Abstract
The goal of this series of experiments was to develop an operant choice procedure to examine rapidly the punishing effects of intravenous drugs in rats. First, the cardiovascular effects of experimenter-administered intravenous histamine, a known aversive drug, were assessed to determine a biologically active dose range. Next, rats responded on each of two levers with concurrently available fixed-ratio 1 schedules of food reinforcement. Intravenous histamine was delivered along with food when responses were made on one of the options, and the lever on which both food and histamine were contingent was switched on a regular basis. A dose of 1.0 mg/kg/inj of histamine was effective in moving responding to the alternate lever, whereas saline, 0.1, or 0.3 mg/kg/inj of histamine were not. Histamine injections produced reliable selection of the alternate lever when they were presented on the same lever for three consecutive sessions, but not when they were switched between levers on each session. In addition, histamine produced greater selection of the alternate lever when it was presented with shorter intertrial interval durations. These findings indicate that, with appropriate parameters, the aversive effects of histamine and perhaps other drugs can be established rapidly using a concurrent choice procedure.
Keywords: choice, punishment, aversive stimuli, acquisition, reversal learning, histamine, rat, lever press
A limitation of standard drug self-administration procedures is that only two outcomes are possible: drugs either function as reinforcers or they do not. If an animal does not self-administer a drug, this could be because the drug is behaviorally inactive or because the drug is aversive. Because both effects are reflected by the same pattern of little or no self-administration, the standard self-administration procedure does not readily allow one to make a distinction between inactive and aversive drug effects. The ability to characterize aversive properties of drugs is important to understand more completely how drugs impact behavior. Yet, compared with our substantial understanding of the reinforcing effects of drugs, relatively little is known about how aversive drugs impact behavior.
One approach for examining the aversive effects of drugs has been to assess the punishing effects of response-contingent intravenous drugs on steady-state operant behavior. In one procedure using squirrel monkeys, food reinforcement was available on fixed-ratio (FR) 30 schedules in two components of a multiple schedule and a drug was injected contingent on responding in one component (e.g., Katz & Goldberg, 1986; Goldberg, 1980; Goldberg & Spealman, 1983; Takada, Barrett, Allen, Cook, & Katz, 1992). For instance, Katz and Goldberg found that responding was suppressed only in the drug component when the first response following food reinforcement resulted in an injection of histamine. Katz and Goldberg found that the suppression of responding by histamine was dose dependent in a pattern similar to that found when different intensities of shock were presented.
Choice procedures have considerable potential for evaluating the aversive effects of drugs. Unlike other models used to infer aversive drug effects with response-independent drug injections (e.g., place conditioning), operant choice procedures have few interpretive problems with respect to understanding the relation between behavior and drug (see Bardo & Bevins, 2000, for a discussion). In addition, the response-decreasing effects of shock punishment have been shown to be more effective in choice procedures than when simple schedules are used. For instance, Azrin and Holz (1966) found that a response resulting in both food and shock decreased much faster and more completely when an alternative “nonpunished” response was available (see also Herman & Azrin, 1964; Holz, Azrin, & Ayllon, 1963; Karsh, 1971; Rachlin, 1967). Woolverton (2003) developed a choice procedure to study the aversive effects of drugs on steady-state behavior. Responding was maintained on two concurrently available variable-ratio (VR) 10 schedules of food reinforcement in rhesus monkeys; histamine injections were introduced contingent on left or right lever responses across conditions. Woolverton found relatively less responding on the lever producing food plus histamine across a range of histamine doses (0.0015 – 0.006 mg/kg/inj). This suggests that the aversive effects of intravenous drugs can be examined through their selective punishing effects on operant choice behavior.
The overarching goal of the present series of experiments was to develop an operant choice procedure with rats that could rapidly assess the aversive effects of drugs. In the present series of experiments, rats with intravenous indwelling catheters responded on two concurrently available FR 1 schedules of food reinforcement. Responding on one lever also produced an intravenous injection of histamine. Histamine is an endogenous neurotransmitter involved in itch and inflammation (see Hough, 2001) and has punishing effects as described above (e.g., Katz & Goldberg, 1986). As in Woolverton (2003), a punishing effect of histamine was indicated if fewer responses occurred on the lever producing both food and histamine than on the lever producing food alone. In addition, the nonhistamine lever was used to indicate whether the direct effects of histamine suppressed behavior in general or functioned primarily as a punisher (i.e., were selective to the histamine-contingent lever).
EXPERIMENT 1
The range of active histamine doses in rats has not been thoroughly evaluated. In restrained squirrel monkeys, Goldberg (1980) found that intravenous histamine injections increased heart rate and decreased blood pressure at doses that produced punishing effects (0.03 – 0.3 mg/kg/injection). We therefore assumed that doses producing strong cardiovascular effects would also produce punishing effects and initially determined a range of histamine doses that produced cardiovascular effects in rats.
Method
Subjects
Ten male Sprague-Dawley rats obtained from Harlan (Indianapolis, IN), weighing approximately 300 g before experimentation, were maintained in a temperature- and humidity-controlled environment on a 12-h light/dark cycle with lights on at 7:00 a.m. Standard laboratory chow and water were provided freely throughout the experiment. All studies were carried out in accordance with the Guide for Care and Use of Laboratory Animals as adopted by the National Institutes of Health. University of Michigan's Committee on the Use and Care of Animals approved all experimental protocols.
Surgery: cardiovascular study
Four rats were anesthetized with ketamine (100 mg/kg i.m.) and xylazine (10 mg/kg i.m.). The femoral vein and artery were exposed with a longitudinal incision. A catheter of Micro-Renathane tubing (Braintree Scientific, Inc., Braintree, MA) was inserted into the vein and the pressure sensor tip of the telemetry device (Data Sciences International, St. Paul, MN) was inserted into the artery. The telemetry device was placed subcutaneously by the hind leg. The distal end of the intravenous catheter was run subcutaneously and exited through the back, where it was secured. The distal end of the catheter was covered with a metal obturator when not in use. Rats were given a week to recover from surgery, during which time catheters were flushed daily with saline.
Surgery: behavioral study
Six rats were surgically implanted with chronic indwelling catheters. Rats were anesthetized with ketamine (100 mg/kg i.m.) and xylazine (10 mg/kg i.m.) before a longitudinal incision was made to expose the femoral vein into which a catheter made of Micro-Renathane tubing was inserted. This tubing was connected to a metal cannula attached to a mesh backplate that was secured subcutaneously. The metal cannula exited the skin and connected to a plastic cap. Catheters were flushed daily with saline to maintain patency.
Apparatus
Cardiovascular equipment
Implantable telemetry devices (PA-C40) and receivers (RPC-1) were used for recording heart rate and blood pressure (Data Sciences International, St. Paul, MN). Data recording was conducted with DataQuestART (Data Sciences International, St. Paul, MN).
Operant chamber
Six Med Associates® (St. Albans, VT, USA) operant conditioning chambers were used. Each chamber was approximately 30 cm long, 24 cm wide, and 21 cm high, and housed in a sound-attenuating cubicle. The front panel of each chamber was equipped with two retractable response levers 6.8 cm above the grid floor and 1.3 cm from the side walls with a horizontal array of red, yellow, and green LEDs above each lever. A 28-V DC houselight was located at the top center of the opposite panel. Between the two levers was a pellet receptacle with its bottom edge 2 cm above a grid floor in which 45-mg sucrose pellets (Bio-Serv, Frenchtown, NJ) could be presented. Injections were delivered silently through i.v. catheters attached to a tether joined by a swivel to a syringe pump (MED Associates, St. Albans, VT) located outside of the sound attenuating cubicle. A chamber ventilation fan masked extraneous noise. Therefore, it is unlikely the rats could hear injections. Injection duration was based on weight (g) of each rat divided by drug-delivery pump flow rate (0.0722 ml per s), yielding an injection of 4.16 s for a 300-g rat. Swivels were held in place by a counterbalanced arm (MED Associates). Control of experimental events and data recording were conducted with Med Associates interfacing and programming.
Cardiovascular Procedure
Rats were placed in a covered Plexiglas® cage (28 cm long × 19 cm wide × 20 cm high) with corncob bedding. The distal end of the catheter, which exited through the rat's back, was connected to a tether that exited from the top of the cage. The end of the tether was connected to a 1.0 ml syringe used to inject drugs. For experimental sessions, telemetry devices were activated and cages were placed on top of the receivers; recording began when the cage was placed on the receiver. Approximately 30 min after data recording began, a saline injection (0.8 ml, i.v.) was delivered. After approximately 20 min, histamine injections were delivered in the following order: 0.01, 0.03, 0.1, 0.3, 1.0, 0.1 mg/kg/inj. Each histamine injection was immediately followed by a saline flush (0.5 ml). Histamine injections were separated by at least 20 min. Injections were 1 ml per kg injected across 5 s. Data was sampled every 10 s and collection lasted approximately 5 hr.
