Abstract
Context:
Our objectives were to investigate the timeliness of formulary decision-making in Atlantic Canada, including the Common Drug Review (CDR) process and the adoption of positive CDR recommendations by Atlantic Canadian provincial public drug plans, and to determine the degree of cost shifting to private payers.
Methods:
Dates of formulary listing decisions from Atlantic Canadian provincial drug plan formularies and utilization analyses from Medavie Blue Cross were used to calculate the timeliness of decisions and cost shifting from public payers to a private payer.
Results:
The median time period between the issuance of a positive CDR recommendation and the addition of a drug to an Atlantic Canadian provincial drug plan was 26.7 weeks (σ=19.1). Cost shifting to employer-sponsored health plans provided by Medavie Blue Cross was minimal.
Discussion:
There is significant variation in the timing of provincial drug formulary listings among the four Atlantic Canadian provinces and the uptake of CDR recommendations.
Conclusion:
Atlantic Canadian provincial governments should support the mandate of the CDR by aiming for a more timely consideration of recommendations.
Abstract
Contexte :
Nos objectifs étaient d'étudier les délais dans les décisions touchant au formulaire pharmaceutique dans le Canada atlantique, notamment pour les processus du Programme commun d'évaluation des médicaments (PCEM) et pour l'adoption des recommandations favorables du PCEM par les régimes d'assurance médicaments des provinces du Canada atlantique. Nous visions également à déterminer le degré des transferts de coûts aux tiers payant privés.
Méthodologie :
Les dates des décisions d'inscription au formulaire par les régimes d'assurance médicaments des provinces du Canada atlantique, ainsi que les analyses d'utilisation de la Croix Bleue Medavie, ont été employées pour calculer les délais des décisions et les transferts de coûts des contribuables aux tiers payant privés.
Résultats :
Le temps médian entre la diffusion d'une recommandation favorable du PCEM et l'ajout d'un médicament aux régimes d'assurance médicaments des provinces du Canada atlantique était de 26,7 semaines (σ=19,1). Les transferts de coûts vers les régimes d'assurance maladie des employeurs fournis par la Croix Bleue Medavie étaient minimaux.
Discussion :
Il y a une variation significative, entre les quatre provinces du Canada atlantique, dans les délais d'inscription au formulaire pharmaceutique et dans l'adoption des recommandations du PCEM.
Conclusion :
Les gouvernements des provinces du Canada atlantique devraient appuyer le mandat du PCEM en visant des délais plus opportuns pour la prise en compte des recommandations.
The Common Drug Review (CDR) was established in 2002 in response to a growing concern over duplication, inefficiency and inconsistency in the public payer drug formulary review process in Canada. Housed within the Canadian Agency for Drugs and Technologies in Health (CADTH), the CDR acts as a centralized drug review process for all federal, provincial and territorial drug plans in Canada (except Quebec) (McMahon et al. 2006). The mandate of the CDR is to provide advice to participating drug plans on which new drugs should be added to public payer formularies. This advice is primarily based on the evaluation of a drug's cost-effectiveness when compared to existing and similar treatments using both internal and external clinical and pharmaco-economic reviewers (Tierney et al. 2008).
The CDR review process begins with a submission by either a drug manufacturer, a federal, territorial or provincial drug plan or the Advisory Committee on Pharmaceuticals (ACP). A submitted drug must receive its Notice of Compliance (NOC) from Health Canada prior to submission. A NOC is issued when a drug is deemed satisfactory and compliant with the safety, efficacy and quality standards as required under the Food and Drugs Act and Regulations (Health Canada 2008). Once the drug has been submitted, a review team evaluates it based upon a developed protocol using a systematic literature search and a review of all relevant clinical and pharmaco-economic information, including unpublished information provided by the manufacturer and a manufacturer-generated pharmaco-economic evaluation. The CDR's compiled evaluation, including all reviews and comments from the respective drug manufacturer, is reviewed by the Canadian Expert drug Advisory Committee (CEDAC), which provides the final recommendation to all participating drug plans on whether or not to list the drug. The CEDAC committee comprises 12 members and a chair with varying clinical and healthcare backgrounds and expertise in methodology, health technology assessment, drug policy and economics (Tierney et al. 2008), with two spots reserved for public members. Submissions are accepted on an ongoing basis and are queued for review on a first-come, first-served basis, with some exceptions for priority status (CADTH 2008a). While the aims of the CDR compare favourably with those of its counterparts in Australia and the united kingdom (Tierney et al. 2008), the effectiveness and timeliness of centralized drug review in Canada have been criticized in the five years since its establishment (Canadian diabetes Association 2007; Dhalla and Laupacis 2008; Standing Committee on Health 2007).
