Abstract
Recent high-resolution studies of kinetochore structure have transformed the way researchers think about this crucial macro-molecular complex, which is essential for ensuring chromosome segregation occurs faithfully during cell division. Kinetochores mediate the interaction between chromosomes and the plus-ends of dynamic spindle microtubules and control the timing of anaphase onset by regulating the spindle assembly checkpoint (SAC). There is much debate in the SAC research community as to whether mitotic cells sense only microtubule attachment at the kinetochore, or both attachment and tension, before committing to anaphase. In this Commentary, we present a brief history of the tension-versus-attachment debate, summarize recent advances in our understanding of kinetochore structure and focus on the implications of a phenomenon known as intrakinetochore stretch for SAC regulation. We also hypothesize how intrakinetochore stretch might impact SAC function by regulating both microtubule attachment stability and the localization and activity of checkpoint components at the kinetochore.
Keywords: Mitosis, Kinetochore, Tension, Spindle-assembly checkpoint
Introduction
During cell division, dynamic microtubules attach to and align chromosomes within the mitotic spindle before segregating the replicated sister chromatids equally between two daughter cells. A general prerequisite for precise distribution of the genome is chromosome biorientation, whereby each sister kinetochore is attached to the plus-ends of microtubules that emanate from opposing spindle poles. Cells deploy a highly sensitive surveillance mechanism that is capable of delaying anaphase if every chromosome has not achieved biorientation within the spindle. This ‘surveillance mechanism’ is known as the spindle assembly checkpoint (SAC) (Musacchio and Salmon, 2007).
In addition to its crucial role in attaching chromosomes to microtubules, the kinetochore is also the physical site at which the localization and activities of signaling molecules are integrated into a ‘wait-anaphase’ signal when chromosomes are not bioriented (Rieder et al., 1994). Normally, anaphase onset is triggered by the activation of an E3 ubiquitin ligase called the anaphase-promoting complex/cyclosome (APC/C) (King et al., 1995; Sudakin et al., 1995), which targets key mitotic substrates for degradation, including cyclin B and securin (Fig. 1A, right) (Peters, 2006). Degradation of these two substrates leads to loss of maturation-promoting factor (MPF) activity (because the MPF complex comprises Cdk1 and its activator cyclin B) as well as activation of the cohesin-cleaving activity of separase through proteolysis of its inhibitor securin (Ciosk et al., 1998; Glotzer et al., 1991). In the presence of unaligned chromosomes, the SAC pathway generates an inhibitory signal that blocks APC/C function (Fig. 1, left). Molecularly speaking, the most potent inhibitor of the APC/C is a four-protein complex known as the mitotic checkpoint complex (MCC), which consists of the checkpoint proteins Mad2, BubR1, Bub3 and the APC/C regulator Cdc20 (Sudakin et al., 2001). All four of the MCC components are enriched at unattached kinetochores and a significant fraction of each protein turns over rapidly (Howell et al., 2000; Howell et al., 2004; Kallio et al., 2002a; Shah et al., 2004). The kinetochore is postulated to be the site where the four key components of the inhibitory SAC signal are brought together to assemble soluble MCC that promotes inhibition of the APC/C. [For other models and further discussion of this topic, there are several recently published reviews (Burke and Stukenberg, 2008; Ciliberto and Shah, 2009; Musacchio and Salmon, 2007).]
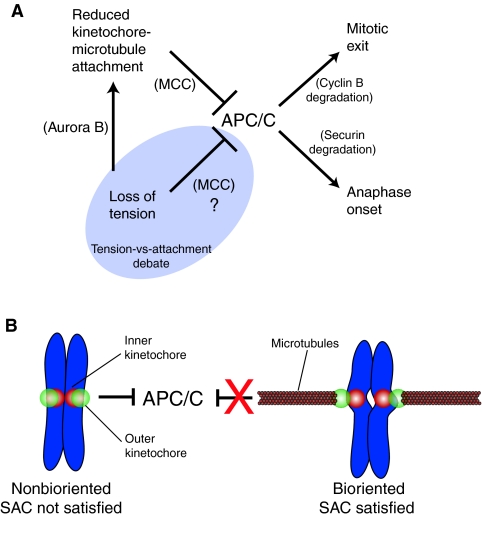
Fig. 1.
Schematic of the spindle assembly checkpoint (SAC) pathway and a ‘macro view’ of kinetochore-microtubule attachment and chromosome biorientation. (A) The anaphase-promoting complex/cyclosome (APC/C) promotes mitotic exit and anaphase onset by targeting cyclin B and securin for degradation by the proteasome. If even a single chromosome is improperly aligned within the spindle then the SAC inhibits the APC/C and the cell delays in mitosis to provide more time to correct alignment errors. Reduced kinetochore-microtubule attachment inhibits the APC/C by generating a soluble inhibitor called the MCC, which is a four-protein complex consisting of Mad2, BubR1, Bub3 and Cdc20. Reduced tension also inhibits the APC/C, although there is debate as to how exactly this is achieved. It is generally agreed that one way in which reduced tension inhibits the APC/C is by reducing kinetochore-microtubule attachment through the action of the Aurora B kinase. The more contentious issue in the field, highlighted in the shaded ‘Tension-vs-attachment’ region, is whether reduced tension can also directly inhibit the APC/C. (B) Chromosomes that are not bioriented generate a wait-anaphase signal that inhibits the APC/C and prevents SAC satisfaction. Chromosome biorientation occurs when attachment factors in the outer kinetochore (green) of each sister chromatid engage microtubules emanating from opposite spindle poles. This generates centromere stretch as evidenced by the increased distance between the inner kinetochores (red) of each sister as well as intrakinetochore stretch, which is an increase in the distance between the inner and outer kinetochore. In the bioriented configuration a wait-anaphase signal is no longer generated (represented by the red X) and the APC/C is free to target its substrates for degradation.
In this Commentary, we begin by reviewing the history of an ongoing discussion in the SAC field: the tension-versus-attachment debate. We then focus on new findings that have revealed a possible role for mechanical changes within the kinetochore structure, known as intrakinetochore stretch, in regulating SAC function. In concluding, we consider several mechanistic models that could explain how these mechanical changes affect microtubule attachment stability and SAC signaling.
Silencing the SAC: the tension-versus-attachment debate
It has been hypothesized that two inputs control the wait-anaphase signal: first, kinetochore-microtubule attachment and, second, the tension generated by stretching of centromeric chromatin between sister kinetochores (Fig. 1). Proponents of a ‘partitioned-checkpoint’ hypothesis hold that specific signaling molecules directly and distinctly generate a wait-anaphase signal in response to the state of the two inputs. According to this model, there is an unattached signal that is mediated by Mad2 and its kinetochore-associated receptor, Mad1, both of which become highly enriched at unattached kinetochores by a mechanism that is poorly understood (Waters et al., 1998). There is also a low-tension signal that is associated with kinetochore localization of BubR1, Bub3 and Bub1 (depending on the model system), as well as kinetochore phosphorylation that can be experimentally detected by phospho-specific BubR1 antibodies and the 3F3/2 antibody, which recognizes a kinetochore-associated phospho-epitope(s), one of which is reported to be phosphorylated BubR1 (Elowe et al., 2007; Essex et al., 2009; Gorbsky and Ricketts, 1993; Logarinho et al., 2004; Skoufias et al., 2001; Wong and Fang, 2007). Critics of the partitioned-checkpoint theory propose that tension, at best, contributes to SAC satisfaction indirectly by stabilizing kinetochore-microtubule attachment (Fig. 1).