Behavioral Procedure
Preliminary training
Rats were provided with a dish of sucrose pellets in their home cage across successive days until all rats consumed the pellets (1 or 2 days). Next, rats were placed in the operant chamber with the houselight on and pellets were delivered response independently on a random-time (RT) 60-s schedule. Under all conditions, the houselight was turned off for 0.25-s during each pellet presentation. During the next two sessions, rats were trained to respond on the levers using a modified autoshaping procedure (see Brown & Jenkins, 1968; Peterson, Ackil, Frommer, & Hearst, 1972). A trial began when one lever was randomly chosen and extended into the chamber and the LEDs over the lever were illuminated. If the lever was pressed within 8 s, the lever was retracted, LEDs turned off, and a pellet was presented. If no response occurred, the lever was retracted, LEDs turned off, and a pellet was presented following 8 s from trial onset. Intertrial intervals (ITIs) were 60 s in duration (time between end of sucrose-pellet presentation and lever extension). Sessions remained in effect for 1 hr.
In the final training session, rats were presented with both levers extended and LEDs turned on above both levers. A response on either lever produced a food pellet and both levers were retracted and LEDs turned off. If no response occurred within 60 s, both levers were retracted and LEDs turned off. Trials were initiated 5 s after the levers were retracted on the previous trial (i.e., 5-s ITI). All sessions were initiated with two forced-choice trials in which one lever was randomly selected and extended into the chamber. A response produced a food pellet and the same stimuli and ITI as described. Sessions remained in effect for 1 hr or until 100 trials were completed (including the initial forced-choice trials). Rats were surgically catheterized following the final training session and given one week to recover.
Baseline condition
The baseline condition was identical to the final session of the preliminary sucrose-pellet training with an exception. Following a response on one lever, an intravenous injection accompanied the sucrose-pellet presentation in both forced-choice and standard trials. A response on the other lever produced only a sucrose pellet. Histamine injections were contingent on responses on the lever on which a majority of responses occurred during the last sucrose-training session. Condition changes typically occurred following three sessions, although occasionally four sessions were arranged when no differential responding was present across levers. Upon changes in condition, all injections were made contingent on the lever with a greater number of responses in the last session of the previous condition. For instance, if 1.0 mg/kg/inj dose of histamine decreased left-lever responding more than right-lever responding and the dose was changed to 0.3 mg/kg/inj, the new dose would be made contingent on right-lever responses.
Drugs
Histamine hydrochloride (Sigma Chemical Co., St. Louis, MO) was dissolved in 0.9% saline solution. Doses of histamine are expressed as weights of the salt.
Results
All statistical tests used an alpha set at .05.
Cardiovascular Effects
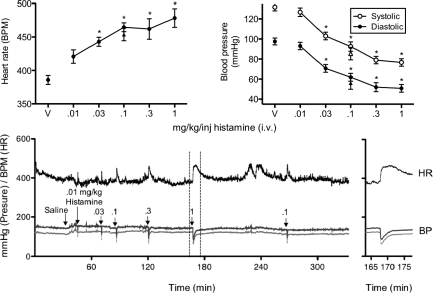
Figure 1 shows the peak effects of histamine on heart rate and blood pressure. Heart rate increased (top-left panel) while systolic and diastolic blood pressure decreased (top-right panel) with increases in histamine dose. These findings were supported by one-way repeated-measures analysis of variance (ANOVA) showing significant increases in heart rate, F(5, 30) = 16.42, and decreases in systolic, F(5, 30) = 135.50, and diastolic, F(5, 30) = 119.40, blood pressure. Bonferroni post hoc analyses show that all three cardiovascular measures differed from vehicle at all doses except 0.01 mg/kg/inj. Replication of the 0.1 mg/kg/inj histamine (triangle symbols) produced results that fell within range of the initial injection of that dose for all cardiovascular measures.
Fig 1.
The top-left panel shows mean (±SEM) peak effect of heart rate as beats per min (BPM) as a function of i.v. saline vehicle (V) and histamine dose (mg/kg/inj). The top-right panel shows mean (±SEM) peak effect of systolic and diastolic blood pressure as millimeters of mercury (mmHg) as a function of i.v. saline vehicle and histamine dose. Triangle symbols indicate replications of the 0.1 mg/kg/inj dose. * = significantly different from vehicle (p < .05). The bottom-left panel shows real-time measurement of blood pressure (BP) and heart rate (HR) with i.v. saline vehicle and different doses of histamine in a representative rat. The bottom-right panel shows the effect of the 1.0 mg/kg/inj dose of histamine in greater detail. The histamine injection occurred immediately prior to onset of effects.
The bottom panel of Figure 1 shows data from a representative rat in which blood pressure and heart rate were assessed. The size of effect on blood pressure and heart rate increased with increasing histamine dose. In addition, the duration of action also increases with larger doses. Onset of changes in these cardiovascular measures occurred approximately 5 s following all histamine doses (detailed data not shown). The x-axis for the 1.0 mg/kg/inj dose of histamine is expanded and shown in the right-bottom panel. Heart rate remained elevated for over 10 min while changes in blood pressure approached baseline levels after about 2.5 min. Given that the 1.0 mg/kg/inj dose reliably affected cardiovascular measures, the 1.0 mg/kg/inj dose was used as the starting dose in the choice procedure.
Behavioral Effects
Figure 2 shows the number of responses on the sucrose + injection and sucrose-alone levers in all 60-min sessions. Note that the graphs for Rats G2 and G3 are both in the second row. Conditions in which the injection was switched from one lever to the other are noted by adding “+Sw” to the injected dose or solution on the condition labels (e.g., saline+Sw). During training (T) with sucrose pellets on both levers, rats responded on both levers. In the next session, 1.0 mg/kg/inj histamine was introduced on the preferred lever. For all rats, responses on the sucrose + injection lever decreased relative to responses on the sucrose-alone lever and, with the exception of Rat G2, responses increased on the sucrose-alone lever. When the sucrose + injection lever was switched for Rats G2 to G5, responding on that lever was lower than on the sucrose lever by the third histamine session for all rats.
Fig 2.
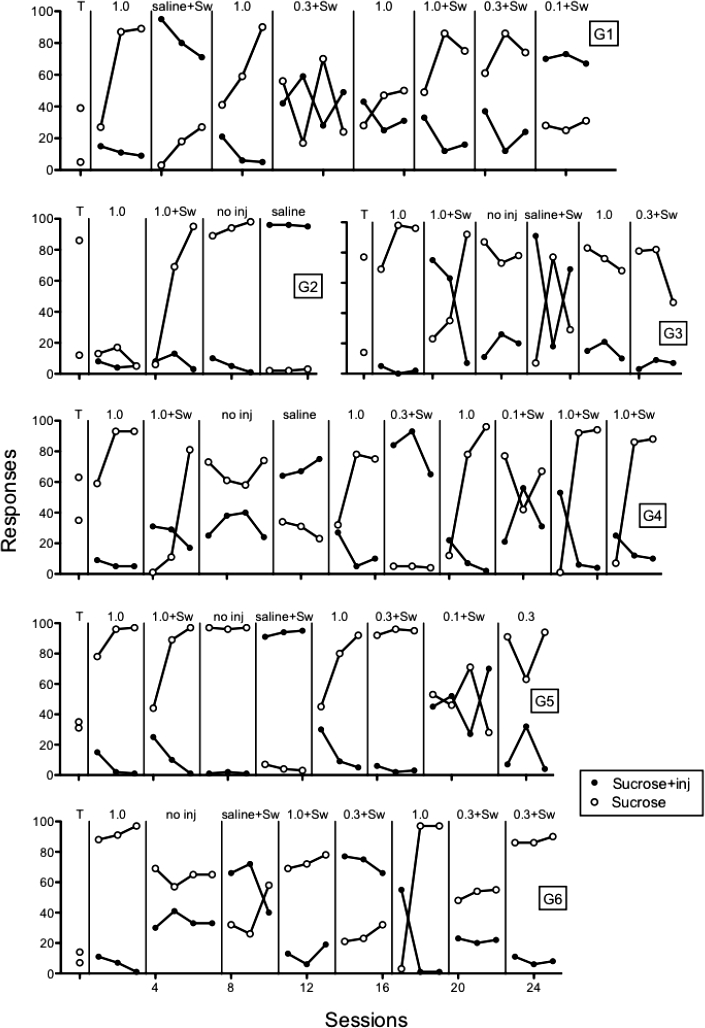
Number of responses on the sucrose + injection (inj) lever and sucrose lever across preliminary sucrose-pellet training (T) and various doses of histamine (mg/kg/inj), saline vehicle, and no injections (no inj). Individual panels show data of individual rats (G1-G6). Lever switches (when the consequences are reversed) are indicated by “Sw.”
