Abstract
Aims
The basis for individual variation in gastroduodenal vulnerability to NSAIDs is not well understood. We aimed to assess whether a gene expression signature was associated with susceptibility to gastroduodenal ulcerations.
Methods
Twenty-five H pylori negative adults were treated for 7 days with naproxen 500 mg BID. Subjects underwent baseline and post-treatment endoscopy, during which biopsies were taken from antrum and duodenum. RNA extraction and cDNA synthesis were performed, followed by PCR of 23 genes relevant to mucosal injury and repair. Fold changes in gene expression were compared between subjects who developed ulcers and those who did not.
Results
Compared to subjects who did not develop ulcers (n=18), subjects who developed antral ulcers (n=7) had significantly greater mucosal up-regulation of interleukin-8 [Fold change = 33.5 (SEM = 18.5) versus −7.7 (3.2)] and of cyclo-oxygenase-2 [2.3 (1.7) versus −10.8 (2.2)]. Conversely, non-ulcer subjects had significantly greater up-regulation of toll-like receptor-4, cyclo-oxygenase-1, and hepatocyte growth factor [14.0 (2.2) vs. −0.8 (1.0), 9.8 (2.4) vs. 0.0 (0.7), and 8.2 (2.6) vs. −2.2 (0.3), respectively].
Conclusions
NSAID-induced antral ulcers are associated with a specific pattern of gastroduodenal mucosal gene expression. These patterns may provide insight into the molecular basis of individual susceptibility to mucosal injury.
INTRODUCTION
NSAID-induced gastroduodenal injury remains an important cause of morbidity and mortality (1). Strategies to minimize NSAID-related adverse events include the use of lower drug doses or of less toxic NSAIDs such as COX-2-selective inhibitors, the co-administration and co-packaging of NSAIDs with gastro-protective agents, and the stratification of patients based on risk factors for bleeding (2–8). Unfortunately, these strategies have been only partially effective: patient and physician adherence with experts’ prescribing recommendations is only fair (9), while the GI-safety advantage of the COX-2 inhibitors is mitigated by such factors as concurrent aspirin use (10) and cardiovascular safety concerns (11).
It has been observed across different studies and in clinical practice that individuals vary in their vulnerability to gastroduodenal NSAID injury. Although certain individuals tolerate many different non-selective NSAIDs, others experience repeated adverse events with different agents. Clinical characteristics such as age and co-morbidity only partly explain these differences. Animal models have suggested several molecular pathways that may influence NSAID-related mucosal injury and repair (12–15). In humans, certain polymorphisms in common cytochrome P450 genes may increase NSAID-related adverse events (16). However, there exist few studies in humans regarding the molecular mechanisms underlying the observed differences in individual vulnerability to NSAID injury (16–18).
We hypothesized that mucosal gene expression during NSAID treatment differs between individuals who develop mucosal injury and those who do not, and that these differences may help explain ulcer pathogenesis. To test our hypothesis, we preselected for analysis a panel of 23 genes that encode proteins that are important in mucosal inflammation and repair. The primary aim of this study was to compare changes in mucosal expression of these 23 genes during one week of naproxen treatment in healthy volunteers who developed antral ulcers versus those who did not.
SUBJECTS AND METHODS
Study Population
The current gene expression study was performed in a sub-group of patients who participated in a clinical study of the effectiveness of omeprazole in preventing naproxen-induced injury (19).
In the clinical study, 70 subjects were divided into two treatment arms (naproxen plus placebo, naproxen plus omeprazole), and 4 subjects were treated with placebo alone. Major inclusion criteria included: age 50 to 75 years; generally good health; willingness to sign the informed consent. Major exclusion criteria included: (1) use of any NSAID (including aspirin) within past 2 weeks, or history of chronic NSAID use; (2) use of antacids, H-2 blocker within past 2 weeks, or PPI within past 30 days; (3) use of any corticosteroid within past 60 days; (4) history of bleeding tendencies or warfarin use within past 60 days; (5) history of previous bleeding ulcer; (6) consumption of 3 or more alcoholic beverages a day; (7) hypersensitivity or allergy to NSAIDs or other contraindications to their use; (8) baseline abdominal pain, nausea and/or cramping; and (9) the presence of one or more gastroduodenal mucosal breaks (erosions or ulcerations) at a baseline endoscopy. As part of the clinical trial, patients provided consent for biopsies. The demographics for the study population are presented in Table 2.
Table 2.
Subject Demographics
| Subject Characteristics | Ulcer (N=7) | No Ulcer (N=18) |
|---|---|---|
| Age, y | ||
| Mean (SD) | 57.4 (7.25) | 57.6 (8.19) |
| Range | 50–68 | 50–75 |
| Sex, no. (%) | ||
| Male | 2 (28.6) | 8 (44.4) |
| Race, no (%) | ||
| White | 5 (71.4) | 14 (77.8) |
| Black | 0 (0) | 2 (11.1) |
| Hispanic | 2 (28.6) | 1 (5.6) |
| Asian | 0 (0) | 1 (5.6) |
For the current primary analysis, subjects treated with omeprazole and/or infected with H pylori (n=41) were excluded in order to eliminate confounding by these two variables. All other subjects from the clinical study were eligible for inclusion in the current study.
In the exploratory analysis of gene regulation at the ulcer margin, we studied tissue from 6 subjects from the sub-population described above, plus tissue from 1 H pylori infected subject.
Study Design
The study was approved by an independent institutional review board. The study consisted of three visits:
Visit 1, Screening
During Visit 1 (Day -10 to -1), subjects read and signed the informed consent, underwent a physical exam, and were screened for inclusion and exclusion criteria.
Visit 2, Baseline Endoscopy
During Visit 2 (Day 0), upper GI endoscopy was performed using standard methods by one of three investigators (JA, LC, and KM). Biopsies were taken for H pylori analysis (HPFast, GI SUPPLY, Windcrest, TX). If the subject had no evidence of gastroduodenal mucosal breaks (erosions or ulcers, see definition below) or of H pylori infection the subject was eligible for the study.
To assess baseline gene expression, 4 forceps biopsies were obtained from normal-appearing mucosa in the duodenal bulb, gastric antrum, and the gastric body (12 biopsies in total). In order to help distinguish healing biopsy sites from NSAID-related lesions during the final endoscopy, the antral biopsies were taken approximately one cm proximal to the pyloric orifice at the 12, 3, 6 and 9 o’clock positions. In addition, a “washout” period of 7 days was provided before the study drug was started in order to allow the biopsy sites heal. All biopsies were obtained utilizing a standard biopsy forceps (Olympus FB 36K-1). Samples were immediately submerged in Trizol, snap frozen with liquid nitrogen, and stored at −80° C until processing for RNA extraction.
