ex vivo, we used paramagnetic beads coated with anti-CD3 and anti-CD28 antibodies, a method that has proven to be highly efficient for the expansion of human and rhesus macaque T cells [8,10,16]. As shown in Figure 1A, analysis by flow cytometry indicates that cells stimulated with the immunobeads and rhIL-2 for 14 days are CD3+ and CD4+, and that a significant proportion also express CD8. Furthermore, the analysis shows that these cells express reduced levels of the HIV/SIV co-receptor CCR5. With this approach the cells can be expanded between 300- to 6000-fold over a period of 24 days, after which the expansion rate decreases (Figure 1B).
Figure 1. Characterization of M. nemestrina primary CD4+ T-cells expanded in vitro.
Cells cultured for 14 days with immunobeads and rhIL-2 express surface markers CD3, CD4, CD8 and CCR5 (A) can be expanded greater than a 6000-fold in a period of 24 days (B), and they can be efficiently transduced with a lentiviral vector encoding GFP (MOI values indicated in the bottom right of each graph) (C). The expression of GFP in transduced cells is consistent over time (D). Of note, the graph in panel B represents the estimated fold-expansion and takes into consideration the cells discarded at each passage. These results correspond to a representative sample.
One advantage of the pigtailed macaque model is that unlike rhesus macaques HIV-1-derived vectors efficiently transduce pigtailed macaque cells [7] due to the absence of a functional TRIM5α [17] and HIV-1 has been shown to replicate in pigtailed macaque cells [18]. We thus evaluated whether these expanded cells could be efficiently transduced with lentiviral vectors. As illustrated in Figure 1C, primary CD4+ T cells stimulated with immunobeads and rhIL-2 and transduced with a VSV-pseudotyped lentiviral vector encoding GFP express the fluorescent marker in a MOI-dependent fashion. At higher MOIs the proliferative potential of the cells decreases (Figure 1B). GFP expression was stable during the four weeks it was evaluated (Figure 1D).
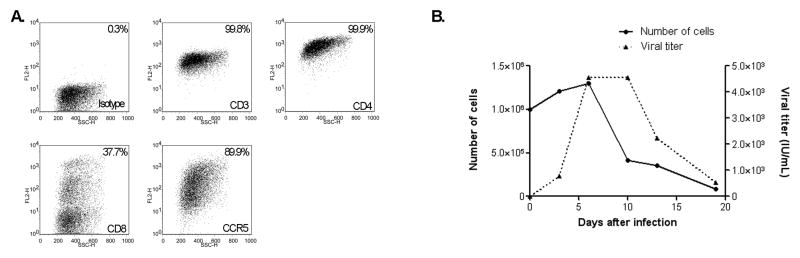
We also explored whether we could transform these lymphocytes for applications that require long-term amplification of functional and phenotypically stable T cells. Cells from two different animals were stimulated with immunobeads and rhIL-2 for 3 days after isolation and then infected with H. saimiri. After several weeks of stable growth, one cell line from each animal was then infected with SIVmac239. We found that H. saimiri infection of M. nemestrina primary CD4+ T cells supports the generation of cell lines that can be expanded for several months. These cell lines can then be productively infected with SIVmac239 as demonstrated in Figure 2. One of the cell lines can also be productively infected with an HIV-1 derivative that contains the SIVmac239 sequences of the vif gene and the Cyclophilin A (CypA) binding loop [19] (Daryl Humes and Julie Overbaugh, personal communication). Interestingly, the expression of CCR5 was considerably higher in the cell lines in comparison to the primary cultures (Figure 1A). This observation is consistent with previous studies that indicate that stimulation of CD4+ T cells with immobilized anti-CD3 and anti-CD28 antibodies reduces the expression of the chemokine receptor in human [20] and rhesus macaque cells [8]. In terms of the H. saimiri producing status of these cells, PCR analysis of the viral gene ORF3 in OMK cells cultured with cell-free conditioned medium from the transformed T cells indicated that the T cells release infectious particles. This result differs from what has been previously reported for cell lines derived from another Old World monkey, Macaca mulatta, in which infectious particles were not detected after transformation with H. saimiri [21].