Responding on both levers was suppressed to varying degrees across rats in the first 1.0 mg/kg/inj histamine condition for Rats G1 and G2 and following the switch in sucrose + injection lever for Rats G2, G4, and G5. Thus, responding sometimes was low on both levers and not just the lever producing intravenous injections of histamine.
In the following conditions, histamine was removed by turning off the infusion pump (no inj) and/or by injecting saline. Black data points in the No Injection conditions indicate the sucrose + injection lever in the previous condition. The pattern of responding tended to continue under both of these conditions, suggesting a conditioned punishing effect, with the exception of the saline condition for Rat G3, in which responding was not systematic across sessions. Shortly after the saline condition, Rat G2 irreparably lost its catheter. Following saline injections and at various points throughout the experiment, 1.0 mg/kg/inj of histamine was reintroduced and responding consistently decreased on the sucrose + injection lever and increased on the sucrose-alone lever.
When the dose was decreased to 0.3 mg/kg/inj for all rats, the effects were mixed. Responding on the sucrose + injection lever decreased below the sucrose lever for Rats G3 and G5. Responding on the sucrose + injection lever did not decrease below the sucrose lever in the first determinations for Rats G1, G4, or G6 but did in later determinations for Rats G1 and G6. The 0.1 mg/kg/inj dose did not systematically decrease responding for any rat tested at that dose (i.e., G1, G4, and G5). The patterns of responding with the 0.1 mg/kg/inj dose resembled those found during the Saline or No Injection conditions.
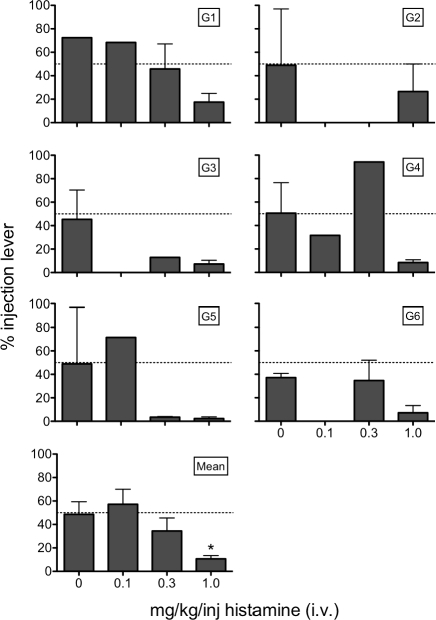
Figure 3 shows the effects of histamine dose as percent sucrose + injection-lever responses across the 6 rats in the choice procedure. Each data point is the mean of the last session of a condition for all determinations of a condition for all rats. No Injection and Saline sessions are combined under 0 mg/kg/inj on the x-axis. In general, the percentage of responding on the sucrose + injection lever decreased with increasing dose, which is supported by a significant main effect of dose, F(3, 40) = 7.17, using a repeated-measures ANOVA. Bonferroni post hoc comparisons show that the 1.0 mg/kg/inj dose is the only dose that differed significantly from the 0 mg/kg/inj.
Fig 3.
The percent responses on the sucrose + injection lever as a function of dose of i.v. histamine (mg/kg/inj). In the panel showing mean data, there are 11 values at 0 mg/kg/inj, 3 values at 0.1 mg/kg/inj, 9 values at 0.3 mg/kg/inj, and 21 values at 1.0 mg/kg/inj. * = significantly different from 0 (p < .05).
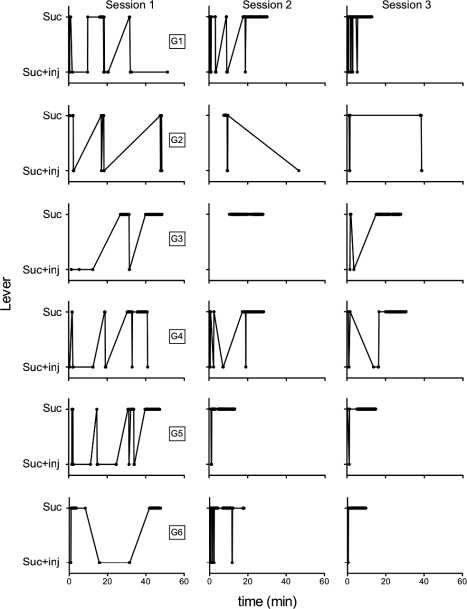
To provide a better picture of how histamine injections impacted choice responding within and across sessions, Figure 4 shows within-session responding in the three sessions of the first condition with 1.0 mg/kg/inj of histamine as a function of time in session. Successive responses on the sucrose + injection and sucrose-alone lever are connected by the line. Thus, the line aids in observing time between responses and switches between levers. Reinforcement and ITI times are included. Session duration tended to decrease from Session 1 to Sessions 2 and 3, as indicated by data terminating farther to the left along the x-axis across sessions. This finding is supported by a one-way repeated-measures ANOVA, F(2, 10) = 8.57, with Bonferroni post hoc analyses showing that Sessions 2 and 3 differed from Session 1 but not from each other.
Fig 4.
Within-session pattern of responding across the first three sessions of the first condition of 1.0 mg/kg/inj dose of histamine as a function of time (min) in the session.
Figure 4 also shows that the pattern of responding on the two levers changed across sessions. In Session 1, responses on the sucrose + injection lever were interspersed throughout the session. In Sessions 2 and 3, conversely, most sucrose + injection responses tended to occur more towards the beginning of the session (except for Rat G2). Given that session duration decreased, it is not entirely clear from Figure 4 whether the distribution of sucrose + injection responses changed or whether the shorter sessions produce this impression. To best convey this effect, the time of the last sucrose + injection and sucrose-alone responses were normalized as a function of total session time. Thus, if the last response occurred 30 min into a 60-min session, that response would be coded as 50%. The last response on both the sucrose + injection lever and the sucrose-alone lever similarly occurred toward the end of the session during Session 1. Across Sessions 2 and 3, the last response on the sucrose-alone lever continued to occur toward the end of those sessions, whereas the last response on the sucrose + injection lever tended to occur more toward the beginning of those sessions. A two-way repeated-measures ANOVA (lever x session) confirmed a significant effect of lever, F(1, 14) = 25.98, and session, F(2, 14) = 9.71, but not a lever x session interaction, F(2, 14) = 14.75. Bonferroni post hoc analyses show that lever differed significantly only during Session 3.
Another pattern apparent from Figure 4 is that latency to respond on either lever appears to be longer following responses on the sucrose + injection lever than following responses on the sucrose-alone lever. This was assessed by examining response speed (1/ latency [s]) as a function of session. Although the effect was not large, response speed tended to be faster on the sucrose-alone lever. Using a repeated-measures ANOVA (lever x session), this effect was supported by a significant main effect of lever, F(1, 14) = 10.84, but not session, F(2, 14) = 13.78, nor their interaction, F(2, 14) = 2.44. Thus, injections of histamine decreased both overall responses on that lever and increased the time to make the next response.
Discussion
The present experiment showed that, when sucrose pellets maintained rats' responding on two concurrently available responses, presenting intravenous injections of histamine contingent on one response decreased that response. Therefore, the findings from the present experiment resemble other studies with primates showing that intravenous histamine is an effective punisher of operant behavior (e.g., Katz & Goldberg, 1986; Goldberg, 1980; Goldberg & Spealman, 1983; Takada et al., 1992; Woolverton, 2003) and extend those findings to a concurrent schedule in rats. Importantly, experimental control over histamine's punishing effects was demonstrated when switching the response in which histamine injections were contingent resulted in a corresponding change in responding. In addition, control over the punishing effects of histamine could be established rapidly—within three sessions of exposure to histamine injections. Therefore, the present procedure provides a rapid method for examining the punishing effects of an intravenous drug on choice behavior by rats.
In addition to simply decreasing the number of responses on the lever producing histamine, the speed of producing another response decreased and overall responding on both levers occasionally tended to decrease as well. One possibility is that these decreases are a result of the direct pharmacological effects of histamine. However, Katz and Goldberg (1986) found longer pauses (decreased response speed) on a histamine lever even before the first response produced a histamine injection. In addition, when responses on the histamine lever were selectively suppressed, responding on the lever presenting only sucrose often occurred at maximal levels. Finally, both decreases in response speed (e.g., Dardano & Sauerbrunn, 1964) and overall responding (Bolles, Holtz, Dunn, & Hill, 1980; Karsh, 1970) have been shown with punishment by electric shock and, therefore, these effects are more likely attributable to a response-suppressing effect of punishing stimuli.
