Abstract
Since liver fatty acid binding protein (L-FABP) facilitates uptake/oxidation of long-chain fatty acids in cultured transfected cells and primary hepatocytes, loss of L-FABP was expected to exacerbate weight gain and/or obesity in response to high dietary fat. Male and female wild-type (WT) and L-FABP gene-ablated mice, pair-fed a defined isocaloric control or high fat diet for 12 weeks, consumed equal amounts of food by weight and kcal. Male WT mice gained weight faster than their female WT counterparts regardless of diet. L-FABP gene ablation enhanced weight gain more in female than male mice—an effect exacerbated by high fat diet. Dual emission X-ray absorptiometry revealed high-fat fed male and female WT mice gained mostly fat tissue mass (FTM). L-FABP gene ablation increased FTM in female, but not male, mice—an effect also exacerbated by high fat diet. Concomitantly, L-FABP gene ablation decreased serum β-hydroxybutyrate in male and female mice fed the control diet and, even more so, on the high-fat diet. Thus, L-FABP gene ablation decreased fat oxidation and sensitized all mice to weight gain as whole body FTM and LTM—with the most gain observed in FTM of control vs high-fat fed female L-FABP null mice. Taken together, these results indicate loss of L-FABP exacerbates weight gain and/or obesity in response to high dietary fat.
Keywords: Liver, Fatty acid binding protein, Fat, Oxidation, Body weight, Fat/lean tissue mass
Introduction
Liver fatty acid binding protein (L-FABP) is a small 14-kDa soluble protein highly expressed in tissues active in long chain fatty acid (LCFA) uptake, oxidation and metabolism—especially liver, intestine, and kidney (0.1–0.4 mM; rev. in [1]). L-FABP is unique among the fatty acid binding protein family in that it has two known (rather than one) ligand binding sites, a broad range/promiscuity for a variety of ligands, and high affinity for long chain fatty acids (LCFAs) or their CoA thioesters (LCFA-CoAs) [1–6]. Demonstration of LCFA and LCFA-CoA binding to L-FABP provided the first insight that this protein may be involved in LCFA uptake and intracellular targeting to metabolic organelles for oxidation and esterification. This expectation has since been borne out by multiple studies performed in vitro, in cultured cells, and mice as follows:
Purified L-FABP extracts LCFA/LCFA-CoA from membranes and protects LCFA-CoAs from hydrolysis [7–11]. Also in vitro, L-FABP removes palmitoyl CoA induced substrate inhibition of carnitine palmitoyltransferase-1, the rate limiting step in mitochondrial LCFA oxidation [12, 13], and serves as a LCFA donor protein for peroxisomal and LCFA-CoA donor protein for mitochondrial LCFA oxidation [14, 15]. Finally, L-FABP facilitates presentation of bound LCFA-CoA for esterification to phosphatidic acid and cholesteryl ester by purified liver microsomes [7–11]. Thus it was expected that L-FABP should alter LCFA oxidation and esterification in living cells and in animals.
In L-FABP overexpressing transformed cells, L-FABP enhanced LCFA uptake [1, 16–19], intracellular transport/diffusion [1, 17], oxidation (especially with branched-chain fatty acids selectively oxidized in peroxisomes [20], and esterification [16, 18, 21]. Initial physiologic studies suggested that L-FABP may function in LCFA metabolism. For example, L-FABP concentration in rat tissues was directly related to the oxidative capacity (liver or other L-FABP expressing tissues) [22, 23]. Further, peroxisomal proliferators induced expression of L-FABP and peroxisomal β-oxidation in primary rat hepatocyte cultures [24, 25]. More recently, the relevance of L-FABP to LCFA/LCFA-CoA oxidation and esterification was more firmly established by studies with genetically engineered mice, in which L-FABP gene ablation inhibited hepatic LCFA uptake and LCFA oxidation in vivo, and reduced hepatic cytosol binding capacity for LCFA and LCFA-CoA [26–29]. Cultured primary mouse hepatocytes from L-FABP knockout (KO) and wild-type (WT) mice also confirmed that loss of L-FABP reduced LCFA uptake, intracellular transport/diffusion, and oxidation [30].
Thus in-vitro, cultured-cell, and animal studies have indicated that L-FABP is involved in hepatic uptake of LCFAs derived from diet or adipose tissue. In the liver, L-FABP may target bound LCFA and/or LCFA-CoA to intracellular organelles for oxidation (mitochondria, peroxisomes) or esterification [endoplasmic reticulum (ER)] for storage (lipid droplets) or secretion (VLDL). Since the net effect of L-FABP would elicit rapid hepatic uptake and removal of LCFAs—primarily by oxidation since hepatic storage of esterified LCFAs is limited—it was hypothesized that L-FABP gene ablation would enhance high fat diet induced weight gain and obesity. This hypothesis was tested in male and female and L-FABP KO and WT mice pair-fed a defined isocaloric high fat versus control diet. Absence of L-FABP exacerbated weight gain and obesity induced by high dietary fat.
Materials and Methods
Materials
RNeasy mini kits were from Qiagen (Valencia, CA). Probes and primers used in the real-time PCR experiments on mouse liver RNA were purchased from Applied Biosystems (Foster City, CA). Protein was quantified by Protein Assay Dye Reagent (Bio-Rad Laboratories, Hercules, CA).
Animals
L-FABP gene-ablated mice were generated using a construct strategy that deleted all four exons of the L-FABP gene as described earlier [26]. Since the L-FABP KO mice were backcrossed to normal C57BL/6NCr mice (Charles River Wilmington, MA obtained through the National Cancer Institute, Frederick Cancer Research and Development Center, Frederick, MD) to the N6 generation (98.4% genetic homogeneity), C57BL/6NCr mice of matching sex/age were used as control animals for the feeding studies. Mice were housed in ventilated microisolator cages, temperature was maintained at 25 °C, and lighting was a constant 12-h light/dark cycle. For the first 7 weeks of life mice were given water and fed standard chow (4.4% gm% fat, Harlan Teklad Rodent Diet 8604, Madison, WI) ad libitum. The Animal Care and Use Committee of Texas A&M University approved all animal protocols.
Composition of Experimental Diets
Defined high fat and control pelleted diets were obtained commercially (Research Diets, Inc., New Brunswick, NJ). The defined control diet (# D12450B, Research Diets, Inc., New Brunswick, NJ) was comprised of 20, 70, and 10 kcal% protein, carbohydrate and fat, respectively, with caloric value of 4,057 kcal/1,055 g or 3.85 kcal/g diet (Table 1). The control diet contained 10 kcal% (4.3 gm%) as fat comprised of soybean oil and lard in nearly equal weight proportions. The isocaloric high-fat diet (# D12451, Research Diets, New Brunswick, NJ) was comprised of 20, 35, and 45 kcal% protein, carbohydrate and fat, respectively, with caloric value of 4,057 kcal/858 g or 4.73 kcal/g (Table 1). Fat was increased to 45 kcal% (24 gm%) by increasing the amount of lard (but not soybean oil) and decreasing the amount of carbohydrate. Both control and high-fat diets had very low cholesterol, 0.002 and 0.02%, respectively, by weight. Fatty acids in the control diet were 25.1, 34.7, and 40.2% saturated, monounsaturated, and polyunsaturated fatty acids, respectively (Table 1), while fatty acid composition in the high-fat diet was comprised of 36.3, 45.3, and 18.5% saturated, monounsaturated, and polyunsaturated fatty acids, respectively (Table 1). Thus, the high-fat diet had more saturated and monounsaturated concomitant with less polyunsaturated fatty acids.
Table 1.
Ingredient and fatty acid group percent composition in control and high fat diets
| Group | Control diet | High fat diet |
|---|---|---|
| Ingredient gm% (kcal%) | ||
| Protein | 19.2 (20) | 24 (20) |
| Carbohydrate | 67.3 (70) | 41 (35) |
| Fat | 4.3 (10) | 24 (45) |
| Fatty acid (%) | ||
| Saturated | 25.1 | 36.3 |
| Monounsaturated | 34.7 | 45.3 |
| Polyunsaturated | 40.2 | 18.5 |
Dietary Study
One week before beginning the dietary studies, male and female mice (7 weeks old, 20–30 g) were switched to a pelleted defined control diet (10 kcal% fat; # D12450B, Research Diets, Inc., New Brunswick, NJ) described above (Table 1), a diet free of significant amounts of phytoestrogens or cholesterol [31] that could complicate any high-fat effect and sex-based comparisons [32]. Each mouse was housed individually in Tecniplast Sealsafe IVC cages equipped with external water bottles and lid holders with wire bars for holding food pellets. After 1 week of acclimation on the control diet, pair-feeding of the high-fat diet for 12 weeks was accomplished as follows: male and female WT and L-FABP KO mice were divided into four groups of 14 mice each: male L-FABP KO, male L-FABP WT, female L-FABP KO, and female L-FABP WT mice. On day one of the feeding study, the amount of control diet consumed as determined by weight and calorie content was measured in one half of the mice in each group. On day two, the other half of each group were pair-fed (1 day offset) the defined high-fat diet based on the amount of control diet consumed by the first half. Each day in the 12-week study body weights of mice from each group were recorded and the amount of high fat and control diet consumed was measured to ensure that each paired group was given food that by weight and calorie was similar to within a non-significant margin of the control group from the previous day. Briefly, at mid-morning daily, each mouse was weighed, and food intake was measured by weighing remaining pellets in the food holder plus any gathered from bedding. The latter were easily recovered since the food was color-coded (yellow for control food, red for high-fat food). At the end of 12 weeks, mice were fasted overnight 12 h, to assure that liver was not influenced by recent digestion and to give more uniform liver weights. Mice were then anesthetized by intraperitoneal (IP) injection of a mixture of ketamine and xylazine (0.01 mL/g body weight; 10 mg ketamine/mL and 1 mg xylazine/mL in 0.9% saline solution), weighed, and examined for whole body phenotype (see below). The anesthetized mice were then euthanized by cervical dislocation, serum collected, and livers harvested, weighed, snap-frozen on dry ice, and stored at −80 °C for mRNA analyses as described [33].