Treatment
Study medication was started 7 days after baseline endoscopy (Day 8). Subjects included in the primary analysis underwent a 6.5 day treatment of naproxen (NPX) 500 mg BID (n=25). In order to provide an indication of background gene variation, four subjects were treated with placebo. Investigators were blinded to which treatment subjects received. Subjects were given a dosing diary to record daily dosages of medication taken. We chose the one-week duration of treatment based on considerations regarding patient safety, patient compliance, and the known kinetics of naproxen-related mucosal injury.
Visit 3, Post-Treatment Endoscopy
Subjects returned within 2–4 hours of the last study drug dose (day 14) for clinical and endoscopic evaluation. Compliance was assessed by counting the number of pills returned and by reviewing the subject’s dosing diary. The follow-up endoscopy was then performed.
Ulcers, defined as any mucosal break ≥ 3mm with unequivocal depth (20), were counted individually and localized to the duodenum, gastric antrum, or gastric body. When necessary for scoring, the endoscopist performed a side-by-side comparison of the lesion’s size with the size of a biopsy forceps passed through the endoscope working channel during the procedure.
At the post-treatment endoscopy, 4 biopsies for molecular analysis were again obtained from the duodenal bulb, gastric antrum, and the gastric body. In order to minimize the potential for tissue degradation, biopsies for the primary analysis were obtained from endoscopically normal-appearing mucosa (i.e. not from endoscopically inflamed or ulcerated mucosa). In an exploratory analysis, a group of subjects also underwent biopsy of ulcer margins. To assess background variability in intra-individual gene expression, we took biopsies from healthy-appearing antrum in 4 subjects who received only placebo.
At the conclusion of the study, a committee comprised of one gastroenterologist and two research assistants adjudicated the endoscopic findings by reviewing high-resolution digital video-recordings of each subject’s final endoscopy. For the purposes of the primary analysis, each subject was adjudicated as either “ulcer” or “non-ulcer.” The judgments of the committee were binding.
Quantitative Real Time PCR for Measuring Gene Expression (QPCR)
Total RNA was isolated from gastric and duodenal samples using RNA Bee (Tel-Test, Inc., Friendwood, TX) according to the manufacturer’s instructions. RNA quality from all samples was evaluated using the Agilent Bioanalyzer 2100 with a RNA Integrity Score (RIN) assigned to each sample tested. Samples with a RIN score of less than 6.5 were removed from analysis based on degradation based bias in the QPCR results (< 5% of antral samples).1 μg of total RNA was used as the template for single-strand complementary DNA (cDNA) synthesis using the Transcriptor Fast Strand cDNA Synthesis Kit (Roche, Indianapolis, IN) according to the manufacturer’s instructions. Quantitative real-time PCR (QPCR) reactions were performed in triplicate for 23 genes using TaqMan chemistry (Applied Biosystems [ABI], Foster City, CA) on preconfigured TaqMan Low Density Arrays (TLDA) utilizing ABI Assay on Demand assays. The 23 targets chosen by the investigators (Table 1) represent a panel of genes that from previous studies have demonstrated to be important in the mucosal response to injury. (21). More specifically, these individual gene targets have found to be either up- or down-regulated in rodent models of gastro-duodenal ulcer formation or healing (21). The primers and probes are commercially available through ABI’s Assay on Demand program (Applied Biosystems, Foster City, CA), and can be found online. Quantitative real time PCR was performed using ABI Master Mix on an ABI Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA), programmed for 95°C for 10 minutes, then 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. The plates were configured to provide duplicate reactions for each sample (for every assay) to account for pipetting and well-to-well variation.
Table 1.
Genes Analyzed by Quantitative PCR of Tissue Obtained at Baseline and Post-Treatment Endoscopy
| Functional Category | Gene | ABI Assay ID |
|---|---|---|
| Immune Response/Pro-Inflammatory | Chemokine (C-X-C motif) Receptor 4 (CXCR-4) | Hs00607978_s1 |
| Cyclo-oxygenase 2 ( COX-2) | Hs00153133_m1 | |
| Interleukin 1 beta (IL1B) | Hs00174097_m1 | |
| Interleukin 8 (IL-8) | Hs00174103_m1 | |
| Tumor Necrosis Factor (TNF) | Hs00174128_m1 | |
| Growth Factors/Mucosal healing | Cyclo-oxygenase 1(COX-1) | Hs00168776_m1 |
| Endothelial Growth factor | Hs00153181_m1 | |
| Hepatocyte Growth Factor (HGF) | Hs00300159_m1 | |
| Platelet Derived Growth Factor C (PDGFC) | Hs00211916_m1 | |
| Platelet Derived Growth Factor D (PDGFD) | Hs00937332_m1 | |
| Toll Like Receptor 2 (TLR-2) | Hs01872448_s1 | |
| Toll Like Receptor 4 (TLR-4) | Hs00152939_m1 | |
| Toll Like Receptor 5 (TLR-5) | Hs00152825_m1 | |
| Transforming Growth Factor Alpha (TGFA) | Hs00177401_m1 | |
| Transforming Growth Factor Beta (TGFB2) | Hs00234244_m1 | |
| Vascular Epidermal Growth Factor (VEGF) | Hs00900054_m1 | |
| Miscellaneous | Angiopoietin 1 (ANGPT-1) | Hs00375823_m1 |
| Annexin-1 (ANXA-1) | Hs00167549_m1 | |
| Annexin-2 (ANXA-2) | Hs00743063_s1 | |
| Hydroxyprostaglandin Dehydrogenase (HPGD) | Hs00168359_m1 | |
| Inducible Nitric Oxide Synthetase (INOS) | Hs00167257_m1 | |
| Trefoil Factor 1 (TFF-1) | Hs00170216_m1 | |
| Trefoil Factor 2 (TFF-2) | Hs00193719_m1 |
All raw data was analyzed using sequence detection systems (SDS) 2.3 software (Applied Biosystems). Differential gene expression was calculated using the “delta-delta CT (cycle threshold)” method (22). In brief, first the average of the triplicate results for the cycle threshold (CT) for each gene was calculated. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene expression CT value (also run in triplicate) was then used to calculate a normalized Ct (delta CT ) value for each target gene. The fold change (FC) of expression between baseline and post-treatment biopsy specimens was then calculated as previously described for each test gene in each subject (22). For each of the 23 genes studied, the differences between baseline and post-treatment expression were compared between the ulcer population and the non-ulcer populations.