The doses required to produce punishing effects with histamine in the present experiment (i.e., 0.3 - 1.0 mg/kg/inj) were higher than those needed in previous studies with monkeys (e.g., Goldberg, 1980; Katz & Goldberg, 1986; Woolverton, 2003). It is unclear whether the differences in potency of histamine are a result of species and/or procedural differences, although choice procedures typically have been more sensitive than simple schedules for detecting punishing effects of stimuli (e.g., Azrin & Holz, 1966). Relatively small procedural differences have been shown to impact the potency of histamine on the responding of squirrel monkeys on multiple schedules. For instance, Goldberg found punishing effects of histamine doses ranging from 0.03 to 0.1 mg/kg/inj when the 11th and 22nd response after FR-30 food reinforcement resulted in histamine injections, but Katz and Goldberg found that slightly larger doses at 0.1 and 0.3 mg/kg/inj were required to achieve punishing effects when only the first response after food reinforcement resulted in a histamine injection. Perhaps further suggesting that procedure modulates the behavioral effects of histamine, the same doses (0.03 – 1.0 mg/kg/inj) produced cardiovascular effects in both the present experiment with rats and Goldberg's cardiovascular studies with squirrel monkeys. Although likely, at this point it is unclear whether rats are less sensitive to histamine than primates.
Another potentially important difference between the present procedure and those used by Goldberg and colleagues (e.g., Goldberg, 1980; Katz & Goldberg, 1986) is that, in their procedure, histamine injections were always signaled by a distinct stimulus change (i.e., light change). Perhaps the light change took on conditioned aversive properties of its own when paired with histamine and enhanced that effect (see Hake & Azrin, 1965, for related findings). In fact, Katz and Goldberg found that response rates often did not return to control levels when saline was substituted for histamine injections. Such stimuli are not necessary to observe punishing effects of low doses of histamine, however. Using a choice procedure with food and intravenous histamine or saline across two response alternatives, Woolverton (2003) found punishing effects of histamine in rhesus monkeys at even smaller doses (0.0015 – 0.006 mg/kg/inj) than Goldberg and colleagues, but without pairing histamine injections with a stimulus change distinct from saline injections.
Although the dose needed to produce a punishing effect in the present experiment was larger than those in other experiments, this might have been a result of our allowing only three sessions per condition. If responding were allowed to continue to stability, as was the case in the other experiments examining the punishing effects of histamine (e.g., Katz & Goldberg, 1986; Goldberg, 1980; Goldberg & Spealman, 1983; Takada et al., 1983; Woolverton, 2003), smaller doses might have punished responding as well. Support for this supposition lies in the fact that the 0.3 mg/kg/inj dose of histamine produced a punishing effect only in a second exposure to that dose for Rats G1 and G6. Thus, more extensive experimental experience might have increased the rats' sensitivity to the punishing effects of histamine in this procedure. The mechanism for this increased sensitivity, whether it be behavioral, pharmacological, or both, is unclear. Nonetheless, the findings from the present experiment show that the aversive effects of intravenous histamine can be examined as a punisher in an operant choice procedure in rats.
EXPERIMENT 2
Experiment 2 attempted to assess whether the punishing effects of 1.0 mg/kg of histamine could be examined even more rapidly than in Experiment 1 by switching the sucrose + injection and sucrose-alone levers across daily experimental sessions. If performance was sensitive to session-by-session changes, experimental manipulations (e.g., dose-effect curves) could be assessed very rapidly within individual subjects.
Method
Subjects and Apparatus
Six male Sprague-Dawley rats were obtained and maintained in the same manner as those used in the behavioral experiment in Experiment 1. The operant chambers also were the same as those used in Experiment 1.
Procedure
Preliminary training with sucrose pellets was the same as in Experiment 1. After training, two conditions of three sessions were arranged with 1.0 mg/kg/inj histamine. Next, the sucrose + injection lever was switched across several daily sessions. Two more conditions then were arranged with the sucrose + injection lever maintained for three consecutive sessions. Finally, for the surviving rats, daily switches of the sucrose + injection lever were arranged.
Results
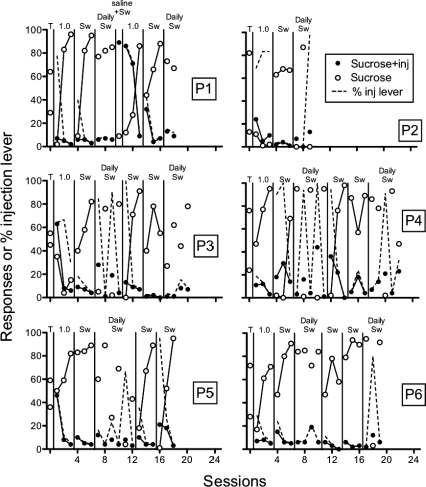
Figure 5 shows the number of responses on the sucrose + injection and sucrose-alone levers, and the percentage of responses on the sucrose + injection lever. When three sessions per condition were arranged, responding resembled patterns seen with the 1.0 mg/kg/inj dose in Experiment 1. However, performance varied across rats with daily switches. Some rats consistently responded more on the sucrose-alone lever (e.g., P1) whereas others alternated between responding more on the sucrose-alone lever and suppression on both levers (e.g., P4). Interestingly, this alternating pattern during initial daily switches could be predicted by comparing the amount of suppression in the two conditions prior to daily switches. In the final condition of daily switches following additional training with three-session conditions, performance continued to vary across rats but not necessarily in the same pattern as during initial daily switches (e.g., P3 & P6).
Fig 5.
Number of responses on the sucrose + injection (inj) lever, sucrose lever, and percent responses on the injection lever across preliminary sucrose-pellet training (T), three-session conditions, and conditions with daily switching of the sucrose + injection lever. Individual panels show data of individual rats (P1-P6). Lever switches are indicated by “Sw.”
Discussion
As in Experiment 1, 1.0 mg/kg/inj histamine punished responding when examined across three-session conditions. However, performance varied under daily lever switches and showed no clear signs of improvement either with additional daily lever switches or following additional three-session conditions. Relative response rates in operant choice situations have been shown to be sensitive to daily or within-session changes in both reinforcement (Davison & Baum, 2000; Mazur, 1992; Mazur & Ratti, 1991) and punishment contingencies (e.g., Karsh, 1970). It is possible that rats would begin to switch reliably given more training with daily switches. The goal of the present experiment, however, was not to determine the amount of training needed to reach such performance but simply to determine whether daily switches with drug punishment could be used as a tool for more rapid assessment of drug effects. At least with these specific procedures, the present experiment suggests they cannot.
EXPERIMENT 3
Experiments 1 and 2 showed that intravenous injections of 1.0 mg/kg of histamine function as a reliable punisher of operant choice behavior in rats and that multiple sessions are important for the punishment effect to be demonstrated reliably. The goal of Experiment 3 was to assess how the punishing effects of the 1.0 mg/kg/inj of histamine can be modified by changes to the temporal context. One such manipulation that has been shown to impact behavior in a variety of Pavlovian and operant procedures is changing the time between trials (i.e., massed vs. spaced trials). Generally speaking, longer ITIs tend to increase or enhance performance in both Pavlovian (e.g., Gibbon, Baldock, Locurto, Gold, & Terrace, 1977) and operant tasks (e.g., Grant, 1975). Across conditions of the present experiment, the duration of the ITI varied from 6 s to 120 s.
Method
Subjects and Apparatus
Five male Sprague-Dawley rats were obtained and maintained in the same manner as those used in the behavioral experiments in Experiments 1 and 2. The operant chambers also were the same as those used in Experiments 1 and 2.
Procedure
The training and baseline procedures were identical to those used in Experiments 1 and 2, except for differences in ITI duration during preliminary training with concurrent FR 1 schedules of sucrose-pellet delivery and following the introduction of 1.0 mg/kg/inj of histamine. For Rats B1, B2, and B3, the ITI duration was 120 s with a maximum session time of 60 min. Thus, rats could complete a total of approximately 30 trials (including forced-choice trials) in a session. For Rats B4 and B5, the ITI duration was 30 s with a maximum session time of 60 min. If sufficiently high rates of trial completion occurred, 100 trials (including forced-choice trials) could be completed in a session. Following 3-4 sessions per condition, ITI durations were changed across conditions with or without a switch in the sucrose + injection lever. The ITI values investigated were 6 s, 30 s, 60 s, 90 s, and 120 s. All ITI values were timed from lever press and the retraction of the levers to the beginning of the next trial (in which the levers were reintroduced into the chamber).
