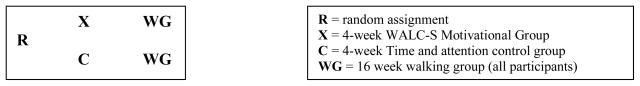Abstract
Persons with schizophrenia spectrum disorders (SSDs) are not only at risk because of disabling disease symptoms but because necessary medications create health risks associated with high rates of obesity. Despite the well-known benefits of exercise, persons with SSDs rarely adhere to such regimens; few interventions to motivate exercise behavior have been tested in this group.
The purpose of this study is to examine effects of the Walk, Address sensations, Learn about exercise, Cue exercise behavior for persons with SSDs (WALC-S) motivational intervention upon exercise behavior. We will recruit a total of eighty outpatients 18–68 years, meeting these criteria: 1) chart diagnosis of schizophrenia, any subtype, schizoaffective disorder or schizophreniform disorder, according to the criteria described in the Diagnostic and Statistical Manual for Mental Disorders, 2) English speaking, 3) Stable medication regimen (defined as no medication changes within the last month), and 4) medical clearance for moderate exercise in writing from primary care provider. Participants will be randomly assigned to the experimental (4-week WALC-S motivational intervention), or the control group (4-week time and attention control). After the first 4 weeks, all participants will attend a 16-week walking group.
The primary measures of the effectiveness of the WALC-S are attendance, persistence and compliance to the 16-week walking group. The study will be completed in approximately January 2010. In addition to hypothesis testing, this study will provide information to estimate effect sizes to calculate power and determine appropriate sample sizes for future inquiries. This paper describes the rationale and design of the study.
Keywords: Schizophrenia, exercise, motivation, psychosocial interventions
Introduction
Schizophrenia spectrum disorders (SSDs) include schizophrenia, schizoaffective disorder and schizophreniform disorder. The diagnostic overlap between the three is well known (1); recent research indicates that persons with these disorders have similar psychiatric symptoms (2) and cognitive deficits (3,4). Another commonality is the increased risk for adverse physical health outcomes shared by persons with these illnesses.
Death rates from diabetes, respiratory/cardiovascular, and other obesity-related illnesses are significantly higher among the over 2 million Americans with SSDs than in the general population (5). The most effective medications for managing SSDs (second generation antipsychotics such as clozapine and olanzapine) are associated with weight gain, glucose dysregulation and diabetes (6,7,8). Despite the well-known benefits of exercise and the health dangers associated with obesity, persons with SSDs rarely adhere to exercise regimens and few exercise adherence interventions have been tested in this group.
Few published studies have examined the effects of exercise in persons with SSDs (9,10,11,12,13,14,15) and only two addressed exercise motivation (16,17). All of the studies examining psychiatric outcomes reported symptom improvement (9,10,11,12); three of four studies addressing physical health outcomes reported positive changes (12,13,14,15). Drop out rates ranged from 5 to 38% over periods from 8–16 weeks in length. Attendance at exercise sessions ranged from 23% to 91% with attendance being positively correlated with degree of improvement. Archie et al., (16) provided free access to a fitness facility to 20 outpatients with schizophrenia for 6 months and monitored their exercise behavior. Dropout rates were 40% after 4 months, 70% after 5 months and 90% after 6 months. These rates compare unfavorably with exercise cessation in the general population, which is approximately 50% after 6 months (24). The most common reason given for poor attendance at the exercise facility was lack of motivation (16). Menza and colleagues (17) tested a one-year weight control program in 31 outpatients with SSDs. The program incorporated exercise as well as behavioral interventions, and compared BMI and weight with a control group of 20 non-intervention patients. Weight and BMI decreased significantly in the intervention group, with 20 of 31 persons completing the study, for a one-year retention rate of 66% (69% of sessions were attended). The study intervention consisted of nutritional counseling, exercise, and behavioral interventions incorporating motivational counseling techniques. Motivational counseling consisted of professional support, encouragement, and strategies to improve exercise habits. This study shows that interventions can increase exercise adherence in persons with SSDs. However, because the control group had no opportunity to participate in an exercise program, it is not possible to separate the motivational effects of the intervention from the motivational effects of exercise alone. Our study addresses this limitation by offering an exercise group to every participant.