Whole-Body Phenotype
The whole body phenotype was analyzed at the beginning and end of the study by dual-energy X-ray absorptiometry (DEXA) utilizing a Lunar PIXImus densitometer (Lunar Corp., Madison, WI). DEXA determined the relative proportion of fat tissue mass (FTM) and bone-free lean tissue mass (LTM) as described earlier [34]. Prior to PIXImus analysis each mouse was anesthetized as described in the previous section. To facilitate recovery after the procedure, mice were injected with yohimbine (0.11 μg/g body weight), as well as with warm saline solution for rehydration. Mice were kept warm during recovery with heat pads to minimize heat loss, and checked every 10 min until recovery was complete. Whole body composition was determined by exposing the mouse (except the head region) to sequential beams of low- and high-energy X-rays with image acquisition of the X-rays impacting a luminescent panel. Bone mass was separated from soft tissue mass by measurement of the ratios of signal attenuation at the different energy levels. Soft tissue mass was further resolved into lean tissue mass (LTM) and fat tissue mass (FTM) for accurate measurement of whole body composition. Calibration was performed using a phantom mouse with known bone mineral density and FTM as described in Refs. [33, 34]. Data were expressed as gain in FTM and LTM at the end of the dietary study compared to the beginning as described earlier [34].
Serum β-Hydroxybutyrate Measurement
Serum levels of the ketone body β-hydroxybutyrate, a product of acetyl CoA produced from fatty oxidation, can be used to measure fatty acid oxidation in the liver [26, 28, 29, 34]. Serum levels of β-hydroxybutyrate (3-HB) were measured with high sensitivity and specificity according to the manufacturer’s directions (Autokit 3HB, Wako Diagnostics, Richmond, VA) by measuring the rate of Thio-NADH (β-thionicotinamide adenine dinucleotide) production spectrophotometrically at 405 nm upon oxidation of 3-HB.
Liver Lipid Content
Liver lipids were extracted, resolved into individual lipid classes, and quantitated as described earlier [34].
Real-Time PCR of Mouse Liver RNA
Real-time PCR (rtPCR) was performed on total RNA from liver isolated and purified with RNeasy mini kits (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Total concentration of RNA extracts was determined spectrophotometrically followed by confirmation of RNA integrity by agarose electrophoresis and ethidium bromide staining. Expression patterns were analyzed by quantitative real-time PCR using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA), TaqMan® One Step PCR Master Mix Reagent kit, as well as gene specific TaqMan® PCR probes and primers. Thermal cycler protocol was as follows: 48 °C for 30 min, 95 °C for 10 min before the first cycle, 95 °C for 15 s, and 60 °C for 1 min, repeated 40 times. TaqMan® One Step chemistry was used for total RNA reverse-transcription in the first step of the thermal cycler protocol (48 °C for 30 min) prior to amplification. Specific probes and primers were Assay-on-Demand® products for mouse: (i) Peroxisomal fatty acid oxidative enzymes: palmitoyl CoA oxidase-1 (ACO-1, Mm00443579_m1), branched chain CoA oxidase-2 (ACO-2, Mm00446408_m1), pristanoyl CoA oxidase-3 (ACO-3, Mm00446122_m1), and L-peroxisomal bifunctional enzyme (PBE, Mm00470091_m1); (ii) Mitochondrial fatty acid oxidative enzymes: carnitine palmitoyl CoA acyltransferase 1 (CpT1, Mm00550438_m1) and mitochondrial medium chain acyl CoA dehydrogenase (MCAD; Mm00431617_m1); and (iii) ER lipid metabolic enzymes including cytochrome p450, family 4, subfamily A, polypeptide 3 (CYP4A3, Mm00484132_m1). Probes and primers were from Applied Biosystems (Foster City, CA). Experiments were performed in triplicate and analyzed with ABI Prism 7000 SDS software (Applied Biosystems) to determine the threshold cycle (CT) from each well. Primer concentrations and cycle number were optimized to ensure that reactions were analyzed in the linear phase of amplification. The mRNA expression levels of ACO-1, ACO-2, ACO-3, PBE, CPT-1, MCAD, and CYP4A3 determined in all groups were normalized to the housekeeping gene coding for 18S RNA. All mRNA data were expressed relative to the control male wild type mouse group on control diet). Relative expression levels were determined by the comparative 2−ΔΔCT method [35] where ΔΔCT = [CT of target gene − CT of 18 s]different mice groups − [CT of target gene − CT of 18 s]control mouse group as described in User Bulletin 2, ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA).
Statistical Analysis
Data analysis was performed on eight dietary groups consisting of seven mice/group: male control-fed WT, female control-fed WT, male control-fed L-FABP KO, female control-fed L-FABP KO, male high fat control WT, female high fat control WT, male high fat L-FABP KO, and female high fat L-FABP KO mice. All data were analyzed by analysis of variance (ANOVA) combined with the Newman-Keuls multiple comparisons test (GraphPad Prism, San Diego, CA). Values represent the mean ± SEM, with p < 0.05 considered statistically significant.
Results
Effect of High-Fat Diet and L-FABP Gene Ablation on Food Consumption
To ensure that any alterations in weight gain were not due to potential differences in food preference, mice were pair-fed as described in “Materials and Methods”. Under pair-feeding conditions, one half of the mice in each group were provided access to the same amount of the high-fat diet as consumed each previous day by the other half of the mice on the control diet. The overall result was that total food consumption was similar whether the mice were fed the high-fat or the control diet.
Since the caloric densities of the control and high fat diet were similar, each paired group was given food that by weight and calorie content was similar to within a non-significant margin of the control group from the previous day. Total food consumption of each mouse was measured daily and summed over the 12-week dietary to show that total gram mass and kcal of control or high-fat diets consumed by WT males did not differ from that of WT females (data not shown). Likewise, L-FABP gene ablation did not significantly affect total gram mass or total kcal of control or high-fat diet consumed by either male or females. Thus, all groups consumed the same amount of food and any differences in weight gain or obesity were not due to differences in food consumption.
Effect of High-Fat Diet and Sex on Weight Gain and Body Weight in Wild-Type Mice
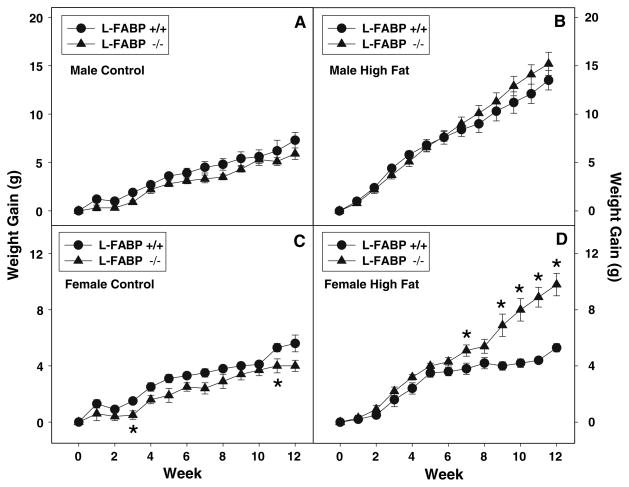
While control-fed WT male (Fig. 1a, solid circles) and female (Fig. 1c, solid circles) mice gained weight with increasing time on the control defined diet, weights were higher for males (0.093 ± 0.008 g/day) than females (0.067 ± 0.007 g/day). The final body weight of WT males (Fig. 2a) was 1.3-fold significantly higher than that of females (Fig. 2b), but males started with higher initial body weights such that there was only a slight difference in weight gain when expressed as a percent gain (Fig. 2c).
Fig. 1.