Results
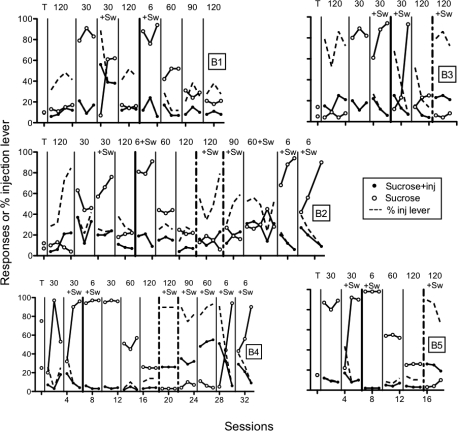
Figure 6 shows individual rats' responding in the choice procedure as number of sucrose + injection responses, sucrose-alone responses, and percent histamine lever responding. For rats beginning with a 120-s ITI (Rats B1, B2, and B3), responding was similar across the two levers for Rat B1, and higher on the sucrose + injection lever for Rats B2 and B3. Thus, with 120-s ITIs, histamine injections did not function as a punisher. With 30-s ITIs, all rats responded more on the sucrose-alone lever. Switching the histamine injection to the other lever produced a switch in responding for all rats. Returning to the 120-s ITI while keeping histamine injections contingent on the same lever resulted in no systematic effect for Rat B1 but fewer responses on the sucrose + injection lever for Rat B2.
Fig 6.
Number of responses on the sucrose + injection (inj) lever, sucrose lever, and percent responses on the injection lever across preliminary sucrose-pellet training (T) and various ITI durations. Individual panels show data of individual rats (B1-B5). Lever switches are indicated by “Sw.”
Next, the histamine lever was switched and ITI duration was changed to 6 s for all rats, as indicated by the thick, vertical condition-change line on each panel, except for Rat B3 whose ITI remained at 30 s. The ITI duration was increased across conditions to 120 s for all rats with no change in histamine lever. As can be seen, responding was reliably lower on the sucrose + injection lever at each condition. When the histamine lever switched for Rats B2, B3, B4, and B5 at the 120-s ITI, as indicated by the thick and vertical dash-dotted lines, in no case did responding reliably decrease on the sucrose + injection lever. Thus, low levels of responding on the sucrose + injection lever with increases in ITI but without lever switches in the previous conditions likely were a function of a sustained pattern of responding from when ITI duration was shorter. For Rats B2 and B4, ITI duration was decreased with a switch in histamine lever across conditions. Although responding was not lower on the sucrose + injection lever at 90-s or 60-s ITIs, responding was reliably decreased on the sucrose + injection lever when returned to the 6-s ITI.
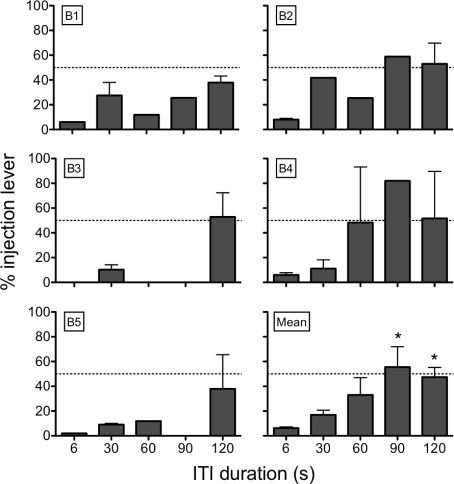
Figure 7 shows percent responding on the sucrose + injection lever as a function of ITI duration across all conditions and all rats. In general, the percentage of responding on the sucrose + injection lever increased with longer ITIs. Using a repeated-measures ANOVA, percent responding on the sucrose + injection lever was significantly increased with increasing ITI, F(4, 38) = 6.00. Bonferroni post hoc comparisons show that the 90-s and 120-s ITIs differed from the 6-s ITI. Overall, these findings suggest that shorter ITIs of 6 or 30 s resulted in a smaller proportion of responses allocated to the sucrose + injection lever than when longer ITIs of 60, 90, or 120 s were in effect.
Fig 7.
The percent responses on the sucrose + injection lever as a function ITI duration (s). In the panel showing mean data, there are 8 values at the 6-s ITI, 12 values at the 30-s ITI, 6 values at the 60-s ITI, 3 values at the 90-s ITI, and 14 values at the 120-s ITI. * = significantly different from the 6-s ITI (p < .05).
Discussion
The findings from Experiment 3 suggest that the punishing effect of histamine is attenuated with longer ITIs. However, the duration of the session was maintained at a maximum of 60 min regardless of ITI duration. Thus, when ITI duration is long, there were fewer possible trials per session. Instead of 98 trials, only 28 trials could be completed in the 60-min session. Therefore, instead of the increased responding on the histamine lever being a function of ITI duration, performance might have been a result of fewer trials.
One pattern from the present experiment suggests that ITI duration might have directly impacted performance. Although not statistically significant, there was a tendency for histamine responding to be greater during sessions with 30-s ITIs compared to 6-s ITIs. This finding is potentially meaningful because 98 trials could be completed with both these ITI durations within the 60-min session. This finding was supported by an unpaired t test on the mean number of trials per condition across all conditions and rats, t(12) = 1.261, n.s. Thus, the present findings suggest that the punishing effects of histamine decreased with longer ITIs.
EXPERIMENT 4
Experiment 4 held the number of trials per session constant with different groups of rats receiving short versus long ITIs. In addition, conditions continued until clear patterns of performance were established during conditions of both acquisition and reversal learning (i.e., lever switch). One group had a short ITI (5 s) with 28 possible trials per session; the other group had a long ITI (120 s) with 28 possible trials per session. If longer ITI durations disrupt performance, as suggested by the findings of Experiment 3, acquisition should be faster in the group with short ITIs. In addition, the effects of a change in the sucrose + injection lever were assessed following acquisition. A large body of literature suggests that conditions facilitating acquisition do not necessarily facilitate reversals in learning (e.g., Boulougouris, Castañé, & Robbins, 2009).
Method
Subjects and Apparatus
Twelve male Sprague-Dawley rats were obtained and maintained in the same manner as those used in the choice procedures of Experiments 1, 2, and 3. The operant chambers also were the same as those used in Experiments 1, 2, and 3.
Procedure
The procedures were similar to those used in Experiments 1, 2, and 3. Preliminary training was identical to that described in Experiments 1 and 2 with 5-s ITIs and 100 trials (including forced-choice trials) during the concurrent FR 1 schedule of sucrose reinforcement. The two groups of 6 rats differed when histamine was introduced on the preferred lever from the previous training session. Six rats were assigned to the Short ITI group with 5-s ITIs and 6 rats were assigned to the Long ITI group with 120-s ITIs. All sessions were capped at 60 min and both groups of rats could complete a maximum of 28 trials per session (including forced-choice trials). For both groups, the sucrose + injection lever was switched when there were at least two consecutive sessions with less than 30 percent of responses occurring on the sucrose + injection lever or 15 consecutive sessions with no systematic differences in responding across the levers.
Results
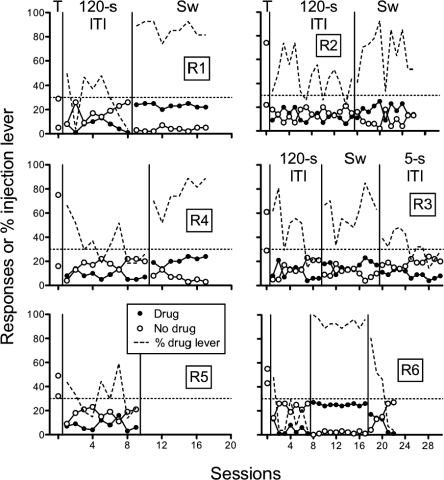
Figure 8 shows the number of sucrose + injection responses, the number of sucrose-alone responses, and the percent sucrose + injection-lever responding for individual rats in the Short ITI group. Responding increased on the sucrose-alone lever across sessions while responding on the sucrose + injection lever tended to remain low throughout. The sucrose + injection lever was switched following four (Br2) to nine (Br1) sessions across rats. Following the lever switch, a similar pattern of low responding on both levers occurred followed by increased responding on the sucrose-alone lever.
Fig 8.
