Abstract
The survival of rat Purkinje cell (PCs) cerebellar cultures was used to test the hypothesis that progesterone is protective againstoxygen–glucose deprivation through potentiation of GABAA receptor activity. Electrophysiological recordings confirm that PCs develop robust excitatory and inhibitory synapses in culture. Exposure of cultured PCs to increasing concentrations of progesterone during oxygen–glucose deprivation revealed a concentration-dependent protection by progesterone, with significant protection observed at physiological concentrations, as low as 10 nm. The concurrent application of the GABAA receptor antagonist picrotoxin (100 µm) completely abolished the neuroprotection afforded by progesterone, indicating that progesterone is neuroprotective through activation of GABAA receptors. Progesterone potentiates GABAA receptor activity indirectly through its metabolites, such as allopregnanolone (ALLO). Therefore, ALLO was applied to PC cultures and was observed to produce significant protection at all concentrations tested, from 10 to 1000 nm. Finally, the inhibition of progesterone metabolism with finasteride abolished the protection afforded by progesterone without having any effect on the neuroprotection caused by ALLO. These data indicate that progesterone protects cerebellar PCs at physiological concentrations through a GABA-active metabolite.
Keywords: allopregnanolone, excitotoxicity, GABAA receptor, ischemia
Introduction
It is now well known that the progesterone family of steroids reduce brain injury after focal and global cerebral ischemia and after traumatic brain injury (TBI; for review see Stein, 2005). Progesterone has been reported to be effective in models of middle cerebral artery occlusion in rodents of both sexes (Jiang et al., 1996; Murphy et al., 2002; Gibson & Murphy, 2004), and in global ischemia models in rodents and cats (Gonzalez-Vidal et al., 1998; Cervantes et al., 2002; Morali et al., 2005). The steroid interacts with a variety of beneficial cellular and molecular signalling pathways in brain. For example, progesterone acts as an antioxidant to reduce lipid peroxidation (Roof et al., 1997), reduces brain oedema following TBI (Roof et al., 1996) and has anti-inflammatory properties (He et al., 2004a; Gibson et al., 2005). Best known is progesterone’s ability to increase GABAergic activity, resulting in decreased neuronal excitability and consequent protection from excitotoxicity. It is likely that progesterone increases GABAergic activity indirectly, through metabolites that potentiate GABAA receptor activity. Progesterone is readily metabolised in the brain to 5α-dihydroprogesterone (DHP) by 5α-reductase, then further reduced to 3α,5α-tetrahyroprogesterone (ALLO) by the enzyme 3α hydroxysteroid reductase (for reviews see Celotti et al., 1992; Mellon & Vaudry, 2001; Stoffel-Wagner, 2001; Belelli & Lambert, 2005). While there is little direct evidence that progesterone’s protective actions are dependent on its metabolism, (Allopregnanolone; ALLO) has been demonstrated to reduce ischemic damage and decrease glutamate excitotoxicity in vitro and in vivo (Lockhart et al., 2002; Ciriza et al., 2004, 2006; He et al., 2004b). Furthermore, a recent report indicates that ALLO is a more potent neuroprotectant than its parent compound in the middle cerebral artery occlusion model of transient ischemia, emphasizing that progesterone could act through its conversion to ALLO (Sayeed et al., 2006).
It has long been recognised that cerebellar Purkinje cells (PCs) are particularly sensitive to ischemic damage in vivo (Pulsinelli et al., 1982; Sato et al., 1990; Horn & Schlote, 1992; Brasko et al., 1995; Fonnum & Lock, 2000). The cellular mechanism and functional significance of this observation remains understudied. However, these cells are well-positioned neurochemically and synaptically to be vulnerable to ischemic challenges. PCs are the largest neurons in the cerebellar cortex and receive inhibitory inputs from Golgi, basket and stellate cells. PCs also receive excitatory inputs from granule cells (parallel fibers) and the inferior olive (climbing fibers). The enormity of excitatory input from both climbing and parallel fibers may make PCs susceptible to glutamate excitotoxicity through excessive activation of Ca2+-permeable AMPA receptors (Brorson et al., 1994; Welsh et al., 2002; Slemmer et al., 2005). Accordingly, the AMPA receptor antagonist NBQX protects PCs after ischemia in vivo (Brasko et al., 1995). Elimination of climbing fiber input from the inferior olive also protects PCs from ischemic damage (Welsh et al., 2002). In addition, PCs receive large inhibitory inputs from interneurons. Therefore, the cerebellar cortex represents an ideal network in which to assess the effects of ischemia-induced excitotoxicity and the neuroprotective potential of compounds that potentiate GABAergic neurotransmission.
Using a novel PC culture system that preserves intact excitatory and inhibitory signalling (Gruol, 1983; Hirano et al., 1986; Hirano & Kasono, 1993), we set out to evaluate cerebellar PCs during oxygen and glucose deprivation (OGD) as a means of simulating cerebellar ischemia and testing the potential for GABAergic progesterone metabolites to protect these cells. We tested the hypothesis that progesterone’s protective actions require its metabolism to ALLO and potentiation of GABAA receptors. Because recent reports suggest that male and female cells respond differently to toxic stimuli and to OGD (Liu et al., 2006; Du et al., 2004), the current study uniquely employed sex-specific neuronal cultures to evaluate whether genetic sex influences outcome and mechanisms of progesterone neuroprotection.
Materials and methods
Sex-specific cerebellar cultures
Neurons were cultured from the cerebellum of embryonic day (E)18 Sprague–Dawley rats in accordance with National Institute of Health guidelines and experimental protocols approved by the institutional IACUC committee. Male and female fetuses were identified as described previously (Liu et al., 2006). Briefly, embryos were sexed by visual inspection of the genital papilla, the presence or absence of testicular artery and sex chords and by measurement of the ano-genital space (larger in male). Polymerase chain reaction for the presence or absence of the Y-chromosome gene sry has been performed previously to verify 100% agreement with visual identification (Liu et al., 2006). Culture techniques were as previously described (Linden et al., 1991), briefly described here. Timed-pregnant Sprague–Dawley rats were killed with CO2 and E18 fetuses removed by caesarean section and placed in ice-cold Hank’s saline. Cerebelli were dissected and chopped into ~1 mm pieces before being digested for 30 min in trypsin at 37 °C in Hank’s saline. Digestion was halted by the addition of 10% fetal calf serum. The digested pieces were then triturated and filtered through a 70-µm nylon mesh. The cell suspension was then spun at 500 g and resuspended in neurobasal + B27 supplements (Invitrogen, Carlsbad, CA, USA). This culture medium contained no oestrogens and low-nm progesterone. Cells were plated on 12-mm round coverslips coated with poly l-lysine in 24-well culture dishes and maintained in a humidified atmosphere. Half the medium was replaced with fresh medium every 3–4 days. PCs are easily identified by their unique morphology (the largest neuron in the cerebellar cortex) and their immunoreactivity to the calcium binding protein calbindin; see Fig. 2 and Baimbridge & Miller (1982). Each culture yields a total of 18–24 coverslips of each gender with 30–50 PCs per coverslip. PCs represent < 5% of the neurons in culture, with the small non-PC neurons being a mixture of excitatory granule cells and inhibitory interneurons such as stellate and basket cells.