Using public information from the Atlantic Canada provinces (Nova Scotia, New Brunswick, Prince Edward Island and Newfoundland and Labrador), our first objective was to investigate the time taken from a drug's submission to the CDR until the adoption of a positive recommendation by Atlantic Canadian provincial drug plans. This time period is divided into two segments: the time taken by the CDR to reach a decision, and the time from when the decision is made until a drug has been listed. Our second objective was to determine how the use of non-binding recommendations for new drugs in Canada has affected the private sector through public payer–private payer cost shifting in the funding of new drugs.
Methods
Timeliness analysis
We used the online CDR database (CADTH 2008b) to identify drugs reviewed by the CDR for which recommendations were released between January 1, 2005 and may 1, 2008. Drugs that were recommended as “List,” “List with criteria/conditions” or “List in a similar manner to other drugs in class” were included in the study. Drugs with recommendations of “Do not list” were not included within the study, as it was unlikely that provinces would choose to list them (McMahon et al. 2006).
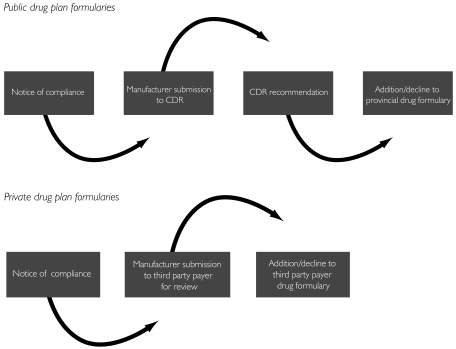
To determine whether or when these drugs were added to Atlantic Canadian provincial drug programs, we reviewed online formularies (Nova Scotia Department of Health 2008; New Brunswick Department of Health 2007; PEI Department of Social Services and Seniors 2006; Newfoundland and Labrador Department of Health and Community Services 2008) along with formulary updates. When a drug did not have an exact date of coverage (mm/dd/yyyy), the date was determined to be the first of the month in which a drug appeared in an update (e.g., if the drug was included in the february 2008 bulletin, the drug was considered to be added on February 1, 2008). For drugs that had still not been added to a provincial formulary at the time of this study, a cut-off date of may 1, 2008 was used in order to perform timeliness calculations. To ensure accuracy, a drug program representative from each of the four Atlantic provinces confirmed our interpretation of the formulary decisions. Timeliness was calculated in two instances: (1) the median length of time between the submission of a drug to the CDR and the issuance of a recommendation and (2) the median length of time between the issuance of a recommendation by the CDR and the addition of a drug to an Atlantic Canadian provincial drug plan formulary (Figure 1). All calculations were performed in microsoft Excel.
FIGURE 1.
Stages in the drug formulary decision-making process in the public and private sectors
Medavie Blue Cross and Atlantic Canadian provincial drug plans
Medavie Blue Cross is the largest private benefits carrier in Atlantic Canada. It provides group health benefits coverage to employers located in New Brunswick, Nova Scotia, Newfoundland and Labrador, Prince Edward Island, Quebec and Ontario, as well as the company's employees throughout Canada, and personal products to individuals residing in Atlantic Canada. All four Atlantic Canadian provinces provide public prescription drug programs on an income-related basis and to individuals who require treatment for specific diseases, with the exception of Prince Edward Island, which administers a seniors' prescription drug program irrespective of income. We anticipated that these conditions would have an impact on the level of public–private cost shifting, with an increased number of cost-shifting possibilities for PEI claims due to its universal Seniors Drug Cost Assistance Plan. This reasoning was based on a greater number of PEI residents being covered under an employer-sponsored benefits plan from Medavie Blue Cross who would also be eligible in the Seniors Drug Cost Assistance Plan because of its universal eligibility.