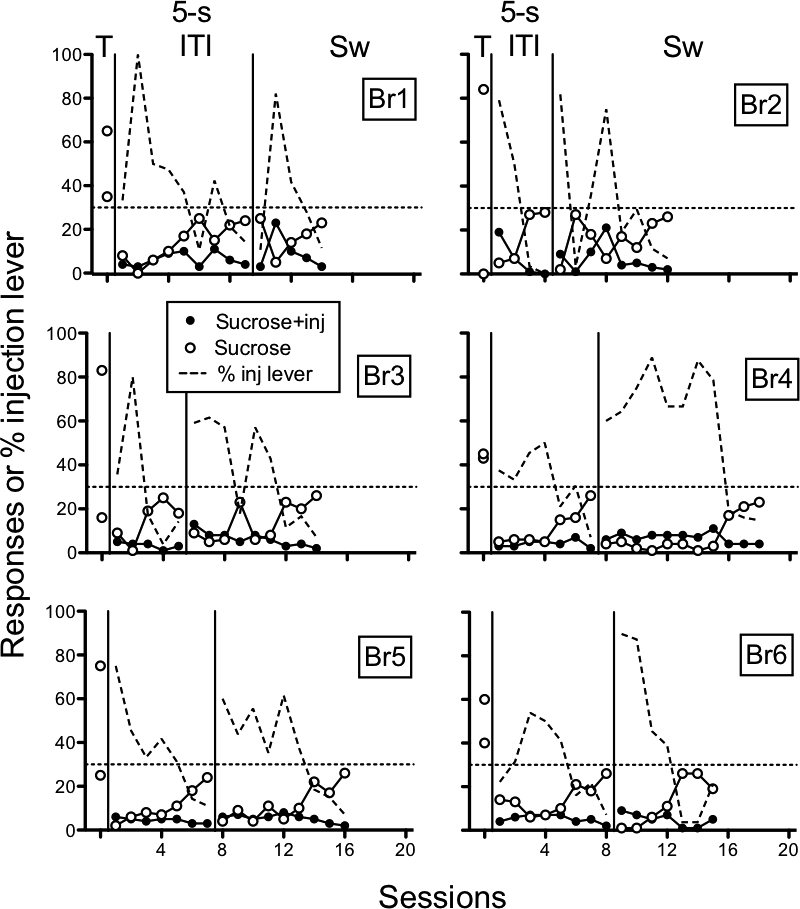
Short ITI group. Number of responses on the sucrose + injection (inj) lever, sucrose lever, and percent responses on the injection lever across preliminary sucrose-pellet training (T), acquisition, and lever switch. Individual panels show data of individual rats (Br1-Br6). Lever switches are indicated by “Sw.”
Figure 9 shows the number of sucrose + injection responses, the number of sucrose-alone responses, and the percent sucrose + injection lever responding for individual rats in the Long ITI group. As with rats in the Short ITI group, responding generally increased on the sucrose-alone lever relative to responding on the sucrose + injection lever, with the exception of Rat R2. The sucrose + injection lever was switched between 7 (R6) to 10 (R4) sessions for all rats except Rat R2. For Rat R2, responding tended to decrease across 15 sessions but not enough to meet the criteria for a lever change. Rat R5's catheter failed shortly following a lever switch. Following the lever switch in the remaining rats, responding occurred either nondifferentially (Rats R2 and R3) or tended to persist on what was the sucrose-alone lever in the previous condition (Rats R1, R4, and R6). The ITI duration was decreased to 5 s for Rats R3 and R6 and responding on the sucrose + injection lever decreased relative to the sucrose-alone lever for both rats. These findings suggest that performance improved with a decrease in ITI duration, although a confound with additional training precludes any definitive conclusions based on this condition alone.
Fig 9.
Long ITI group. Number of responses on the sucrose + injection (inj) lever, sucrose lever, and percent responses on the injection lever across preliminary sucrose-pellet training (T), acquisition, lever switch, and change in ITI duration. Individual panels show data of individual rats (R1-R6). Lever switches are indicated by “Sw.”
In both the Short and Long ITI groups, histamine functioned as a punisher of responding during initial acquisition; however, those effects differed between the groups following a reversal of the contingencies. Thus, histamine functioned as a punisher during acquisition regardless of ITI duration but only when the ITI was short following a change in sucrose + injection lever.
Discussion
Experiment 3 showed that histamine functioned as a punisher when ITIs were short (i.e., 5 s – 30 s) but not when ITIs were long (90 – 120 s). The ITI duration and trials per session, however, were confounded. Controlling for this confound, the present experiment partially supported the findings from Experiment 3. Although most rats in both the Short and Long ITI groups responded less on the sucrose + injection lever during initial acquisition, only responding by the Short ITI group switched when the sucrose + injection lever was changed. The findings from Experiment 4 suggest that the failure to acquire the punishment effect with 120-s ITIs in Experiment 3 likely was a result of too few sessions in that condition. However, failures to reverse performance when the histamine lever was switched with longer ITIs likely were at least partially a function of the longer ITI durations.
Better performance, as defined by more sucrose-alone lever responding, might be expected with short ITIs under the conditions of the present experiments. A leading explanation for poorer performance with shorter ITIs in operant conditional-discrimination procedures is proactive interference (see Grant, 1975). According to proactive interference, performance on previous trials (n - 1, n - 2, etc.) interferes with performance on the current trial (n). If a left response is reinforced on one trial, for instance, there is a tendency to choose left on subsequent trials regardless of the stimulus configurations of those trials. Given that consequences for responding on both levers were identical from trial to trial within conditions of the present experiment, previous trials influencing responding on the current trial could only improve performance on subsequent trials (see Grant, 1975, 2000; Roberts, 1980, for relevant findings). Thus, increasing “interference” from one trial to the next with shorter ITIs could only improve performance in the present experiment, consistent with the present findings.
In addition, shorter ITIs might improve performance (i.e., decrease sucrose + injection responding) by increasing accumulated histamine from one trial to the next, thereby increasing any aversive effects produced by histamine. Prior to differential responding across levers in the Short ITI group, an accumulation of the aversive effects of histamine might be indicated by the initial suppression of responding on both levers only in the Short ITI group. This effect was not readily observed in the Long ITI group. Thus, the effect of accumulated histamine initially might have decreased the number of trials per session with short ITIs but later enhanced the ability of histamine to selectively decrease responding on the sucrose + injection lever.
The effects of ITI duration on reversal learning (e.g., lever switch) have been mixed when rats and pigeons have been trained to discriminate between a response producing food and a different response producing no food. Consistent with the present findings, several researchers have found improved reversal learning with shorter ITIs (e.g., Williams, 1971). Conversely, reversal-learning performance and ITI duration also have been shown to be positively related (Sarason, Sarason, Miller, & Mahmoud, 1956) and unrelated (North, 1950a, 1950b). Williams has noted that, with some exceptions, differences in ITI duration systematically impact reversal learning when ITIs are short (between 0 – 60 s) and responses on the nonreinforced option asymptote beyond 60 s. The findings from Experiment 3 (see Figure 7) and the present experiment generally support Williams's findings.
Finally, the fact that changes in ITI duration impacted performance in the present procedure suggests that altering other procedural variables (e.g., FR value, open vs. closed economy, histamine dose, etc.) could produce different patterns of results. For instance, changing the overall magnitude or quality of reinforcement might affect sensitivity to the punishing effects of intravenous histamine (cf. Ferraro & Perkins, 1968). Another question of interest is whether longer-acting histaminergic or other aversive drugs might result in improved reversal learning with longer ITIs.
GENERAL DISCUSSION
The present series of experiments found that histamine functioned as a punisher of operant behavior in a choice situation in rats. Experiment 1 showed that 1.0 mg/kg/inj histamine was a more reliable punisher than vehicle and smaller histamine doses (0.1 and 0.3 mg/kg/inj). Experiment 2 showed that daily changes in the histamine lever were too frequent to produce reliable switching in responding, even when responding reliably changed following three-session conditions. Finally, Experiments 3 and 4 showed stronger punishing effects of histamine with shorter ITI durations.
The present findings support previous findings that intravenous histamine is an effective punisher of operant behavior in monkeys (e.g., Goldberg, 1980; Negus, 2005; Woolverton, 2003). We extended the findings of Negus and Woolverton in showing that the punishing effects of drugs can be studied in relatively standard operant choice situations in rodents. Thus, the present findings suggest that the punishing effects of intravenous drugs in rodents could be studied in any laboratory equipped with standard self-administration equipment. These findings open the way to examining the aversive effects of drugs in rodents in other situations such as in traditional free-operant concurrent variable-interval (VI) schedules of reinforcement. Studying punishment with drugs in free-operant procedures would confer the advantage of generating a large amount of behavioral data (perhaps with fewer drug injections) but this would most likely be at the expense of such a rapid assessment of drug effects. Nonetheless, the ability to study aversive stimuli, including the punishing effects of drugs in rodents, is important given the disparity between the prevalent use of aversive contingencies in natural settings (e.g., physical and verbal reprimands, timeout) and the need for studies examining basic processes governing the aversive effects of stimuli (Lerman & Vorndran, 2002).