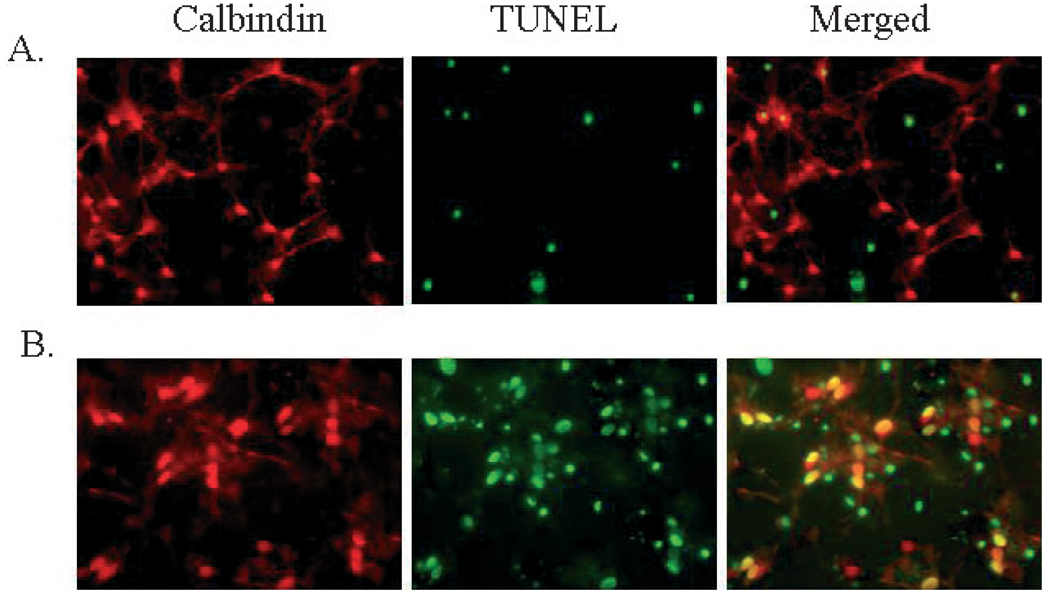
Fig. 2.
Exposure of cultured cerebellar PCs to 2 h OGD resulted in significant cell death as indicated by TUNEL staining. (A) Representative images of control cultures double-stained with anticalbindin (red) to identify PCs and TUNEL (green) to indicate DNA damage. (B) Representative images of the significant damage observed in PCs following 2 h OGD, as indicated by the high number of yellow (merged) cells.
Immunocytochemistry
At 11–14 days in vitro (DIV) the growth medium was removed from coverslips and cerebellar neurons were washed with phosphate-buffered saline (PBS) immediately prior to being incubated for 20 min in freshly prepared 4% paraformaldehyde (Sigma-Aldrich, St Louis, MO, USA). Cells were then washed with PBS and incubated in asolution of PBS containing 0.2% Triton X-100 for 1 h in order to permeabilize the cells. Following three 10-min washes with PBS, cells were incubated for 1 h in blocking solution containing 5% goat serum, then incubated with a 1 : 1000 dilution of anticalbindin antibody (diluted in blocking solution) for 1 h at room temperature (Sigma monoclonal antibody C28K). Following four 10-min washes with PBS, cells were incubated with a 1 : 500 dilution of Cy3-conjugated goat antimouse IgG secondary antibody (Jackson Immuno, West Grove, PA, USA) for 1 h at room temperature before being washed and stained for terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick-end labelling (TUNEL; see below) according to the manufacturer’s instructions (Roche, Basel, Switzerland), and finally mounted on slides with Prolong antifade reagent (Molecular Probes, Eugene, OR, USA). Fluorescence imaging was performed on an upright Leica DMIRE2 microscope using Open Laboratory software (Improvision, Lexington, MA, USA) to capture and analyse images.
OGD and cell viability
OGD was induced by replacing the sex-steroid-free culture media with glucose-free deoxygenated saline (described below) solution and placing the cultures in an anaerobic incubator containing 5% CO2 and 95% N2 at 37 °C (Coy Laboratories Products, Grass Lake, MI, USA; contains catalyst ensuring oxygen levels < 1 p.p.m.) for 60–120 min and then returned to normoxia and normal culture media. We used TUNEL reactions to identify cells with fragmented DNA (Roche, Basel, Switzerland). It is important to note that the TUNEL assay does not distinguish among cell death mechanisms (necrosis or apoptosis); however, it is useful for detecting damaged cells with light or fluorescence microscopy at an early time point. Cell viability was assessed by visual inspection of damaged PCs stained with TUNEL and double-labelled with anti-C28K antibody to evaluate PC damage. Data are presented as percentages of TUNEL-positive PCs (TUNEL- and C28K-positive cells / total C28K-positive cells) on two coverslips from the same culture. The experimenter counted > 75 cells per experiment and was blinded to the exposure condition of each slide.
Electrophysiology
After 12–14 DIV, cultures were transferred to a recording chamber mounted on the stage of an inverted microscope equipped with a harmonic component system (Leica DM IL, Houston, TX, USA). PCs were selected by their unique morphology, large soma and prominent dendritic arbor. Whole-cell voltage-clamp experiments were made from the somas of PCs using an Axopatch 200B amplifier (Axon Instruments, Union City, CA, USA) interfaced to a Dell computer (Dell, Round Rock, TX, USA). Data were collected at a sample frequency of 20 kHz and analysed using pCLAMP9 software (Axon Instruments). Electrodes pulled from borosilicate glass capillaries, with inner filament, had resistances of 1–3 MΩ when filled with the internal solution (described below). Whole-cell capacitance and series resistance (60–80%) were electronically compensated. Spontaneous event detection was performed using a threshold crossing routine on 3-min sections of data. The total number of events observed in each 3-min section of data was used to calculate frequency. Twenty-five inhibitory postsynaptic currents (IPSCs) or excitatory postsynaptic currents (EPSCs) from each cell were randomly chosen and fitted with a sum of two or three exponentials to determine the average rise and decay time constants.
Solutions and drugs
The composition of the bathing saline solution was (in mm): NaCl, 140; KCl, 5; MgCl2, 0.8; CaCl2, 1; HEPES, 10; and glucose, 10; pH 7.35 with NaOH. PCs were recorded using an internal pipette solution consisting of (in mm): CsCl, 140; EGTA, 1; HEPES, 10; MgCl2, 1; MgATP, 5; pH 7.3 with CsOH. CNQX and picrotoxin were obtained from Tocris (Ellisville, MO, USA). Allopregnanolone and finasteride were obtained from Sigma-Aldrich (St Louis, MO, USA) and progesterone was obtained from American Pharmaceutical Partners (Schaumberg, IL, USA). CNQX was dissolved in DMSO at a stock concentration of 50 mm. Picrotoxin was dissolved in EtOH at a stock concentration of 100 mm. Allopregnanolone was dissolved in DMSO at a stock concentration of 10 mm. Finasteride was dissolved in DMSO at a stock concentration of 10 mm.
Data analysis and statistics
Analysis of cell viability following OGD and treatment were performed blind; the identity of the treatment group was concealed and coded by a separate investigator. Each n represents a separate experiment performed on cultured cells obtained from a different pregnant rat. All data are presented as mean ± SEM. Statistical significance was determined with Student’s t-test.