Methods
This paper reviews the rationale and design of our study. The purpose of this study is to test the effects of the Walk, Address sensations, Learn about exercise, Cue exercise behavior for persons with SSDs (WALC-S) motivational intervention on the following three aspects of exercise behavior:
Attendance-The ratio of the total number of exercise sessions attended to the total number of exercise sessions offered;
Persistence-The number of consecutive weeks that the participant attends at least one exercise session; and
Compliance-The performance of activities during exercise sessions.
We hypothesize that experimental participants will have higher: 1) attendance rates, 2) persistence rates and 3) compliance rates at the exercise program than controls.
Design
This experimental study is testing the WALC-S motivational intervention by comparing the attendance, persistence and compliance of an experimental group of persons with SSDs (who receive the WALC-S intervention) with the exercise attendance, persistence and compliance of a time-and-attention control group (not receiving the WALC-S intervention). The WALC-S and the time and attention control will be provided prior to a 16-week walking group that will be offered to all participants. (See Figure 1).
Figure 1.
Study Design
Motivational intervention
The Walk, Address sensations, Learn about exercise, Cue exercise behavior (WALC) was originally designed to motivate community-dwelling older adults to exercise. The WALC is based upon self-efficacy theory and has been theoretically and empirically validated (18–23). Persons with SSDs face many similar exercise barriers as elderly persons. Research has shown that both elderly persons and those with SSDs lack exercise motivation (16,23,24). Further, elderly persons perceive the following barriers to exercise: fear of pain (18), problems with goal setting (25), and lack of knowledge regarding the positive benefits of exercise (15, 24–26). Problems with goal setting may be partially related to cognitive deficits associated with aging (27, 28). The literature supports the presence of similar exercise barriers among persons with SSDs. Persons with SSDs have documented cognitive deficits that lead to difficulties setting goals and carrying out goal directed plans (29). In one study, over 33% of persons cited fear of pain as a barrier to exercise participation and participants reported that health benefits experienced were unexpected (12), highlighting the similarities with elderly persons who lack knowledge as to the strong relationship of health benefits and exercise (25). We believe these similarities make the use of the WALC a reasonable starting point in our search for interventions to increase exercise behavior in persons with SSDs.
Enrollment criteria
The sample will be selected from persons with SSDs aged 18–68 years, receiving outpatient care at a community agency and meeting the following criteria: 1) a chart diagnosis of schizophrenia (any subtype), schizoaffective disorder or schizophreniform disorder, according to the criteria described in the Diagnostic and Statistical Manual-IV for Mental Disorders (1), 2) English speaking, 3) Stable medication regimen (defined as no medication changes within the last month), and 4) medical clearance for moderate exercise in writing from primary care provider.
Exclusion criteria include chart documentation of mental retardation, developmental delay, uncorrected visual, hearing impairments or any physiological condition preventing safe participation. Persons with the following physical conditions per chart documentation are excluded: 1) hospitalization within the past 12 months for angina pectoris, myocardial infarction, or cardiac surgery of any kind, 2) congestive heart failure, 3) cardiac pacemaker, 4) heart rate > 100 or < 50 at rest, 5) uncontrolled hypertension defined as a blood pressure exceeding 140/90 on 3 consecutive readings despite adequate treatment, 6) history of spinal or hip fractures or hip or knee arthroplasty, 7) neuromuscular or orthopedic limitations to normal, unassisted ambulation, and 8) other medical conditions that, in the opinion of the primary care provider or investigators, involve risk exceeding the potential benefit of participation.