Because chromosome biorientation is the geometry that best ensures equal segregation of the genome, it has been postulated that cells would be well-served to detect biorientation by monitoring the elevated tension state of centromeric chromatin and the kinetochores (McIntosh, 1991). This ‘tension hypothesis’ garnered strong support from a classic set of micromanipulation studies in which praying mantid spermatocytes that delayed anaphase in the presence of a single tensionless chromosome could be coaxed to complete cell division by artificially applying tension to the chromosome with a microneedle (Li and Nicklas, 1995). It was also demonstrated that application of tension to chromosomes reduced the phosphorylation state of a kinetochore component implicated in regulating cell division — the kinetochore-associated phospho-epitope recognized by the 3F3/2 antibody (Nicklas et al., 1998; Nicklas et al., 1995). It had previously been shown that microinjection of the 3F3/2 antibody blocked dephosphorylation of the epitope and arrested mammalian cells in metaphase (Campbell and Gorbsky, 1995). Accumulation of the 3F3/2 phospho-epitope, hyperphosphorylation of BubR1 and mitotic delay could all be induced pharmacologically by reducing tension at kinetochores with the microtubule-stabilizing drug taxol (Elowe et al., 2007; Waters et al., 1998). Significant support for the existence of separate attachment- and tension-sensing pathways of the SAC came from a study in which HeLa cells treated with low doses of the microtubule-depolymerizing drug vinblastine became arrested in mitosis with bioriented chromosomes exhibiting reduced tension, but without apparent defects in microtubule attachment or detectable levels of kinetochore-associated Mad2 (Skoufias et al., 2001). More evidence in support of a role for tension in regulating SAC signaling came from studies in budding yeast in which the presence of unreplicated mitotic chromatids, and thus the complete absence of centromere stretch, were found to prevent SAC satisfaction (Stern and Murray, 2001). Similarly, the presence of monopolar spindles in which chromosome biorientation cannot be established was also found to delay tissue cells in mitosis (Kapoor et al., 2000).
Although the evidence indicating that lack of tension could stimulate production of a wait-anaphase signal appeared to be growing, there were many experimental caveats and contemporaneous findings suggesting that tension might not have such a direct role in SAC regulation. This focused the attention of researchers back onto attachment as the primary regulator of SAC signaling. The SAC pathway is so sensitive to kinetochore-microtubule attachment that the presence of a single unattached kinetochore is sufficient to prevent anaphase onset (Rieder et al., 1994). Laser ablation experiments in PtK cells demonstrated that this mitotic delay was mediated by an inhibitory signal generated by the unattached kinetochore (Rieder et al., 1995). This work also hinted that lack of tension was not sufficient for generating the wait-anaphase signal, because cells containing a mono-oriented chromosome in which the unattached kinetochore was ablated exited mitosis shortly after ablation.
Experimentation also began pointing towards a role for tension in regulating microtubule attachment stability (Fig. 1A, left). Micromanipulation experiments in spermatocytes revealed that tension increased the number of kinetochore microtubules, probably by stabilizing attachment (King and Nicklas, 2000). In addition, exposure to tension-reducing drugs such as taxol or monastrol, which inhibits the kinesin 5 family member Eg5 resulting in monopolar spindles, yielded chromosomes with detectable levels of Mad2, a hallmark of unattached kinetochores (Kapoor et al., 2000; Waters et al., 1998). Furthermore, the effects of taxol and monastrol could be over-ridden by microinjection of an inhibitory Mad2 antibody (Canman et al., 2003; Waters et al., 1998).
These experiments precipitated questions as to how tension could regulate microtubule attachment at the molecular level. Initial insights into the molecular mechanism linking tension to kinetochore-microtubule attachment stability came from studies of budding yeast. The checkpoint response to lack of tension in budding yeast was found to be dependent on the activity of the Aurora B kinase homologue Ipl1 (Biggins and Murray, 2001). There is now evidence that Aurora B (Ipl1 in yeast), which phosphorylates kinetochore proteins that bind microtubules and reduces their binding affinity (Cheeseman et al., 2002; Cheeseman et al., 2006; DeLuca et al., 2006), primarily contributes to the SAC pathway by creating unattached kinetochores (Fig. 1A, left) (Pinsky et al., 2006).
Manipulating the function of Aurora B kinase has been used by SAC researchers to probe the relative contributions of tension and attachment to SAC signaling. Inhibition of this kinase activity stabilizes kinetochore-microtubule attachments (Cimini et al., 2006) and reduces the duration of mitotic delays that are associated with lack of tension. In general, these data are interpreted to mean that the wait-anaphase signal that is generated under ‘low-tension’ conditions must be derived, not from reduced tension, but from defects in microtubule attachment. However, a survey of Aurora B inhibition studies reveals that this conclusion is not so clear cut (summarized in Table 1). Most notable is that the checkpoint response to microtubule depolymerization, which undoubtedly produces unattached kinetochores on every chromosome, is also compromised in response to Aurora B inhibition in a number of different cell types. Furthermore, Aurora B perturbation has pleiotropic effects, which include defects in kinetochore assembly that negatively impact recruitment of checkpoint proteins to the kinetochore (Table 1) (Musacchio and Salmon, 2007). As Aurora B function is required for kinetochore assembly and generally for maintaining SAC activity, perturbations of this kinase are a blunt rather than a precise tool for dissecting the relative contribution of tension and attachment to SAC signaling.
Table 1.
Survey of published Aurora B inhibition results
The tension-versus-attachment debate has been difficult to resolve owing to the entanglement of the two central players (Nicklas et al., 2001; Pinsky and Biggins, 2005). In short, the debate boils down to the question of whether lack of tension directly produces a wait-anaphase signal or whether it indirectly does so by reducing kinetochore-microtubule attachment through the activity of Aurora B (Fig. 1A). Recent work characterizing mechanical changes within the kinetochore itself, which we refer to as ‘intrakinetochore stretch’, has implications for both of these possibilities — introducing a new kind of tension to the debate.
Intrakinetochore stretch and the SAC
Three recent studies in which inner and outer kinetochore constituents were labeled with different colored fluorophores and subjected to live-cell imaging or fixed-cell analysis revealed that the distance between centromere protein-A (CENP-A) within the inner kinetochore and proteins of the outer kinetochore (Ndc80 and Mis12) increased as chromosomes interacted with spindle microtubules (Maresca and Salmon, 2009; Uchida et al., 2009; Wan et al., 2009). Hence, in addition to centromere stretch, intrakinetochore stretch is also introduced during mitosis. Despite the fact that these studies were carried out with different cell types (Drosophila melanogaster S2 cells and HeLa cells), they each concluded that SAC signaling correlates not with the extent of centromere stretch but rather with the state of intrakinetochore stretch. More specifically, low intrakinetochore stretch was associated with generation of a wait-anaphase signal, whereas increased intrakinetochore stretch correlated with SAC satisfaction.
There is supporting evidence for the concept that the wait-anaphase signal is not directly triggered in response to reduced centromere stretch. A study in Saccharomyces cerevisiae showed that unreplicated dicentric chromosomes, which support assembly of two kinetochores on the same chromosome, are capable of aligning and satisfying the SAC, suggesting that introduction of conventional centromere stretch is not essential for anaphase-onset in yeast (Dewar et al., 2004). Another more recent study in human cells demonstrated that the SAC is satisfied in mitosis with unreplicated genomes (MUGs), another condition in which conventional centromere stretch cannot be generated because the bulk of centromeric DNA is absent and sister kinetochores, which do not exist, are not connected to each other (O'Connell et al., 2008). In these experimental conditions, merotelic attachment of individual kinetochores to microtubules from opposite spindle poles (an aberrant form of attachment that does not produce a wait-anaphase signal) (Cimini et al., 2001), is a potential source of attachment-site tension or intrakinetochore stretch.
Hence, if the definition of tension is limited to centromere stretch, then it could be reasonably argued that the SAC can be satisfied without tension. However, these findings do not completely rule out tension as a contributor to SAC signaling. In fact, an alternative explanation of these data is that they have focused the search for potential tension-regulated SAC mechanisms away from interkinetochore centromere stretch and towards intrakinetochore stretch.
Several high-resolution structural studies of the kinetochore offer a variety of potential explanations for how intrakinetochore stretch occurs (Fig. 2). In one electron tomography study, the outer plate of the kinetochore was found to consist of a flexible and fibrous network of proteins that extended outward onto attached microtubules (Dong et al., 2007). In a second electron tomography study, kinetochore fibrils were observed to extend outward and connect to bending protofilaments of kinetochore-associated microtubules (McIntosh et al., 2008). This finding complements a previous observation, also made using electron tomography, that most kinetochore-associated microtubules in metaphase are embedded in the outer plate and consist of curved protofilaments — indicative of a depolymerizing state (VandenBeldt et al., 2006). Considering the molecular makeup of the outer kinetochore, it was hypothesized in these electron tomography studies that the Ndc80 complex, a component of the core microtubule attachment site at the kinetochore, could be a component of fibrous linkages to the microtubule. Thus, extension of fibrils consisting of Ndc80 attached to the ends of kinetochore microtubules is a possible explanation for the measured increase in the distance between Ndc80 and CENP-A (Fig. 2A).