Cost-shift analysis
The first step of the cost-shift analysis was to determine the drug programs offered in each Atlantic Canadian province and the relevant eligibility requirements. The eligibility of individuals covered under Medavie Blue Cross who may also be covered under an Atlantic Canadian provincial drug program was then determined through the assessment of eligibility criteria provided on governmental websites. In essence, private drug coverage is intended to reimburse clients for specified products and services not available through public programs to avoid costly duplication. This approach ensures that there is minimal duplication between private coverage and existing public drug benefit programs. Eligible Atlantic Canadian provincial drug programs were determined on the basis that they provided universal access to all residents, and thus would be providing coverage to Medavie clients. This step was performed to ensure that true cost shifting took place. For the purpose of this paper, cost shifting is defined as the impact on employer-sponsored benefit plans caused by excessive time lags between a positive CDR recommendation and the addition of new drugs to provincial formularies. To capture CDR-recommended drugs that had yet to be added to one province's drug formulary as of may 1, 2008, we undertook an analysis of the formularies in the other three Atlantic provinces. If the drug was covered under any of the other eligible Atlantic Canadian provincial drug programs, then it was included in the analysis.
A list of CDR-recommended drugs covered under eligible Atlantic Canadian provincial drug programs was then compiled along with the respective time lapse from CDR recommendation to public formulary addition for each Atlantic Canadian province. Private drug data supplied by Medavie Blue Cross were analyzed in three-month intervals, where possible, to facilitate the subtraction of a three-month grace period. We utilized a grace period to account for reasonable time lags in the uptake of CDR recommendations by public drug plans. A three-month grace period was determined to be sufficient time after consulting literature and international examples of centralized drug review (McMahon et al. 2006; Morgan et al. 2006). A sensitivity analysis was performed by expanding the grace period to six months.
Cost shifting was calculated on the basis of Medavie Blue Cross's paying for a CDR-recommended drug during the time lapse between the recommendation and the date it was added to the formulary (or May 1, 2008 if the drug had still not been added to the provincial formulary) minus the three-month grace period. We calculated the actual cost shift using information provided by Medavie Blue Cross regarding the total amount paid, total co-insurance paid and total number of claims submitted.
Results
Timeliness
A total of 35 drugs were considered eligible for this study based on a CDR recommendation to “List,” “List with criteria/conditions” or “List in a similar manner to other drugs in class,” with a recommendation date between January 1, 2005 and may 1, 2008. The first measure of timeliness was the change in the length of the CDR process itself over the study period. Results indicated there was no improvement in the timeliness of the CDR review process over the three-year period (Table 1). For drugs recommended in 2005, the median length of time between the submission of a drug and the issuance of a CDR recommendation was 20.3 weeks. This time reached 21.9 weeks in 2006, representing an increase of 7.8%. In 2007, the length of time increased again to 24.1, an increase of 10.0% from 2006 and an increase of 18.7% over 2005.
TABLE 1.