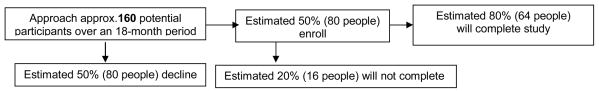
Our enrollment target is 80 participants. Allowing for a 20% attrition rate, it is estimated that approximately 64 participants (32 per group) will complete the study. (See Figure 2). We use a computer-generated random number table to assign patients to either the control or the intervention group in a 1:1 ratio. The randomization process is expected to result in equal distribution of important sociodemographic and illness variables between the two groups. The sample will not be stratified by gender, ethnicity, or other variables, since no data are available at this time to indicate differential response to the intervention based upon differences between participants.
Figure 2.
Estimated Study Enrollment and Completion Rates
Study Interventions
Upon study entry, participants are randomly assigned to either the experimental or control group. Experimental participants (8–9 persons per group) attend the one-hour WALC-S motivational group weekly for 4 weeks prior to their 16-week walking group. The WALC-S was designed to address cognitive deficits common to persons with SSDs. While variability exists between individuals, cognitive impairments are present to some degree in virtually every SSD (30). Specific deficits include mild-to-moderate impairment in attention and memory and severe impairment in executive functioning (31–32). Executive functioning deficits involve frontal lobe dysfunction in the areas of planning, organization, and mental flexibility (30) that impede problem solving and decision making processes (33). We used a number of strategies to offset these difficulties. For example, we addressed memory deficits (34) through repetition of information during WALC-S sessions, and by providing written and verbal reminders of WALC-S groups. To assist participants to sustain attention during WALC-S groups, information was presented in a variety of formats: first verbally, then pictorially with visual aids, and finally demonstrated in person. Research staff assisted with goal setting and identifying ways to overcome exercise barriers in order to offset difficulties with problem solving and decision making (35). Finally, because participants in prior studies cited the importance of the social aspects of exercise (36), we designed the WALC-S as a one-hour group intervention (8–9 participants per group) that met weekly for four weeks.
The WALC-S intervention is not a walking group; rather, it is a forum for discussion of the basics of walking for exercise supplemented by a booklet provided to every experimental participant. The group practices a series of basic stretches at each meeting. Study personnel assist with goal setting, a process that is difficult for persons with schizophrenia due to limitations in executive functioning (35,37). Study personnel provide suggestions on reducing common exercise discomforts, such as using heat or massage for muscular soreness. Study personnel provide instruction on potential exercise benefits and overcoming barriers to exercise. Participants are provided with reminder calendars to track WALC-S attendance and any other exercises performed independently. Activities for each weekly group are listed in Table 1.
Table 1.
Activities and materials for the WALC-S.
| Week | Activities and Materials |
|---|---|
| One | Introductions of research staff and group members Review section of booklet on basics of exercising safely Perform stretches: Poster with photograph of each stretch, demonstration by research staff and return demonstration by group members |
| Two | Introductions and Check-in with each member Review section of booklet on basics of exercising safely Review section of booklet on exercise benefits Perform stretches: Poster with photograph of each stretch, demonstration by research staff and return demonstration by group members |
| Three | Introductions and Check-in with each member Review section of booklet on basics of exercising safely Review section of booklet on exercise benefits Barriers and solutions: Discuss and write suggestions for dealing with common exercise barriers on blackboard. Perform stretches: Poster with photograph of each stretch, demonstration by research staff and return demonstration by group members |
| Four | Introductions and Check-in with each member Review section of booklet on basics of exercising safely Review section of booklet on exercise benefits Barriers and solutions: Discuss and write suggestions for dealing with common exercise barriers on blackboard. Set individual goals Provide reminder calendars to cue exercise Perform stretches: Poster with photograph of each stretch, demonstration by research staff and return demonstration by group members |
Control participants (8–9 persons per group) attend the one-hour time-and-attention control group weekly for 4 weeks prior to their 16-week walking group. Control groups are conducted by the same research staff as the WALC-S groups, and meet on the same day in the same location for an identical period of time. Control groups focus on healthy behaviors such as medication adherence, smoking cessation and progressive muscle relaxation.
