Abstract
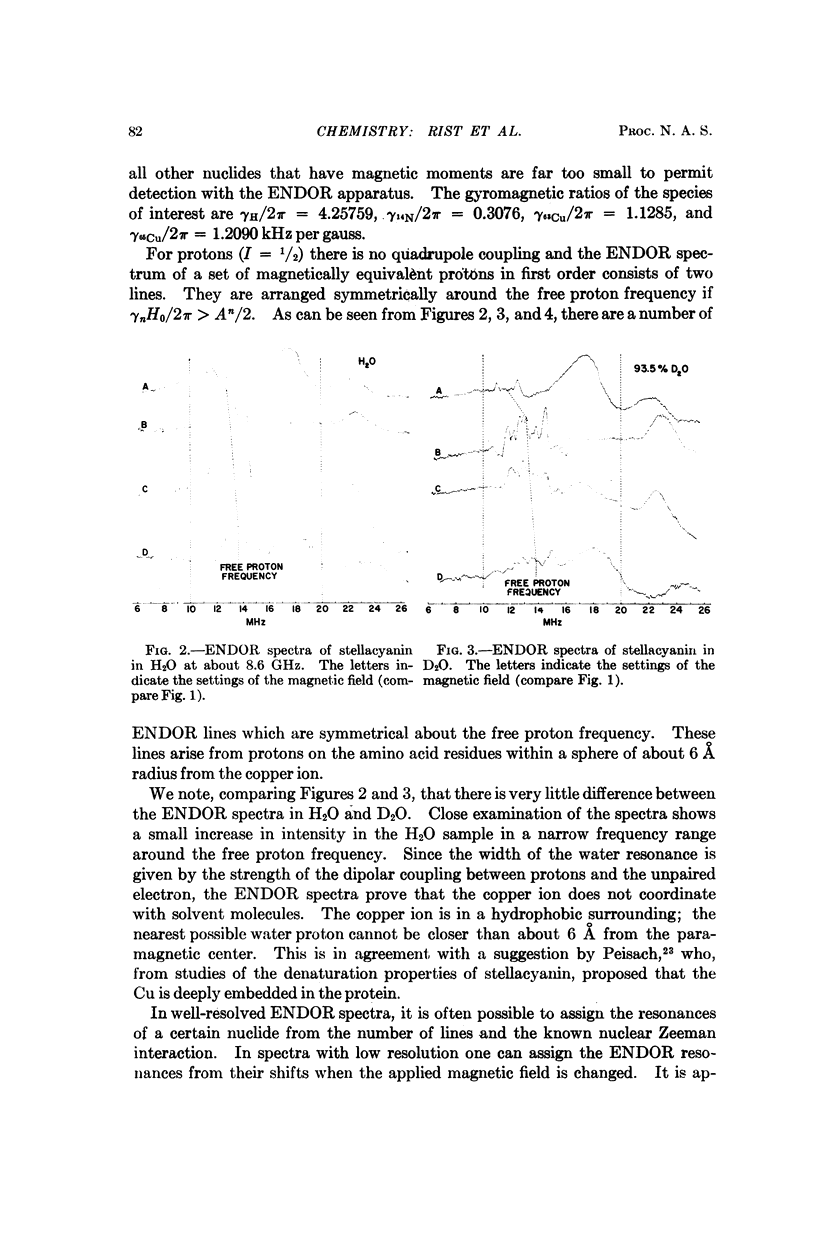
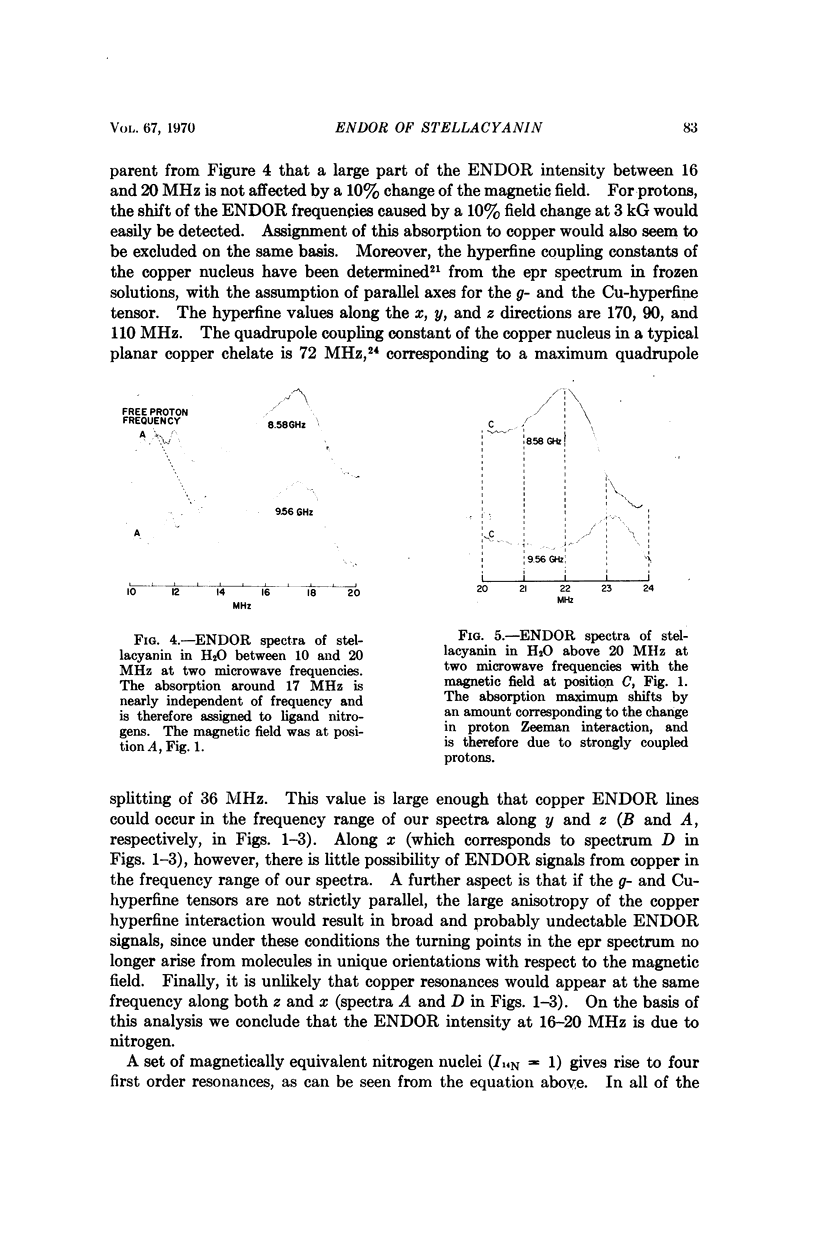
Electron-nuclear double resonance has been used to study ligand hyperfine interactions of the copper (II) complex in a frozen solution of the blue protein stellacyanin. It is shown that the copper ion coordinates with at least one nitrogen ligand, and probably more than one, and that the copper ion is in a hydrophobic environment.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg W. E., Peisach J. The optical and magnetic properties of copper in Chenopodium album plastocyanin. Biochim Biophys Acta. 1966 Oct 10;126(2):269–273. doi: 10.1016/0926-6585(66)90063-x. [DOI] [PubMed] [Google Scholar]
- Brill A. S., Bryce G. F. Cupric ion in blue proteins. J Chem Phys. 1968 May 15;48(10):4398–4404. doi: 10.1063/1.1668007. [DOI] [PubMed] [Google Scholar]
- Ehrenberg A., Eriksson L. E., Hyde J. S. Electron-nuclear double resonance from flavin free radicals in NADPH dehydrogenase ("old yellow enzyme"). Biochim Biophys Acta. 1968 Oct 8;167(2):482–484. doi: 10.1016/0005-2744(68)90234-9. [DOI] [PubMed] [Google Scholar]
- Eisenberger P., Pershan P. S. Magnetic resonance studies of met-myoglobin and myoglobin azide. J Chem Phys. 1967 Nov 1;47(9):3327–3333. doi: 10.1063/1.1712394. [DOI] [PubMed] [Google Scholar]