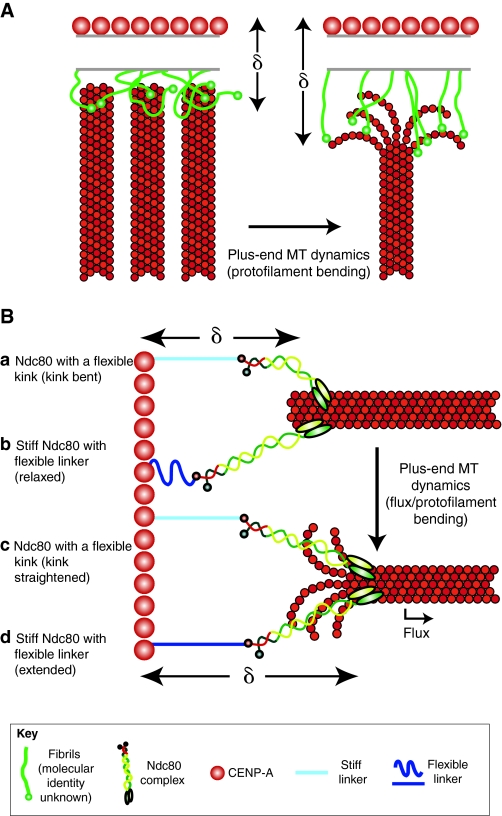
Fig. 2.
There are numerous, non-mutually exclusive, explanations for how intrakinetochore stretch could be generated. The term delta (δ) represents the distance between the inner kinetochore component CENP-A and the outer kinetochore component Ndc80. (A) The fibril model, adapted with permission from McIntosh et al. (McIntosh et al., 2008), proposes that filamentous elements bind to bending protofilaments at the plus-end of kinetochore microtubules (MTs). In the absence of bent protofilaments (left), the fibrils would not be extended; however, association with bent protofilaments (right) extends the fibrils and δ increases. The identity of these fibrils is unknown, although Ndc80 has been proposed as a candidate. (B) Lattice-binding models for intrakinetochore stretch. It has been proposed that the Ndc80 complex has a flexible kink (a), which is supported by data from studies of yeast, and that there are compliant elements internal to Ndc80 within the kinetochore (b), which was suggested from mapping human kinetochores. Both may be true, but for simplicity, each example highlights one of these possibilities. Association of Ndc80 with either fluxing kinetochore microtubules or bending protofilaments increases δ by straightening the Ndc80 complex (c), by extending a movable element internal to Ndc80 in the kinetochore (d), or both.
If the fibrils comprise the Ndc80 complex, then the fibril model places the Ndc80 molecule at the very end of the microtubule, which is ideal for coupling force production by microtubule dynamics to chromosome movement. However, measuring the separation between differentially labeled kinetochore proteins at nanometer accuracy has produced a different view of the kinetochore, at least with regards to Ndc80. Rather than being localized at the end of the microtubule as predicted by the fibril model, the microtubule-binding domain of Ndc80 was mapped to >50 nm interior of the plus-ends of cold-stable kinetochore microtubules in metaphase PtK cells (Wan et al., 2009). This suggests that many of the kinetochore-associated Ndc80 complexes associate with the side of the microtubule lattice, as has been shown in vitro (Cheeseman et al., 2006). Lattice-binding models of intrakinetochore stretch in which the Ndc80 complex binds to the side of microtubules are proposed below.
Direct imaging of the Ndc80 complex by electron microscopy revealed bending at the site of a conserved break in the coiled-coil domain of Ndc80 (Wang et al., 2008). Consequently, straightening of the Ndc80 complex itself could account for increased intrakinetochore stretch (Fig. 2B). This possibility is supported by a nanometer-scale protein map of the budding yeast kinetochore, in which the Ndc80 complex was found to shorten, relative to its metaphase length, in late anaphase — when tension should be lost (Joglekar et al., 2009). Although tension-mediated straightening of the Ndc80 complex is an exciting new hypothesis, live-cell studies in tissue cells that measured intrakinetochore stretch used tags that were positioned internal to the Ndc80 kink site; therefore, changes in the distance between the fluorescent markers could not reflect straightening of the complex at the bend. Furthermore, the super-resolution protein map of the human kinetochore indicated that the Ndc80 complex does not change its length or orientation relative to the microtubule lattice in either the presence or absence of tension (Wan et al., 2009).
The super-resolution protein map of the HeLa cell kinetochore also identified two moveable elements that are localized between the Ndc80 complex and CENP-A. The first was a compliant element identified as the peripheral centromeric chromatin, consisting of CENP-A-containing nucleosomes and the inner kinetochore component CENP-C. The second was a moveable or flexible structural element, the identity of which is unknown, which allowed a stiff Ndc80 complex and one end of an outer kinetochore component called the Mis12 complex to shift inward by ~15 nm towards the inner kinetochore component CENP-I in the presence of taxol. Reduction of centromere stretch during chromosome oscillations in metaphase neither generates a wait-anaphase signal nor reduces the distance between Ndc80 and CENP-I. Therefore, the position of the entire Ndc80 complex and part of the Mis12 complex relative to the inner kinetochore — rather than changes in the compliant centromeric component — appears to be the most crucial element linking intrakinetochore stretch to the SAC. In support of this idea, near-complete loss of centromere stretch in Drosophila S2 cells following addition of 10 nm taxol neither prevented SAC satisfaction and anaphase-onset nor significantly reduced intrakinetochore stretch (Maresca and Salmon, 2009).
Intrakinetochore stretch could position Aurora B substrates within the kinetochore with high spatial resolution
One of the first mechanistic models to explain how tension could affect microtubule attachment stability came from work on the budding yeast homologues of inner-centromere protein INCENP (Sli15 in yeast) and Aurora B (Ipl1), two components of the chromosomal passenger complex (CPC) (Tanaka et al., 2002). This model proposed that tension-dependent movement of kinetochore substrates away from Aurora B during biorientation leads to reduced phosphorylation of microtubule regulators and, consequently, stable microtubule attachment (Andrews et al., 2004; Tanaka et al., 2002) (reviewed by Kelly and Funabiki, 2009). The first iteration of this model, which persists today, depicts the CPC in a fixed position between sister centromeres regardless of stretch state (Fig. 3A). Introduction of centromere stretch moves the kinetochore substrate away from the stationary population of CPC, thereby reducing the likelihood of phosphorylation by Aurora B. However, this picture is not true to life, because the CPC stretches along with the centromeric chromatin (Beardmore et al., 2004). Furthermore, a recent study found that artificially positioning Aurora B closer to the outer kinetochore destabilized microtubule attachment and delayed anaphase onset (Liu et al., 2009). The researchers concluded that increased spatial separation of outer kinetochore substrates relative to Aurora B stabilizes microtubule attachment and promotes SAC silencing. It is difficult to envision how a stiff kinetochore structure built upon a centromeric interface to which the CPC is always localized can provide the spatial resolution that these models evoke. Rather, we would argue that kinetochore substrates cannot be spatially separated from the source of phosphorylation unless they undergo a relative movement away from the centromeric chromatin — or unless there is intrakinetochore stretch (Fig. 4A).
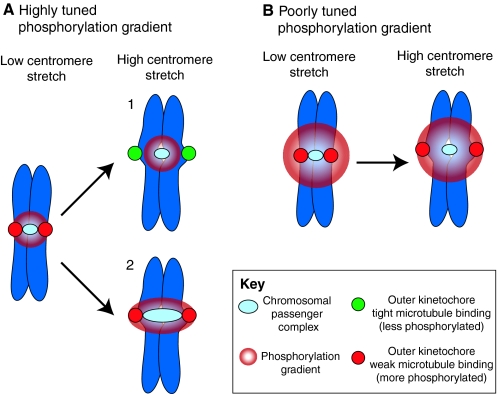
Fig. 3.