Sixteen-week walking group (all participants). After the 4-week experimental or control group is completed, all participants participate in a 16-week walking group. Walking groups are conducted by different research personnel than those who led the WALC-S group or the time-and-attention control group to minimize the effects of relationship with group leader alone upon outcomes. Each walking group begins with a series of warm up stretches (38). Warm up is followed by walking, beginning with 5 minutes and gradually increasing to 30 minutes over the first four weeks. Interaction during the walking groups is social rather than therapeutic (e.g. discussion of daily activities, family members, recreational plans, and the like). Walking group research personnel do not initiate any discussions of WALC-S content during walking groups. Should any participant initiate discussion or questions regarding WALC-S concepts, group leaders respond with appropriate education, goal setting or reinforcement. For example, if a participant remarks that he or she is experiencing less depression since beginning the walking group, the group leader reinforces information regarding the benefits of exercise on mood, but refrains from initiating any such discussions. Research personnel note that this has occurred on only one occasion; thus, we estimate that the likelihood of the blind being broken as a result of discussion of WALC-S training during the walking groups, appears to be minimal. Each walking session concludes with a series of cool down stretches. Walking sessions occur outdoors (weather permitting) at the recruitment facility on Monday, Wednesday, and Friday, either in the morning or afternoon. Control and experimental participants do not attend the same walking group, in order to prevent cross-contamination.
Treatment Fidelity
Two different research personnel blinded to treatment group are providing walking groups to experimental and control participants. The first author conducted an 8-hour training session before any walking groups; both personnel scored 100% on a knowledge test after the training. The first author provides at least 2 hours of supervision each week, and attends 50% of the walking groups. A measure of concurrence with the walking group training is generated by completion of a fidelity form once a month. The form provides an objective measure of the provision of each aspect of the walking group, ranging from 1-none of the time to 5-all of the time. The first author provides remediation for any area that scores below 4-most of the time. Remediation is organized accordingly: (1) identification of specific concerns or questions, (2) item-by-item discussion of walking group procedures, (3) provision of feedback and clarification, and (4) immediate correction of deficiencies. These activities ensure that the walking groups are implemented as stated, increasing uniformity and reducing alternative explanations based upon differences in walking group procedures.
Outcomes
Walking group attendance will be measured as a ratio of number of exercise sessions attended to total number of exercise sessions offered. For example, a person attending 24 exercise group sessions of the total 48 sessions offered would have an attendance rate of 50%. Walking group persistence will be measured as the number of consecutive weeks that the participant attends at least one walking group. Walking group compliance is measured by pedometer. The number of steps walked per group will be used to generate an ordinal measure of compliance as follows: Full-The number of steps equals or exceeds that of the previous walking group; Partial-The number of steps is not more than 10% less than that of the previous walking group; None-The number of steps is more than 10% less than that of the pervious walking group.
Data Collection will occur over a two-year period. Data regarding sociodemographic characteristics, living arrangements, prescribed medications and medication and/or dosage changes will be collected on all participants at study entry. Attendance, persistence, and compliance are documented at each walking group. Sixty-four participants have been recruited; this partial sample consists mostly of female (n = 36, 56.3%) Caucasians (n = 42, 65.6%) from 24–68 years with a mean age of 47.88 (SD 8.85). The majority live with family members (n = 30, 46.9%). Forty-eight participants have completed all study activities including descriptive measures, the WALC-S or the time-and-attention control group, and the 16-week walking group with its associated measures of attendance, persistence and compliance.