Length of time for CDR recommendations, by year of recommendation*
| Generic drug name (brand name) | CDR submission | CDR recommendation | Time lapse (weeks) | σ |
|---|---|---|---|---|
| dutasteride (Avodart®) | 24/08/2004 | 20/01/2005 | 21.3 | |
| adalimumab (Humira® – rheumatoid arthritis) | 24/09/2004 | 11/02/2005 | 20.0 | |
| abacavir/lamivudine (Kivexa®) | 26/07/2005 | 07/12/2005 | 19.1 | |
| mycophenolate sodium (Myfortic®) | 03/03/2005 | 08/07/2005 | 18.1 | |
| erlotinib (Tarceva®) | 19/07/2005 | 07/12/2005 | 20.1 | |
| fosamprenavir calcium (Telzir®) | 24/01/2005 | 16/06/2005 | 20.4 | |
| voriconazole (Vfend® – aspergillus) | 25/10/2004 | 14/04/2005 | 24.4 | |
| drospirenone / ethinyl estradiol (Yasmin®) | 20/01/2005 | 16/06/2005 | 21.0 | |
| Median | 20.3 | 1.9 | ||
| niacin / lovastatin (Advicor®) | 18/10/2005 | 26/04/2006 | 27.1 | |
| ciclesonide (Alvesco®) | 24/07/2006 | 20/12/2006 | 21.3 | |
| tipranavir (Aptivus®) | 25/12/2005 | 17/05/2006 | 20.4 | |
| amlodipine besylate / atorvastatin calcium (Caduet®) | 25/12/2005 | 17/05/2006 | 20.4 | |
| travoprost and timolol maleate (Duotrav®) | 24/03/2006 | 24/08/2006 | 21.9 | |
| adalimumab (Humira® – psoriatic arthritis) | 21/06/2006 | 29/11/2006 | 23.0 | |
| quinagolide hydrochloride (Norprolac®) | 23/11/2005 | 17/05/2006 | 25.0 | |
| etonogestrel / ethinyl estradiol (Nuvaring®) | 05/05/2006 | 29/11/2006 | 29.7 | |
| pantoprazole magnesium (Pantoloc M®) | 17/03/2006 | 20/07/2006 | 17.9 | |
| efalizumab (Raptiva®) | 25/10/2005 | 24/08/2006 | 43.3 | |
| treprostinil sodium (Remodulin®) | 24/02/2006 | 20/07/2006 | 20.9 | |
| triptorelin pamoate (Trelstar®) | 27/02/2006 | 20/07/2006 | 20.4 | |
| trospium chloride (Trosec®) | 24/03/2006 | 24/08/2006 | 21.9 | |
| emtricitabine / tenofovir disoproxil fumarate (Truvada®) | 29/05/2006 | 25/10/2006 | 21.3 | |
| voriconazole (Vfend® – candidemia) | 24/03/2006 | 25/10/2006 | 30.7 | |
| tenofovir disoproxil fumarate (Viread®) | 11/08/2005 | 15/03/2006 | 30.9 | |
| Median | 21.9 | 6.4 | ||
| ramipril / hydrochlorothiazide (Altace HCT®) | 26/03/2007 | 14/06/2007 | 11.4 | |
| entecavir (Baraclude®) | 12/12/2006 | 28/11/2007 | 50.1 | |
| varenicline tartrate (Champix®) | 21/03/2007 | 16/08/2007 | 21.1 | |
| ciprofloxacin hydrochloride & dexamethasone otic suspension (Ciprodex®) | 15/06/2007 | 18/10/2007 | 17.9 | |
| deferasirox (Exjade®) | 26/10/2006 | 19/04/2007 | 25.0 | |
| adalimumab (Humira® – ankylosing spondylitis) | 12/07/2007 | 19/12/2007 | 22.9 | |
| adalimumab (Humira® – crohn's disease) | 12/07/2007 | 19/12/2007 | 22.9 | |
| adefovir dipivoxil (Hepsera®) | 29/06/2007 | 18/10/2007 | 15.9 | |
| alglucosidase (Myozyme®) | 10/10/2006 | 14/06/2007 | 35.3 | |
| abatacept (Orencia®) | 26/10/2006 | 27/06/2007 | 34.9 | |
| darunavir (Prezista®) | 29/08/2006 | 14/02/2007 | 24.1 | |
| sildenafil citrate (Revatio®) | 20/09/2006 | 14/02/2007 | 21.0 | |
| rituximab (Rituxan®) | 26/06/2006 | 14/02/2007 | 33.3 | |
| lanreotide acetate (Somatuline Autogel®) | 20/02/2007 | 19/07/2007 | 21.3 | |
| sunitinib malate (Sutent®) | 20/07/2006 | 28/03/2007 | 35.9 | |
| Median | 24.1 | 10.0 |
Based on drugs with a recommendation of “List,” “List with criteria/conditions” and “list in a similar manner to other drugs in class” issued between January 1, 2005 and May 1, 2008.
With regard to the time lapse between the issuance of a positive CDR recommendation and the addition of a drug to a provincial drug formulary, timeliness was improved over the three-year study period in both New Brunswick and Prince Edward Island; however, timeliness was not improved in Nova Scotia and Newfoundland and Labrador (Table 2).
TABLE 2.