Real time PCR data is represented both numerically (Tables 3 and 4) and using a “heat map” (Figure 1), which visually depicts patterns in gene expression for individual subjects across multiple genes. Briefly, a color scale is created using the largest gene expression fold changes as the low and high settings of the color scale. Red colors represent genes that are up-regulated, with the warmer color corresponding to more strongly up-regulated signals; green colors represent genes that are down-regulated, with the cooler colors corresponding to more strongly down-regulated signals. Each individual subject is depicted in one column and each individual gene is depicted in one row. This representation allows for the visual detection of gene expression patterns in individual subjects that otherwise might not be observed if represented in table format as statistical average of all subjects being measured.
Table 3.
A comparison of fold changes (baseline versus post-treatment) in genes in naproxen-treated patients between the ulcer and the non-ulcer populations. Tissue samples were obtained from normal-appearing antral mucosa. The 5/23 genes are presented for which significant differences between the ulcer and non-ulcer groups were observed. In one non-ulcer patient, RNA degradation in the post-endoscopy specimen prevented analysis of gene expression, and this patient was excluded from the analysis.
| Gene | Ulcer (N = 7) | No Ulcer (N = 17) | Placebo (N=4) |
|---|---|---|---|
| Hepatocyte Growth Factor | −2.2 (0.3) | 8.2 (2.6) | −0.9 (0.7) |
| Cyclo-oxygenase 1 | 0.0 (0.7) | 9.8 (2.4) | 0.3 (0.8) |
| Toll-Like Receptor-4 | −0.8 (1.0) | 14.0 (2.2) | −0.2 (1.4) |
| Cyclo-oxygenase-2 | 2.3 (1.7) | −10.8 (2.2) | −0.7 (1.2) |
| Interleukin-8 | 33.5 (18.5) | −7.7 (3.2) | −1.8 (1.1) |
Data are expressed as mean (SEM). P= 0.042 by Wilcoxon matched pairs signed ranks test.
Table 4.
A comparison of fold changes (baseline versus post-treatment) in genes in duodenal mucosa in naproxen-treated patients between antral ulcer and the non antral-ulcer populations. Unlike in antral tissue, differences between the ulcer and non-ulcer populations are not significant. All biopsies were taken from normal-appearing mucosa. Six subjects were excluded because of lost sample/poor RNA quality. PCR assays for inducible nitric oxide synthetase and endothelial growth factor (EGF) did not produce interpretable data.
| Gene | Ulcer (N = 5) | No Ulcer (N = 14) |
|---|---|---|
| Hepatocyte Growth Factor | −3.40 (2.95) | −0.49 (2.15) |
| Cyclo-oxygenase 1 | −0.27 (1.24) | 2.10 (1.52) |
| Toll-Like Receptor-4 | −0.34 (2.59) | −1.01 (1.76) |
| Cyclo-oxygenase-2 | 3.46 (0.57) | 4.23 (1.53) |
| Interleukin-8 | 57.05 (38.46) | 21.03 (10.7) |
Data are expressed as mean (SEM). P= 0.038 by Wilcoxon matched pairs signed ranks test.
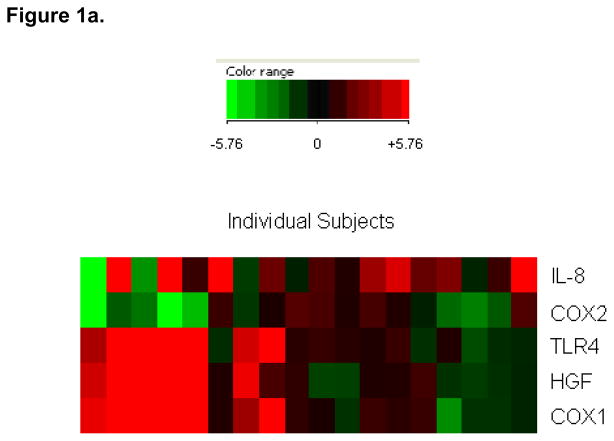
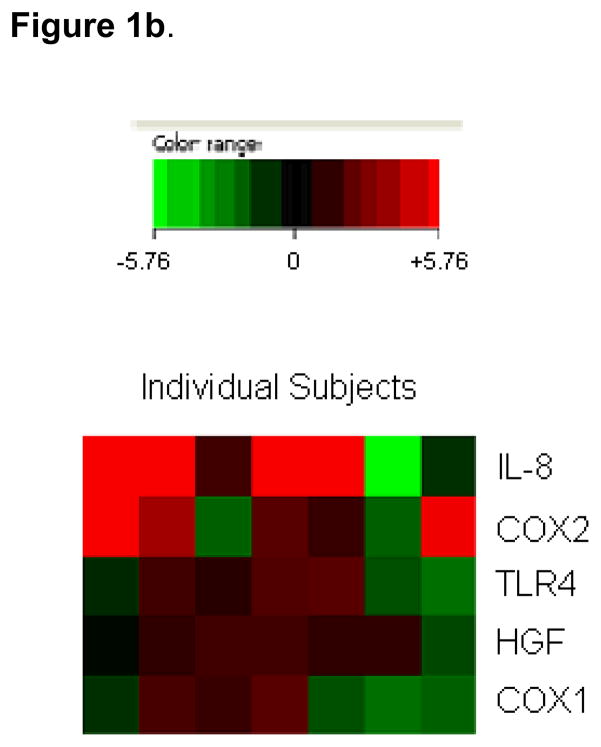
Figure 1.
“Heat maps” depicting a subject-by-subject comparison of antral gene fold changes. Each subject is depicted by one column. Each gene is depicted by one row. Only the five genes for which significant differences between ulcer and non-ulcer populations were noted are depicted. Data are presented on a scale from strong up-regulation (bright red) to strong down regulation (bright green) (see more detailed description in Methods).
Figure 1a. Non-ulcer subjects
Figure 1b. Ulcer subjects.
“Heat map” depicting a subject-by-subject comparison of gene fold changes for subjects who developed ulcers.
Endpoints
For the primary endpoint, we calculated fold changes in individual target genes in normal-appearing antral mucosa in healthy subjects treated with 7 days of naproxen.
Our primary objective was to compare gene fold changes in naproxen-treated healthy subjects in the antral ulcer versus the non-ulcer populations. Our secondary objectives were to evaluate: (1) fold changes in subjects treated with placebo; (2) fold changes in duodenal mucosa in patients with antral ulcers compared to fold changes in the duodenum in non-ulcer subjects; and (3) fold changes at the ulcer margin.