Can kinetochore phosphorylation and microtubule-attachment stability be sufficiently regulated by a phosphorylation gradient and centromere stretch? (A) A highly tuned Aurora B phosphorylation gradient extends from the chromosomal passenger complex (CPC) (light blue), localized to the inner centromere, to just beyond the kinetochores (red) under conditions of low centromere stretch. When centromere stretch is introduced there are two possibilities: (1) the most commonly drawn version of this model suggests that the CPC is maintained in a fixed position upon introduction of centromere stretch extending the kinetochores (now green) beyond the range of the highly tuned phosphorylation gradient. This decreases the likelihood of kinetochore phosphorylation and increases its affinity for kinetochore microtubules. (2) Experimental evidence has shown that the CPC is stretched along with the centromeric chromatin. As the position of the kinetochore (red) has not changed relative to the source of the gradient, centromere stretch cannot move the kinetochores beyond the range of the highly tuned phosphorylation gradient and tight binding cannot be promoted. (B) A poorly tuned phosphorylation gradient extends well beyond the sister kinetochores and introduction of centromere stretch is not sufficient to position kinetochores outside its reach. In this case, it would not matter whether the CPC is stretched, so only one possibility is shown. The only example that provides the spatial resolution required for precise regulation of phosphorylation state and attachment stability is model 1 in A, and experimental evidence suggests that the CPC is not maintained in a fixed position when the centromeric chromatin is stretched. Thus, alternative (related) models should also be considered.
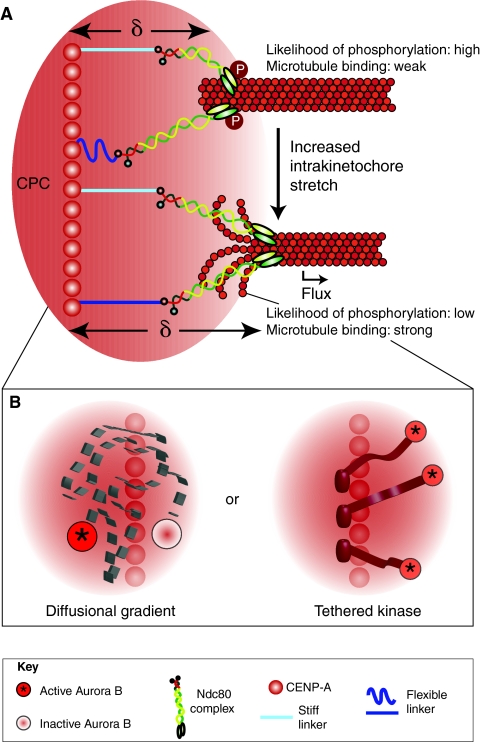
Fig. 4.
Intrakinetochore stretch can provide the spatial resolution necessary to efficiently regulate kinetochore phosphorylation and attachment stability. (A) The CPC, which is localized in the inner centromere (underlying CENP-A), generates a functional range of Aurora-B-kinase-mediated phosphorylation (red gradient). Increasing delta (δ) and introduction of intrakinetochore stretch positions the outer kinetochore outside of the working distance of Aurora B and therefore promotes stronger binding to the microtubule because of reduced phosphorylation. Unlike the centromere-stretch model outlined in Fig. 3A, the intrakinetochore-stretch model allows for movement of microtubule attachment factors such as Ndc80 relative to the source of phosphorylation. (B) How is the working distance of Aurora B defined? One possibility is that it acts through a diffusional gradient whereby Aurora B becomes activated (asterisk) at a point-source (centromeric chromatin) and then diffuses away before it deactivates (no asterisk). Alternatively, active Aurora B kinase (asterisk) might be tethered to the centromeric chromatin, with its working distance within the kinetochore space defined by the length of this physical linkage (Santaguida and Musacchio, 2009).
The spatial positioning of Aurora B substrates requires that the kinase has a defined sphere of influence whereby it phosphorylates substrates within range, but cannot modify targets that are out of range. How is such a working distance defined? Most interpretations envision a diffusion-based gradient of Aurora B kinase activity emanating from the centromeric chromatin (Fig. 3). Such a gradient would have to be highly spatially tuned and steep enough so that it could differentially regulate kinetochore substrates that move less than 40 nm from the source. This gradient would be very sensitive to perturbations in the dynamic properties and lifetimes of active Aurora B molecules; thus it might not be the most reliable mechanism by which to regulate such an important process. Furthermore, the gradient model absolutely depends on the presence of a dynamic and diffusible population of Aurora B originating from the centromere. Interestingly, the dynamic properties of centromere-associated Aurora B are still somewhat controversial, as one study found that it was a stable component of the inner centromere in all stages of mitosis, whereas another found it to be dynamic (Delacour-Larose et al., 2004; Murata-Hori and Wang, 2002). It is crucial for the diffusible-gradient model that this discrepancy is addressed.
It has recently been postulated that Aurora B is tethered to the centromeric chromatin within the inner kinetochore by a flexible linker (INCENP) creating a physically defined working distance for the molecule (Santaguida and Musacchio, 2009). As discussed above, Aurora B that is localized at or originating from the very periphery of the centromeric chromatin might be the most crucial population of the kinase for regulating microtubule attachment. Although both the tethered kinase model and the soluble gradient model could generate something akin to a phosphorylation gradient within the kinetochore space, it will be important to differentiate whether this is achieved by physically tethering Aurora B to the peripheral centromere or by a purely diffusion-based mechanism (Fig. 4B). Although there is mounting evidence in support of spatial positioning models for Aurora B phosphorylation at the kinetochore, it has also been proposed that, upon chromosome biorientation, the CPC undergoes conformational changes that are similar to intrakinetochore stretch, which prevent activation of Aurora B kinase by INCENP (Sandall et al., 2006). Thus, intrakinetochore stretch could regulate Aurora-B-mediated phosphorylation indirectly, by positioning its substrates within the kinetochore, and/or directly, by regulating its kinase activity.
The case for intrakinetochore stretch as a SAC regulator
The state of intrakinetochore stretch correlates with the state of the SAC (Maresca and Salmon, 2009; Uchida et al., 2009; Wan et al., 2009). Correlation alone does not prove that introduction of intrakinetochore stretch directly silences the SAC; however, a careful examination of the literature offers a compelling case that reduced intrakinetochore stretch could stimulate a wait-anaphase signal independently of defects in microtubule attachment (Fig. 5).
Fig. 5.
The case for reduced intrakinetochore stretch in generating a wait-anaphase signal. (A) Schematic adaptation of data from Clute and Pines (adapted with permission) (Clute and Pines, 1999). Cyclin-B1—GFP levels begin declining after alignment of the last chromosome, indicating SAC satisfaction, and anaphase onset ensues (blue line). However, even after the SAC has been satisfied, addition of 10 μM taxol reinitiates an active wait-anaphase signal and cyclin-B1—GFP degradation ceases within minutes (red line). (B) However, data from an electron microscopy study by McEwen et al. (reproduced with permission) (McEwen et al., 1997) concluded that taxol treatment did not cause a significant decrease in kinetochore microtubule number and, in fact, slightly more kinetochore microtubules were observed after a 10 minute treatment with 1 μM taxol. (C) High-resolution mapping of the kinetochore by Wan et al. (Wan et al., 2009) found that delta (δ) was reduced within 5 minutes of addition of 10 μM taxol. In this schematic, we highlight the response of the same Ndc80 attachment site (with examples of either a kinked Ndc80 molecule or a stiff Ndc80 complex with a flexible linker — for details, refer to key in Fig. 2) under conditions of full intrakinetochore stretch, relaxing to reduced intrakinetochore stretch following a 5 minute taxol treatment. We envision that this reduction of intrakinetochore stretch is a result of reduced flux and/or straightening of the microtubule lattice owing to loss of bending protofilaments. Thus, reduction of intrakinetochore stretch, not kinetochore microtubule number, occurs on the same timescale (minutes) as generation of a wait-anaphase signal in response to taxol.
A thought-provoking study in which levels of GFP-labeled cyclin B1 were monitored throughout mitosis revealed that the onset of cyclin B1 degradation, which indicates SAC satisfaction, begins shortly after the last chromosome becomes aligned onto the metaphase plate (which correlates with loss of Mad2 and the 3F3/2 epitope at the last attached kinetochore) (Gorbsky and Ricketts, 1993; Howell et al., 2000). Interestingly, degradation of cyclin-B1—GFP at late metaphase was halted within 1 minute of introducing 10 μM taxol to either PtK cells or HeLa cells. Thus, this study revealed that, even after the SAC has been satisfied, a wait-anaphase signal can be reinitiated rapidly by taxol addition (Clute and Pines, 1999). It is unlikely that the wait-anaphase signal produced under these conditions is a consequence of microtubule attachment defects, because kinetochore microtubule number has been observed by electron microscopy to increase slightly following a 10 minute treatment of PtK cells with 1 μM taxol (McEwen et al., 1997). By contrast, intrakinetochore stretch in HeLa cells was lost rapidly (within 5 minutes) following addition of 10 μM taxol (Wan et al., 2009). Thus, the case for intrakinetochore stretch is simple: a measurable reduction in intrakinetochore stretch, but not microtubule number, occurs on a comparable timescale as generation of the wait-anaphase signal in response to taxol.