Results
Cultured PCs
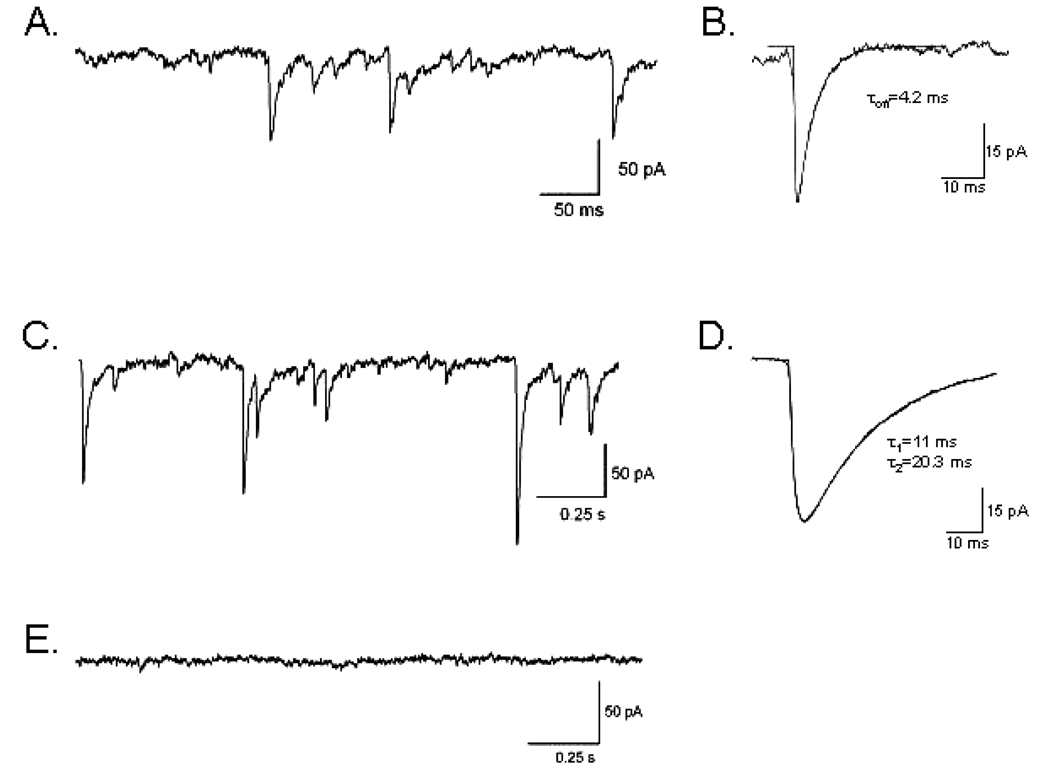
Whole-cell voltage-clamp recordings obtained from cerebellar PCs contained inward spontaneous synaptic events corresponding to a mixture of inhibitory (GABAergic) and excitatory (glutamatergic) synaptic currents, in agreement with previous reports (Gruol, 1983; Hirano et al., 1986; Hirano & Kasono, 1993). The application of the GABAA receptor antagonist picrotoxin (100 µm) eliminated GABA-mediated currents and isolated glutamatergic spontaneous excitatory postsynaptic currents (sEPSCs). The complete inhibition of synaptic currents by the additional presence of CNQX confirmed their identity as AMPA receptor-mediated EPSCs (Fig. 1). A high degree of variability in the frequency and temporal pattern of sEPSCs was observed. In four out of six cells bursts of high-frequency sEPSCs (> 20 Hz) with lower frequency activity between bursts (6–12 Hz) were observed. Isolated sEPSCs were analysed to determine amplitude and kinetics. Spontaneous EPSCs had a mean amplitude of 27.6 ± 7.0 pA and exhibited rapid decay kinetics (τoff = 5.1 ± 1.2 ms). Application of CNQX (5 lm) eliminated AMPA-mediated currents and isolated GABAergic spontaneous inhibitory postsynaptic currents (sIPSCs). In contrast to sEPSCs, sIPSCs were more regular in their temporal pattern. The average frequency of sIPSCs was 6.4 ± 1.5 Hz (n = 4), with a mean amplitude of 74.3 ± 11.5 pA (n = 4). Consistent with sIPSCs recorded from PCs in cerebellar brain slices (Konnerth et al., 1990; Southan & Robertson, 1998), the sIPSCs recorded from cultured PCs had a decay that was best fitted with a double exponential (τ1 = 4.8 ± 2.1 ms and τ2 = 17.5 ± 2.4 ms). These data clearly demonstrate the presence of intact excitatory and inhibitory synapses onto cultured PCs, making this a good model for studying cerebellar responses to OGD in the context of an intact synaptic network.
Fig. 1.
Cultured cerebellar PCs generate intact inhibitory and excitatory synapses. (A) Representative trace of sEPSCs recorded in the presence of 100 µm picrotoxin. (B) Average of multiple events fitted to determine rise and decay time constants. (C) Representative trace of spontaneous inhibitory postsynaptic currents recorded in the presence of 5 µm CNQX. Note different timescale from trace A. (D) Average of multiple events fitted to determine rise and decay time constants. (E) Trace recorded in the presence of both picrotoxin and CNQX.
PC sensitivity to ischemia and reperfusion
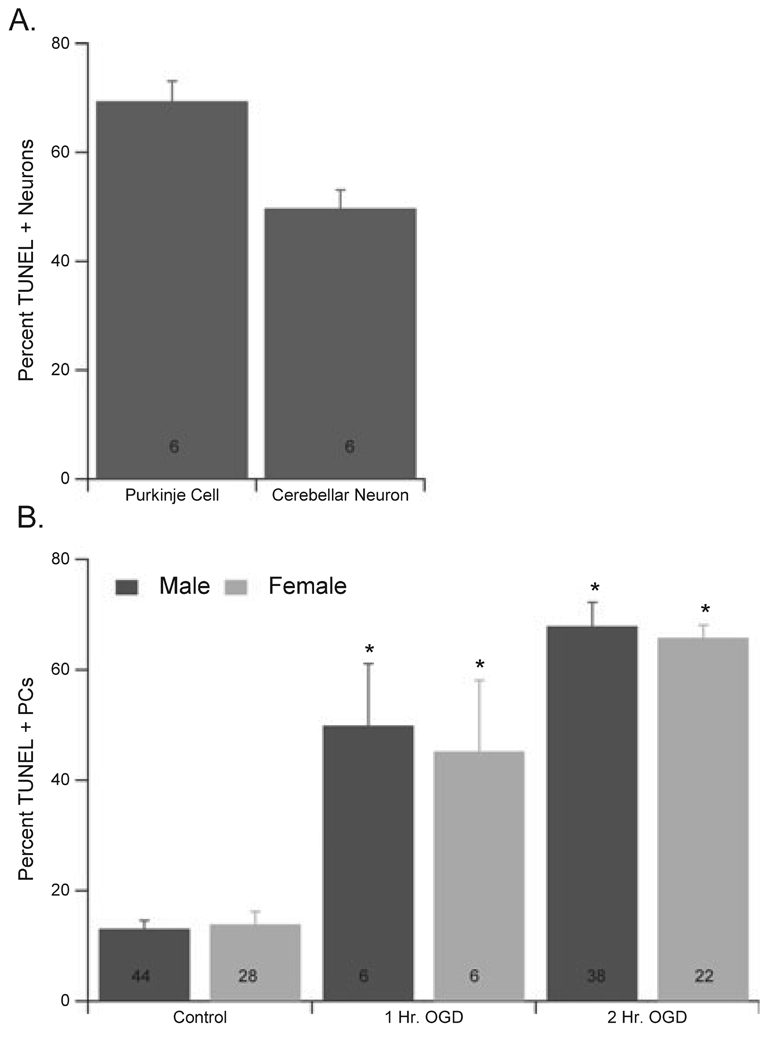
Experiments were performed using cerebellar cultures to assess the differential sensitivity of PCs to OGD. TUNEL reactions were used to identify cells with fragmented DNA. Cultures were treated with OGD for 2 h and cell viability was assessed 3 h after returning to normoxia (3 h reoxygenation). PCs were labelled with anticalbindin antibody (C28K; Fig. 2, Red) and damage assessed by fluorescent TUNEL staining to identify cells with DNA damage (Fig. 2, Green), with damaged cells appearing yellow in the merged image (Fig. 2, Merge). PCs were damaged to a significantly greater extent following OGD (69.4 ± 3.7% TUNEL-positive; n = 6) than non-PCs (49.7 ± 3.4%; n = 6; P < 0.05) in culture (Fig. 3a). Therefore, this study has focused on the response of PCs to OGD.