Statistical Analysis
The statistical methods utilized for the clinical study have been previously reported (19). The sample size for the current pilot study was determined by available resources and by the number of the subjects in the clinical study who qualified for inclusion. Statistical analysis of the real time PCR data was performed using methodology described previously (23–25). For all CT value triplicates, a mean and standard error was calculated. For each gene analyzed, a student’s T-test was used in order to test the significance of the differences in regulation in response to treatment (normalized delta CT value) between the ulcer and non-ulcer populations. The descriptive statistics for only the genes for which the fold change differences between the ulcer and non-ulcer populations had a p-value <0.05 by the student’s T-test are reported. Aggregate statistics for each gene (mean and standard error of mean) across subjects were calculated, and compared between the ulcer and non-ulcer groups. Additionally, a Wilcoxon matched pairs signed ranks test was performed to address the small and unequal sample sizes for the groups compared. The results of this analysis yielded a p-value of 0.042 in the naproxen treated baseline vs. post-treatment group and a p-value of 0.038 in the same treatment group in duodenal samples (see Results). The significant p-value rejects the hypothesis that the differences measured are coincidence.
RESULTS
Demographics and Endoscopic Findings
A total of 25 naproxen-treated subjects met the inclusion and exclusion criteria. Of these subjects, 7/25 (28%) developed an antral ulcer. Two of these 7 subjects developed a duodenal ulcer in addition to an antral ulcer. No subject developed a duodenal ulcer alone. All subjects in the antral ulcer and the non-ulcer groups developed small erosions. No patient developed clinically evident bleeding or other significant adverse events. The demographic characteristics were similar between the groups (Table 2).
Antral Gene Expression in Placebo-Treated Subjects
None of the 4 placebo-treated subjects had mucosal breaks at the follow-up endoscopy. When comparing gene expression in antral and duodenal tissue obtained at the baseline and post-treatment endoscopies in subjects who received placebo, no significant changes were detected for the 23 genes tested (selected data included in Table 3). These data demonstrate that changes in gene expression are not due to background intra-individual variation due to other causes.
Antral Gene Expression in the Naproxen-Treated Subjects
The primary endpoint was the change in gene expression in tissue obtained from biopsies of normal-appearing antral mucosa after 7 days of naproxen treatment. Significant differences in gene expression between the ulcer and non-ulcer groups were detected for five of the 23 genes tested (Table 3). Specifically, IL-8 and COX-2 were significantly up-regulated in the ulcer group compared to the non-ulcer group. Indeed, the expression of these two genes was down-regulated in the non-ulcer group. Conversely, COX-1, TLR4 and HGF were significantly up-regulated in the non-ulcer group relative to the ulcer group.
Gene Expression in Tissue Obtained from the Antral Ulcer Margin
In an exploratory analysis, gene expression at the ulcer margins was tested in 7 subjects who developed antral ulcers after 7 days of treatment. RNA quality in tissue obtained from biopsies of the ulcer margins was found to be comparable to RNA quality from tissue obtained from biopsies of normal-appearing mucosa. In the biopsies from the ulcer margins, up-regulation of IL-8 (FC=14.7, SEM=4.1) and COX-2 (FC=3.8, SEM=1.0) was detected. Significant fold changes were not detected for the other 21 genes tested (data not shown).
Gene Expression in Duodenum after Naproxen Treatment
Based on the antral results, we subsequently analyzed expression of IL-8, COX-2, COX-1, TLR-4, and HGF genes in normal-appearing duodenal mucosa after naproxen treatment. In 6 of 25 cases, RNA quality was inadequate.
In naproxen-treated patients, significant up-regulation of IL-8 and COX-2 was observed (Table 4). Unlike in the antrum, there were no significant differences between the ulcer and non-ulcer groups. In 3 subjects who also developed duodenal ulcers, the trends in duodenal mucosal gene expression were similar to results in subjects who did not develop duodenal ulcers (data not shown).
Gene Expression in Individual Subjects after Treatment with Naproxen
In order to detect patterns of gene expression, fold changes were next analyzed subject-by subject. In the non-ulcer group, it was noted that the 7 subjects who had strong up-regulation of HGF also had strong up-regulation of COX-1 and TLR4 (Figure 1a). Conversely, none of the other non-ulcer patients (n=11) had up-regulation (FC>2.0) of any of these three genes. Of the 7 subjects who strongly up-regulated HGF, COX-1 and TLR-4, 5 strongly down-regulated COX-2. In the subject-by-subject analysis, we did not observe any significant correlation between gene expression in the antrum and duodenum (data not shown).
When NSAID-associated antral expression of different genes was compared within individual subjects in the ulcer group, it was noted that that 4/5 subjects who had antral up-regulation of IL-8 also had strong up-regulation of COX-2 (Figure 1b). Of the other two ulcer patients, one had up-regulation of COX-2 but not IL-8, while the other had up-regulation of neither.
DISCUSSION
NSAIDs injure the gastrointestinal mucosa by direct topical toxicity and by blocking the activity of mucosal COX, the rate-limiting enzyme in prostaglandin production (26). Prostaglandins mediate mucosal protection by decreasing acid production, increasing mucus and bicarbonate secretion, enhancing mucosal blood flow, and regulating genes that are important to mucosal homeostasis (27). In addition to injuring the mucosa, NSAIDs inhibit mucosal healing (28, 29). The chemistry, duration and dose of the NSAID as well as clinical characteristics of the patient influence the susceptibility to ulcer development (3–7). However, the biological basis for individual-to-individual differences in vulnerability to NSAID injury is poorly understood. Rodent studies suggest that analyzing mucosal gene expression during NSAID treatment may yield insights into the pathogenesis of injury (30, 31).
Given the frequency of NSAID-related ulcers and the limitations to methods of ulcer prevention, we aimed to identify molecular markers associated with ulceration. Ideally, we could identify patterns of gene expression that predict who will develop an ulcer. The principal finding in the current study is that healthy human subjects who develop antral ulcers during treatment with a non-selective COX inhibitor display a distinct pattern of mucosal gene expression. Specifically, subjects who developed antral ulcers up-regulated COX-2 and IL-8, compared with subjects who did not develop ulcers. Conversely, individuals who did not develop NSAID injury showed increased expression of TLR-4, COX-1, and HGF.
IL-8, a member of the CXC family of chemokines, induces neutrophils to adhere to the endothelium at areas of tissue injury, translocate across the endothelium, and perform phagocytosis and local tissue destruction (32). Crabtree et al have shown that in rodents neutrophils mediate H pylori-related gastroduodenal injury (33). Wallace et al have shown that NSAID-related injury causes adherence of neutrophils to gastric venules, and that NSAID injury is decreased in neutropenic rats (34, 35). Several signals have been proposed to mediate neutrophil recruitment by IL-8, including prostaglandins, TNF-alpha, and leukotrienes (36). In humans, up-regulation of IL-8 may be associated with H pylori-related ulcer disease (37), while proton pump inhibitors suppress IL-8 production by cell lines and abrogate IL-8-mediated actions (38). Our data demonstrating striking IL-8 up-regulation in individuals who developed ulcers suggest that IL-8 dependent pathways are also important in human NSAID-related injury and repair.