Figure 2. Characterization of a M. nemestrina transformed H. saimiri T-cell line.
Following H. saimiri transformation and culture for 4 months, the cells express surface markers CD3, CD4, CD8 and CCR5 (A) and can be efficiently infected with SIVmac239 (B). Two cell lines from different animals were established with similar results.
DISCUSSION
Through the use of paramagnetic beads coated with antibodies specific for the cell surface proteins CD4, CD3, and CD28, we have been able to efficiently isolate and expand primary M. nemestrina CD4+ T cells from whole peripheral blood. Previous reports have described the ex vivo expansion of M. mulatta CD4+ T cells [8,10]. In those studies the efficiency of expansion varied considerably, and the best expansion rates were observed when feeder cells were used [10]. However, considering the large numbers of T cells required for adoptive immunotherapy protocols, such an approach is expensive and impractical. Among the expansion protocols that do not involve the use of feeder cells, paramagnetic beads coated with stimulatory CD3 and CD28 antibodies rendered expansion rates of less than 200 and could not be sustained beyond 14 days [8]. Under the feeder-free conditions we have established here for M. nemestrina samples, CD4+-enriched cells can be expanded beyond the level reported with other methods for M. mulatta samples and for a longer period of time. Additionally, using these conditions these cells can be efficiently marked using lentiviral vectors, which would allow for the cells to be easily tracked after reinfusion into animals. Even though we have not attempted to expand cells from other species with this method, it is likely that the conditions we have established will also work with cells from other Macaca species, since both the CD3 and CD28 antibodies used here have been reported to cross-react with M. fascicularis and M. mulatta (NIH Nonhuman Primate Reagent Resource).
Adoptive transfer of T cells has shown great promise in a number of animal studies and clinical trials for different diseases; however, the in vivo persistence of the transferred cells, especially that of T cell clones, varies and may be short [4,22]. Some strains of H. saimiri are capable of transforming human and macaque T cells allowing stable growth in culture, and it has been demonstrated that the transformed T cells maintain the antigen specificity and surface marker expression of the parental cells [21,23]. Importantly, it has been shown that when H. saimiri transformed autologous cells are reinfused, these cells are well tolerated and do not cause pathological changes in M. mulatta individuals even one year after infusion [24].
The M. nemestrina expansion and transformation studies we present here have additional practical applications. Because of the susceptibility of the H. saimiri transformed lymphocytes to HIV and SIV infection, these cells are valuable reagents for HIV studies. For example, the expansion method can be used to expand gene-modified lymphocytes ex vivo, and challenge these cells to evaluate resistance to infection in adoptively transferred, or in gene-modified stem cell-derived lymphocytes. The transformed lymphocyte cell line will also be a useful tool to study aspects of viral infection that may be limited in primary cells, including studies to help characterize host factors that enhance or restrict infection. In summary, here we have developed a transformed T cell line for HIV research, and a method to expand primary M. nemestrina cells to facilitate adoptive T cell immunotherapy and also anti-HIV gene therapy pre-clinical studies.
Acknowledgments
This work was supported by NIH grants HL084345, AI042552, and DK056465, Bethesda, MD. HPK is a Markey Molecular Medicine Investigator and the recipient of the José Carreras/E. Donnall Thomas Endowed Chair for Cancer Research.