Fig. 3.
Male and female PCs were equally sensitive to OGD. (A) Quantification of the differential sensitivity of cerebellar neurons exposed to 2 h of OGD, indicating that PCs are significantly more sensitive to OGD than non-PC cerebellar neurons. (B) Quantification of damage observed in male and female PCs exposed to 1 or 2 h of OGD. The percentage of TUNEL-positive PCs was assessed following 3 h reoxygenation. Numbers in each bar represent number of experiments performed, each on a different culture. Data are mean ± SEM. *P < 0.05 vs. vehicle-treated cells (2 h OGD).
Experiments were performed on cultures from male and female embryos grown in the absence of background sex steroids in order to assess the effect of genetic sex on sensitivity of PCs to OGD. No sex difference in baseline sensitivity to OGD was observed (Fig. 3b). Significant PC damage was observed in both male cultures (49.9 ± 11.2% TUNEL-positive PCs, compared to 14.1 ± 2.3% control; n = 6) and female cultures (45.2 ± 12.9% TUNEL-positive PCs, compared to 17.1 ± 6.0% control; n = 6) following 1 h OGD. Similarly, 2 h OGD caused significant and more consistent levels of damage in both male (69.5 ± 2.3%; n = 38; P < 0.05) and female (64.8 ± 2.3%; n = 22; P < 0.05) cultures (Fig. 3), and therefore all further experiments were performed using 2 h OGD.
Progesterone neuroprotection of PCs
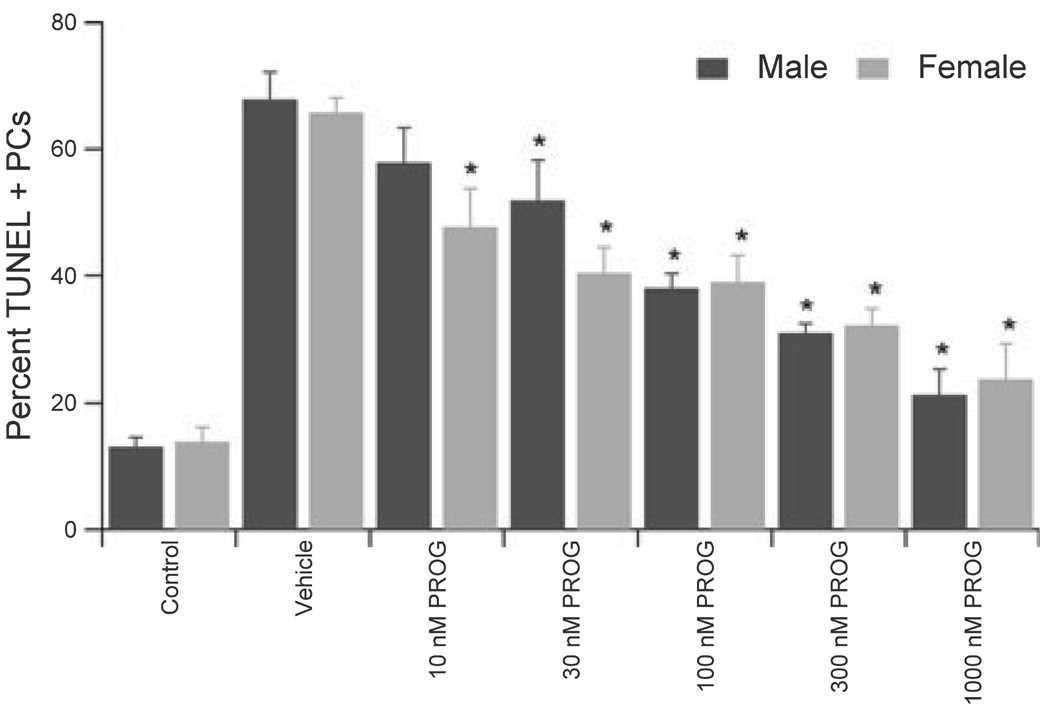
Progesterone has recently been demonstrated to be protective in animal models of cerebral ischemia (for review see Stein & Hoffman, 2003); however, little is known of the neuroprotective potential of progesterone in the cerebellum. The application of a pharmacological dose of progesterone (1 µm) 15 min prior to OGD, and maintained throughout OGD and reoxygenation, resulted in significant protection of PCs from OGD, independent of sex, decreasing damage from 58.4 ± 6.8% to 21.3 ± 4.1% (n = 7; P < 0.05) in male cultures and from 61.9 ± 4.9% to 23.8 ± 5.6% (n = 5; P < 0.05) in female cultures. Furthermore, there was a dose-dependent protection of PCs by progesterone (Fig. 4), with significant protection observed at all concentrations tested in female cultures and significant protection observed at concentrations ≥ 30 nm in male cells.
Fig. 4.
Progesterone protected PCs in a concentration-dependent manner. Male and female PCs were exposed to the various concentrations of progesterone for 15 min prior to OGD and maintained throughout reoxygenation. Data are mean ± SEM. *P < 0.05 vs. vehicle-treated cells (2 h OGD) from the same sex.