Weekly weight gain in response to high-fat diet and L-FABP gene ablation. Weight gain of male (a, b) and female (c, d) WT and L-FABP KO mice fed a defined control-diet (a, c) or high-fat diet (b, d) was measured daily and summed weekly as described in “Materials and Methods”. Solid circles refer to WT mice while solid triangles refer to L-FABP KO mice. Values represent the mean ± sem, n = 5–7. Statistical analysis was as follows: * p ≤ 0.05 versus WT mice on high fat diet
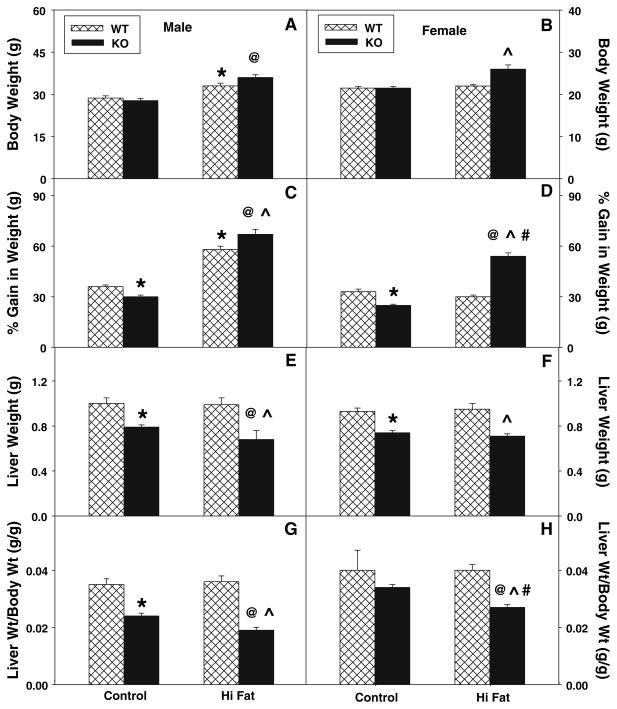
Fig. 2.
Effect of L-FABP gene ablation and high-fat diet on final body weight and liver weight. Final body weight (a, b) and % gain in body weight (c, d) were determined for male (a, c) and female (b, d) WT (hatched bar) and L-FABP KO (solid bar) mice fed defined control-diet or high-fat diet for 12 weeks as described in “Materials and Methods”. After killing, liver weights (e, f) and liver weight/body weight (g, h) were determined for male (e, g) and female (f, h) WT and L-FABP KO mice as in “Materials and Methods”. Values represent the mean ± sem, n = 5–7. Statistical analysis was as follows: * p ≤ 0.05 versus WT mice on control-diet; @ p ≤ 0.001 versus L-FABP KO mice on control-diet; ^ p ≤ 0.001 versus WT mice on high-fat diet; and # p ≤ 0.003 versus male L-FABP KO on high-fat diet
On the high-fat diet, both WT males (Fig. 1b, solid circles) and females (Fig. 1d, solid circles) also gained weight with increasing time, with males gaining markedly more weight than on control food. WT males on the high-fat diet gained weight at nearly twofold faster rate (0.18 ± 0.01 g/day) than on the control diet (0.09 ± 0.01 g/day). In contrast, WT females on the high-fat diet gained weight at a similar rate (0.063 ± 0.004 g/day) to control diet (0.067 ± 0.007 g/day). Consequently, at the end of the 12-week study, WT males weighed much more, regardless of whether expressed as total body weight (Fig. 2a) or as % change in weight (Fig. 2c), than their WT female counterparts (Fig. 2b, d). However, these changes were not due to differences in food consumption (data not shown). Thus, WT males gained weight faster, regardless of control or high-fat diet, and were more sensitive to weight gain induced by high-fat diet than their WT female counterparts.
Effect of High-Fat Diet and Sex on Weight Gain and Body Weight in L-FABP Knockout Mice
L-FABP gene ablation did not significantly affect the rate of weight gain in males (Fig. 1a, solid triangles) fed the control diet. While L-FABP gene ablation trended to slightly decrease the rate of weight gain in females (Fig. 1c, solid triangles) on the control diet, this effect did not achieve statistical significance. The lack of effect of L-FABP gene ablation on rate of weight gain on control diet was reflected in only slight or no differences in final body weight (Fig. 2a, b) and % change in body weight (Fig. 2c, d) in male or female L-FABP KO mice as compared to their WT counterparts. In contrast, L-FABP gene ablation significantly increased weight gain in response to the high fat diet, most prominently in females (Fig. 1d, solid triangles) where significant differences were observed in weeks 9–12 between the high-fat fed female KO versus WT mice. However, while weight gain in L-FABP KO males trended higher than for WT males, the rate did not achieve statistical significance at p < 0.05 (Fig. 1b). In contrast, L-FABP KO females gained weight nearly 1.8-fold faster than WT females on the same high fat, 12-week diet (Fig. 1d). As a result, at the end of the 12-week high-fat diet, L-FABP KO females weighed significantly more than their WT female counterparts, expressed as total body weight (Fig. 2b) and as % change in weight (Fig. 2d). However, as previously noted, because the dietary study was pair-fed, weight gain differences were not due to differences in food consumption.
Taken together, these data indicate that L-FABP gene ablation increases sensitivity, particularly of female mice, to high-fat diet induced weight gain.
Effect of High-Fat Diet and Sex on Liver Weight
While high-fat diet did not affect liver weights (Fig. 2e, f) or the ratio of liver weight/body weight (Fig. 2g, h) in either male or female WT mice, L-FABP gene ablation significantly altered these parameters. In control-fed mice, L-FABP gene ablation decreased the liver weight in both males and females (Fig. 2e, f), and the ratio of liver weight/body weight was also decreased in males (Fig. 2g) as compared to their WT counterparts. The high-fat diet further decreased liver weight (Fig. 2e, f) slightly and decreased the ratio of liver weight/body weight (Fig. 2g, h) in both L-FABP KO males and females as compared to their respective control-fed groups. Thus, L-FABP gene ablation decreased absolute liver mass (g) in both males and females on the control diet. The high-fat diet exacerbated these decreases in both L-FABP KO males and females.
Although high fat diet and/or L-FABP gene ablation elicited small changes in content of several individual lipid classes, there was little effect on the total level of liver lipids indicative of steatosis, primarily the neutral lipids (triglyceride + cholesterol ester). Neither isocaloric pair feeding of high fat diet nor L-FABP gene ablation significantly elevated the total level of neutral lipids indicative of steatosis in male mice (Table 2). Neutral lipid content of livers from female mice, regardless of diet or genotype, was generally about twofold higher in female than male mice (Table 2). However, neither isocaloric pair feeding of high fat diet nor L-FABP gene ablation significantly elevated these lipids in female mice (Table 2). Thus, pair-feeding of isocaloric high fat diet did not induce hepatic steatosis in either male or female mice. Likewise, L-FABP gene ablation did not induce hepatic steatosis—regardless of the diet.
Table 2.
Effect of high fat diet and L-FABP gene ablation on liver lipid content
| Sex | Gene type | Diet | Lipid mass (nmol/mg protein) |
|||||
|---|---|---|---|---|---|---|---|---|
| PL | C | FFA | TG | CE | Neutral lipids | |||
| Male | WT | Control | 433 ± 23 | 32 ± 3 | 45 ± 4 | 454 ± 45 | 30 ± 4 | 484 ± 45 |
| Male | KO | Control | 392 ± 30 | 25 ± 4 | 30 ± 5 | 353 ± 33 | 28 ± 4 | 381 ± 33 |
| Male | WT | High fat | 417 ± 22 | 36 ± 4 | 23 ± 2 | 429 ± 28 | 46 ± 5 | 476 ± 29 |
| Male | KO | High fat | 446 ± 43 | 37 ± 3 | 25 ± 2 | 469 ± 37 | 46 ± 6 | 515 ± 38 |
| Female | WT | Control | 486 ± 45 | 30 ± 3 | 35 ± 5 | 907 ± 144 | 104 ± 14 | 1,012 ± 145 |
| Female | KO | Control | 546 ± 63 | 43 ± 5 | 31 ± 4 | 720 ± 62 | 135 ± 25 | 856 ± 67 |
| Female | WT | High fat | 467 ± 33 | 37 ± 3 | 19 ± 2 | 885 ± 53 | 101 ± 8 | 987 ± 54 |
| Female | KO | High fat | 459 ± 22 | 43 ± 3 | 24 ± 2 | 1,069 ± 112 | 143 ± 14 | 1,212 ± 113 |
Lipids were extracted and composition determined as described in “Materials and Methods”. Values represent the mean ± SE (n = 5–8) PL phospholipid, C cholesterol, FFA unesterified (free) fatty acid, TG triacylglyceride, CE cholesterol ester, Neutral Lipids TG + CE
Effect of High-Fat Diet and Sex on Fat and Lean Tissue Mass in Wild-Type Mice
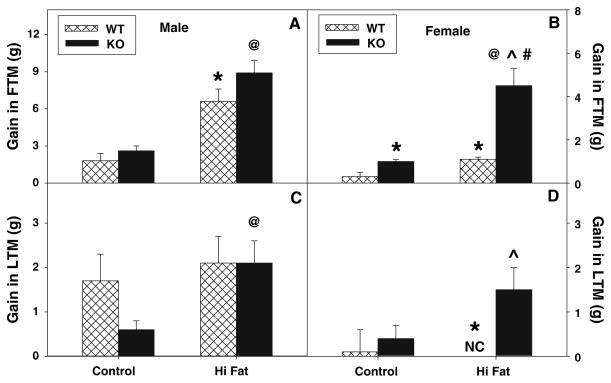
To determine if the above changes in body weight were due to altered fat tissue mass (FTM), lean tissue mass (LTM), or both, dual emission X-ray absorptiometry (DEXA) was performed on each mouse at the beginning and end of the dietary study as described in “Materials and Methods”. Quantitative analysis and comparison of multiple DEXA images taken at the end versus the beginning of the dietary study showed that control-fed male WT mice (Fig. 3a) exhibited a greater proportion of weight gain as FTM than their female counterparts (Fig. 3b). Furthermore, high-fat fed WT males and females had 3.6- and 3.7-fold higher fat gain, respectively (Fig. 3a), as compared to control diet animals. In addition, the control-fed wild-type males gained much more LTM than their female counterparts (Fig. 3d).