The punishing effects of intravenous histamine in rodents have been demonstrated previously, although the procedure was not a standard operant procedure. Sharpless (1961) arranged for intravenous injections of histamine to be contingent on rats entering one goal box of a T-maze when both goal boxes were baited with food. As in the present experiments, histamine was shown to punish the response resulting in histamine injections. Unlike the present data, however, a very large dose of histamine (8.75 mg/kg/inj) was used. Perhaps more important, it is unclear the extent to which the runway paradigm reflects Pavlovian conditioned approach responses versus an operant response (see Farwell & Ayres, 1979, and Boakes, 1979, for relevant discussions). Bardo and Bevins (2000) reviewed a series of experiments in which response-independent drug injections produced disparate behavioral and neurological changes compared with when drugs were presented response dependently. Similar arguments might be extended to the use of single-goal runway procedures that have been used to assess the aversive effects of drugs, with the anxiogenic effects of intravenous cocaine in particular (see Ettenberg, 2009, for a review).
Histamine injections always were presented response dependently on one of two available levers in the present experiments. In addition to suppressing responses on the histamine-contingent lever, responding on both levers occasionally was suppressed. Bolles et al. (1980) found similar effects under a number of conditions. For instance, two response topographies produced food on a single manipulandum, but only one topography resulted in shock. When shock was introduced in the beginning of a session, both topographies were suppressed. By the end of a session, the topography that was not shocked came to predominate. Bolles et al. suggested that these different patterns early and late in sessions with shock reveal a change in learning from a stimulus–stimulus association (i.e., lever–shock) to a more refined response–stimulus (i.e., topography–shock) association. Along these lines, in the present experiments, suppressed responding on both levers reflected associations between the intelligence panel and histamine injections (stimulus–stimulus learning). Following more extended training, rats learned that histamine injections were specific to responding on one lever (response–stimulus learning).
Experiment 4 extended the findings of Bolles et al. (1980) by suggesting that learning responses resulting in punishing stimuli is dependent on previous experience and ITI duration. Differential responding across levers occurred during acquisition with long and short ITIs, but reversal learning was evident only when ITIs were short. Relevant to these findings, Karsh (1970, 1971) directly assessed reversal learning in choice situations with shock punishment in rats. When one lever produced food reinforcement and the other lever produced no consequence (extinction) in one group or extinction plus shock in another group, responding in both groups rapidly switched between those levers when the contingencies were reversed. However, in another group, food was presented on one lever and shock plus food were presented on another lever. In this group, rats repeatedly failed to respond more on the lever not producing shock when shock alternated between levers across daily sessions (Karsh, 1970) or multisession conditions (Karsh, 1971). Across reversals, responding perseverated on the original food-alone lever arranged during acquisition, regardless of whether shock currently was contingent on that lever or the other lever. The present findings and others (Negus, 2005; Woolverton, 2003) suggest that conflict situations (food + punishment) do not necessarily result in perseverative behavior. Moreover, the finding that reversal learning was found when ITI durations were short but perseverative responding was found when ITI durations were long suggests the effects reported by Karsh might be modulated by procedural variables, including ITI duration. Interestingly, Karsh used 60-s ITI durations, which according to Williams (1971) is the ITI duration at which reversal-learning performance with food reinforcement begins to degrade.
In light of the effect of ITI duration modulating the punishing effects of intravenous histamine, it is interesting to consider the relation between the cardiovascular effects and behavioral effects of histamine. Histamine likely decreased blood pressure by dilating small blood vessels causing a baroreceptor reflex that increased heart rate (see Brown & Roberts, 2001). Caution must be used when comparing cardiovascular and behavioral effects because histamine was presented response independently when assessing cardiovascular effects and response dependently when assessing behavioral effects (see Bardo & Bevins, 2000, for a related discussion of such differences in rewarding drug effects). Nonetheless, one potential relation between the effects of histamine is that the cardiovascular effects directly mediated the punishing effects.
Several points of evidence, however, suggest that cardiovascular effects do not mediate the punishing effects of histamine. First, despite successful acquisition in Experiment 4 with 120-s ITIs, those rats failed to reverse their performance when the histamine lever was switched. Presumably, the lever switch did not affect the duration and/or intensity of the cardiovascular effects. Second, the 0.1 and 0.3 mg/kg/inj dose resulted in clear cardiovascular changes but did not always produce clear punishing effects. Finally, Goldberg (1980) found that the H2-receptor antagonist cimetidine blocked cardiovascular effects of histamine but not the punishing effects. The punishing, but not cardiovascular, effects of histamine were blocked by the H1-receptor antagonist diphenhydramine. Together, these findings suggest that the punishing effects produced by intravenous histamine generally do not depend on cardiovascular changes. Unfortunately, the physiological mechanisms producing the aversive effects of histamine in Goldberg's and the present experiments are unknown. Some insight comes from studies of human participants receiving intravenous infusions of liberators of endogenous histamine (e.g., Compound 48/80; see Brown & Roberts, 2001). These human participants reported burning and itching sensations in the hands, face, scalp, and ears, along with other feelings of discomfort (e.g., colic, nausea). Similar aversive effects resulting from histamine also might occur in rats and potentially be the source of the punishing effects observed in the present experiment.
Given that cardiovascular effects likely do not mediate the punishing effects of histamine, the question remains as to what does mediate the punishing effects of histamine. Katz and Goldberg (1986) showed that a range of H1-receptor antagonists attenuated the punishing effects of histamine, but not the punishing effects of shock (cf. Bergman & Spealman, 1986). Conversely, Katz and Goldberg also showed that drugs that reduce anxiety in humans (e.g., benzodiazepines) attenuate the punishing effects of both shock and histamine (see Cook & Davidson, 1973; Kleven & Koek, 1999; Rowlett, Lelas, Tornatzky, & Licata, 2006, for relative potencies of a range of benzodiazepines in humans and laboratory animals). In addition, other researchers have shown that anxiolytics reduce the punishing effects of a range of other intravenous drugs (e.g., nicotine, Goldberg & Spealman, 1983; beta carboline, Takada et al., 1992), as well as other forms of punishment (e.g., signaled timeout, van Haaren & Anderson, 1997). Given the general finding that the effects of punishing stimuli are attenuated with anxiolytics, perhaps all punishing effects of stimuli are mediated by the emotional, anxiety-producing effects of those stimuli. The neuropsychopharmacological systems that mediate the emotional effects of punishing stimuli are likely to differ among stimuli that punish operant behavior. Regardless of the substrate that mediates the process of punishment, the findings from the present experiments suggest that those effects can be modulated by environmental context.
A future direction in this line of research is to determine whether the present procedure can be used to discriminate between drugs producing true punishing effects and drugs that are self-administered but at relatively low levels. For instance, the dose of nicotine shown to produce the highest levels of self-administration (0.03 mg/kg/inj, free base) on FR 1 schedules of reinforcement in rats maintains approximately 30 responses per 1-hr session (Donny, Caggiula, Mielke, Jacobs, Rose, & Sved, 1998). If similar levels of nicotine self-administration were to be maintained with the present choice procedure and the remaining responses allocated to the sucrose-alone lever, only approximately one third of total responses would be allocated to the drug lever. According to our definition of punishment (i.e., fewer responses on the drug lever) nicotine would have functioned as a punisher under these circumstances. Therefore, the present findings with histamine suggest this choice procedure can effectively assess the punishing effects of aversive drugs; however, its validation for assessing reinforcing drug effects remains a goal for future experiments.
Acknowledgments
The authors would like to thank Adam Kynaston, Davina Barron, Yong Gong Shi, A.J. Flores, and Jessica Priebe for their technical assistance and Gail Winger for her helpful comments on a previous version of this manuscript. CAP was supported by the National Institutes of Health under Ruth L. Kirschstein National Service Research Service Award T32 DA007268. CJ was supported by the National Institutes of Health under Ruth L. Kirschstein National Service Research Service Award T32 DA007267. The University of Michigan Substance Abuse Research Center (UMSARC) Innovative Approaches to Investigate Aspect of Drug Use and Abuse Grant (U026035) to CAP funded this study.