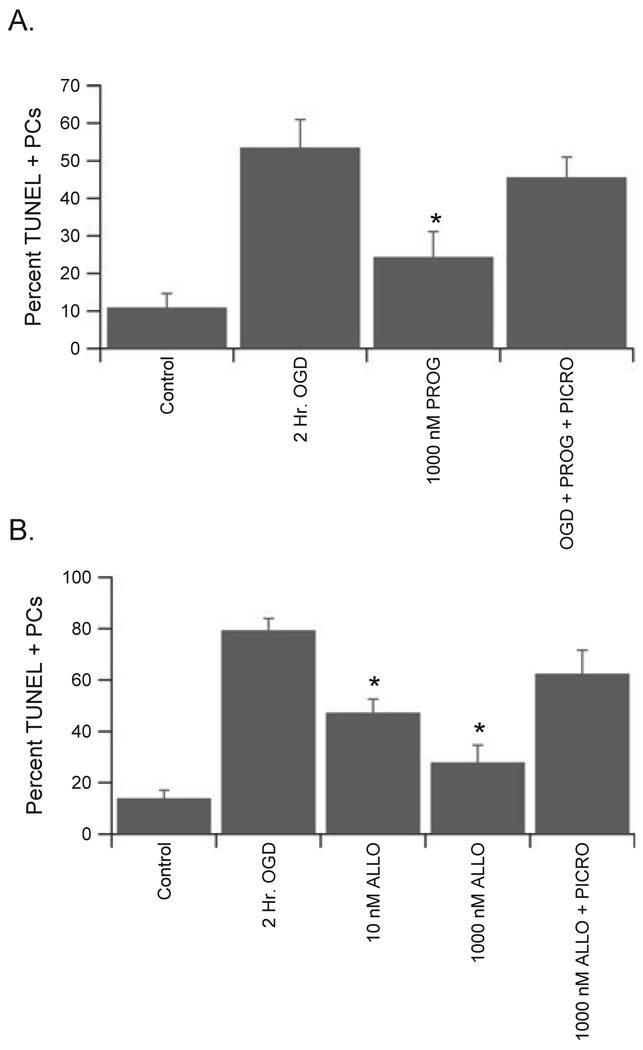
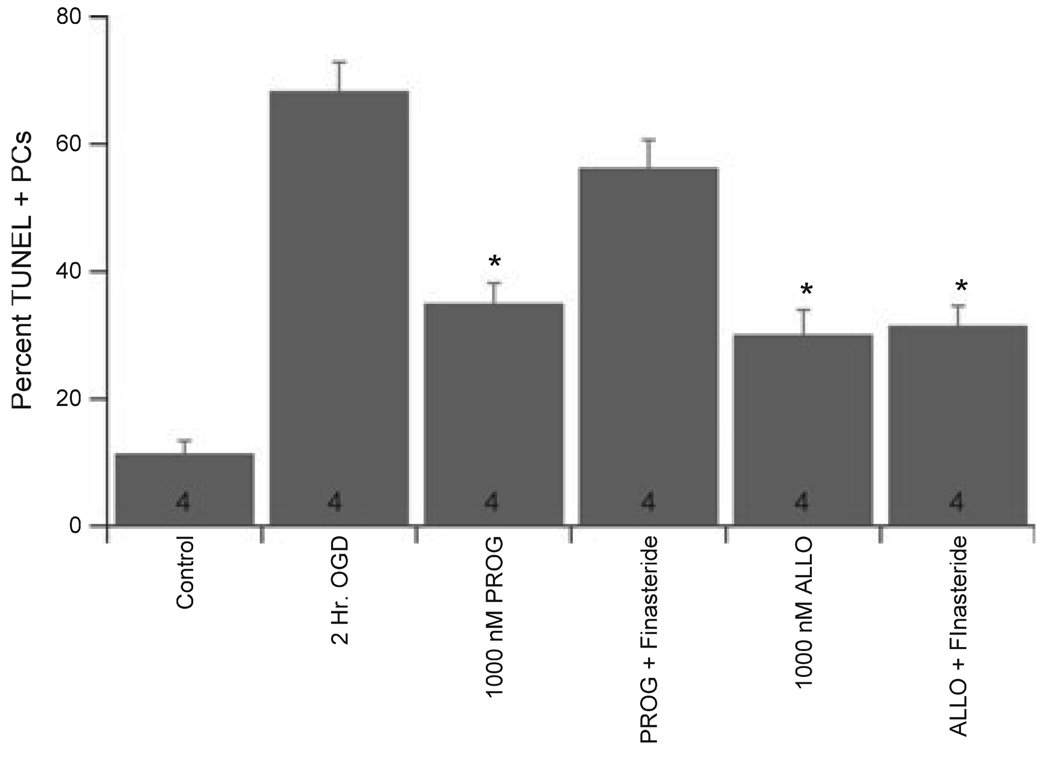
In order to test the hypothesis that progesterone is neuroprotective through interaction with GABAA receptors, the GABAA receptor antagonist picrotoxin was applied to cerebellar cultures. The additional presence of 100 µm picrotoxin (a concentration known to completely inhibit GABAA receptor activity in these cells; see Fig. 1) prevented the protection afforded by progesterone to male PCs, increasing the damage from 24.4 ± 6.8% in progesterone alone (1 µm) to 45.6 ± 5.4% in progesterone + picrotoxin (Fig. 5; n = 4; P < 0.05). Picrotoxin did not effect PC survival on its own (data not shown). Similar data were obtained in female cultures (data not shown). Progesterone does not directly modulate the activity of GABAA receptors, while the neuroactive metabolite ALLO is among the most potent positive modulators of GABAA receptor activity. Therefore, the ability of ALLO to protect PCs from OGD-induced damage was tested. Similar to progesterone, ALLO was protective at concentrations as low as 10 nm in both male and female cultures (Fig. 5) and picrotoxin reversed the protection afforded by high levels of ALLO (1 µm), increasing the damage from 27.9 ± 6.7% to 62.5 ± 9.2% in male cultures (Fig. 5; n = 4; P < 0.05). Finally, the 5α-reductase inhibitor finasteride was used to determine whether progesterone protection of PCs requires its conversion to an active metabolite. In agreement with this hypothesis, coapplication of 10 µm finasteride reversed the protection afforded by pharmacological doses (1 µm) of progesterone (Fig. 6). Importantly, finasteride had no effect on the protection provided by ALLO (Fig. 6).
Fig. 5.
Progesterone and its metabolite ALLO protected PCs via activation of GABAA receptors. (A) Quantification of the neuroprotective effect of exposure to 1 µm progesterone (PROG) for 15 min prior to OGD and maintained throughout reoxygenation. The additional presence of 100 µm picrotoxin prevented the protection afforded by progesterone. (B) Quantification of the neuroprotective effect of exposure to 1 µm ALLO for 15 min prior to OGD and maintained throughout reoxygenation. The additional presence of 100 µm picrotoxin prevented the protection afforded by ALLO. Data are mean ± SEM. *P < 0.05 vs. vehicle-treated cells (2 h OGD).
Fig. 6.
Progesterone neuroprotection required its metabolism. Quantification of the ability of finasteride to prevent progesterone neuroprotection. PCs were preincubated with 10 µm finasteride for 15 min, then exposed to either 1 µm progesterone or ALLO for another 15 min prior to OGD and maintained throughout reperfusion. Data are mean ± SEM. *P < 0.05 vs. vehicle-treated cells (2 h OGD).