Fig. 3.
Effect of L-FABP gene ablation and high-fat diet on whole body phenotype: Gain and percent gain in fat tissue mass (FTM) and lean tissue mass (LTM). At the beginning and end of the 12-week dietary study, FTM and LTM was determined by dual emission X-ray absorptiometry (DEXA) in male and female WT (hatched bar) and L-FABP KO (solid bar) mice fed control- and high-fat diets as described in “Materials and Methods”. Changes in initial and final FTM (a, b) and LTM (c, d) for male (a, c) and female (b, d) WT and L-FABP KO mice were reported as gain in respective tissue mass. Values represent the mean ± sem, n = 5–7. Statistical analysis was as follows: * p ≤ 0.02 versus WT mice on control-diet; @ p ≤ 0.02 versus L-FABP KO mice on control-diet; ^ p ≤ 0.006 versus WT mice on high-fat diet; and # p ≤ 0.001 versus male L-FABP KO mice on high-fat diet
In summary, these data indicated that both WT male and female mice were more susceptible to fat weight gain on a high-fat diet than control diet.
Effect of L-FABP Gene Ablation on Fat and Lean Tissue Mass in Response to A High-Fat Diet
DEXA analysis revealed that the gain in FTM in L-FABP KO male and female mice exhibited a similar qualitative pattern as their WT counterparts on the control diet (Fig. 3), but L-FABP gene ablation increased FTM in control and high-fat fed female mice with a trend towards increase observed for the male KO mice (Fig. 3a). LTM was also increased in the control and high-fat female (Fig. 3d) but not male (Fig 3c) KO mice.
Thus with a control diet, L-FABP gene ablation increased both fat and lean tissue mass in both males and females as compared to WT mice on the same diet. In response to high-fat diet, both L-FABP KO males and females gained more weight as fat, as well as lean tissue mass than their WT counterparts, with more gain observed in females (Fig. 3b) than males (Fig. 3a). The absolute gain observed in FTM was highest for high-fat fed WT and L-FABP KO males (Fig. 3a). In contrast, the largest fold increase in FTM was measured in high-fat fed L-FABP null females (4.5-fold) when compared to fold increases observed with male counterparts (3.4–3.7-fold). In LTM, the high-fat fed L-FABP KO males and females gained similar amounts as compared to control-fed L-FABP KO counterparts.
Effect of High Fat Diet and L-FABP Gene Ablation on Fatty Acid Oxidation: Serum β-Hydroxybutyrate Level
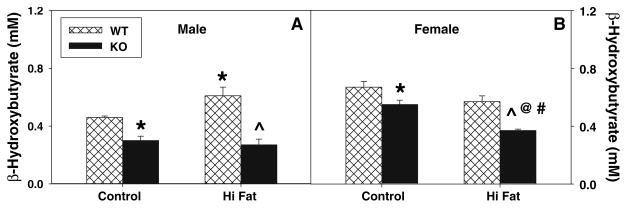
In control-fed WT females, serum β-hydroxybutyrate levels were about 50% higher than males (Fig. 4a), indicating higher hepatic fatty acid oxidation. The high-fat diet increased serum β-hydroxybutyrate levels in male mice to levels indistinguishable from control-fed WT female mice, but similar results were not observed with high-fat fed WT females (Fig. 4b). In control-fed mice, L-FABP gene ablation resulted in decreased serum β-hydroxybutyrate levels in both males and females (Fig. 4), and the high-fat diet produced further decreases, most markedly in females.
Fig. 4.
Effect of L-FABP gene ablation and high-fat diet on serum β-hydroxybutyrate levels. At the end of the dietary study, serum was collected and levels of β-hydroxybutyrate were determined for male (a) and female (b) WT and L-FABP KO mice as described in “Materials and Methods”. Cross-hatched and solid bars refer to WT and KO mice, respectively. Values represent the mean ± sem, n = 5–7. Statistical analysis was as follows: * p ≤ 0.03 versus WT mice on control-diet; @ p ≤ 0.002 versus L-FABP KO mice on control-diet; ^ p ≤ 0.007 versus WT mice on high-fat diet; and # p ≤ 0.05 versus male L-FABP KO mice on high-fat diet
Taken together, these data indicated that L-FABP gene ablation decreased fatty acid oxidation—an effect magnified by a high-fat diet.
Effect of High-Fat Diet and L-FABP Gene Ablation on Hepatic Levels of Peroxisomal Enzymes Involved in Fatty Acid Oxidation
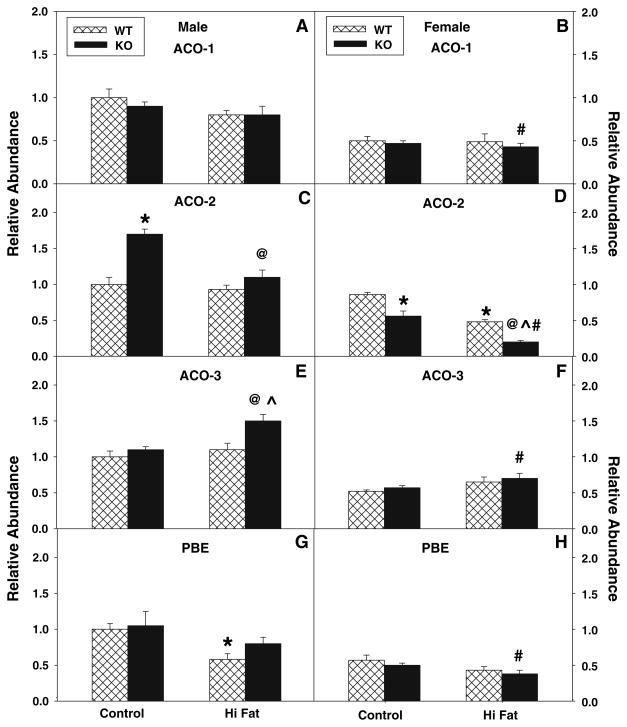
Since decreased serum levels of β-hydroxybutyrate indicated that L-FABP gene ablation reduced fatty acid oxidation, especially in response to high-fat diet, mRNA levels of four key enzymes involved in peroxisomal fatty acid oxidation were examined: acyl CoA oxidase-1 (ACO-1, the key enzyme in peroxisomal β-oxidation of straight-chain fatty acids); acyl CoA oxidase-2 (ACO-2, involved in branched-chain fatty acid oxidation); acyl CoA oxidase-3 (ACO-3, involved in oxidation of pristanoyl-CoA—a branched-chain acyl CoA), and peroxisomal bifunctional enzyme (PBE). In control-fed wild-type mice, peroxisomal levels of ACO-1 (Fig. 5a, b), ACO-3 (Fig. 5c, d) and PBE (Fig. 5g, h) were significantly lower (about twofold each) in females versus males. The high-fat diet did not increase the levels of these proteins in either sex as compared to their control-fed, sex-matched counterparts.
Fig. 5.
Effect of L-FABP gene ablation and high-fat diet on key enzymes in hepatic peroxisomal fatty acid oxidation. At the end of the 12-week dietary study, livers were harvested and expression of the key peroxisomal enzymes involved in peroxisomal fatty acid oxidation was measured by real time PCR as described in “Materials and Methods”. Acyl CoA oxidase-1 (ACO-1, a, b); acyl CoA oxidase-2 (ACO-2, c, d); acyl CoA oxidase-3 (ACO-3, e, f); and bifunctional enzyme (PBE, g, h) were determined for male (a, c, e, g) and female (b, d, f, h) mice as described in “Materials and Methods”. Cross-hatched and solid bars refer to WT and KO mice, respectively. Values represent the mean ± sem, n = 5–7. Statistical analysis was as follows: * p ≤ 0.02 versus WT mice on control-diet; @ p ≤ 0.002 versus L-FABP KO mice on control-diet; ^ p ≤ 0.03 versus WT mice on high-fat diet; and # p ≤ 0.01 versus male L-FABP KO mice on high-fat diet
L-FABP gene ablation did not significantly alter levels of ACO1 in control-fed or high-fat fed mice (Fig. 5a, b). In contrast, L-FABP gene ablation increased the level of ACO-2 in control-fed male mice (Fig. 5c), but decreased the level of ACO-2 in control-fed and high-fat fed females (Fig. 5d) when compared to the correspondingly fed WT mice. ACO-3 level was increased by L-FABP gene ablation in high-fat fed L-FABP KO male mice (Fig. 5e). Levels of PBE were not significantly altered by L-FABP gene ablation in either male (Fig. 5g) or female (Fig. 5h) mice fed control (or high-fat) diet as compared to similar diet fed wild-type mice.