TLR-4, an innate immune receptor, has been identified in non-inflamed human gastric epithelium and in gastric mucosal leukocytes and dendritic cells (39, 40). Hold et al demonstrated that in H pylori infection a polymorphism in the TLR-4 gene increases risk of gastric carcinoma (41). Rodent studies suggest that TLR-4 activation increases intestinal susceptibility to NSAID-related injury (15, 42). Up-regulation of TLR-4 in the stomach during NSAID treatment may result from topical injury (43), or from exposure of injured mucosa to luminal bacteria or to the by-products of tissue injury (44). In the current study subjects who did not develop ulcers up-regulated TLR-4. In previous studies, we have shown that TLR-4 is required for colonic mucosal healing, and that a relationship exists between TLR-4 activation and COX activation (45), Although is plausible that a similar pathway exists during NSAID-induced damage, the mechanism by which TLR-4 activation favors in this setting is unknown.
After gastric injury, damaged cells are sloughed and replaced by healthy cells that proliferate, migrate, and organize into glands. In vitro studies have shown that HGF mediates restitution (46, 47). In vivo human studies have suggested that HGF expression is increased in hypertrophic gastritis, ulcerative colitis, and H pylori gastritis (48–50). Production of HGF by lamina propria macrophages, neutrophils, and fibroblasts has been shown to be up-regulated by mucosal prostaglandins (13, 46). In our study, HGF was up-regulated in response to naproxen in individuals who did not develop ulcers, suggesting that it may mediate protection or repair in certain individuals. In the H pylori infected stomach, IL-1β has been shown to increase HGF release (48), but in the current study IL-1β was not found to be up-regulated. Unlike in vitro studies by Takahashi (46), our experiments did not detect HGF up-regulation at the ulcer margin, which may be relevant to failure of restitution.
Previous studies investigating the impact of NSAID administration on COX biosynthesis and enzymatic activity have produced heterogeneous results (51–53). In electron microscopic studies, Jackson et al showed strong COX-1 and COX-2 staining in parietal cells of the normal human stomach, and increased COX-2 at the rims of ulcers (54). In rats, “compensatory” up-regulation of COX-2 in the stomach mucosa has been shown in response to COX-1 inhibition (12, 55). McCarthy et al showed that COX-2 immunostaining was present in epithelial cells and parietal cells in H pylori infected individuals (56). In our study subjects who did not develop ulcers up-regulated COX-1 and down-regulated COX-2. The finding of COX-1 up-regulation in a feedback response to blockade of COX-1 by naproxen corroborates the finding of Bhandari et al (57). Although early studies suggested that in the normal stomach COX-2 is not constitutively expressed (58), our findings and those of several other groups suggest that in certain populations COX-2 is constitutively present (54, 59). Our data demonstrating up-regulation of COX-2 at the margin of ulcers, presumably promoting ulcer healing, is consistent with previous studies (54, 57). In our study the non-ulcer population down-regulated COX-2, suggesting that resistance to injury was not COX-2-mediated. Further studies measuring COX-2 protein, prostaglandins, and RNA copy number will address this question further.
Like prostaglandins, nitric oxide (NO) mediates mucosal protection, via its effects on mucosal blood flow, leukocyte adherence, and other properties (60). NO is released from vascular endothelial cells, epithelial cells and sensory nerves in gut mucosa, and is regulated by constitutive and inducible nitric oxide synthetase (NOS). Because of its importance in mucosal protection, NO donating NSAIDs are currently in human trials (61). Multiple studies have suggested that COX inhibition results in an increase in local production of NO. For example, two recent healthy human volunteer studies showed a local increase in inducible NOS at the protein level in the gastric corpus in subjects treated with ibuprofen or aspirin for three days (51, 52). A parallel increase in constitutive NOS was not seen, and in one of these studies up-regulation of inducible NOS was noted only in subjects with adverse reactions or endoscopic lesions related to NSAIDs. Up-regulation was greater in H. pylori-infected subjects. We did not demonstrate up-regulation of inducible NOS in response to COX inhibition. This discrepancy likely reflects methodological differences. For example, we utilized 7-day rather than 3-day treatment, and naproxen rather than aspirin or ibuprofen. In addition, we obtained tissue from the gastric antrum, which is the predominant site of mucosal NSAID damage, rather than the gastric corpus. Lastly, we measured gene expression rather than protein expression. Focused and detailed molecular analysis of the impact of NSAID treatment on constitutive and inducible NOS under a variety of study conditions will be required to clarify these differences.
A subject-by-subject analysis of the non-ulcer population showed that the same individuals induced HGF, COX-1 and TLR4 (Figure 1a). It is unclear whether or not these changes are regulated dependently or independently. However, previous animal studies have shown a relationship between TLR-4 and COX gene regulation in colitis (45) as well as of prostaglandin biosynthesis and HGF regulation (13).
There are several limitations to this pilot study. First, the number of subjects in each arm is small, and the findings require confirmation in larger populations. Second, in order to minimize risk to our healthy volunteer subjects, we limited the treatment duration to 1 week. It is unproven that molecular changes seen after one week mirror changes occurring during long-term NSAID treatment. This question can be addressed in studies of patients requiring chronic analgesic use. Third, the value of endoscopic ulcers as a “surrogate marker” for adverse clinical events remains controversial, although widely accepted (20). Of note, the antral and duodenal ulcer rates were 28% and 8% respectively in our study, somewhat higher than those reported in the literature. We believe this reflects the fact that this was a single site study and that we enforced a strict width definition by sizing lesions with the biopsy forceps and by using blinded adjudication. As expected, no subjects in the placebo group developed ulcers. Also reassuringly, no significant gene changes occurred in the placebo-treated population, suggesting that neither meals nor other environmental factors on the gastric mucosa confounded our results.
The results of our pilot study suggest future directions for research. First, our findings require corroboration in other, larger populations. Second, studies of protein expression will help clarify the functional significance of the genetic changes that we observed. Third, studies of gene copy number can provide absolute quantitative data for gene activation. Fourth, studies of gene families (e.g. using microarrays) can help delineate molecular pathways of gene activation in the stomach in response to NSAIDs.