Median lengths of time, in weeks, for drug approval process for Atlantic Canada provinces, by year*
| CDR Submission – CDR recommendation | σ | CDR Recommendation – Provincial formulary | σ | Total | ||
|---|---|---|---|---|---|---|
| New Brunswick | ||||||
| 2005 | 20.3 | 1.9 | 45.1 | 22.7 | 65.4 | |
| 2006 | 21.9 | 6.4 | 31.9 | 12.6 | 53.8 | |
| 2007 | 24.1 | 10.0 | 22.0 | 8.1 | 46.1 | |
| Nova Scotia | ||||||
| 2005 | 20.3 | 1.9 | 6.9 | 7.0 | 27.2 | |
| 2006 | 21.9 | 6.4 | 18.6 | 32.9 | 40.5 | |
| 2007 | 24.1 | 10.0 | 12.1 | 12.9 | 36.2 | |
| Newfoundland & Labrador | ||||||
| 2005 | 20.3 | 1.9 | 10.1 | 36.5 | 30.4 | |
| 2006 | 21.9 | 6.4 | 35.9 | 21.4 | 57.8 | |
| 2007 | 24.1 | 10.0 | 13.5 | 13.6 | 37.6 | |
| Prince Edward Island | ||||||
| 2005 | 20.3 | 1.9 | 125.1 | 39.7 | 145.4 | |
| 2006 | 21.9 | 6.4 | 76.6 | 27.6 | 98.5 | |
| 2007 | 24.1 | 10.0 | 44.1 | 16.0 | 68.2 | |
Based on information for 35 drugs with a recommendation of “List,” “List with criteria/conditions” and “list in a similar manner to other drugs in class” issued between January 1, 2005 and May 1, 2008.
In fact, although the length of time for the adoption of a positive CDR recommendation fell by roughly 19 weeks in New Brunswick, it increased by nine weeks in Nova Scotia over the study period. The median length of time for all four Atlantic Canadian provinces between the issuance of a positive CDR recommendation and the addition of a CDR-recommended drug to a provincial drug formulary was 27.0 weeks (σ=34.2). With respect to the overall drug review and listing process, results were similar to those of the previous calculation, with New Brunswick and Prince Edward Island improving on their timeliness over the study period. The overall median length of time for drug approval in all four Atlantic Canadian provinces was 50.0 weeks (σ=33.7), representing a time lapse of nearly one year between the submission of a drug to the CDR and the uptake of a positive CDR recommendation in Atlantic Canada. Variation in provincial timeliness and listing was consistent throughout the study period.
Cost shifting
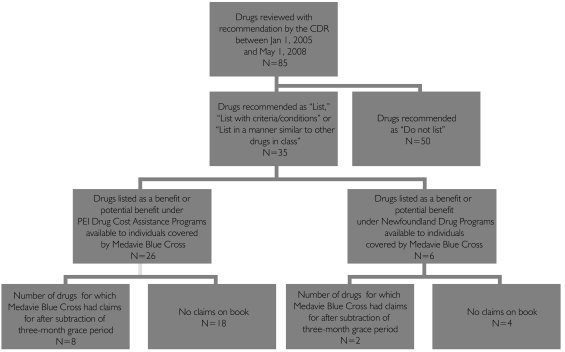
Of the 35 drugs included in this analysis, 26 (74.3%) were eligible under PEI Drug Cost Assistance, 6 (17%) were eligible under the Newfoundland and Labrador Prescription Drug Program, 6 (17%) were eligible under the Nova Scotia drug Program and 7 (20%) were eligible under the New Brunswick Prescription Drug Program. After running a utilization analysis and accounting for the three-month grace period, Medavie Blue Cross had received claims from enrolees to reimburse costs for eight drugs in PEI and two drugs in Newfoundland and Labrador (Figure 2).
FIGURE 2.
Drugs included in the cost-shift analysis
The utilization analysis revealed that a cost shift occurred in Prince Edward Island and Newfoundland and Labrador (Table 3). In PEI, public–private cost shifting with respect to Medavie Blue Cross equaled $46,922.51 after factoring in a three-month grace period. Cost shifting related to prescription claims paid for Caduet® represented the greatest cost to Medavie Blue Cross, with a total amount paid of $16,342.20. Vfend® and Tarceva® represented the highest costs per claim, with an average of $4,655.33 and $2,735.40, respectively.