Taken together, these data indicated that, regardless of diet, the male WT and L-FABP KO mice exhibited a greater abundance of several key peroxisomal enzymes (ACO-1, ACO3, PBE) involved in fatty acid oxidation as compared to their female counterparts. L-FABP gene ablation upregulated the expression of several of these oxidative enzymes (ACO-2 in control-fed males; ACO-3 in high-fat fed males), but did not change or slightly decreased the levels of these and other fatty acid oxidative enzymes depending on diet. Thus, the decreased fatty acid oxidation indicated by decreased serum levels of β-hydroxybutyrate in L-FABP KO mice was not associated with decreased expression of most peroxisomal proteins involved in fatty acid oxidation.
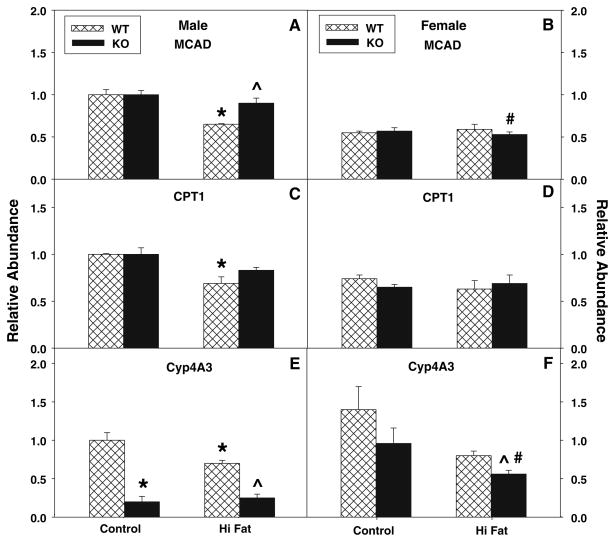
Effect of High-Fat Diet and L-FABP Gene Ablation on Hepatic Levels of Mitochondrial Enzymes Involved in Fatty Acid Oxidation
Carnitine palmitoyl transferase-1 (CPT-1, located at the outer mitochondrial membrane), is the rate-limiting enzyme involved in import of straight-chain fatty acids into mitochondria for β-oxidation, while medium chain acyl CoA dehydrogenase (MCAD) is a key enzyme involved in β-oxidation of straight-chain fatty acids within mitochondria. MCAD (Fig. 6a, b) and CPT-1 (Fig. 6c, d) levels were substantially lower in control-fed WT females than males. The high-fat diet decreased expression of MCAD (Fig. 6a) and CPT1 (Fig. 6c) in males, but not females. L-FABP gene ablation did not significantly affect MCAD (Fig. 6a, b) or CPT-1 (Fig. 6c, d) in control-fed males or females. On the high-fat diet, similar findings were obtained with L-FABP KO females, while MCAD (Fig. 6a), but not CPT-1 (Fig. 6c), was increased in the L-FABP KO males. Therefore, decreased fatty acid oxidation shown as decreased serum levels of β-hydroxybutyrate in L-FABP gene-ablated mice on control and high-fat diets was also not associated with diminution of key enzymes involved in mitochondrial fatty acid β-oxidation.
Fig. 6.
Effect of L-FABP gene ablation and high-fat diet on liver levels of key enzymes in mitochondrial fatty acid oxidation and microsomal lipid oxidation. At the end of the 12-week dietary study, livers were harvested and expression levels of two key enzymes involved in mitochondrial fatty acid oxidation: medium chain acyl CoA dehydrogenase (MCAD, a, b), carnitine palmitoyl transferase-1 (CPT-1, c, d), and a key microsomal enzyme involved in lipid oxidation: cytochrome p4A3 (CYP4A3, e, f) were measured by real time PCR in male (a, c, e) and female (b, d, f) mice as described in “Materials and Methods”. Cross-hatched and solid bars refer to WT and KO mice, respectively. Values represent the mean ± sem, n = 5–7. Statistical analysis was as follows: * p ≤ 0.04 versus WT mice on control-diet; ^ p ≤ 0.009 versus WT mice on high-fat diet; and # p ≤ 0.005 versus male L-FABP KO mice on high-fat diet
Effect of High-Fat Diet and L-FABP Gene Ablation on Hepatic Levels of Microsomal Enzymes Involved in Lipid Metabolism
The relative abundance of Cytochrome P450 4A3 (CYP4A3), an important ER enzyme participating in microsomal lipid oxidation, was also measured. High-fat diet and L-FABP gene ablation decreased levels of CYP4A3 in both male and female mice (Fig. 6e, f) showing that L-FABP gene ablation did not increase levels of a key ER enzyme involved in lipid oxidation.
Discussion
A major role of liver is to take up and effectively metabolize dietary long chain fatty acids (LCFAs) after intestinal absorption (rev. in [1, 22, 23]). LCFAs as well as their CoA thioesters are known to be potent detergents that dissolve membranes and inhibit the action of multiple receptors and enzymes (rev. in [1, 7, 36, 37]). To prevent these adverse effects, mammals have evolved a family of cytosolic proteins with sufficiently high affinity for these ligands that intracellular levels of unbound free fatty acid and fatty acyl CoA are maintained at a low level, i.e. nM range (rev. in [1, 37–40]). High levels of liver fatty acid binding protein (L-FABP) representing 2–5% of cytosolic protein (0.1–0.4 mM) are expressed in tissues very active in long chain fatty acid (LCFA) uptake, oxidation and metabolism—especially liver, intestine, and kidney (rev. in [1]). Studies performed in vitro show that L-FABP facilitates uptake of fatty acids from membranes [7–11], removes substrate inhibition of carnitine palmitoyltransferase-1 (rate limiting step in mitochondrial LCFA oxidation) by palmitoyl CoA [12, 13], and functions as a LCFA donor protein for both peroxisomal and mitochondrial LCFA oxidation in vitro [14, 15]. Other studies with L-FABP overexpressing transformed cells have further established the potential functional significance of L-FABP in facilitating LCFA uptake [1, 16–19], intracellular transport/diffusion [1, 17], oxidation [20], and esterification [16, 18, 21]. The physiological significance and relevance of L-FABP to LCFA and LCFA-CoA oxidation and esterification was confirmed by numerous studies utilizing L-FABP gene-ablated mice where hepatic LCFA uptake in vivo and in cultured hepatocytes exhibited reduced hepatic cytosolic binding capacity for LCFAs and LCFA-CoAs, reduced intracellular LCFA transport/diffusion, and decreased hepatic LCFA oxidation [26–30]. Since liver stores relatively little esterified LCFA as lipid droplets, the net effect of these actions of L-FABP would be to facilitate rapid removal and oxidation. On this basis it was hypothesized that L-FABP gene ablation would enhance weight gain and obesity in a high-fat diet situation. To avoid potential complications due to preference for high fat diet, this hypothesis was addressed by pair feeding L-FABP KO mice compared to WT mice on a defined control diet versus isocaloric high-fat diet for 12 weeks as described in “Materials and Methods”. The results provided the following new insights:
First, isocaloric pair feeding of high fat diet elicited sex-dependent body weight gain in male, but not female, mice. Because the mice were pair fed, the greater weight gain of male vs female mice was not due to differences in food consumption. While it was previously established that ad libitum feeding high fat diet itself markedly increases food consumption (as much as 40%) and consequently body weight gain [41–47], the present findings clearly established that weight gain was also greater (at least in males) when the mice were pair fed an isocaloric high fat diet. Thus, consuming the same number of calories as fat (high fat diet) rather than carbohydrate (low fat diet) resulted in increased weight gain in male mice. The greater increase in male mouse body mass was associated with about threefold greater increase in FTM than LTM while female mice isocaloric pair fed high fat diet exhibited increased FTM but not LTM. The molecular basis for the differential response of male versus female mice to isocaloric pair fed high fat diet may be related in part to the sex dependent difference in hepatic L-FABP expression in rodents [31, 48]. Consistent with these findings, sex dependent differences in response to other types of diet (e.g. phytol rich, cholesterol rich, cholestatic, etc.) have also been observed in mice [31, 42–45, 49]. Taken together, these data indicated that the male wild type mice were more efficient in converting both control diet and isocaloric pair fed high fat diet into increased body mass, both FTM and less so LTM.