In conclusion, our results suggest that NSAID-induced antral ulcers are associated with a specific pattern of mucosal gene regulation. Up-regulation of HGF-1, COX-1, and TLR-4 are associated with decreased NSAID injury, while up-regulation of IL-8 may represent a mechanism of injury. Detailed analysis of pathways involving HGF-1, COX-1, COX-2, TLR-4, and IL-8 using tissue obtained from endoscopic biopsy specimens may yield useful information regarding the mechanisms of NSAID-related injury.
Acknowledgments
The authors thank Colleen D’Souza, Liliana Morales, Jennifer Page, and Maya Voynarovska, Joanna Sypula, Michael DiCola, without whom this study could not have been accomplished.
Footnotes
Statement of Interests:
1. Author’s declaration of personal interest
(i) Dr Aisenberg has performed sponsored research and consulting for Pfizer, Inc. and for NiCox.
2. Declaration of funding interests:
(i) This study was funded in part by in part by an investigator initiated grant by Pfizer Inc. and in part by a grant from the Digestive Diseases Research Foundation.
References
- 1.Lanas A, Perez-Aisa MA, Feu F, Ponce J, Saperas E, Santolaria S, et al. A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal antiinflammatory drug use.[see comment] American Journal of Gastroenterology. 2005 Aug;100(8):1685–93. doi: 10.1111/j.1572-0241.2005.41833.x. [DOI] [PubMed] [Google Scholar]
- 2.Henry D, Robertson J. Nonsteroidal anti-inflammatory drugs and peptic ulcer hospitalization rates in New South Wales. Gastroenterology. 1993 Apr;104(4):1083–91. doi: 10.1016/0016-5085(93)90277-j. [DOI] [PubMed] [Google Scholar]
- 3.Griffin MR, Piper JM, Daugherty JR, Snowden M, Ray WA. Nonsteroidal anti-inflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Annals of Internal Medicine. 1991 Feb 15;114(4):257–63. doi: 10.7326/0003-4819-114-4-257. [DOI] [PubMed] [Google Scholar]
- 4.Farkouh ME, Kirshner H, Harrington RA, Ruland S, Verheugt FWA, Schnitzer TJ, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet. 2004 Aug 21–27;364(9435):675–84. doi: 10.1016/S0140-6736(04)16894-3. [see comment] [DOI] [PubMed] [Google Scholar]
- 5.Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study.[see comment] JAMA. 2000 Sep 13;284(10):1247–55. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 6.Hawkey C, Talley NJ, Yeomans ND, Jones R, Sung JJY, Langstrom G, et al. Improvements with esomeprazole in patients with upper gastrointestinal symptoms taking non-steroidal antiinflammatory drugs, including selective COX-2 inhibitors. American Journal of Gastroenterology. 2005 May;100(5):1028–36. doi: 10.1111/j.1572-0241.2005.41465.x. [DOI] [PubMed] [Google Scholar]
- 7.Hooper L, Brown TJ, Elliott R, Payne K, Roberts C, Symmons D. The effectiveness of five strategies for the prevention of gastrointestinal toxicity induced by non-steroidal anti-inflammatory drugs: systematic review.[see comment] BMJ. 2004 Oct 23;329(7472):948. doi: 10.1136/bmj.38232.680567.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheiman JM, Yeomans ND, Talley NJ, Vakil N, Chan FKL, Tulassay Z, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors.[see comment] American Journal of Gastroenterology. 2006 Apr;101(4):701–10. doi: 10.1111/j.1572-0241.2006.00499.x. [DOI] [PubMed] [Google Scholar]
- 9.Ferrana M, DiMario F, Battaglia G, Vianello F, Salandin S, Bo ND, et al. Compliance with therapy for ulcer disease: clinical experience and review of the literature. Advances in Therapy. 1994 Mar-Apr;11(2):52–7. [PubMed] [Google Scholar]
- 10.Lanas A, Garcia-Rodriguez LA, Arroyo MT, Gomollon F, Feu F, Gonzalez-Perez A, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut. 2006 Dec;55(12):1731–8. doi: 10.1136/gut.2005.080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahme E, Nedjar H. Risks and benefits of COX-2 inhibitors vs non-selective NSAIDs: does their cardiovascular risk exceed their gastrointestinal benefit? A retrospective cohort study.[see comment] Rheumatology. 2007 Mar;46(3):435–8. doi: 10.1093/rheumatology/kel428. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka A, Araki H, Hase S, Komoike Y, Takeuchi K. Up-regulation of COX-2 by inhibition of COX-1 in the rat: a key to NSAID-induced gastric injury. Alimentary Pharmacology & Therapeutics. 2002 Apr;16( Suppl 2):90–101. doi: 10.1046/j.1365-2036.16.s2.22.x. [DOI] [PubMed] [Google Scholar]
- 13.Bamba H, Ota S, Kato A, Matsuzaki F. Nonsteroidal anti-inflammatory drugs may delay the repair of gastric mucosa by suppressing prostaglandin-mediated increase of hepatocyte growth factor production. Biochemical & Biophysical Research Communications. 1998 Apr 17;245(2):567–71. doi: 10.1006/bbrc.1998.8436. [DOI] [PubMed] [Google Scholar]
- 14.Mizuno H, Sakamoto C, Matsuda K, Wada K, Uchida T, Noguchi H, et al. Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice.[see comment] Gastroenterology. 1997 Feb;112(2):387–97. doi: 10.1053/gast.1997.v112.pm9024292. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe T, Higuchi K, Kobata A, Nishio H, Tanigawa T, Shiba M, et al. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent.[see comment] Gut. 2008 Feb;57(2):181–7. doi: 10.1136/gut.2007.125963. [DOI] [PubMed] [Google Scholar]
- 16.Pilotto A, Seripa D, Franceschi M, Scarcelli C, Colaizzo D, Grandone E, et al. Genetic susceptibility to nonsteroidal anti-inflammatory drug-related gastroduodenal bleeding: role of cytochrome P450 2C9 polymorphisms. Gastroenterology. 2007 Aug;133(2):465–71. doi: 10.1053/j.gastro.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Blanco G, Martinez C, Ladero JM, Garcia-Martin E, Taxonera C, Gamito FG, et al. Interaction of CYP2C8 and CYP2C9 genotypes modifies the risk for nonsteroidal anti-inflammatory drugs-related acute gastrointestinal bleeding. Pharmacogenetics & Genomics. 2008 Jan;18(1):37–43. doi: 10.1097/FPC.0b013e3282f305a9. [DOI] [PubMed] [Google Scholar]