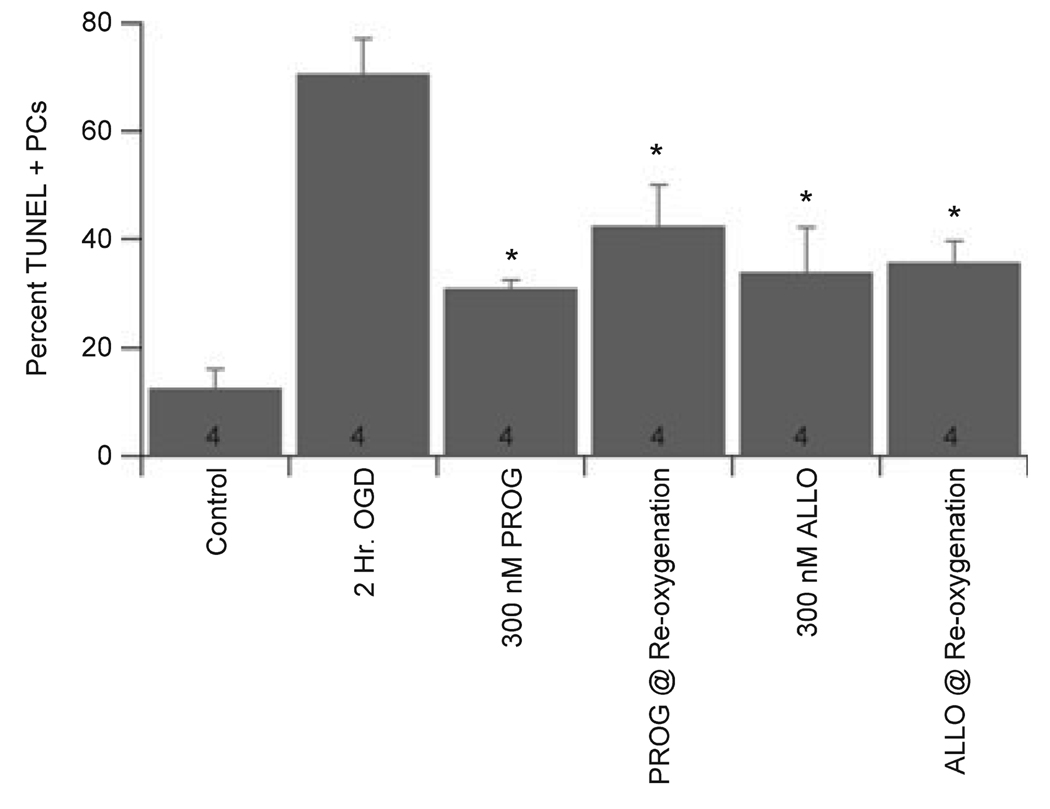
The ability of both progesterone and ALLO to protect PCs from OGD-induced damage when applied after OGD was examined. In male cultures, 300 nm progesterone and 300 nm ALLO caused significant protection when applied concurrently with reoxygenation (decreased damage from 70.6 ± 6.5% to 42.4 ± 7.6% and 35.9 ± 3.7% for progesterone and ALLO, respectively; Fig. 7; n = 4). Similar results were found for female cultures, with 300 nm progesterone decreasing damage from 69.8 ± 6.5% to 48.3 ± 10.5% (n = 4; P < 0.1) and ALLO decreasing damage from 69.8 ± 6.5% to 36.5 ± 14.6% (n = 4; P < 0.05) when applied immediately following OGD. These data indicate that progesterone and ALLO are able to protect PCs when applied following the ischemic event, suggesting a potential for therapeutic benefit.
Fig. 7.
Progesterone and ALLO effectively protected PCs when applied after OGD. Progesterone and ALLO were applied to PC cultures concurrent with reoxygenation (PROG or ALLO @reoxygenation). Data are mean ± SEM. *P < 0.05 vs. vehicle-treated cells (2 h OGD).
Discussion
In this study cerebellar cultures obtained from male and female embryos were used to examine the sensitivity of male and female PCs to OGD and to test whether progesterone protects PCs from OGD through active metabolites that increase GABAergic activity. The data indicate that PCs are more sensitive than other cerebellar neurons to experimental ischemia (OGD), but that there is no difference in the sensitivity of male and female PCs to OGD. This study also demonstrated that physiological concentrations of progesterone protect male and female PCs from OGD and that the progesterone protection was reversed by the inhibition of GABAA receptors. Further experiments revealed that progesterone neuroprotection is probably mediated through a metabolite that directly potentiates GABAA receptor activity.
The cerebellum is well suited as a model for studying cell death, because damage leads to easily recognised phenotypes such as ataxia and impaired motor coordination (Trouillas et al., 1997). As a result, a large number of cerebellar disorders have been identified in humans and mice, and all involve PC compromise (for review see Sarna & Hawkes, 2003). Because the efferent pathway from PCs to deep cerebellar nuclei is the sole output of the cerebellar cortex, PC loss results in a functional lesion of the cerebellum. The anatomy and neuronal circuitry of the cerebellum is well defined and PCs are the largest neuron in the cerebellar cortex, receiving excitatory input from granule cells and inhibitory inputs from Golgi, basket and stellate cells. In agreement with earlier findings, we demonstrated that cultured cerebellar PCs receive robust excitatory inputs from granule cells as well as inhibitory inputs from interneurons (Gruol, 1983; Hirano et al., 1986; Hirano & Kasono, 1993). This mixed cerebellar neuron culture offers important advantages over typical homogenous neuronal cultures. First, PCs are known to be hypersensitive to stress and ischemia–hypoxia, but relatively few studies have attempted to elucidate the mechanism behind their sensitivity or potential therapeutic interventions. Secondly, the presence of multiple cerebellar neuronal cell types allows for the generation of a network in vitro containing both inhibitory and excitatory neurons, enabling us to assess the effects of ischemia on the balance between excitatory and inhibitory transmission and the enhancement of endogenous inhibition as a mode of neuroprotection. Consistent with in vivo reports, this report demonstrated that cultured PCs are the most sensitive neuron to OGD in the cerebellum. To our knowledge this is the first neuronal culture model demonstrating PC sensitivity to ischemia–reperfusion.
There is emerging evidence that cellular responses to stresses causing cell death are sex-specific, independent of exposure to sex steroids (Lieb et al., 1995; Du et al., 2004; Liu et al., 2004, 2006). However, we did not observe any difference in sensitivity to OGD between male and female PCs. This may indicate that the neuronal response to such complex insults as ischemia is not sex-specific, in contrast to that observed for non-neuronal brain cells (Liu et al., 2006). Alternatively, there may be regional differences, such that primary cerebellar neurons do not respond in a sexually dimorphic manner, but neurons from other brain regions may. Despite the negative result reported here, this remains an interesting new topic that merits further investigation.
Progesterone has been demonstrated to be neuroprotective in a variety of experimental animal models of focal and global ischemia, including TBI (Stein & Hoffman, 2003), middle cerebral artery occlusion (Roof & Hall, 2000; Murphy et al., 2002; Gibson & Murphy, 2004) and cardiac arrest (Gonzalez-Vidal et al., 1998; Cervantes et al., 2002). However, the mechanism of neuroprotection remains in question. The concentration of progesterone in the brain is thought to closely follow circulating serum levels; therefore we tested the protection afforded by acute progesterone across a concentration range (10–200 nm) consistent with the levels measured in rat serum (Butcher et al., 1974; Nequin et al., 1979) as well as pharmacological doses up to 1 µm. Results in the present study indicate that concentrations of progesterone consistent with low physiological levels in the rat (10 nm) protect both male and female cerebellar PCs in culture against OGD. Progesterone is readily metabolised into compounds such as ALLO which directly interact with inhibitory GABAA ionotropic receptors (for reviews see Mellon & Vaudry, 2001; Stoffel-Wagner, 2001; Belelli & Lambert, 2005). In order to isolate the rapid nongenomic effects of progesterone, primary neuronal cultures were grown in the absence of sex steroids and progesterone was acutely applied immediately prior to exposure to OGD. Three separate experiments were performed to directly test the hypothesis that progesterone neuroprotection is mediated via an active metabolite that potentiates GABAergic neurotransmission. Firstly, inhibition of GABAA receptors with picrotoxin prevented the protection afforded by progesterone, indicating that GABAA receptor activity is required for progesterone neuroprotection. Secondly, the GABA-active progesterone metabolite ALLO protects PCs, and again neuroprotection was prevented by picrotoxin, indicating a central role for GABAA receptors in progesterone and ALLO neuroprotection. Finally, inhibition of progesterone metabolism with a 5α-reductase inhibitor (finasteride) reversed the protection afforded by progesterone while having no effect on ALLO-mediated neuroprotection. These data, taken together, indicate that the progesterone protection of cerebellar PCs is mediated through a GABA-active metabolite, probably ALLO, and that intact GABAA receptors are required for neuroprotection.