There are some acknowledged limitations to our case. First, extended treatment (24 hours) of PtK cells with nanomolar concentrations of taxol was recently reported to reduce kinetochore fiber fluorescence intensity suggesting that taxol can compromise microtubule attachment (Rizk et al., 2009). A second drawback is that the three cited observations that support this theory were made in different cell types (HeLa or PtK cells) with variable concentrations of taxol (1 μM versus 10 μM). A standardized approach using identical taxol concentrations in the same cell line combined with a careful examination of microtubule occupancy under such conditions would further clarify whether loss of intrakinetochore stretch directly regulates production of the wait-anaphase signal.
Translating reduced intrakinetochore stretch into a wait-anaphase signal at a mechano-molecular level
How can reduction of intrakinetochore stretch contribute to generation of a wait-anaphase signal? Data from the Drosophila S2 cell study suggest that intrakinetochore stretch acts upstream of kinetochore-bound Mad1-Mad2, meaning that any signal derived from a reduction in intrakinetochore stretch depends on recruitment of Mad1-Mad2 to the kinetochore (Maresca and Salmon, 2009). Furthermore, reduction of intrakinetochore stretch in S2 cells was associated with increased phosphorylation of the 3F3/2 epitope. We have already discussed how changes in intrakinetochore stretch could impact phosphorylation of Aurora B substrates; however, the fact that the 3F3/2 phospho-epitope is generated by polo kinase (Ahonen et al., 2005; Wong and Fang, 2005) indicates that other kinase-substrate pairings might also be regulated by intrakinetochore stretch. Thus, Mad1-Mad2 localization and kinetochore phosphorylation represent an ideal starting point for building regulatory models.
Similarly to the Aurora B hypothesis, the key feature of these models is the relative movement of two structural elements. For the Aurora B model, this means movement of a kinetochore component relative to the peripheral centromeric chromatin. But what if the kinase and substrate are both located within the kinetochore structure? In other words, can relative movement occur within the kinetochore structure?
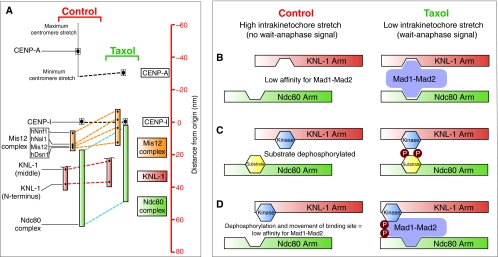
The nanometer-scale map of the human kinetochore says ‘yes’. The KMN protein network, which consists of the two microtubule-binding factors KNL-1 (also known as AF15Q14, hSpc105 and Blinkin; hereafter referred to as KNL-1 for simplicity) and the Ndc80 complex linked together by the Mis12 complex, makes up the core microtubule attachment site at the outer kinetochore (Cheeseman et al., 2006). Interestingly, two elements of the KMN network, which were called the Ndc80 and KNL-1 arms, can move relative to each other within the kinetochore (Wan et al., 2009). In the presence of taxol, the Ndc80 arm, accompanied by one end of the Mis12 complex, shifted inward towards the inner kinetochore component CENP-I by ~15 nm, whereas the KNL-1 arm and the other end of the Mis12 complex did not (Fig. 6A). A conformational change in the Mis12 complex could contribute to the movement of the Ndc80 arm relative to KNL-1; however, it is possible that a structural reorganization of the Mis12 complex is also a crucial component of the regulatory mechanism at work. Although this is an interesting concept, we focus in the following section on mechano-molecular models that explain how the movement of the Ndc80 complex relative to KNL-1 could impact SAC signaling (Fig. 6B-D).
Fig. 6.
Speculative models for translating the relative movement of two mechanical elements within the kinetochore into a wait-anaphase signal. (A)A high-resolution map of the kinetochore reveals two mechanical arms within the kinetochore (KNL-1 arm and Ndc80 arm) that move relative to each other (Wan et al., 2009). Taxol treatment reduces intrakinetochore stretch in part by causing the Ndc80 arm (green) to move inward (represented by blue dashed line) toward the inner kinetochore component CENP-I (set as the origin, distance=0 nm) relative to the KNL-1 arm (green), which maintains a relatively constant distance from CENP-I (red dashed line). The linkage between these two arms is the Mis12 complex (orange) consisting of four components: hNnf1, hNsl1, Mis12 and hDsn1. This complex undergoes a conformational change in the presence of taxol, whereby one end of the complex (hNnf1) shifts inwards while the other end (Dsn1) does not. The C-terminus of KNL-1 is not shown because it was not mapped; however, it has been reported to interact with the Mis12 complex (Kiyomitsu et al., 2007). The CENP-I mark probably represents the periphery of the centromeric chromatin (Wan et al., 2009). (B-D) The geometrical arrangement of the two arms could affect SAC signaling by regulating the localization and/or phosphorylation of checkpoint components. (B) A lock-and-key model for Mad1-Mad2 localization to the kinetochore. Low intrakinetochore stretch promotes Mad1-Mad2 binding at the kinetochore by positioning multiple low-affinity binding sites near each other. The affinity of Mad1-Mad2 is reduced upon introduction of intrakinetochore stretch and the SAC is satisfied. The schematic drawing of Mad1-Mad2 is not meant to convey information about the structural organization of the components but is simply meant to show kinetochore binding sites on the complex that, for example, might only be present in Mad1. (C) Relative positioning of a kinase and its substrate promotes phosphorylation of the substrate under low intrakinetochore stretch and reduced phosphorylation of the substrate upon repositioning of the two components when intrakinetochore stretch increases. (D) A model combining lock-and-key with phospho-regulation. Mad1-Mad2 affinity for the kinetochore is promoted by localized phosphorylation near its binding site or of Mad1-Mad2 itself. The affinity of Mad1-Mad2 for the kinetochore is reduced by increased intrakinetochore stretch because of repositioning of the binding site and dephosphorylation.
The identity of the Mad1 receptor at the kinetochore is unknown. Perhaps, rather than one strong kinetochore-associated binding partner, there are several low-affinity binding sites located on the different mechanical arms of the kinetochore (Fig. 6B). When intrakinetochore stretch is low, the binding sites could be positioned to increase the affinity of Mad1 for the kinetochore. However, introduction of intrakinetochore stretch would position the binding sites further apart, decreasing affinity of Mad1-Mad2 for the kinetochore, thereby extinguishing the SAC.
Changes in the relative positioning of the two arms could also regulate the phosphorylation state of kinetochore components (Fig. 6C). In this model, localizing the kinase to one arm and the substrate to the other would allow translation of intrakinetochore stretch into a particular phosphorylation state of a kinetochore component. Of course, positioning substrates relative to a kinetochore-bound phosphatase has also been proposed to affect kinetochore function (Andrews et al., 2004; Pinsky et al., 2009; Tanaka et al., 2002; Vanoosthuyse and Hardwick, 2009). It will be interesting to identify kinetochore-associated kinases and phosphatases that localize to one arm while their substrates localize to the other.
Syntelic attachments are a result of both sister kinetochores being attached to microtubules from the same pole — an aberrant configuration that generates a wait-anaphase signal. Furthermore, this type of attachment must be corrected by detaching one of the kinetochores from its microtubules so that proper biorientation can occur. It is possible that reduced intrakinetochore stretch in one or both of the kinetochores contributes to the attachment correction and wait-anaphase signaling mechanism(s) associated with syntelic attachments. Thus, a closer look at intrakinetochore stretch in the syntelic configuration would be of significant interest.
Are there two populations of Mad1-Mad2, and what is the kinetochore receptor?