Data Analysis Plan
The Statistical Package for the Social Sciences (SPSS) and the Statistical Analysis System (SAS) will be used to analyze the data. Data analysis will begin with data plots and basic descriptive statistics appropriate for the level of measurement. Groups will be compared for pretreatment equivalence on sociodemographic characteristics, as well as type and dosage of prescribed medications. Expected mean squares will be calculated and the appropriate combination will be used for hypothesis tests with specific functions of the repeated measures. General linear model analyses in SAS (GLM and MIXED procedures) will be used to examine the effects of time, treatment, and time by treatment interaction. For hypotheses 1 and 2, a sample size of 32 in each group will have 80% power to detect an effect size of 0.629 using a two-group t-test with a 0.05 one-sided significance level. For hypothesis 3, a sample size of 32 in each group will have 80% power to detect a probability of 0.679 that an observation in the control group is less than an observation in the experimental group using a Wilcoxon rank-sum test with a 0.05 one-sided significance level.
Summary
In addition to hypothesis testing, this study will provide information to estimate effect sizes to calculate power and determine appropriate sample sizes for future inquiries. This work is the first step toward our ultimate goal of developing and empirically evaluating exercise interventions to enhance health for persons with SSDs.
Acknowledgments
This project is supported by a grant from the National Institutes of Health, R03 MH079047-01.
Contributor Information
Lora Humphrey Beebe, Email: lbeebe1@utk.edu, 1200 Volunteer Blvd, University of Tennessee, College of Nursing Knoxville, TN 37996, Tel (865) 974-3978, Fax (865) 974 3569.
R. Burk, University of Tennessee.
K. McIntyre, InterFaith Health Clinic.
K. Smith, Tennessee Wesleyan College.
D. Velligan, University of Texas Health Science Center at San Antonio.
B. Resnick, University of Maryland.
A. Tavakoli, University of South Carolina.
C. Tennison, Helen Ross McNabb Center.
O. Dessieux, University of Tennessee.
References
- 1.American Psychiatric Association (APA) Diagnostic and statistical manual of Mental Disorders. 4. Washington, DC: 2000. [Google Scholar]
- 2.Kopelowicz A, Ventura J, Liberman PA, Mintz J. Consistency of Brief Psychiatric Rating Scale factor structure across a broad spectrum of schizophrenia patients. Psychopathology. 2008;41(2):77–84. doi: 10.1159/000111551. [DOI] [PubMed] [Google Scholar]
- 3.Premkumar P, Cooke MA, Fannon D, Peters S, Michel TM, Aasen I, Murray RM, Kuipers E, Kumari V. Misattribution bias of threat related facial expression is related to a longer duration of illness and poor executive function in schizophrenia and schizoaffective disorder. Eur Psychiatry. 2008;23(1):14–19. doi: 10.1016/j.eurpsy.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacchetti F, Galluzzo A, Panariello A, Parrinello G, Cappa SF. Self-ordered pointing and visual conditioning associated learning tasks in drug free schizophrenia spectrum disorder patients. BMC Psychiatry. 2008;23(8):6. doi: 10.1186/1471-244X-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleischhacker WW, Cetkovick-Bakmas M, DeHert M, Hennekens CH, Lambert M, Leucht S, Maj M, McIntyre RS, Naber D, Newcomer JW, Olfson M, Osby U, Sartorius N, Lieberman JA. Comorbid somatic illnesses inpatients with severe mental disorders: Clinical, policy and research challenges. J Clin Psychiatry. 2008;69(4):514–519. doi: 10.4088/jcp.v69n0401. [DOI] [PubMed] [Google Scholar]