The conversion of progesterone to ALLO in the brain takes place predominantly within glial cells (Celotti et al., 1997). Glia perform a variety of functions that are critical for neuronal health, including glutamate uptake and release, water transport and hormone metabolism; they thus play a significant role in the response to ischemia (for review see Chen & Swanson, 2003). In particular, glial cells within the cerebellum appear to alter the response and sensitivity of neurons to ischemia and excitotoxicity (Beaman-Hall et al., 1998; Barakat et al., 2002; Welsh et al., 2002; Yamashita et al., 2006). Therefore, progesterone neuroprotection in vivo may involve interaction with glial cells, neurons or other cell types. The use of a pure neuronal culture in the present study demonstrates that progesterone neuroprotection in the cerebellum involves direct interaction with neurons. Importantly, cerebellar PCs are unique neurons that express the enzymes (5α-reductase and 3α-hydroxysteroid dehydrogenase) necessary to metabolise progesterone into ALLO (Tsutsui et al., 2003), allowing progesterone neuroprotection via interconversion to ALLO in the absence of supporting glial cells.
GABA is the predominant inhibitory neurotransmitter in the brain and is therefore an appealing target for counteracting the excessive excitatory stimulation observed following ischemia. While many studies have demonstrated protection with GABA modulators, it is important to note that multiple studies have failed to observe protection with similar compounds (for review see Schwartz-Bloom & Sah, 2001). Why then have so many studies failed to see a robust protection following application of GABA-active molecules? The answer is probably in the detailed analysis of regional differences. That is, networks that have large inhibitory inputs are likely to be protected by GABA potentiation, while regions comprised of predominantly excitatory inputs will not benefit from such treatment. The enormity of the excitatory input from both climbing and parallel fibers may make cerebellar PCs particularly vulnerable to excitotoxicity. In agreement with this hypothesis, the AMPA receptor antagonist NBQX partially protects PCs from ischemia in vivo (Brasko et al., 1995). Similarly, we observed that the AMPA receptor antagonist CNQX afforded robust protection against OGD in our cultured PC model (data not shown). The cerebellum is also unique in the strength of inhibitory drive exhibited on the PC. For example, in vivo recordings demonstrate that the addition of the GABA agonist muscimol completely inhibits spontaneous PC activity (Caesar et al., 2003). Therefore, the cerebellum represents an ideal brain region in which to study the ability of inhibitory synaptic activity to suppress excitotoxicity. In addition, while the cerebellum has long been known to be a region of the brain particularly vulnerable to a variety of insults, it is relatively understudied. The data presented in this study indicate that progesterone, through its neuroactive metabolite ALLO, increases GABAA receptor activity and protects cultured PCs from OGD, suggesting that GABA modulators may hold therapeutic potential, at least as it pertains to the cerebellum. Therefore, it would be of interest to test the ability of progesterone to protect cerebellar PCs in in vivo models of cerebral ischemia and neurodegeneration involving the cerebellum.
Acknowledgements
We would like to thank Dr Sufang Yang for her advice and assistance with the establishment of cerebellar cultures. This work was supported by NIH grant 1 R21 NS052591.
Abbreviations
- ALLO
3α,5α-tetrahyroprogesterone
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate
- DHP
5α-dihydroprogesterone
- E
embryonic day
- EPSC
excitatory postsynaptic current
- IPSC
inhibitory postsynaptic current
- NBQX
2,3-dioxo-6-nitro-1,2,3,4- tetrahydrobenzo[f]quinoxaline-7-sulphonamide
- OGD
oxygen and glucose deprivation
- PBS
phosphate-buffered saline
- PC
Purkinje cell
- sEPSC
spontaneous EPSC
- sIPSC
spontaneous IPSC
- TBI
traumatic brain injury
- TUNEL
terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick-end labeling
References
- Baimbridge KG, Miller JJ. Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res. 1982;245:223–229. doi: 10.1016/0006-8993(82)90804-6. [DOI] [PubMed] [Google Scholar]
- Barakat L, Wang D, Bordey A. Carrier-mediated uptake and release of taurine from Bergmann glia in rat cerebellar slices. J. Physiol. (Lond.) 2002;541:753–767. doi: 10.1113/jphysiol.2001.015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman-Hall CM, Leahy JC, Benmansour S, Vallano ML. Glia modulate NMDA-mediated signaling in primary cultures of cerebellar granule cells. J. Neurochem. 1998;71:1993–2005. doi: 10.1046/j.1471-4159.1998.71051993.x. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA (A) receptor. Nat. Rev. Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Brasko J, Rai P, Sabol MK, Patrikios P, Ross DT. The AMPA antagonist NBQX provides partial protection of rat cerebellar Purkinje cells after cardiac arrest and resuscitation. Brain Res. 1995;699:133–138. doi: 10.1016/0006-8993(95)01015-n. [DOI] [PubMed] [Google Scholar]
- Brorson JR, Manzolillo PA, Miller RJ. Ca2+ entry via AMPA / KA receptors and excitotoxicity in cultured cerebellar Purkinje cells. J. Neurosci. 1994;14:187–197. doi: 10.1523/JNEUROSCI.14-01-00187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Caesar K, Thomsen K, Lauritzen M. Dissociation of spikes, synaptic activity, and activity-dependent increments in rat cerebellar blood flow by tonic synaptic inhibition. Proc. Natl Acad. Sci. USA. 2003;100:16000–16005. doi: 10.1073/pnas.2635195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celotti F, Melcangi RC, Martini L. The 5 alpha-reductase in the brain: molecular aspects and relation to brain function. Front. Neuroendocrinol. 1992;13:163–215. [PubMed] [Google Scholar]
- Celotti F, Negri-Cesi P, Poletti A. Steroid metabolism in the mammalian brain: 5alpha-reduction and aromatization. Brain Res. Bull. 1997;44:365–375. doi: 10.1016/s0361-9230(97)00216-5. [DOI] [PubMed] [Google Scholar]
- Cervantes M, Gonzalez-Vidal MD, Ruelas R, Escobar A, Morali G. Neuroprotective effects of progesterone on damage elicited by acute global cerebral ischemia in neurons of the caudate nucleus. Arch. Med. 2002;33:6–14. doi: 10.1016/s0188-4409(01)00347-2. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Azcoitia I, Garcia-Segura LM. Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J. Neuroendocrinol. 2004;16:58–63. doi: 10.1111/j.1365-2826.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Carrero P, Frye CA, Garcia-Segura LM. Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J. Neurobiol. 2006;66:916–928. doi: 10.1002/neu.20293. [DOI] [PubMed] [Google Scholar]
- Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RS. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J. Biol. Chem. 2004;279:38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Lock EA. Cerebellum as a target for toxic substances. Toxicol. Lett. 2000;112–113:9–16. doi: 10.1016/s0378-4274(99)00246-5. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Constantin D, Prior MJ, Bath PM, Murphy SP. Progesterone suppresses the inflammatory response and nitric oxide synthase-2 expression following cerebral ischemia. Exp. Neurol. 2005;193:522–530. doi: 10.1016/j.expneurol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J. Cereb. Blood Flow Metab. 2004;24:805–813. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Vidal MD, Cervera-Gaviria M, Ruelas R, Escobar A, Morali G, Cervantes M. Progesterone: protective effects on the cat hippocampal neuronal damage due to acute global cerebral ischemia. Arch. Med. 1998;29:117–124. [PubMed] [Google Scholar]