The corona is a transient formation, which appears in electron micrographs as long fibrous elements that extend ~100-200 nm beyond the outer kinetochore plate, where Ndc80 is localized (DeLuca et al., 2005). The molecular make-up of the corona is not entirely known but motor proteins — including CENP-E and dynein, as well as checkpoint regulators such as Mad1, Mad2 and BubR1 — are highly enriched in the corona (Hoffman et al., 2001). Under normal conditions, the corona is rapidly depleted upon encountering microtubules (Cimini et al., 2003) and a dense corona only persists in the absence of microtubules. The corona acts both as an antenna, transmitting a robust wait-anaphase signal via its enriched checkpoint proteins, and as a molecular grappling hook that grabs hold of nearby microtubules through motor proteins such as CENP-E and dynein. Kinetochore-associated dynein streams off the kinetochore, stripping checkpoint regulators along with it (Howell et al., 2001). Therefore, dynein has a central role in silencing the SAC. Microtubule attachment leads to a reduction of kinetochore-bound Mad1-Mad2 to levels below detection by conventional microscopy. Unlike Mad1-Mad2, BubR1 remains at lower but detectable levels at metaphase kinetochores until just before anaphase (Hoffman et al., 2001; Howell et al., 2004). Bub1 and BubR1 have also been shown to interact with the outer kinetochore component KNL-1 (Kiyomitsu et al., 2007) and therefore can localize interior to the corona (Jablonski et al., 1998).
The mechanical model we propose depends on the existence of a population of Mad1-Mad2, which, similarly to Bub1 and BubR1, can associate with the core-microtubule attachment site and is recruited to kinetochores owing to a reduction of intrakinetochore stretch. Furthermore, this internal population of Mad1-Mad2 must be capable of generating a wait-anaphase signal despite the fact that it might be present at very low levels.
Ndc80 was identified in a yeast two-hybrid screen as a Mad1-interacting protein, and depletion of Ndc80 compromises Mad1-Mad2 localization to the kinetochore in several systems (DeLuca et al., 2003; Martin-Lluesma et al., 2002; McCleland et al., 2003; Meraldi et al., 2004). More specifically, SAC function, and presumably Mad1-Mad2 localization, is dependent on the calponin homology (CH) domain of Ndc80 (Guimaraes et al., 2008). However, the case for Ndc80 as the Mad1-Mad2 receptor is not airtight; Mad2 was found to localize normally to kinetochores in Ndc80-depleted PtK cells following nocodazole treatment, and these cells remained arrested in mitosis for as long as control cells (Guimaraes et al., 2008). These conflicting findings can be explained by the presence of two distinct populations of Mad1-Mad2 — one within the core microtubule attachment site and one within the corona. We hypothesize that localization of the internal Mad1-Mad2 population requires the CH domain of Ndc80, perhaps via direct interaction. Localization of Mad1-Mad2 at the kinetochore also depends on the RZZ (rod, zwilch, ZW10) complex (Basto et al., 2000; Buffin et al., 2005). Therefore, the RZZ complex might comprise one of the binding sites for Mad1-Mad2 within the core microtubule-attachment site. Alternatively, RZZ or the kinetochore component Spindly, which depends on RZZ for its localization (Chan et al., 2009; Gassmann et al., 2008; Griffis et al., 2007; Yamamoto et al., 2008), might be the Mad1-Mad2 receptor at the kinetochore, as reported for the Spindly homologue in Caenorhabditis elegans (Yamamoto et al., 2008). However, unlike the RZZ complex, the role of Spindly in recruiting Mad1 does not appear to be conserved, because knockdown of Spindly expression in Drosophila S2 cells led to accumulation of Mad2 (and presumably Mad1) at kinetochores (Griffis et al., 2007). Interestingly, taxol treatment has been shown to cause an increase in kinetochore-associated Rod and ZW10 (Famulski and Chan, 2007). Thus, reduction of intrakinetochore stretch could lead to recruitment of Mad1-Mad2 by first recruiting a Mad1-Mad2 receptor such as RZZ.
It has been proposed that the checkpoint functions as a simple two-state switch whereby association of the kinetochore with either microtubules or Mad1-Mad2 is mutually exclusive (reviewed by Burke and Stukenberg, 2008). Along these lines, it is conceivable that association of Ndc80 with the microtubule, which requires the CH domain (Ciferri et al., 2008; Wei et al., 2007), induces a conformational change in this region that reduces its affinity for Mad1-Mad2. However, recent evidence from studies of S. cerevisiae suggests that a more complicated mechanism is at work: expression of phospho-mimetic mutants of Ndc80 at several Mps1 phosphorylation sites delayed anaphase onset and constitutively localized Mad2 to kinetochores independently of their microtubule-attachment state (Kemmler et al., 2009). However, similarly to Aurora B, Mps1 activity also appears to be required for SAC signaling in general, because the mitotic delay induced by the Ndc80 mutant could be overcome in the absence of Mps1 activity. This argues against the idea that microtubule attachment absolutely precludes Mad1-Mad2 localization. In light of this finding, we believe that the CH domain of Ndc80 in a particular phosphorylation state could regulate localization of Mad1-Mad2 to the kinetochore: by direct interaction; in concert with additional Mad1-Mad2 binding factors; or by localizing a downstream Mad1-Mad2 receptor such as the RZZ complex.
Dynein and intrakinetochore stretch
Microtubule attachment and plus-end dynamics clearly affect intrakinetochore stretch; however, the contribution of kinetochore motors to intrakinetochore stretch is poorly understood. Dynein is of particular interest because of its directionality and its proposed roles in SAC silencing and in promoting end-coupled microtubule attachment and intrakinetochore tension (Gassmann et al., 2008; Howell et al., 2001; Varma et al., 2008; Yang et al., 2007). In dynein-inhibited cells, centromere stretch is either partially reduced or unaffected, and kinetochore-microtubule attachment can be normal even though high levels of Mad2 are retained at kinetochores and the cells delay in mitosis (Griffis et al., 2007; Howell et al., 2000; Maresca and Salmon, 2009). Because intrakinetochore stretch was partially reduced but highly variable following dynein depletion in Drosophila S2 cells (Maresca and Salmon, 2009), the contribution of dynein to intrakinetochore stretch remains unclear. A careful examination of intrakinetochore stretch following dynein inhibition would be a worthwhile endeavor. We propose that kinetochore-associated dynein could contribute to silencing the SAC in three ways: first, by removing coronal Mad1-Mad2 in early prometaphase; second, by increasing intrakinetochore stretch and thereby reducing affinity of Mad1-Mad2 for the core-microtubule attachment site; and third, by stripping this lower-affinity population of Mad1-Mad2 from the core attachment site.
Conclusions and perspectives
Generation of the wait-anaphase signal correlates with reduced intrakinetochore stretch rather than reduced interkinetochore centromere stretch. What aspect of reduced intrakinetochore stretch is crucial for SAC signaling? The nanometer-resolution map of the HeLa cell kinetochore revealed that the distance between the Ndc80 complex and CENP-A can increase as a result of the extension of two compliant or flexible components: the first is peripheral centromeric chromatin containing CENP-A and CENP-C, and the second is an unknown element between the Ndc80 complex and the inner kinetochore component CENP-I. Unlike the relationship between the Ndc80 arm and one end of the Mis12 complex, the KNL-1 arm and the other end of the Mis12 complex maintain a constant distance from CENP-I in the presence and absence of tension.
We postulate that movement of the Ndc80 arm closer to the inner kinetochore contributes to the generation of a wait-anaphase signal by two possible mechanisms. First, positioning of the Ndc80 arm closer to Aurora B at the inner centromere might produce bona fide unattached kinetochores by reducing the affinity of the kinetochore for microtubules. Second, a conformational change in the Mis12 complex could position the Ndc80 complex relative to KNL-1 within the KMN network in a manner that catalyzes assembly of the MCC by mechanisms that are not yet understood.
In considering these possibilities, we realized that, although kinetochore-microtubule attachment is a commonly used phrase in the field, in reality, it is a murky concept. Electron microscopy can be used to assay microtubule occupancy at the kinetochore; however, even if a microtubule occupies the kinetochore space, it is not possible to determine the extent to which the attachment machinery at the kinetochore is actively engaging that microtubule. What might be mechanistically most important is not microtubule occupancy but rather the residence or dwell time of attachment factors such as the Ndc80 complex on the microtubule lattice. Shorter dwell times of Ndc80 complexes could be sufficient to reduce intrakinetochore stretch and generate a wait-anaphase signal without compromising the ability of the kinetochore, as a whole, to remain attached to the kinetochore fiber. The development of experimental approaches that can more quantitatively assay the degree to which microtubules are physically engaged with attachment factors at the kinetochore would greatly advance our mechanistic understanding of how the SAC pathway is regulated by kinetochore mechanics.