- 18.Martinez C, Blanco G, Ladero JM, Garcia-Martin E, Taxonera C, Gamito FG, et al. Genetic predisposition to acute gastrointestinal bleeding after NSAIDs use. British Journal of Pharmacology. 2004 Jan;141(2):205–8. doi: 10.1038/sj.bjp.0705623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai J, Sanyal S, Goo T, Benson A, Bodian C, Miller K, et al. Primary Prevention of Adverse Gastroduodenal Effects from Short-Term Use of Non-Steroidal Anti-Inflammatory Drugs by Omeprazole 20 mg in Healthy Subjects: A Randomized, Double-Blind, Placebo-Controlled Study. Dig Dis Sci. 2008;53(8):2059–65. doi: 10.1007/s10620-007-0127-4. 2008/8. [DOI] [PubMed] [Google Scholar]
- 20.Yeomans NDNJ. Systematic review: ulcer definition in NSAID ulcer prevention trials. Aliment Pharmacol Ther. 2008;15(27):465–72. doi: 10.1111/j.1365-2036.2008.03610.x. [DOI] [PubMed] [Google Scholar]
- 21.Naito Y, Ito M, Watanabe T, Suzuki H. Biomarkers in patients with gastric inflammation: a systematic review. Digestion. 2005;72(2–3):164–80. doi: 10.1159/000088396. [DOI] [PubMed] [Google Scholar]
- 22.Klugmann M, Leichtlein CB, Symes CW, Klaussner BC, Brooks AI, Young D, et al. A novel role of circadian transcription factor DBP in hippocampal plasticity. Mol Cell Neurosci. 2006 Feb;31(2):303–14. doi: 10.1016/j.mcn.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Wong M, Medrano J. Real-time PCR for mRNA quantitation. Biotechniques. 2005;39(1):484–5. doi: 10.2144/05391RV01. 10/2005. [DOI] [PubMed] [Google Scholar]
- 24.Ramakers C, Ruijter J, Deprez R, Moorman A. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neuroscience letters. 2003;339(1):62–6. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 25.Luu-The V, Pauquet N, Calvo E, Cumps J. Improved real-time RT-PCR method for high-throughput measurements using second derivative calculation and double correction. Biotechniques. 2005;38(2):287–93. doi: 10.2144/05382RR05. [DOI] [PubMed] [Google Scholar]
- 26.Wallace JL. COX-2: a pivotal enzyme in mucosal protection and resolution of inflammation. Thescientificworldjournal. 2006;6:577–88. doi: 10.1100/tsw.2006.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace JL. Nonsteroidal anti-inflammatory drugs and the gastrointestinal tract. Mechanisms of protection and healing: current knowledge and future research. American Journal of Medicine. 2001 Jan 8;110(1A):19S–23S. doi: 10.1016/s0002-9343(00)00631-8. [DOI] [PubMed] [Google Scholar]
- 28.Dorta G, Nicolet M, Vouillamoz D, Margalith D, Blum AL, Armstrong D, et al. Effects of misoprostol on healing and prevention of biopsy-induced gastroduodenal lesions occurring during the administration of diclofenac to volunteers.[erratum appears in Aliment Pharmacol Ther 1996 Oct;10(5):833] Alimentary Pharmacology & Therapeutics. 1996 Aug;10(4):563–9. doi: 10.1046/j.1365-2036.1996.29171000.x. [DOI] [PubMed] [Google Scholar]
- 29.Dorta G, Nicolet M, Vouillamoz D, Margalith D, Saraga E, Bouzourene H, et al. The effects of omeprazole on healing and appearance of small gastric and duodenal lesions during dosing with diclofenac in healthy subjects. Alimentary Pharmacology & Therapeutics. 2000 May;14(5):535–41. doi: 10.1046/j.1365-2036.2000.00737.x. [DOI] [PubMed] [Google Scholar]
- 30.Wallace JL, Ma L. Inflammatory mediators in gastrointestinal defense and injury. Experimental Biology & Medicine. 2001 Dec;226(11):1003–15. doi: 10.1177/153537020122601107. [DOI] [PubMed] [Google Scholar]
- 31.Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Digestive Diseases & Sciences. 2005 Oct;50( Suppl 1):S24–33. doi: 10.1007/s10620-005-2803-6. [DOI] [PubMed] [Google Scholar]
- 32.Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997 Oct 31;91(3):385–95. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 33.Crabtree JE. Gastric mucosal inflammatory responses to Helicobacter pylori. Alimentary Pharmacology & Therapeutics. 1996 Apr;10( Suppl 1):29–37. doi: 10.1046/j.1365-2036.1996.22164003.x. [DOI] [PubMed] [Google Scholar]
- 34.Wallace JL, Arfors KE, McKnight GW. A monoclonal antibody against the CD18 leukocyte adhesion molecule prevents indomethacin-induced gastric damage in the rabbit. Gastroenterology. 1991 Apr;100(4):878–83. doi: 10.1016/0016-5085(91)90259-n. [DOI] [PubMed] [Google Scholar]
- 35.Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Baillieres Best Pract Res Clin Gastroenterol. 2001 Oct;15(5):691–703. doi: 10.1053/bega.2001.0229. [DOI] [PubMed] [Google Scholar]
- 36.Hudson N, Balsitis M, Everitt S, Hawkey CJ. Enhanced gastric mucosal leukotriene B4 synthesis in patients taking non-steroidal anti-inflammatory drugs. Gut. 1993 Jun;34(6):742–7. doi: 10.1136/gut.34.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crabtree JE. Role of cytokines in pathogenesis of Helicobacter pylori-induced mucosal damage. Digestive Diseases & Sciences. 1998 Sep;43(9 Suppl):46S–55S. [PubMed] [Google Scholar]
- 38.Handa O, Yoshida N, Fujita N, Tanaka Y, Ueda M, Takagi T, et al. Molecular mechanisms involved in anti-inflammatory effects of proton pump inhibitors. Inflammation Research. 2006 Nov;55(11):476–80. doi: 10.1007/s00011-006-6056-4. [DOI] [PubMed] [Google Scholar]