TABLE 3.
Cost-shift analysis from public payer to private payer
| Drug name | Province | CDR recommendation date | Date added to provincial formulary | Total amount paid* |
|---|---|---|---|---|
| ramipril / hydrochlorothiazide (Altace HCT®) | PEI | 14/6/2007 | 3/3/2008 | 1,174.52 |
| dexamethasone otic suspension (Ciprodex®) | PEI | 18/10/2007 | 3/3/2008 | 1,882.91 |
| travoprost and timolol maleate (Duotrav®) | PEI | 24/8/2006 | 28/5/2007 | 85.82 |
| ciclesonide (Alvesco®) | PEI | 12/12/2006 | 28/5/2007 | 190.96 |
| dutasteride (Avodart®) | PEI | 20/1/2005 | 1/5/2008 | 8,685.90 |
| amlodipine besylate / atorvastatin calcium (Caduet®) | PEI | 17/5/2006 | 1/5/2008 | 16,342.20 |
| erlotinib (Tarceva®) | PEI | 7/12/2005 | 1/5/2008 | 9,249.55 |
| voriconazole (Vfend®) | PEI | 14/4/2005 | 1/5/2008 | 9,310.65 |
| 46,922.51 | ||||
| erlotinib (Tarceva®) | NL | 7/12/2005 | 13/2/2007 | 14,167.77 |
| tenofovir disoproxil fumarate (Viread®) | NL | 15/3/2006 | 13/2/2007 | 2,987.53 |
| 17,155.30 | ||||
| TOTAL | 64,077.81 |
Total amount paid out by Medavie Blue Cross for claims on each drug during the time period between the date the drug was recommended by the CDR and the date it was added to the provincial drug formulary minus a grace period of 3 months.
In Newfoundland and Labrador, public–private cost shifting amounted to $17,155.30 after the three-month grace period. Tarceva® accounted for the greatest cost, with a total amount paid of $14,167.77; however, Viread® accounted for the greatest co-insurance costs to enrolees ($746.90). If a six-month grace period is used instead of three months, the total public–private cost-shifting amount is $34,703.34 and $17,155.30 in PEI and Newfoundland and Labrador, respectively. There was no evidence of cost shifting (as defined in this study) in Nova Scotia and New Brunswick.
Discussion
The results of this study suggest that centralized drug review did little to reduce variation in the listing of new drugs to Atlantic provincial formularies during the study period. The CDR process itself did not show any improvement in timeliness over the study period, with an overall increase of nearly four weeks. Timeliness in provincial decision-making regarding CDR recommendations was equally problematic, with significant variation in the listing of drugs between all four Atlantic Canadian provinces over the study period. Our results are in agreement with other studies which have shown that there has been much heterogeneity in provincial and interprovincial adoption of CDR recommendations (Grootendorst 2002; Marra et al. 2006). With a “no means no” and “yes means maybe” mentality among Canadian provincial drug plan decision-makers, the CDR remains in a strictly advisory capacity, without the authority to enforce compliance (Marra et al. 2006; McMahon et al. 2006).
As demonstrated in Prince Edward Island, while some CDR-recommended drugs were added within a few months of recommendation, many had still not been added years later. When this variability in response time exists within one province, it is easy to understand the scope of variability across provinces. Some provinces may not have the financial resources and capacity to add some CDR-recommended drugs in a timely manner, or may choose to delay or reject the addition of drugs owing to specific population needs. While such constraints are certainly understandable, provinces are not currently held accountable for delays in adopting recommendations, and there is little transparency as to why delays occur. While this issue is not currently within the scope of the CDR, some experts contend that the current situation is unacceptable and argue that provinces should willingly and openly provide a rationale for delays in drug coverage (Dhalla and Laupacis 2008).