Acknowledgments
The authors are grateful to all members of the Salmon lab, past and present, especially Jay Gatlin, Ajit Joglekar, Ryan O'Quinn, Dileep Varma and Xiaohu Wan for sharing many insightful discussions. We apologize to anyone whose work we could not highlight due to word limitations. T.J.M. thanks Green Day for inspiring the title of the manuscript. This work was supported by the American Cancer Society (T.J.M., PF0711401) and the NIH (E.D.S., R37-GM24364). Deposited in PMC for release after 12 months.
References
- Ahonen L. J., Kallio M. J., Daum J. R., Bolton M., Manke I. A., Yaffe M. B., Stukenberg P. T., Gorbsky G. J. (2005). Polo-like kinase 1 creates the tension-sensing 3F3/2 phosphoepitope and modulates the association of spindle-checkpoint proteins at kinetochores. Curr. Biol. 15, 1078-1089 [DOI] [PubMed] [Google Scholar]
- Andrews P. D., Ovechkina Y., Morrice N., Wagenbach M., Duncan K., Wordeman L., Swedlow J. R. (2004). Aurora B regulates MCAK at the mitotic centromere. Dev. Cell 6, 253-268 [DOI] [PubMed] [Google Scholar]
- Basto R., Gomes R., Karess R. E. (2000). Rough deal and Zw10 are required for the metaphase checkpoint in Drosophila. Nat. Cell Biol. 2, 939-943 [DOI] [PubMed] [Google Scholar]
- Beardmore V. A., Ahonen L. J., Gorbsky G. J., Kallio M. J. (2004). Survivin dynamics increases at centromeres during G2/M phase transition and is regulated by microtubule-attachment and Aurora B kinase activity. J. Cell Sci. 117, 4033-4042 [DOI] [PubMed] [Google Scholar]
- Biggins S., Murray A. W. (2001). The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 15, 3118-3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffin E., Lefebvre C., Huang J., Gagou M. E., Karess R. E. (2005). Recruitment of Mad2 to the kinetochore requires the Rod/Zw10 complex. Curr. Biol. 15, 856-8561 [DOI] [PubMed] [Google Scholar]
- Burke D. J., Stukenberg P. T. (2008). Linking kinetochore-microtubule binding to the spindle checkpoint. Dev. Cell 14, 474-479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. S., Gorbsky G. J. (1995). Microinjection of mitotic cells with the 3F3/2 anti-phosphoepitope antibody delays the onset of anaphase. J. Cell Biol. 129, 1195-1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canman J. C., Cameron L. A., Maddox P. S., Straight A., Tirnauer J. S., Mitchison T. J., Fang G., Kapoor T. M., Salmon E. D. (2003). Determining the position of the cell division plane. Nature 424, 1074-1078 [DOI] [PubMed] [Google Scholar]
- Chan Y. W., Fava L. L., Uldschmid A., Schmitz M. H., Gerlich D. W., Nigg E. A., Santamaria A. (2009). Mitotic control of kinetochore-associated dynein and spindle orientation by human Spindly. J. Cell Biol. 185, 859-874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman I. M., Anderson S., Jwa M., Green E. M., Kang J., Yates J. R., 3rd, Chan C. S., Drubin D. G., Barnes G. (2002). Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell 111, 163-172 [DOI] [PubMed] [Google Scholar]
- Cheeseman I. M., Chappie J. S., Wilson-Kubalek E. M., Desai A. (2006). The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127, 983-997 [DOI] [PubMed] [Google Scholar]
- Ciferri C., Pasqualato S., Screpanti E., Varetti G., Santaguida S., Dos Reis G., Maiolica A., Polka J., De Luca J. G., De Wulf P., et al. (2008). Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell 133, 427-439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliberto A., Shah J. V. (2009). A quantitative systems view of the spindle assembly checkpoint. EMBO J. 28, 2162-2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini D., Howell B., Maddox P., Khodjakov A., Degrassi F., Salmon E. D. (2001). Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J. Cell Biol. 153, 517-527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini D., Moree B., Canman J. C., Salmon E. D. (2003). Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J. Cell Sci. 116, 4213-4225 [DOI] [PubMed] [Google Scholar]
- Cimini D., Wan X., Hirel C. B., Salmon E. D. (2006). Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr. Biol. 16, 1711-1718 [DOI] [PubMed] [Google Scholar]
- Ciosk R., Zachariae W., Michaelis C., Shevchenko A., Mann M., Nasmyth K. (1998). An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell 93, 1067-1076 [DOI] [PubMed] [Google Scholar]
- Clute P., Pines J. (1999). Temporal and spatial control of cyclin B1 destruction in metaphase. Nat. Cell Biol. 1, 82-87 [DOI] [PubMed] [Google Scholar]
- Delacour-Larose M., Molla A., Skoufias D. A., Margolis R. L., Dimitrov S. (2004). Distinct dynamics of Aurora B and Survivin during mitosis. Cell Cycle 3, 1418-1426 [DOI] [PubMed] [Google Scholar]
- DeLuca J. G., Howell B. J., Canman J. C., Hickey J. M., Fang G., Salmon E. D. (2003). Nuf2 and Hec1 are required for retention of the checkpoint proteins Mad1 and Mad2 to kinetochores. Curr. Biol. 13, 2103-2109 [DOI] [PubMed] [Google Scholar]
- DeLuca J. G., Dong Y., Hergert P., Strauss J., Hickey J. M., Salmon E. D., McEwen B. F. (2005). Hec1 and nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol. Biol. Cell 16, 519-531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca J. G., Gall W. E., Ciferri C., Cimini D., Musacchio A., Salmon E. D. (2006). Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 127, 969-982 [DOI] [PubMed] [Google Scholar]
- Dewar H., Tanaka K., Nasmyth K., Tanaka T. U. (2004). Tension between two kinetochores suffices for their bi-orientation on the mitotic spindle. Nature 428, 93-97 [DOI] [PubMed] [Google Scholar]
- Ditchfield C., Johnson V. L., Tighe A., Ellston R., Haworth C., Johnson T., Mortlock A., Keen N., Taylor S. S. (2003). Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161, 267-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Vanden Beldt K. J., Meng X., Khodjakov A., McEwen B. F. (2007). The outer plate in vertebrate kinetochores is a flexible network with multiple microtubule interactions. Nat. Cell Biol. 9, 516-522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowe S., Hummer S., Uldschmid A., Li X., Nigg E. A. (2007). Tension-sensitive Plk1 phosphorylation on BubR1 regulates the stability of kinetochore microtubule interactions. Genes Dev. 21, 2205-2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele M. J., Lan W., Jwa M., Miller S. A., Chan C. S., Stukenberg P. T. (2008). Aurora B kinase and protein phosphatase 1 have opposing roles in modulating kinetochore assembly. J. Cell Biol. 181, 241-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex A., Dammermann A., Lewellyn L., Oegema K., Desai A. (2009). Systematic analysis in Caenorhabditis elegans reveals that the spindle checkpoint is composed of two largely independent branches. Mol. Biol. Cell 20, 1252-1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulski J. K., Chan G. K. (2007). Aurora B kinase-dependent recruitment of hZW10 and hROD to tensionless kinetochores. Curr. Biol. 17, 2143-2149 [DOI] [PubMed] [Google Scholar]
- Gassmann R., Essex A., Hu J. S., Maddox P. S., Motegi F., Sugimoto A., O'Rourke S. M., Bowerman B., McLeod I., Yates J. R., 3rd, et al. (2008). A new mechanism controlling kinetochore-microtubule interactions revealed by comparison of two dynein-targeting components: SPDL-1 and the Rod/Zwilch/Zw10 complex. Genes Dev. 22, 2385-2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. (1991). Cyclin is degraded by the ubiquitin pathway. Nature 349, 132-138 [DOI] [PubMed] [Google Scholar]
- Gorbsky G. J., Ricketts W. A. (1993). Differential expression of a phosphoepitope at the kinetochores of moving chromosomes. J. Cell Biol. 122, 1311-1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis E. R., Stuurman N., Vale R. D. (2007). Spindly, a novel protein essential for silencing the spindle assembly checkpoint, recruits dynein to the kinetochore. J. Cell Biol. 177, 1005-1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes G. J., Dong Y., McEwen B. F., Deluca J. G. (2008). Kinetochore-microtubule attachment relies on the disordered N-terminal tail domain of Hec1. Curr. Biol. 18, 1778-1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf S., Cole R. W., LaTerra S., Zimmer C., Schnapp G., Walter R., Heckel A., van Meel J., Rieder C. L., Peters J. M. (2003). The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 161, 281-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman D. B., Pearson C. G., Yen T. J., Howell B. J., Salmon E. D. (2001). Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol. Biol. Cell 12, 1995-2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B. J., Hoffman D. B., Fang G., Murray A. W., Salmon E. D. (2000). Visualization of Mad2 dynamics at kinetochores, along spindle fibers, and at spindle poles in living cells. J. Cell Biol. 150, 1233-1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B. J., McEwen B. F., Canman J. C., Hoffman D. B., Farrar E. M., Rieder C. L., Salmon E. D. (2001). Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J. Cell Biol. 155, 1159-1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B. J., Moree B., Farrar E. M., Stewart S., Fang G., Salmon E. D. (2004). Spindle checkpoint protein dynamics at kinetochores in living cells. Curr. Biol. 14, 953-964 [DOI] [PubMed] [Google Scholar]