Second, L-FABP gene ablation elicited sex-dependent body weight gain in female more than male mice. L-FABP gene ablation modestly increased weight gain (primarily as FTM) in control-fed female, but not male, mice. A similar pattern of increased weight gain was also observed in older (>6 mo) female L-FABP KO mice fed ad libitum standard rodent chow [43, 45] or pair-fed a different defined control-diet [42, 43]. Isocaloric pair feeding high fat diet dramatically exacerbated this effect in female, but not male, mice. The increased weight gain in high-fat fed female L-FABP KO mice was not due to differences in food consumption. The molecular basis for the gender dependent effect of L-FABP gene ablation may be related in part to the interdependent relationship between L-FABP and PPARα. The promoter of L-FABP contains a PPRE, L-FABP induces expression of PPARα, and L-FABP transports fatty acids into the nucleus to ligand activate PPARα and induce expression of more L-FABP [39, 40, 50, 51]. Roles for PPARs in sex dependent differences in mouse lipid metabolism have also been shown with PPARα null and PPARβ null mice [50, 52, 53].
Third, the effect of L-FABP gene ablation on diet induced obesity and hepatic steatosis appears to be dependent on both feeding regimen and susceptibility of the mouse background strain to diet induced obesity. Isocaloric pair feeding high fat diet (current study) as well as ad libitum feeding standard rodent chow (low fat) or isocaloric pair-feeding different types of low fat defined control diets [42–45] indicated that L-FABP gene ablation did not protect mice from high-fat diet induced weight gain. In addition, isocaloric pair fed high fat diet mice, regardless of sex of L-FABP expression, did not exhibit hepatic steatosis. In contrast to these studies with wild type C57BL/6NJ mice and L-FABP KO mice backcrossed to the C57BL/6NJ mice, ad libitum feeding of high fat diet to the more obesity susceptible C57BL6J strain induced hepatic steatosis [46, 47]. Surprisingly, ad libitum feeding of high fat diet to L-FABP KO mice backcrossed to the more obesity susceptible C57BL6J strain resulted in protection from high fat diet induced obesity and from hepatic steatosis [46, 47]. The marked differences in response to high fat diet between the two L-FABP KO mouse models were not due to differences in back-cross generation number [54]. Instead, these difference in phenotype may be attributed in part to several factors: (i) Wild type mice fed high fat diet ad libitum consume up to 40% more food and gain as much as 50% more weight [46, 47]. While isocaloric pair feeding high fat diet did not upregulate L-FABP in wild type mice determined by western blotting (data not shown), ad libitum feeding high fat diet is known to activate PPARα—a nuclear receptor that induces transcription of multiple genes in fatty acid metabolism including L-FABP (rev. in [50, 55, 56]). Thus, ad libitum feeding high fat diet may upregulate L-FABP expression in the wild type mice, but not the L-FABP KO mice. (ii) The two independently generated L-FABP KO mice were backcrossed to a different C57BL/6 mouse strains differing markedly in susceptibility to high fat diet induced obesity. The C57BL/6J (Jackson Labs) used to backcross independently generated L-FABP KO mice are more susceptible to high fat diet induced obesity (higher weight gain, impaired glucose tolerance) than mice used for backcrossing in the current study (C57BL/SNJ from Charles River) (JAX NOTES, Issue 511, Fall, 2008, http://jaxmice.jax.org/jaxnotes/511/511n.html) [57–59]. The mechanistic basis for the difference in obesity susceptible of these strains is partly due to the C57BL/6J mouse strain exhibiting a null mutation in the NAD nucleotide transhydrogenase (Nnt) gene which codes for an enzyme that regulates NADH/NAD ratio—an important contributor to LCFA synthesis (JAX NOTES, Issue 511, Fall, 2008, http://jaxmice.jax.org/jaxnotes/511/511n.html). (iii) Overexpression of the green fluorescent protein (GFP) in the independently generated L-FABP KO mouse, but not the wild type control [29, 46, 47]. GFP was recently shown to function as a potent electron donor to several cofactors important in lipid metabolism including cytochrome c, FAD, FMN, and NAD in vitro [60]. Taken together, these findings demonstrate the importance of sex, high fat dietary regimen (pair fed, ad libitum fed), L-FABP KO background strain, and overexpression of GFP as each being important potential contributors to the whole body and hepatic phenotype of L-FABP KO mice fed high fat diet.
Fourth, the finding that L-FABP gene ablation did not induce hepatic steatosis in mice fed either control diet or pair fed high fat diet was consistent with the existing literature of known functions established for L-FABP in vitro, in cultured transformed cells, in cultured primary hepatocytes, and mice. L-FABP KO reduces hepatic uptake of dietary LCFA in vivo [26, 28] and in cultured L-FABP KO hepatocytes [30]. LCFA uptake directly correlates with L-FABP expression level in cultured transfected transformed cell lines [16, 19]. Further support comes from the fact that L-FABP gene ablation reduces LCFA oxidation in vivo as shown by reduced serum β-hydroxybutyrate in mice pair fed high fat diet (current study) and in L-FABP KO mice fed control chow [26, 28, 29], by reduced LCFA oxidation in cultured primary hepatocytes from L-FABP KO [30], and by increased LCFA oxidation in cultured transfected transformed cell lines [16, 19]. Regardless of the back-cross strain utilized to generate the two distinct L-FABP KO mice, inhibition of LCFA β-oxidation was observed in response to L-FABP gene ablation and this inhibition was not associated with major changes in levels of most of the key proteins/enzymes involved in peroxisomal and mitochondrial oxidation.
In summary, the present findings demonstrated that ablation of all four exons of the L-FABP gene and backcrossing to the C57BL/6NJ strain (resistant to high fat diet induced obesity) decreased fatty acid β-oxidation and increased weight gain (especially in fat tissue mass) —effects exacerbated by pair-feeding a defined isocaloric high-fat diet. This phenotype was consistent with predictions based on in vitro and cultured cell studies demonstrating L-FABP increases LCFA uptake (rev. in [1, 26]), LCFA intracellular transport [1, 17, 30, 61, 62], and both mitochondrial and peroxisomal LCFA oxidation [20, 30]. Thus, L-FABP gene ablation did not protect mice from weight gain and obesity induced when fed either control diet or isocaloric pair fed high fat diet, but instead exacerbated weight gain and obesity. While L-FABP gene ablation appeared to protect mice fed high fat diet ad libitum, interpretation of this phenotype is more complex due to much greater food consumption and weight gain concomitant with likely L-FABP upregulation in wild type mice, more obesity sensitive background strain, and presence of GFP in the KO but not wild type mice. Resolving the relative contributions of these factors is an important challenge. It is of interest to note that the pattern of higher weight gain and obesity in L-FABP mice fed high fat diet was also exhibited by adipocyte fatty acid binding protein (A-FABP) gene-ablated mice, which show increased body weight gain as compared with their WT counterparts when fed a high-fat diet [63]. Likewise, intestinal fatty acid binding protein (I-FABP) gene ablation resulted in mice with a higher weight gain than their WT counterparts when fed either a low-fat or a high-fat diet [64]. These findings suggested that in L-FABP KO mice pair fed a high-fat diet, LCFAs are more available for storage (weight gain) rather than hepatic uptake and oxidation (or storage).
Acknowledgments
This work was supported in part by the United States Public Health Service National Institutes of Health grants DK41402 (FS and ABK), GM31651 (FS and ABK), and DK70965 (BPA).