It is important to note that the CDR has no control over the quality of the manufacturer's submission itself, including whether or not it will require further information regarding the submission. However, this study indicates that the CDR's response time in reviewing a drug and issuing a recommendation did not improve over the study period; instead, it actually increased by roughly six weeks for the 35 drugs studied. While some may argue that the length of the CDR process could be expected to increase as the number of drug submissions increases, we argue that CADTH should devote enough resources to the CDR to ensure the timely review of future drug submissions. Furthermore, CADTH has released a time frame document for CDR review that states a desired length of 19 to 25 weeks for new drug submission reviews without reconsiderations at the manufacturer's request (CADTH 2007). Timeliness information for the drugs examined in this study indicates that the CDR has been on the longer side of this time frame in recent years. We encourage CADTH to renew commitment to these time frames and to increase resources where needed.
This study has also shown that time delays in the acceptance of CDR recommendations do result in financial impacts both to employer-sponsored health insurance providers and to patients in Atlantic Canada; however, these impacts are not large. It is worth noting that Tarceva® and Vfend® are two drugs that have yet to be added to the PEI formulary at the time of writing this paper, despite both being recommended for inclusion on formularies by the CDR in 2005.
The findings of this study also uphold the criticism that the CDR has not reduced variation among provincial formularies. Our findings support research conducted by Dewa and colleagues (2005) in which Nova Scotia residents had better odds of having prescription drug coverage than PEI residents. As previously stated, because the CDR acts in an advisory capacity only, provinces are able to choose which drugs they wish to add to their formulary based on CDR findings. The impact of these delays and variation on third-party health carriers and their plan sponsors throughout Canada is that some will carry the financial brunt of these delays while others will not feel the impact as much, depending on how quick the response time is. Thus, in its current capacity, the CDR fails to reduce variability, and not only between fTP drug plan formularies: it also fails to reduce variations in cost shifting from drug plans to insurance providers and patients. Ideally, if the CDR were to reduce variability, all provinces and public drug plans would be required to adopt recommendations within a certain time period, as in the united kingdom upon the issuance of guidance by the National Institute for Health and Clinical Excellence (NICE).
Limitations
This study did have several limitations. First, we were limited by the number and type of drugs submitted to and recommended by the CDR in the last few years. Because the onus of submission is on the drug manufacturer, federal, provincial or territorial drug plans or the ACP, the CDR is unable to regulate which drugs it will review and when. The number of drugs recommended, when analyzed against the cohort of Medavie Blue Cross customers, led to only a few drugs remaining eligible for this study. It would be expected that if more disease-specific drugs for multiple sclerosis, cystic fibrosis, and others were recommended by the CDR, then there would have been a greater public–private cost shift. Moreover, this study looked at only one private benefit carrier, albeit the region's largest.
Conclusion
The CDR was intended to create a streamlined process for the submission and review of new drugs in Canada. Although it does provide increased and equitable access to rigorous clinical and pharmaco-economic analysis, it has done little to reduce variation in the listing of new drugs in Atlantic Canada over the three-year study period. This inaction is partly due to variation in the provincial uptake of positive CDR recommendations to provincial drug formularies, primarily as a result of the CDR's status as an advisory body only. CADTH has argued that the CDR has not further delayed the listing of drugs on public formularies (CADTH 2008c); however, this study shows that the CDR failed to improve upon its timeliness over the three-year study period. To enable a more efficient process, Atlantic Canadian provincial governments should support the mandate of the CDR by aiming for a more timely consideration of recommendations. Prior to the implementation of the CDR, the Atlantic Common drug Review functioned to help pool resources for the review of drugs for these smaller provinces. If a pan-Canadian approach to mandate the adoption of CDR recommendations – such as NICE in the united kingdom – is not feasible, then perhaps the Atlantic Common drug Review could be used to ensure that there is a common approach to the adoption of decisions in this fairly homogenous part of the country.
Acknowledgements
The authors would like to acknowledge Sam Lanctin, Theresa Rose and Nancy Vincent for their help in the development of this paper. We also acknowledge the contributions of Elodie Ramos and Ranjita Benerjee in reviewing the manuscript. Andrea scobie's salary for this study was funded as part of a national peer-reviewed research internship funded by MITACS with partnership from Medavie Blue Cross.
Contributor Information
Andrea C. Scobie, College of Pharmacy, Dalhousie University, Halifax, NS.
Neil J. Mackinnon, College of Pharmacy, Dalhousie University, Halifax, NS.