- Gruol DL. Cultured cerebellar neurons: endogenous and exogenous components of Purkinje cell activity and membrane response to putative transmitters. Brain Res. 1983;263:223–241. doi: 10.1016/0006-8993(83)90315-3. [DOI] [PubMed] [Google Scholar]
- He J, Evans CO, Hoffman SW, Oyesiku NM, Stein DG. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp. Neurol. 2004a;189:404–412. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- He J, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor. Neurol. Neurosci. 2004b;22:19–31. [PubMed] [Google Scholar]
- Hirano T, Kasono K. Spatial distribution of excitatory and inhibitory synapses on a Purkinje cell in a rat cerebellar culture. J. Neurophysiol. 1993;70:1316–1325. doi: 10.1152/jn.1993.70.4.1316. [DOI] [PubMed] [Google Scholar]
- Hirano T, Kubo Y, Wu MM. Cerebellar granule cells in culture: monosynaptic connections with Purkinje cells and ionic currents. Proc. Natl Acad. Sci. USA. 1986;83:4957–4961. doi: 10.1073/pnas.83.13.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn M, Schlote W. Delayed neuronal death and delayed neuronal recovery in the human brain following global ischemia. Acta Neuropathol. (Berl.) 1992;85:79–87. doi: 10.1007/BF00304636. [DOI] [PubMed] [Google Scholar]
- Jiang N, Chopp M, Stein D, Feit H. Progesterone is neuroprotective after transient middle cerebral artery occlusion in male rats. Brain Res. 1996;735:101–107. doi: 10.1016/0006-8993(96)00605-1. [DOI] [PubMed] [Google Scholar]
- Konnerth A, Llano I, Armstrong CM. Synaptic currents in cerebellar Purkinje cells. Proc. Natl Acad. Sci. USA. 1990;87:2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb K, Andrae J, Reisert I, Pilgrim C. Neurotoxicity of dopamine and protective effects of the NMDA receptor antagonist AP-5 differ between male and female dopaminergic neurons. Exp. Neurol. 1995;134:222–229. doi: 10.1006/exnr.1995.1052. [DOI] [PubMed] [Google Scholar]
- Linden DJ, Dickinson MH, Smeyne M, Connor JA. A long-term depression of AMPA currents in cultured cerebellar Purkinje neurons. Neuron. 1991;7:81–89. doi: 10.1016/0896-6273(91)90076-c. [DOI] [PubMed] [Google Scholar]
- Liu M, Hurn PD, Alkayed NJ. Sex-specific modulation of astrocyte cell death by inflammatory cytokines. In: Krieglestein J, editor. Pharmacology of Cerebral Ischemia. Stuttgart, Germany: Medpharm Scientific Publishers; 2004. [Google Scholar]
- Liu M, Hurn PD, Roselli CE, Alkayed NJ. Role of P450 aromatase in sex-specific astrocytic cell death. J. Cereb. Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600331. in press [May 17; Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Lockhart EM, Warner DS, Pearlstein RD, Penning DH, Mehrabani S, Boustany RM. Allopregnanolone attenuates N-methyl-D-aspartate- induced excitotoxicity and apoptosis in the human NT2 cell line in culture. Neurosci. Lett. 2002;328:33–36. doi: 10.1016/s0304-3940(02)00448-2. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Vaudry H. Biosynthesis of neurosteroids and regulation of their synthesis. Int. Rev. Neurobiol. 2001;46:33–78. doi: 10.1016/s0074-7742(01)46058-2. [DOI] [PubMed] [Google Scholar]
- Morali G, Letechipia-Vallejo G, Lopez-Loeza E, Montes P, Hernandez-Morales L, Cervantes M. Post-ischemic administration of progesterone in rats exerts neuroprotective effects on the hippocampus. Neurosci. Lett. 2005;382:286–290. doi: 10.1016/j.neulet.2005.03.066. [DOI] [PubMed] [Google Scholar]
- Murphy SJ, Littleton-Kearney MT, Hurn PD. Progesterone administration during reperfusion, but not preischemia alone, reduces injury in ovariectomized rats. J. Cereb. Blood Flow Metab. 2002;22:1181–1188. doi: 10.1097/01.WCB.0000037990.07114.07. [DOI] [PubMed] [Google Scholar]
- Nequin LG, Alvarez J, Schwartz NB. Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biol. Reprod. 1979;20:659–670. doi: 10.1095/biolreprod20.3.659. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann. Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Heyburn JW, Stein DG. Progesterone rapidly decreases brain edema: treatment delayed up to 24 hours is still effective. Exp. Neurol. 1996;138:246–251. doi: 10.1006/exnr.1996.0063. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J. Neurotrauma. 2000;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hoffman SW, Stein DG. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol. Chem. Neuropathol. 1997;31:1–11. doi: 10.1007/BF02815156. [DOI] [PubMed] [Google Scholar]
- Sarna JR, Hawkes R. Patterned Purkinje cell death in the cerebellum. Prog. Neurobiol. 2003;70:473–507. doi: 10.1016/s0301-0082(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Sato M, Hashimoto H, Kosaka F. Histological changes of neuronal damage in vegetative dogs induced by 18 minutes of complete global brain ischemia: two-phase damage of Purkinje cells and hippocampal CA1 pyramidal cells. Acta Neuropathol. (Berl.) 1990;80:527–534. doi: 10.1007/BF00294614. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct Volume after transient middle cerebral artery occlusion. Ann. Emerg. Med. 2006;47:381–389. doi: 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Schwartz-Bloom RD, Sah R. gamma-Aminobutyric acid (A) neurotransmission and cerebral ischemia. J. Neurochem. 2001;77:353–371. doi: 10.1046/j.1471-4159.2001.00274.x. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, De Zeeuw CI, Weber JT. Don’t get too excited: mechanisms of glutamate-mediated Purkinje cell death. Prog. Brain Res. 2005;148:367–390. doi: 10.1016/S0079-6123(04)48029-7. [DOI] [PubMed] [Google Scholar]
- Southan AP, Robertson B. Modulation of inhibitory post-synaptic currents (IPSCs) in mouse cerebellar Purkinje and basket cells by snake and scorpion toxin K+ channel blockers. Br. J. Pharmacol. 1998;125:1375–1381. doi: 10.1038/sj.bjp.0702218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DG. The case for progesterone. Ann. NY Acad. Sci. 2005;1052:152–169. doi: 10.1196/annals.1347.011. [DOI] [PubMed] [Google Scholar]
- Stein DG, Hoffman SW. Estrogen and progesterone as neuroprotective agents in the treatment of acute brain injuries. Pediatr. Rehabil. 2003;6:13–22. doi: 10.1080/1363849031000095279. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B. Neurosteroid metabolism in the human brain. Eur. J. Endocrinol. 2001;145:669–679. doi: 10.1530/eje.0.1450669. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B. International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J. Neurol. Sci. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Sakamoto H, Ukena K. A novel aspect of the cerebellum: biosynthesis of neurosteroids in the Purkinje cell. Cerebellum. 2003;2:215–222. doi: 10.1080/14734220310016169. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Yuen G, Placantonakis DG, Vu TQ, Haiss F, O’Hearn E, Molliver ME, Aicher SA. Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4, and the cerebellar contribution to posthypoxic myoclonus. Adv. Neurol. 2002;89:331–359. [PubMed] [Google Scholar]
- Yamashita A, Makita K, Kuroiwa T, Tanaka K. Glutamate transporters GLAST and EAAT4 regulate postischemic Purkinje cell death: an in vivo study using a cardiac arrest model in mice lacking GLAST or EAAT4. Neurosci. Res. 2006;55:264–270. doi: 10.1016/j.neures.2006.03.007. [DOI] [PubMed] [Google Scholar]