- 39.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Advances in Experimental Medicine & Biology. 2005;560:11–8. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- 40.Schmausser B, Andrulis M, Endrich S, Lee SK, Josenhans C, Muller-Hermelink HK, et al. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clinical & Experimental Immunology. 2004 Jun;136(3):521–6. doi: 10.1111/j.1365-2249.2004.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hold GL, Rabkin CS, Chow W-H, Smith MG, Gammon MD, Risch HA, et al. A functional polymorphism of toll-like receptor 4 gene increases risk of gastric carcinoma and its precursors. Gastroenterology. 2007 Mar;132(3):905–12. doi: 10.1053/j.gastro.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 42.Kato S, Ito Y, Nishio H, Aoi Y, Amagase K, Takeuchi K. Increased susceptibility of small intestine to NSAID-provoked ulceration in rats with adjuvant-induced arthritis: involvement of enhanced expression of TLR4. Life Sciences. 2007 Sep 29;81(16):1309–16. doi: 10.1016/j.lfs.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 43.Ortega-Cava CF, Ishihara S, Rumi MAK, Kawashima K, Ishimura N, Kazumori H, et al. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol. 2003 Apr 15;170(8):3977–85. doi: 10.4049/jimmunol.170.8.3977. [DOI] [PubMed] [Google Scholar]
- 44.Ferrero RL. Innate immune recognition of the extracellular mucosal pathogen, Helicobacter pylori. Mol Immunol. 2005 May;42(8):879–85. doi: 10.1016/j.molimm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006 Sep;131(3):862–77. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi M, Ota S, Hata Y, Mikami Y, Azuma N, Nakamura T, et al. Hepatocyte growth factor as a key to modulate anti-ulcer action of prostaglandins in stomach. Journal of Clinical Investigation. 1996 Dec 1;98(11):2604–11. doi: 10.1172/JCI119080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terano A, Sakata-Horie K, Shimada T, Hiraishi H, Yoshiura K, Yoneda M, et al. The role of cellular migration in the repair process of gastric epithelial cells. Life Sciences. 2001 Nov 9;69(25–26):3083–9. doi: 10.1016/s0024-3205(01)01414-x. [DOI] [PubMed] [Google Scholar]
- 48.Yasunaga Y, Shinomura Y, Kanayama S, Higashimoto Y, Yabu M, Miyazaki Y, et al. Increased production of interleukin 1 beta and hepatocyte growth factor may contribute to foveolar hyperplasia in enlarged fold gastritis.[see comment] Gut. 1996 Dec;39(6):787–94. doi: 10.1136/gut.39.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitamura S, Kondo S, Shinomura Y, Isozaki K, Kanayama S, Higashimoto Y, et al. Expression of hepatocyte growth factor and c-met in ulcerative colitis. Inflammation Research. 2000 Jul;49(7):320–4. doi: 10.1007/PL00000212. [DOI] [PubMed] [Google Scholar]
- 50.Taha AS, Curry GW, Morton R, Park RH, Beattie AD. Gastric mucosal hepatocyte growth factor in Helicobacter pylori gastritis and peptic ulcer disease. American Journal of Gastroenterology. 1996 Jul;91(7):1407–9. [PubMed] [Google Scholar]
- 51.Konturek PC, Kania J, Hahn EG, Konturek JW. Ascorbic acid attenuates aspirin-induced gastric damage: role of inducible nitric oxide synthase. Journal of Physiology & Pharmacology. 2006 Nov;57( Suppl 5):125–36. [PubMed] [Google Scholar]
- 52.Gallego-Sandin S, Novalbos J, Rosado A, Gisbert JP, Galvez-Mugica M-A, Garcia AG, et al. Effect of ibuprofen on cyclooxygenase and nitric oxide synthase of gastric mucosa: correlation with endoscopic lesions and adverse reactions. Digestive Diseases & Sciences. 2004 Sep;49(9):1538–44. doi: 10.1023/b:ddas.0000042261.22387.06. [DOI] [PubMed] [Google Scholar]
- 53.Halter F, Tarnawski AS, Schmassmann A, Peskar BM. Cyclooxygenase 2-implications on maintenance of gastric mucosal integrity and ulcer healing: controversial issues and perspectives. Gut. 2001 Sep;49(3):443–53. doi: 10.1136/gut.49.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jackson LM, Wu KC, Mahida YR, Jenkins D, Hawkey CJ. Cyclooxygenase (COX) 1 and 2 in normal, inflamed, and ulcerated human gastric mucosa. Gut. 2000 Dec;47(6):762–70. doi: 10.1136/gut.47.6.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davies NM, Sharkey KA, Asfaha S, Macnaughton WK, Wallace JL. Aspirin causes rapid up-regulation of cyclo-oxygenase-2 expression in the stomach of rats. Alimentary Pharmacology & Therapeutics. 1997 Dec;11(6):1101–8. doi: 10.1046/j.1365-2036.1997.00247.x. [DOI] [PubMed] [Google Scholar]
- 56.McCarthy CJ, Crofford LJ, Greenson J, Scheiman JM. Cyclooxygenase-2 expression in gastric antral mucosa before and after eradication of Helicobacter pylori infection. American Journal of Gastroenterology. 1999 May;94(5):1218–23. doi: 10.1111/j.1572-0241.1999.01070.x. [DOI] [PubMed] [Google Scholar]
- 57.Bhandari P, Bateman AC, Mehta RL, Patel P. Mucosal expression of cyclooxygenase isoforms 1 and 2 is increased with worsening damage to the gastric mucosa. Histopathology. 2005 Mar;46(3):280–6. doi: 10.1111/j.1365-2559.2005.02053.x. [DOI] [PubMed] [Google Scholar]
- 58.Kargman S, Charleson S, Cartwright M, Frank J, Riendeau D, Mancini J, et al. Characterization of Prostaglandin G/H Synthase 1 and 2 in rat, dog, monkey, and human gastrointestinal tracts. Gastroenterology. 1996 Aug;111(2):445–54. doi: 10.1053/gast.1996.v111.pm8690211. [DOI] [PubMed] [Google Scholar]
- 59.Venerito M, Treiber G, Wex T, Kuester D, Roessner A, Di Mario F, et al. Effects of low-dose aspirin on gastric erosions, cyclooxygenase expression and mucosal prostaglandin-E2 do not depend on Helicobacter pylori infection. Alimentary Pharmacology & Therapeutics. 2006 Apr 15;23(8):1225–33. doi: 10.1111/j.1365-2036.2006.02856.x. [DOI] [PubMed] [Google Scholar]
- 60.Brzozowski T, Konturek PC, Pajdo R, Ptak-Belowska A, Kwiecien S, Pawlik M, et al. Physiological mediators in nonsteroidal anti-inflammatory drugs (NSAIDs)-induced impairment of gastric mucosal defense and adaptation. Focus on nitric oxide and lipoxins. Journal of Physiology & Pharmacology. 2008 Aug;59( Suppl 2):89–102. [PubMed] [Google Scholar]
- 61.Fiorucci S, Santucci L, Distrutti E. NSAIDs, coxibs, CINOD and H2S-releasing NSAIDs: what lies beyond the horizon. Digestive & Liver Disease. 2007 Dec;39(12):1043–51. doi: 10.1016/j.dld.2007.09.001. [DOI] [PubMed] [Google Scholar]