REFERENCES
- Azrin N.H, Holz W.C. Punishment. In: Honig W.K, editor. Operant behavior: Areas of research and application. Appleton-Century-Crofts; New York: 1966. pp. 380–447. [Google Scholar]
- Bardo M.T, Bevins R.A. Conditioned place preference: What does it add to our preclinical understanding of drug reward. Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bergman J, Spealman R.D. Some behavioral effects of Histamine H1 antagonists in squirrel monkeys. Journal of Pharmacology and Experimental Therapeutics. 1986;239:104–110. [PubMed] [Google Scholar]
- Boakes R.A. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian interactions. Lawrence Erlbaum Associates; Hillsdale, NJ: 1979. pp. 67–101. [Google Scholar]
- Bolles R.C, Holtz R, Dunn T, Hill W. Comparisons of stimulus learning and response learning in a punishment situation. Learning and Motivation. 1980;11:78–96. [Google Scholar]
- Boulougouris V, Castañé A, Robbins T.W. Dopamine D2/D3 receptor agonist quinpirole impairs spatial reversal learning in rats: Investigation of D3 receptor involvement in persistent behavior. Psychopharmacology. 2009;202:611–620. doi: 10.1007/s00213-008-1341-2. [DOI] [PubMed] [Google Scholar]
- Brown N.J, Roberts J.R. Histamine, bradykinin, and their antagonists. In: Hardman J.G, Limberd L.E, Goodman Gilman A, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics, 10th ed. McGraw-Hill; New York: 2001. pp. 645–667. [Google Scholar]
- Brown P.L, Jenkins H.M. Autoshaping of the pigeon's key peck. Journal of the Experimental Analysis of Behavior. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook L, Davidson A.B. Effects of behaviorally active drugs in a conflict-punishment procedure in rats. In: Garattini S, Mussini E, Randall L.O, editors. The benzodiazepines. Raven Press; New York: 1973. pp. 604–673. [Google Scholar]
- Dardano J.F, Sauerbrunn D. Selective punishment of concurrent progressive ratio behavior. Journal of the Experimental Analysis of Behavior. 1964;7:51–65. doi: 10.1901/jeab.1964.7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison M, Baum W.M. Choice in a variable environment: Every reinforcer counts. Journal of the Experimental Analysis of Behavior. 2000;74:1–24. doi: 10.1901/jeab.2000.74-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny E.C, Caggiula A.R, Mielke M.M, Jacobs K.S, Rose C, Sved A.F. Acquisition of nicotine self-administration in rats: The effects of dose, feeding schedule, and drug contingency. Psychopharmacology. 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. The runway model of drug self-administration. Pharmacology, Biochemistry, and Behavior. 2009;91:271–277. doi: 10.1016/j.pbb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell B.J, Ayres J.B. Stimulus–reinforcer and response–reinforcer relations in the control of conditioned appetitive headpoking (“goal tracking”) in rats. Learning and Motivation. 1979;10:295–312. [Google Scholar]
- Ferraro D.P, Perkins D. Effects of reinforcement magnitude during successive reinforcement–punishment cycles. Psychonomic Science. 1968;10:183–184. [Google Scholar]
- Gibbon J, Baldock M.D, Locurto C, Gold L, Terrace H.S. Trial and intertrial durations in autoshaping. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:264–284. [Google Scholar]
- Goldberg S.R. Histamine as a punisher in squirrel monkeys: Effects of pentobarbital, chlordiazepoxide and H1- and H2-receptor antagonists on behavior and cardiovascular responses. Journal of Pharmacology and Experimental Therapeutics. 1980;214:726–736. [PubMed] [Google Scholar]
- Goldberg S.R, Spealman R.D. Suppression of behavior by intravenous injections of nicotine or by electric shocks in squirrel monkeys: Effects of chlordiazepoxide and mecamylamine. Journal of Pharmacology and Experimental Therapeutics. 1983;224:334–340. [PubMed] [Google Scholar]
- Grant D.S. Proactive interference in pigeon short-term memory. Journal of Experimental Psychology: Animal Behavior Processes. 1975;1:207–220. [Google Scholar]
- Grant D.S. Influence of intertrial interval duration on the intertrial agreement effect in delayed matching-to-sample with pigeons. Animal Learning & Behavior. 2000;28:288–297. [Google Scholar]
- Hake D.F, Azrin N.H. Conditioned punishment. Journal of the Experimental Analysis of Behavior. 1965;8:279–293. doi: 10.1901/jeab.1965.8-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R.L, Azrin N.H. Punishment by noise in an alternative response situation. Journal of the Experimental Analysis of Behavior. 1964;7:185–188. doi: 10.1901/jeab.1964.7-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz W.C, Azrin N.H, Ayllon T. Elimination of behavior of mental patients by response-produced extinction. Journal of the Experimental Analysis of Behavior. 1963;6:407–412. doi: 10.1901/jeab.1963.6-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough L.B. Genomics meets histamine receptors: New subtypes, new receptors. Molecular Pharmacology. 2001;59:415–419. [PubMed] [Google Scholar]
- Karsh E.B. Fixation produced by conflict. Science. 1970;168:873–875. doi: 10.1126/science.168.3933.873. [DOI] [PubMed] [Google Scholar]
- Karsh E.B. Effects of conflict on choice behavior of rats. Journal of Comparative and Physiological Psychology. 1971;76:505–514. doi: 10.1037/h0031411. [DOI] [PubMed] [Google Scholar]
- Katz J.L, Goldberg S.R. Effects of H1-receptor antagonists on responding punished by histamine injection or electric shock presentation in squirrel monkeys. Psychopharmacology. 1986;90:461–467. doi: 10.1007/BF00174061. [DOI] [PubMed] [Google Scholar]
- Kleven M.S, Koek W. Effects of benzodiazepine agonists on punished responding in pigeons and their relationship with clinical doses in humans. Psychopharmacology. 1999;141:206–212. doi: 10.1007/s002130050826. [DOI] [PubMed] [Google Scholar]
- Lerman D.C, Vorndran C.M. On the status of knowledge for using punishment: Implications for treating behavior disorders. Journal of Applied Behavior Analysis. 2002;35:431–464. doi: 10.1901/jaba.2002.35-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur J.E. Choice behavior in transition: Development of preference with ratio and interval schedules. Journal of Experimental Psychology: Animal Behavior Processes. 1992;18:364–378. doi: 10.1037//0097-7403.18.4.364. [DOI] [PubMed] [Google Scholar]
- Mazur J.E, Ratti T.A. Choice behavior in transition: Development of preference in a free-operant procedure. Animal Learning & Behavior. 1991;19:241–248. [Google Scholar]
- Negus S.S. Effects of punishment on choice between cocaine and food in rhesus monkeys. Psychopharmacology. 2005;181:244–252. doi: 10.1007/s00213-005-2266-7. [DOI] [PubMed] [Google Scholar]
- North A.J. Improvement in successive discrimination reversals. Journal of Comparative and Physiological Psychology. 1950a;43:442–460. doi: 10.1037/h0061372. [DOI] [PubMed] [Google Scholar]
- North A.J. Performance under an extended series of discrimination reversals. Journal of Comparative and Physiological Psychology. 1950b;43:461–470. doi: 10.1037/h0059055. [DOI] [PubMed] [Google Scholar]
- Peterson G.B, Ackil J.E, Frommer G.P, Hearst E.S. Conditioned approach and contact behavior toward signals for food or brain-stimulation reinforcement. Science. 1972;177:1009–1011. doi: 10.1126/science.177.4053.1009. [DOI] [PubMed] [Google Scholar]
- Rachlin H. The effect of shock intensity on concurrent and single-key responding in concurrent-chain schedules. Journal of the Experimental Analysis of Behavior. 1967;10:87–93. doi: 10.1901/jeab.1967.10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W.A. Distribution of trials and intertrial retention in delayed matching to sample with pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1980;3:217–237. doi: 10.1037//0097-7403.6.3.217. [DOI] [PubMed] [Google Scholar]
- Rowlett J.K, Lelas S, Tornatzky W, Licata S.C. Anti-conflict effects of benzodiazepines in rhesus monkeys: Relationship with therapeutic doses in humans and role of GABAA receptors. Psychopharmacology. 2006;184:201–211. doi: 10.1007/s00213-005-0228-8. [DOI] [PubMed] [Google Scholar]
- Sarason I.G, Sarason B.R, Miller M, Mahmoud P. The role of the intertrial interval in discrimination and reversal learning. Journal of Comparative and Physiological Psychology. 1956;49:77–79. doi: 10.1037/h0048488. [DOI] [PubMed] [Google Scholar]
- Sharpless S.K. Effects of intravenous injections of epinephrine and norepinephrine in a choice situation. Journal of Comparative and Physiological Psychology. 1961;54:103–108. [Google Scholar]
- Takada K, Barrett J.E, Allen M.S, Cook J.M, Katz J.L. Punishment of schedule-controlled behavior with ß-carboline injections: Antagonism and comparisons with other compounds. Journal of Pharmacology and Experimental Therapeutics. 1992;261:138–145. [PubMed] [Google Scholar]
- van Haaren F, Anderson K.G. Effects of chlordiazepoxide, buspirone and cocaine on behavior suppressed by timeout presentation. Behavioral Pharmacology. 1997;8:174–182. [PubMed] [Google Scholar]
- Williams B.A. The effects of intertrial interval on discrimination reversal learning in the pigeon. Psychonomic Science. 1971;23:241–243. [Google Scholar]
- Woolverton W.L. A novel choice method for studying drugs as punishers. Pharmacology, Biochemistry, and Behavior. 2003;76:125–131. doi: 10.1016/s0091-3057(03)00219-3. [DOI] [PubMed] [Google Scholar]