Abbreviations
- L-FABP
Liver fatty acid binding protein
- FTM
Fat tissue mass
- LTM
Lean tissue mass
- WT
Wild-type
- DEXA
Dual emission X-ray absorptiometry
- LCFA
Long chain fatty acids
- rtPCR
Real-time PCR
- ACO-1
Palmitoyl CoA oxidase-1
- ACO-2
Branched chain CoA oxidase-2
- ACO-3
Pristanoyl CoA oxidase-3
- PBE
L-peroxisomal bifunctional enzyme
- CpT1-l
Carnitine palmitoyl CoA acyltransferase-1
- MCAD
Mitochondrial medium chain acyl CoA dehydrogenase
- HMG-CoA Syn
HMG-CoA synthase
- CYP4A3
Cytochrome p450, family 4, subfamily A, polypeptide 3
References
- 1.McArthur MJ, Atshaves BP, Frolov A, Foxworth WD, Kier AB, Schroeder F. Cellular uptake and intracellular trafficking of long chain fatty acids. J Lipid Res. 1999;40:1371–1383. [PubMed] [Google Scholar]
- 2.Richieri GV, Ogata RT, Kleinfeld AM. Equilibrium constants for the binding of fatty acids with fatty acid binding proteins from adipocyte, intestine, heart and liver measured with the fluorescent probe ADIFAB. J Biol Chem. 1994;269:23918–23930. [PubMed] [Google Scholar]
- 3.Frolov A, Cho TH, Murphy EJ, Schroeder F. Isoforms of rat liver fatty acid binding protein differ in structure and affinity for fatty acids and fatty acyl CoAs. Biochemistry. 1997;36:6545–6555. doi: 10.1021/bi970205t. [DOI] [PubMed] [Google Scholar]
- 4.Thompson J, Ory J, Reese-Wagoner A, Banaszak L. The liver fatty acid binding protein-comparison of cavity properties of intracellular lipid binding proteins. Mol Cell Biochem. 1999;192:9–16. [PubMed] [Google Scholar]
- 5.Haunerland NH, Spener F. Fatty acid binding proteins–insights from genetic manipulations. Prog Lipid Res. 2004;43:328–349. doi: 10.1016/j.plipres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 6.He Y, Yang X, Wang H, Estephan R, Francis F, Kodukula S, Storch J, Stark RE. Solution-state molecular structure of apo and oleate-liganded liver fatty acid binding protein. Biochemistry. 2007;46:12543–12556. doi: 10.1021/bi701092r. [DOI] [PubMed] [Google Scholar]
- 7.Jolly CA, Hubbell T, Behnke WD, Schroeder F. Fatty acid binding protein: stimulation of microsomal phosphatidic acid formation. Arch Biochem Biophys. 1997;341:112–121. doi: 10.1006/abbi.1997.9957. [DOI] [PubMed] [Google Scholar]
- 8.Jolly CA, Wilton DA, Schroeder F. Microsomal fatty acyl CoA transacylation and hydrolysis: fatty acyl CoA species dependent modulation by liver fatty acyl CoA binding proteins. Biochim Biophys Acta. 2000;1483:185–197. doi: 10.1016/s1388-1981(99)00170-5. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder F, Jolly CA, Cho TH, Frolov AA. Fatty acid binding protein isoforms: structure and function. Chem Phys Lipids. 1998;92:1–25. doi: 10.1016/s0009-3084(98)00003-6. [DOI] [PubMed] [Google Scholar]
- 10.Chao H, Zhou M, McIntosh A, Schroeder F, Kier AB. Acyl CoA binding protein and cholesterol differentially alter fatty acyl CoA utilization by microsomal acyl CoA: cholesterol transferase. J Lipid Res. 2003;44:72–83. doi: 10.1194/jlr.m200191-jlr200. [DOI] [PubMed] [Google Scholar]
- 11.Nemecz G, Schroeder F. Selective binding of cholesterol by recombinant fatty acid-binding proteins. J Biol Chem. 1991;266:17180–17186. [PubMed] [Google Scholar]
- 12.Woldegiorgis G, Bremer J, Shrago E. Substrate inhibition of carnitine palmitoyltransferase by palmitoyl-CoA and activation by phospholipids and proteins. Biochim Biophys Acta. 1985;837:135–140. doi: 10.1016/0005-2760(85)90236-x. [DOI] [PubMed] [Google Scholar]
- 13.Bhuiyan AKMJ, Pande SV. Carnitine palmitoyltransferase activities: effects of serum albumin, acyl-CoA binding protein and fatty acid binding protein. Mol Cell Biochem. 1994;139:109–116. doi: 10.1007/BF01081733. [DOI] [PubMed] [Google Scholar]
- 14.Reubsaet FA, Veerkamp JH, Bruckwilder ML, Trijbels JM, Monnens LA. The involvement of fatty acid-binding protein in peroxisomal fatty acid oxidation. FEBS Lett. 1990;267:229–230. doi: 10.1016/0014-5793(90)80931-8. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen JT, Faergeman NJ, Kristiansen K, Knudsen J. Acyl-CoA-binding protein (ACBP) can mediate intermembrane acyl-CoA transport and donate acyl-CoA for beta-oxidation and glycerolipid synthesis. Biochem J. 1994;299:165–170. doi: 10.1042/bj2990165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy EJ, Prows DR, Jefferson JR, Schroeder F. Liver fatty acid binding protein expression in transfected fibroblasts stimulates fatty acid uptake and metabolism. Biochim Biophys Acta. 1996;1301:191–198. doi: 10.1016/0005-2760(96)00024-0. [DOI] [PubMed] [Google Scholar]
- 17.Murphy EJ. L-FABP and I-FABP expression increase NBD-stearate uptake and cytoplasmic diffusion in L-cells. Am J Physiol. 1998;275:G244–G249. doi: 10.1152/ajpgi.1998.275.2.G244. [DOI] [PubMed] [Google Scholar]
- 18.Prows DR, Murphy EJ, Schroeder F. Intestinal and liver fatty acid binding proteins differentially affect fatty acid uptake and esterification in L-Cells. Lipids. 1995;30:907–910. doi: 10.1007/BF02537481. [DOI] [PubMed] [Google Scholar]
- 19.Wolfrum C, Buhlman C, Rolf B, Borchers T, Spener F. Variation of liver fatty acid binding protein content in the human hepatoma cell line HepG2 by peroxisome proliferators and anti-sense RNA affects the rate of fatty acid uptake. Biochim Biophys Acta. 1999;1437:194–201. doi: 10.1016/s1388-1981(99)00008-6. [DOI] [PubMed] [Google Scholar]
- 20.Atshaves BP, Storey S, Huang H, Schroeder F. Liver fatty acid binding protein expression enhances branched-chain fatty acid metabolism. Mol Cell Biochem. 2004;259:115–129. doi: 10.1023/b:mcbi.0000021357.97765.f2. [DOI] [PubMed] [Google Scholar]
- 21.Jefferson JR, Slotte JP, Nemecz G, Pastuszyn A, Scallen TJ, Schroeder F. Intracellular sterol distribution in transfected mouse L-cell fibroblasts expressing rat liver fatty acid binding protein. J Biol Chem. 1991;266:5486–5496. [PubMed] [Google Scholar]
- 22.Veerkamp JH, van Moerkerk HT. Fatty acid-binding protein and its relation to fatty acid oxidation. Mol Cell Biochem. 1993;123:101–106. doi: 10.1007/BF01076480. [DOI] [PubMed] [Google Scholar]
- 23.Veerkamp JH, van Moerkerk HT. The fatty acid-binding protein content and fatty acid oxidation capacity of rat tissues. Prog Clin Biol Res. 1992;375:205–210. [PubMed] [Google Scholar]
- 24.Vanden Heuvel JP, Sterchele PF, Nesbit DJ, Peterson RE. Coordinate induction of acyl-CoA binding protein, fatty acid binding protein and peroxisomal β-oxidation by peroxisome proliferators. Biochem Biophys Acta. 1993;1177:183–190. doi: 10.1016/0167-4889(93)90039-r. [DOI] [PubMed] [Google Scholar]
- 25.Brandes R, Kaikaus RM, Lysenko N, Ockner RK, Bass NM. Induction of fatty acid binding protein by peroxisome proliferators in primary hepatocyte cultures and its relationship to the induction of peroxisomal beta-oxidation. Biochim Biophys Acta. 1990;1034:53–61. doi: 10.1016/0304-4165(90)90152-m. [DOI] [PubMed] [Google Scholar]
- 26.Martin GG, Danneberg H, Kumar LS, Atshaves BP, Erol E, Bader M, Schroeder F, Binas B. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid binding protein (L-FABP) gene. J Biol Chem. 2003;278:21429–21438. doi: 10.1074/jbc.M300287200. [DOI] [PubMed] [Google Scholar]
- 27.Martin GG, Huang H, Atshaves BP, Binas B, Schroeder F. Ablation of the liver fatty acid binding protein gene decreases fatty acyl CoA binding capacity and alters fatty acyl CoA pool distribution in mouse liver. Biochemistry. 2003;42:11520–11532. doi: 10.1021/bi0346749. [DOI] [PubMed] [Google Scholar]
- 28.Erol E, Kumar LS, Cline GW, Shulman GI, Kelly DP, Binas B. Liver fatty acid-binding protein is required for high rates of hepatic fatty acid oxidation but not for the action of PPAR-a in fasting mice. FASEB J. 2004;18:347–349. doi: 10.1096/fj.03-0330fje. [DOI] [PubMed] [Google Scholar]
- 29.Newberry EP, Xie Y, Kennedy S, Buhman KK, Luo J, Gross RW, Davidson NO. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid binding protein gene. J Biol Chem. 2003;278:51664–51672. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- 30.Atshaves BP, McIntosh AL, Lyuksyutova OI, Zipfel WR, Webb WW, Schroeder F. Liver fatty acid binding protein gene ablation inhibits branched-chain fatty acid metabolism in cultured primary hepatocytes. J Biol Chem. 2004;279:30954–30965. doi: 10.1074/jbc.M313571200. [DOI] [PubMed] [Google Scholar]