- Eriksson L. E., Hyde J. S., Ehrenberg A. Electron-nuclear double resonance from flavin radicals in liquid and polycrystalline phase and conjugated to protein. Biochim Biophys Acta. 1969 Nov 18;192(2):211–230. doi: 10.1016/0304-4165(69)90358-4. [DOI] [PubMed] [Google Scholar]
- KATOH S., TAKAMIYA A. NATURE OF COPPER-PROTEIN BINDING IN SPINACH PLASTOCYANIN. J Biochem. 1964 Apr;55:378–387. doi: 10.1093/oxfordjournals.jbchem.a127898. [DOI] [PubMed] [Google Scholar]
- Malkin R., Malmström R. G., Vänngård T. The requirement of the "non-blue" copper (II) for the activity of fungal laccase. FEBS Lett. 1968 Jul;1(1):50–54. doi: 10.1016/0014-5793(68)80016-x. [DOI] [PubMed] [Google Scholar]
- Malmström B. G., Reinhammar B., Vänngård T. Two forms of copper (II) in fungal laccase. Biochim Biophys Acta. 1968 Feb 1;156(1):67–76. doi: 10.1016/0304-4165(68)90105-0. [DOI] [PubMed] [Google Scholar]
- Peisach J., Levine W. G., Blumberg W. E. Structural properties of stellacyanin, a copper mucoprotein from Rhus vernicifera, the Japanese lac tree. J Biol Chem. 1967 Jun 25;242(12):2847–2858. [PubMed] [Google Scholar]