References
- Canadian Agency for Drugs and Technologies in Health (CADTH). Common Drug Review Time Frames. 2007. Retrieved January 6, 2010. < http://cadth.ca/media/cdr/process/cdr_timeframes_april_e.pdf>.
- Canadian Agency for Drugs and Technologies in Health (CADTH). Procedure for Common Drug Review. Ottawa: Author; 2008a. [Google Scholar]
- Canadian Agency for Drugs and Technologies in Health (CADTH). Search CDR Drug Database. 2008b. Retrieved January 6, 2010. < http://cadth.ca/index.php/en/cdr/search>.
- Canadian Agency for Drugs and Technologies in Health (CADTH). The CADTH Common Drug Review – Myths Versus Facts. Ottawa: Author; 2008c. [Google Scholar]
- Canadian Diabetes Association. The Common Drug Review Isn't Working for Canadians with Diabetes. Ottawa: Author; 2007. Written submission to the House of Commons Standing Committee on Health. [Google Scholar]
- Dewa C.S., Hoch J.S., Steele L. Prescription Drug Benefits and Canada's Uninsured. International Journal of Law and Psychiatry. 2005;28(5):496–513. doi: 10.1016/j.ijlp.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Dhalla I., Laupacis A. Moving from Opacity to Transparency in Pharmaceutical Policy. Canadian Medical Association Journal. 2008;178(4):428–31. doi: 10.1503/cmaj.070799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootendorst P. Beneficiary Cost Sharing Under Canadian Provincial Prescription Drug Benefit Programs: History and Assessment. PharmacoEconomics. 2002;9(2):79–99. [PubMed] [Google Scholar]
- Health Canada. Notice of Compliance. 2008. Retrieved January 6, 2010. < http://www.hc-sc.gc.ca/dhp-mps/prodpharma/notices-avis/index-eng.php>.
- Marra C.A., Lynd L.D., Anis A.H., Esdaile J.M. Approval Process and Access to Prescription Drugs in Canada. Arthritis Care & Research. 2006;55(1):9–11. doi: 10.1002/art.21709. [DOI] [PubMed] [Google Scholar]
- McMahon M., Morgan S., Mitton C. The Common Drug Review: A NICE Start for Canada? Health Policy. 2006;77(3):339–51. doi: 10.1016/j.healthpol.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Morgan S.G., McMahon M., Mitton C., Roughead E., Kirk R., Kanavos P. et al. Centralized Drug Review Processes in Australia, Canada, New Zealand and the United Kingdom. Health Affairs. 2006;25(2):337–47. doi: 10.1377/hlthaff.25.2.337. [DOI] [PubMed] [Google Scholar]
- New Brunswick Department of Health. New Brunswick Prescription Drug Program Formulary March 2008. 2007. Retrieved January 6, 2010. < http://www.gnb.ca/0212/pdf/NBPDP_Formulary/NBPDPFormularyMarch2008english.pdf>.
- Newfoundland and Labrador Department of Health and Community Services. Newfoundland and Labrador Prescription Drug Program Benefit Listing by Drug Identification (DIN) 2008. Retrieved January 6, 2010. < http://www.health.gov.nl.ca/health/nlpdp/nldrug.pdf>.
- Nova Scotia Department of Health. Nova Scotia Formulary. 2008. Retrieved January 6, 2010. < http://www.gov.ns.ca/health/Pharmacare/formulary.pdf>.
- Prince Edward Island Department of Social Services and Seniors. Drug Programs Formulary, July 2006. 2006. Retrieved January 6, 2010 < http://www.gov.pe.ca/photos/original/sss_drugformula.pdf>.
- Standing Committee on Health. Prescription Drugs Part 1 – Common Drug Review: A F/P/T Process. Second report. Ottawa: House of Commons Canada; 2007. [Google Scholar]
- Tierney M., Manns B. Members of the Canadian Expert Drug Advisory Committee. Optimizing the Use of Prescription Drugs in Canada through the Common Drug Review. Canadian Medical Association Journal. 2008;178(4):432–35. doi: 10.1503/cmaj.070713. [DOI] [PMC free article] [PubMed] [Google Scholar]