- 31.Atshaves BP, McIntosh AL, Payne HR, Mackie J, Kier AB, Schroeder F. Effect of branched-chain fatty acid on lipid dynamics in mice lacking liver fatty acid binding protein gene. Am J Physiol. 2005;288:C543–C558. doi: 10.1152/ajpcell.00359.2004. [DOI] [PubMed] [Google Scholar]
- 32.Thigpen JE, Setchell KD, Goelz MF, Forsythe DB. The phyto estrogen content of rodent diets. Envron Health Persp. 1999;107:A182–A183. doi: 10.1289/ehp.107-1566530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atshaves BP, McIntosh AL, Martin GG, Landrock D, Payne HR, Bhuvanendran S, Landrock K, Lyuksyutova OI, Johnson JD, Macfarlane RD, Kier AB, Schroeder F. Overexpression of sterol carrier protein-2 differentially alters hepatic cholesterol accumulation in cholesterol-fed mice. J Lipid Res. 2009;50:1429–1447. doi: 10.1194/jlr.M900020-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atshaves BP, Payne HR, McIntosh AL, Tichy SE, Russell D, Kier AB, Schroeder F. Sexually dimorphic metabolism of branched chain lipids in C57BL/6J mice. J Lipid Res. 2004;45:812–830. doi: 10.1194/jlr.M300408-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Powell GL, Tippett PS, Kiorpes TC, McMillin-Wood J, Coll KE, Schultz H, Tanaka K, Kang ES, Shrago E. Fatty acyl CoA as an effector molecule in metabolism. Fed Proc. 1985;44:81–84. [Google Scholar]
- 37.Gossett RE, Frolov AA, Roths JB, Behnke WD, Kier AB, Schroeder F. Acyl CoA binding proteins: multiplicity and function. Lipids. 1996;31:895–918. doi: 10.1007/BF02522684. [DOI] [PubMed] [Google Scholar]
- 38.Faergeman NJ, Knudsen J. Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem J. 1997;323:1–12. doi: 10.1042/bj3230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang H, Starodub O, McIntosh A, Kier AB, Schroeder F. Liver fatty acid binding protein targets fatty acids to the nucleus: real-time confocal and multiphoton fluorescence imaging in living cells. J Biol Chem. 2002;277:29139–29151. doi: 10.1074/jbc.M202923200. [DOI] [PubMed] [Google Scholar]
- 40.Huang H, Starodub O, McIntosh A, Atshaves BP, Woldegiorgis G, Kier AB, Schroeder F. Liver fatty acid binding protein colocalizes with peroxisome proliferator receptor alpha and enhances ligand distribution to nuclei of living cells. Biochemistry. 2004;43:2484–2500. doi: 10.1021/bi0352318. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Botolin D, Xu J, Christian B, Mitchell E, Jayaprakasam B, Nair M, Peters JM, Busik J, Olson LK, Jump DB. Regulation of fatty acid elongase and desaturase expression in diabetes and obesity. J Lipid Res. 2006;47:2028–2041. doi: 10.1194/jlr.M600177-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) gene ablation alters liver bile acid metabolism in male mice. Biochem J. 2005;391:549–560. doi: 10.1042/BJ20050296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) gene ablation potentiates hepatic cholesterol accumulation in cholesterol-fed female mice. Am J Physiol. 2006;290:G36–G48. doi: 10.1152/ajpgi.00510.2004. [DOI] [PubMed] [Google Scholar]
- 44.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein gene ablated female mice exhibit increased age dependent obesity. J Nutr. 2008;138:1859–1865. doi: 10.1093/jn/138.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein gene ablation enhances age-dependent weight gain in male mice. Mol Cell Biochem. 2009;324:101–115. doi: 10.1007/s11010-008-9989-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newberry EP, Kennedy SM, Xie Y, Sternard BT, Luo J, Davidson NO. Diet-induced obesity and hepatic steatosis in L-FABP−/− mice is abrogated with SF, but not PUFA, feeding and attenuated after cholesterol supplementation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G307–G314. doi: 10.1152/ajpgi.00377.2007. [DOI] [PubMed] [Google Scholar]
- 47.Newberry EP, Xie Y, Kennedy SM, Luo J, Davidson NO. Protection against western diet-induced obesity and hepatic steatosis in liver fatty acid binding protein knockout mice. Hepatology. 2006;44:1191–1205. doi: 10.1002/hep.21369. [DOI] [PubMed] [Google Scholar]
- 48.Pignon J-P, Bailey NC, Baraona E, Lieber CS. Fatty acid-binding protein: a major contributor to the ethanol-induced increase in liver cytosolic proteins in the rat. Hepatology. 1987;7:865–871. doi: 10.1002/hep.1840070512. [DOI] [PubMed] [Google Scholar]
- 49.Mackie JT, Atshaves BP, Payne HR, McIntosh AL, Schroeder F, Kier AB. Phytol-induced hepatotoxicity in mice. Toxicol Pathol. 2009;37:201–208. doi: 10.1177/0192623308330789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schroeder F, Petrescu AD, Huang H, Atshaves BP, McIntosh AL, Martin GG, Hostetler HA, Vespa A, Landrock K, Landrock D, Payne HR, Kier AB. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids. 2008;43:1–17. doi: 10.1007/s11745-007-3111-z. [DOI] [PubMed] [Google Scholar]
- 51.McIntosh AL, Atshaves BP, Hostetler HA, Huang H, Davis J, Lyuksyutova OI, Landrock D, Kier AB, Schroeder F. Liver type fatty acid binding protein (L-FABP) gene ablation reduces nuclear ligand distribution and peroxisome proliferator activated receptor-alpha activity in cultured primary hepatocytes. Arch Biochem Biophys. 2009;485:160–173. doi: 10.1016/j.abb.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Djouadi F, Weinheimer CJ, Saffitz JE, Pitchford C, Bastin J, Gonzalez FJ. A gender-related defect in lipid metabolism and glucose homeostasis in PPARalpha deficient mice. J Clin Inv. 1998;102:1083–1091. doi: 10.1172/JCI3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenberger TA, Hovda JT, Peters JM. Targeted disruption of peroxisome proliferator activated receptor beta (delta) results in distinct gender differences in mouse brain phospholipid and esterified fatty acid levels. Lipids. 2002;37:495–500. doi: 10.1007/s11745-002-0923-1. [DOI] [PubMed] [Google Scholar]
- 54.Martin GG, Atshaves BP, Huang H, McIntosh AL, Williams BW, Russell DH, Kier AB, Schroeder F. Hepatic phenotype of liver fatty acid binding protein (L-FABP) gene ablated mice. Am J Physiol. 2009 doi: 10.1152/ajpgi.00116.2009. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hostetler HA, Petrescu AD, Kier AB, Schroeder F. Peroxisome proliferator activated receptor alpha (PPARalpha) interacts with high affinity and is conformationally responsive to endogenous ligands. J Biol Chem. 2005;280:18667–18682. doi: 10.1074/jbc.M412062200. [DOI] [PubMed] [Google Scholar]
- 56.Hostetler HA, Kier AB, Schroeder F. Very-long-chain and branched-chain fatty acyl CoAs are high affinity ligands for the peroxisome proliferator-activated receptor alpha (PPARalpha) Biochemistry. 2006;45:7669–7681. doi: 10.1021/bi060198l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tallman DL, Noto AD, Taylor CG. Low and high fat diets inconsistently induce obesity in C57BL/6J mice and obesity compromises n-3 fatty acid status. Lipids. 2009;44:577–580. doi: 10.1007/s11745-009-3312-8. [DOI] [PubMed] [Google Scholar]
- 58.Koza RA, Nikonova L, Hogan J, Rim JS, Mendoza T, Faulk C, Skaf J, Kozak LP. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2006;2:e81. doi: 10.1371/journal.pgen.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burcelin R, Crivelli V, Dacosta A, Roy-Tirelli A, Thorens B. Heterogeneous metabolic adaptation of C57BL/6J mice to high fat diet. Am J Physiol Endocrinol Metab. 2002;282:E834–E842. doi: 10.1152/ajpendo.00332.2001. [DOI] [PubMed] [Google Scholar]
- 60.Bogdanov AM, Mishin AS, Yampolsky IV, Belousov VV, Chudakov DM, Subach FV, Verkhusha VV, Lukyanov S, Lukyonov KA. Green fluorescent proteins are light-induced electron donors. Nat Chem Biol. 2009;5:459–461. doi: 10.1038/nchembio.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luxon BA, Weisiger RA. Sex differences in intracellular fatty acid transport: role of cytoplasmic binding proteins. Am J Physiol. 1993;265:G831–G841. doi: 10.1152/ajpgi.1993.265.5.G831. [DOI] [PubMed] [Google Scholar]
- 62.Weisiger RA. Cytosolic fatty acid binding proteins catalyze two distinct steps in intracellular transport of their ligands. Mol Cell Biochem. 2005;239:35–42. [PubMed] [Google Scholar]
- 63.Hotamisligl GS, Johnson RS, Distel RJ, Ellis RF, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274:1377–1379. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- 64.Vassileva G, Huwyler L, Poirer K, Agellon LB, Toth MJ. The intestinal fatty acid binding protein is not essential for dietary fat absorption in mice. FASEB J. 2000;14:2040–2046. doi: 10.1096/fj.99-0959com. [DOI] [PubMed] [Google Scholar]