References
- 1.Peggs KS, Verfuerth S, Pizzey A, Khan N, Guiver M, Moss PA, Mackinnon S. Adoptive Cellular Therapy for Early Cytomegalovirus Infection After Allogeneic Stem-Cell Transplantation With Virus-Specific T-Cell Lines. Lancet. 2003;362:1375–1377. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 2.Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, Srivastava DK, Bowman LC, Krance RA, Brenner MK, Heslop HE. Infusion of Cytotoxic T Cells for the Prevention and Treatment of Epstein-Barr Virus-Induced Lymphoma in Allogeneic Transplant Recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 3.Levine BL, Bernstein WB, Aronson NE, Schlienger K, Cotte J, Perfetto S, Humphries MJ, Ratto-Kim S, Birx DL, Steffens C, Landay A, Carroll RG, June CH. Adoptive Transfer of Costimulated CD4+ T Cells Induces Expansion of Peripheral T Cells and Decreased CCR5 Expression in HIV Infection. Nat Med. 2002;8:47–53. doi: 10.1038/nm0102-47. [DOI] [PubMed] [Google Scholar]
- 4.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T Cell Therapy Using Antigen-Specific CD8+ T Cell Clones for the Treatment of Patients With Metastatic Melanoma: in Vivo Persistence, Migration, and Antitumor Effect of Transferred T Cells. PNAS. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batten CJ, De Rose R, Wilson KM, Agy MB, Chea S, Stratov I, Montefiori DC, Kent SJ. Comparative Evaluation of Simian, Simian-Human, and Human Immunodeficiency Virus Infections in the Pigtail Macaque (Macaca Nemestrina) Model. AIDS Res Hum Retroviruses. 2006;22:580–588. doi: 10.1089/aid.2006.22.580. [DOI] [PubMed] [Google Scholar]
- 6.Berger C, Huang M-L, Greenberg PD, Riddell SR, Kiem H-P. Nonmyeloablative Immunosuppressive Regimen Prolongs in Vivo Persistence of Gene-Modified Autologous T Cells in a Nonhuman Primate Model. J Virol. 2001;75:799–808. doi: 10.1128/JVI.75.2.799-808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trobridge GD, Beard BC, Gooch C, Wohlfahrt M, Olsen P, Fletcher J, Malik P, Kiem H-P. Efficient Transduction of Pigtailed Macaque Hemtopoietic Repopulating Cells With HIV-Based Lentiviral Vectors. Blood. 2008;111:5537–5543. doi: 10.1182/blood-2007-09-115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onlamoon N, Hudson K, Bryan P, Mayne AE, Bonyhadi M, Berenson R, Sundstrom BJ, Bostik P, Ansari AA, Villinger F. Optimization of in Vitro Expansion of Macaque CD4 T Cells Using Anti-CD3 and Co-Stimulation for Autotransfusion Therapy. Journal of Medical Primatology. 2006;35:178–193. doi: 10.1111/j.1600-0684.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- 9.Onlamoon N, Plagman N, Rogers KA, Mayne AE, Bostik P, Pattanapanyasat K, Ansari AA, Villinger F. Anti-CD3/28 Mediated Expansion of Macaque CD4+ T Cells Is Polyclonal and Provides Extended Survival After Adoptive Transfer. Journal of Medical Primatology. 2007;36:206–218. doi: 10.1111/j.1600-0684.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang D, Murakami A, Johnson RP, Sui J, Cheng J, Bai J, Marasco WA. Optimization of Ex Vivo Activation and Expansion of Macaque Primary CD4-Enriched Peripheral Blood Mononuclear Cells for Use in Anti-HIV Immunotherapy and Gene Therapy Strategies. J Acquir Immune Defic Syndr. 2003;32:245–254. doi: 10.1097/00126334-200303010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, Desrosiers R, Authors FN. Induction of AIDS in Rhesus Monkeys by Molecularly Cloned Simian Immunodeficiency Virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 12.Regier DA, Desrosiers RC. The Complete Nucleotide Sequence of a Pathogenic Molecular Clone of Simian Immunodeficiency Virus. AIDS Res Hum Retroviruses. 