- 6.Ananth J, Venkatesh R, Burgoyne K, Gadasalli R, Binford R, Gunatilake S. Atypical antipsychotic induced weight gain: Pathophysiology and management. Ann Clin Psychiatry. 2004;16(2):75–85. doi: 10.1080/10401230490453293. [DOI] [PubMed] [Google Scholar]
- 7.Jin H, Meyer JM, Jeste DV. Atypical antipsychotics and glucose dysregulation: a systematic review. Schizophr Res. 2004;71(2–3):195–212. doi: 10.1016/j.schres.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Newcomer JW. Abnormalities of glucose metabolism associated with atypical antipsychotic drugs. J Clin Psychiatry. 2004;65(Suppl 18):36–46. [PubMed] [Google Scholar]
- 9.Gimino FA, Levin SJ. The effects of aerobic exercise on perceived self-image in post-hospitalized schizophrenic patients. Med Sci Sports Exerc. 1984;16:139. [Google Scholar]
- 10.Chamov S. Positive short-term effects of activity on behavior in chronic schizophrenic patients. British Journal of Clinical Psychiatry. 1986;25:125–133. doi: 10.1111/j.2044-8260.1986.tb00681.x. [DOI] [PubMed] [Google Scholar]
- 11.Pelham TW, Campagna PD, Ritvo PG, Birnie WA. The effects of exercise therapy on clients in a psychiatric rehabilitation program. Psyiatr Rehabil J. 1993;16:75–84. [Google Scholar]
- 12.Beebe LH, Tian L, Goodwin A, Morris N, Swant-Allen S, Kuldau J. Effects of exercise on mental and physical health parameters of persons with schizophrenia. Issues Ment Health Nurs. 2005;26(6):661–676. doi: 10.1080/01612840590959551. [DOI] [PubMed] [Google Scholar]
- 13.Pendlebury J, Haddad P, Dursun S. Evaluation of a behavioral weight management programme for patients with severe mental illness: 3 year results. Hum Psypharrmacol. 2005;20:447–448. doi: 10.1002/hup.707. [DOI] [PubMed] [Google Scholar]
- 14.Ball MP, Coons VB, Buchanan RW. A program for treating olanzapine related weight gain. Psychiatr Serv. 2001;52:967–969. doi: 10.1176/appi.ps.52.7.967. [DOI] [PubMed] [Google Scholar]
- 15.Vreeland B, Minsk S, Menza M, Rigussio-Radler D, Roemheld-Hamm B, Stern R. A program for managing weight gain associated with atypical antipsychotics. Psychiatr Serv. 2003;54(8):1155–1157. doi: 10.1176/appi.ps.54.8.1155. [DOI] [PubMed] [Google Scholar]
- 16.Archie W, Wilson JH, Osborne S, Hobbs H, McNiven J. Pilot study: Access to fitness facility and exercise levels in olanzapine-treated patients. Can J Psychiatry. 2003;48(9):628–632. doi: 10.1177/070674370304800910. [DOI] [PubMed] [Google Scholar]
- 17.Menza M, Vreeland B, Minsky S, Gara M, Radler DR, Sakowitz M. Managing atypical antipsychotic-associated weight gain: 12-month data on a multimodal weight control program. J Clin Psychiatry. 2004;65(4):471–477. [PubMed] [Google Scholar]
- 18.Resnick B. Efficacy beliefs in geriatric rehabilitation. J Ger Nsg. 1998;2:34–45. doi: 10.3928/0098-9134-19980701-08. [DOI] [PubMed] [Google Scholar]
- 19.Resnick B. Testing a model of exercise behavior in older adults. Res Nurs Health. 2001;24:83–92. doi: 10.1002/nur.1011. [DOI] [PubMed] [Google Scholar]
- 20.Resnick B. Testing the impact of the WALC intervention on exercise adherence in older adults. J Gerantol Nurs. 2002;28(6):40–49. doi: 10.3928/0098-9134-20020601-10. [DOI] [PubMed] [Google Scholar]
- 21.Resnick B, Orwig D, Magaziner J, Wynne C. The impact of social support on exercise behavior in older adults. Clin Nurs Res. 2002;11(1):52–70. doi: 10.1177/105477380201100105. [DOI] [PubMed] [Google Scholar]