1990;6:1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- 13.An DS, Donahue RE, Kamata M, Poon B, Metzger M, Mao SH, Bonifacino A, Krouse AE, Darlix JL, Baltimore D, Qin FX, Chen IS. Stable Reduction of CCR5 by RNAi Through Hematopoietic Stem Cell Transplant in Non-Human Primates. PNAS. 2007;104:13110–13115. doi: 10.1073/pnas.0705474104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salter RD, Howell DN, Cresswell P. Genes Regulating HLA Class I Antigen Expression in T-B Lymphoblast Hybrids. Immunogenetics. 1985;21:235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- 15.Chackerian B, Long EM, Luciw PA, Overbaugh J. Human Immunodeficiency Virus Type 1 Coreceptors Participate in Postentry Stages in the Virus Replication Cycle and Function in Simian Immunodeficiency Virus Infection. J Virol. 1997;71:3932–3939. doi: 10.1128/jvi.71.5.3932-3939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine BL, Mosca JD, Riley JL, Carroll RG, Vahey MT, Jagodzinski LL, Wagner KF, Mayers DL, Burke DS, Weislow OS, St Louis DC, June CH. Antiviral Effect and Ex Vivo CD4+ T Cell Proliferation in HIV-Positive Patients As a Result of CD28 Costimulation. Science. 1996;272:1939–1943. doi: 10.1126/science.272.5270.1939. [DOI] [PubMed] [Google Scholar]
- 17.Brennan G, Kozyrev Y, Kodama T, Hu S-L. Novel TRIM5 Isoforms Expressed by Macaca Nemestrina. J Virol. 2007;81:12210–12217. doi: 10.1128/JVI.02499-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agy MB, Schmidt A, Florey MJ, Kennedy BJ, Schaefer G, Katze MG, Corey L, Morton WR, Bosch ML. Serial in Vivo Passage of HIV-1 Infection in Macaca Nemestrina. Virology. 1997;238:336–343. doi: 10.1006/viro.1997.8832. [DOI] [PubMed] [Google Scholar]
- 19.Kamada K, Igarashi T, Martin MA, Khamsri B, Hatcho K, Yamashita T, Fujita M, Uchiyama T, Adachi A. Generation of HIV-1 Derivatives That Productively Infect Macaque Monkey Lymphoid Cells. PNAS. 2006;103:16959–16964. doi: 10.1073/pnas.0608289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll RG, Riley JL, Levine BL, Feng Y, Kaushal S, Ritchey DW, Bernstein W, Weislow OS, Brown CR, Berger EA, June CH, St Louis DC. Differential Regulation of HIV-1 Fusion Cofactor Expression by CD28 Costimulation of CD4+ T Cells. Science. 1997;276:273–276. doi: 10.1126/science.276.5310.273. [DOI] [PubMed] [Google Scholar]
- 21.Meinl E, Fickenscher H, Hoch RM, Malefyt RD, de Waal MR, Hart BA, Wekerle H, Hohlfeld R, Fleckenstein B. Growth Transformation of Antigen-Specific T Cell Lines From Rhesus Monkeys by Herpesvirus Saimiri. Virology. 1997;229:175–182. doi: 10.1006/viro.1996.8427. [DOI] [PubMed] [Google Scholar]
- 22.Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, Schwartzentruber DJ, Hwu P, Marincola FM, Sherry R, Leitman SF, Rosenberg SA. Adoptive Transfer of Cloned Melanoma-Reactive T Lymphocytes for the Treatment of Patients With Metastatic Melanoma. J Immunotherapy. 2001;24:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Weber F, Meinl E, Drexler K, Czlonkowska A, Huber S, Fickenscher H, Muller-Fleckenstein I, Fleckenstein B, Wekerle H, Hohlfeld R. Transformation of Human T-Cell Clones by Herpesvirus Saimiri: Intact Antigen Recognition by Autonomously Growing Myelin Basic Protein-Specific T Cells. PNAS. 1993;90:11049–11053. doi: 10.1073/pnas.90.23.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knappe A, Feldmann G, Dittmer U, Meinl E, Nisslein T, Wittmann S, Matz-Rensing K, Kirchner T, Bodemer W, Fickenscher H. Herpesvirus Saimiri-Transformed Macaque T Cells Are Tolerated and Do Not Cause Lymphoma After Autologous Reinfusion. Blood. 2000;95:3256–3261. [PubMed] [Google Scholar]