- 22.Resnick B, Palmer MH, Jenkins L, Spellbring AM. Path analysis of efficacy expectations and exercise behavior in older adults. J Adv Nurs. 2000;31(6):1309–1315. doi: 10.1046/j.1365-2648.2000.01463.x. [DOI] [PubMed] [Google Scholar]
- 23.Resnick B, Spellbring AM. The factors that influence exercise behavior in older adults. J Gerantol Nurs. 2000;26:34–42. [Google Scholar]
- 24.Dishman R. Increasing and maintaining exercise and physical activity. Behavioral Therapy. 1991;21:345–373. [Google Scholar]
- 25.Resnick B. Across the aging continuum: Motivating older adults to exercise. Advanced Nursing Practice. 2005;13(9):37–40. [PubMed] [Google Scholar]
- 26.Mann M, Pruitt R, Meehan N, Kemper K. Counseling seniors about physical activity. Advanced Nursing Practice. 1997;7:37–40. [PubMed] [Google Scholar]
- 27.Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning- Dixon F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58(3):204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 28.Kiosses DN, Alexopoulos GS. IADL function, cognitive deficits and severity of depression: A preliminary study. Am J Psychiatry. 2005;13(3):244–249. doi: 10.1176/appi.ajgp.13.3.244. [DOI] [PubMed] [Google Scholar]
- 29.Velligan DI, Bow-Thomas CC. Executive function in schizophrenia. Seminar in Clinical Neuropsychiatry. 1999;4(1):24–33. doi: 10.1053/SCNP00400024. [DOI] [PubMed] [Google Scholar]
- 30.Bowie CR, Jaga K. Methods for treating cognitive deficits in schizophrenia. Expert Rev Neurother. 2007;7(3):281–287. doi: 10.1586/14737175.7.3.281. [DOI] [PubMed] [Google Scholar]
- 31.Bilder RM, Reiter G, Bates J, Lencz T, Szeszko P, Goldman RS, Robinson D, Lieberman JA, Kane JM. Cognitive development in schizophrenia: follow- back from the first episode. J Clin Exp Neuropsychol. 2006;28:270–282. doi: 10.1080/13803390500360554. [DOI] [PubMed] [Google Scholar]
- 32.Seidman LJ, Buka SL, Goldstein JM, Tsuang MT. Intellectual decline in schizophrenia: Evidence from a prospective birth cohort 28 year follow up study. J Clin Exp Neuropsychol. 2006;28:225–242. doi: 10.1080/13803390500360471. [DOI] [PubMed] [Google Scholar]
- 33.Vaskinn A, Sundet K, Frits S, Simonsen C, Birkenaes AB, Jonsdottir H, Ringen PA, Andreassen OA. Emotion perception and learning potential: Mediators between neurocognition and social problem solving in schizophrenia? J Int Neuropsychol Soc. 2008;14:279–288. doi: 10.1017/S1355617708080314. [DOI] [PubMed] [Google Scholar]
- 34.Bacon E, Izaute M, Danion JM. Preserved memory monitoring but impaired memory control during episodic encoding in patients with schizophrenia. J Int Neuropsychol Soc. 2007;13(2):219–227. doi: 10.1017/S1355617707070245. [DOI] [PubMed] [Google Scholar]
- 35.Kim H, Lee D, Shin YM, Chey J. Impaired strategic decision making in schizophrenia. Brain Research. 2007 Nov;14:90–100. doi: 10.1016/j.brainres.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 36.Beebe LH. Walking Tall: A person with schizophrenia on a journey to better health. J Psychosoc Nurs Ment Health Serv. 2005;44(6):1–4. doi: 10.3928/02793695-20060601-08. [DOI] [PubMed] [Google Scholar]
- 37.Velligan DI, Kern RS, Gold JM. Cognitive rehabilitation for schizophrenia and the putative role of motivation and expectancies. Schizophr Bull. 2006;32(3):474–485. doi: 10.1093/schbul/sbj071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reid JG, Thomson JM. Exercise prescription for fitness. Englewood Cliffs NJ: Prentice-Hall; 1985. [Google Scholar]