- Jablonski S. A., Chan G. K., Cooke C. A., Earnshaw W. C., Yen T. J. (1998). The hBUB1 and hBUBR1 kinases sequentially assemble onto kinetochores during prophase with hBUBR1 concentrating at the kinetochore plates in mitosis. Chromosoma 107, 386-396 [DOI] [PubMed] [Google Scholar]
- Joglekar A. P., Bloom K., Salmon E. D. (2009). In vivo protein architecture of the eukaryotic kinetochore with nanometer scale accuracy. Curr. Biol. 19, 694-699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio M. J., Beardmore V. A., Weinstein J., Gorbsky G. J. (2002a). Rapid microtubule-independent dynamics of Cdc20 at kinetochores and centrosomes in mammalian cells. J. Cell Biol. 158, 841-847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio M. J., McCleland M. L., Stukenberg P. T., Gorbsky G. J. (2002b). Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr. Biol. 12, 900-905 [DOI] [PubMed] [Google Scholar]
- Kapoor T. M., Mayer T. U., Coughlin M. L., Mitchison T. J. (2000). Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J. Cell Biol. 150, 975-988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A. E., Funabiki H. (2009). Correcting aberrant kinetochore microtubule attachments: an Aurora B-centric view. Curr. Opin. Cell Biol. 21, 51-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmler S., Stach M., Knapp M., Ortiz J., Pfannstiel J., Ruppert T., Lechner J. (2009). Mimicking Ndc80 phosphorylation triggers spindle assembly checkpoint signalling. EMBO J. 28, 1099-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. M., Nicklas R. B. (2000). Tension on chromosomes increases the number of kinetochore microtubules but only within limits. J. Cell Sci. 113Pt 21, 3815-3823 [DOI] [PubMed] [Google Scholar]
- King R. W., Peters J. M., Tugendreich S., Rolfe M., Hieter P., Kirschner M. W. (1995). A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81, 279-288 [DOI] [PubMed] [Google Scholar]
- Kiyomitsu T., Obuse C., Yanagida M. (2007). Human Blinkin/AF15q14 is required for chromosome alignment and the mitotic checkpoint through direct interaction with Bub1 and BubR1. Dev. Cell 13, 663-676 [DOI] [PubMed] [Google Scholar]
- Li X., Nicklas R. B. (1995). Mitotic forces control a cell-cycle checkpoint. Nature 373, 630-632 [DOI] [PubMed] [Google Scholar]
- Liu D., Vader G., Vromans M. J., Lampson M. A., Lens S. M. (2009). Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science 323, 1350-1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logarinho E., Bousbaa H., Dias J. M., Lopes C., Amorim I., Antunes-Martins A., Sunkel C. E. (2004). Different spindle checkpoint proteins monitor microtubule attachment and tension at kinetochores in Drosophila cells. J. Cell Sci. 117, 1757-1771 [DOI] [PubMed] [Google Scholar]
- Maresca T. J., Salmon E. D. (2009). Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J. Cell Biol. 184, 373-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Lluesma S., Stucke V. M., Nigg E. A. (2002). Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science 297, 2267-2270 [DOI] [PubMed] [Google Scholar]
- McCleland M. L., Gardner R. D., Kallio M. J., Daum J. R., Gorbsky G. J., Burke D. J., Stukenberg P. T. (2003). The highly conserved Ndc80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity. Genes Dev. 17, 101-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. F., Heagle A. B., Cassels G. O., Buttle K. F., Rieder C. L. (1997). Kinetochore fiber maturation in PtK1 cells and its implications for the mechanisms of chromosome congression and anaphase onset. J. Cell Biol. 137, 1567-1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh J. R. (1991). Structural and mechanical control of mitotic progression. Cold Spring Harb. Symp. Quant. Biol. 56, 613-619 [DOI] [PubMed] [Google Scholar]
- McIntosh J. R., Grishchuk E. L., Morphew M. K., Efremov A. K., Zhudenkov K., Volkov V. A., Cheeseman I. M., Desai A., Mastronarde D. N., Ataullakhanov F. I. (2008). Fibrils connect microtubule tips with kinetochores: a mechanism to couple tubulin dynamics to chromosome motion. Cell 135, 322-333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P., Draviam V. M., Sorger P. K. (2004). Timing and checkpoints in the regulation of mitotic progression. Dev. Cell 7, 45-60 [DOI] [PubMed] [Google Scholar]
- Murata-Hori M., Wang Y. L. (2002). Both midzone and astral microtubules are involved in the delivery of cytokinesis signals: insights from the mobility of aurora B. J. Cell Biol. 159, 45-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata-Hori M., Tatsuka M., Wang Y. L. (2002). Probing the dynamics and functions of aurora B kinase in living cells during mitosis and cytokinesis. Mol. Biol. Cell 13, 1099-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A., Salmon E. D. (2007). The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8, 379-393 [DOI] [PubMed] [Google Scholar]
- Nicklas R. B., Ward S. C., Gorbsky G. J. (1995). Kinetochore chemistry is sensitive to tension and may link mitotic forces to a cell cycle checkpoint. J. Cell Biol. 130, 929-939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas R. B., Campbell M. S., Ward S. C., Gorbsky G. J. (1998). Tension-sensitive kinetochore phosphorylation in vitro. J. Cell Sci. 111 (Pt 21), 3189-3196 [DOI] [PubMed] [Google Scholar]
- Nicklas R. B., Waters J. C., Salmon E. D., Ward S. C. (2001). Checkpoint signals in grasshopper meiosis are sensitive to microtubule attachment, but tension is still essential. J. Cell Sci. 114, 4173-4183 [DOI] [PubMed] [Google Scholar]
- O'Connell C. B., Loncarek J., Hergert P., Kourtidis A., Conklin D. S., Khodjakov A. (2008). The spindle assembly checkpoint is satisfied in the absence of interkinetochore tension during mitosis with unreplicated genomes. J. Cell Biol. 183, 29-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. M. (2006). The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 7, 644-656 [DOI] [PubMed] [Google Scholar]
- Petersen J., Hagan I. M. (2003). S. pombe aurora kinase/survivin is required for chromosome condensation and the spindle checkpoint attachment response. Curr. Biol. 13, 590-597 [DOI] [PubMed] [Google Scholar]
- Pinsky B. A., Biggins S. (2005). The spindle checkpoint: tension versus attachment. Trends Cell Biol. 15, 486-493 [DOI] [PubMed] [Google Scholar]
- Pinsky B. A., Kung C., Shokat K. M., Biggins S. (2006). The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat. Cell Biol. 8, 78-83 [DOI] [PubMed] [Google Scholar]
- Pinsky B. A., Nelson C. R., Biggins S. (2009). Protein phosphatase 1 regulates exit from the spindle checkpoint in budding yeast. Curr. Biol. 19, 1182-1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C. L., Schultz A., Cole R., Sluder G. (1994). Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J. Cell Biol. 127, 1301-1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C. L., Cole R. W., Khodjakov A., Sluder G. (1995). The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 130, 941-948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk R. S., Bohannon K. P., Wetzel L. A., Powers J., Shaw S. L., Walczak C. E. (2009). MCAK and paclitaxel have differential effects on spindle microtubule organization and dynamics. Mol. Biol. Cell 20, 1639-1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandall S., Severin F., McLeod I. X., Yates J. R., 3rd, Oegema K., Hyman A., Desai A. (2006). A Bir1-Sli15 complex connects centromeres to microtubules and is required to sense kinetochore tension. Cell 127, 1179-1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida S., Musacchio A. (2009). The life and miracles of kinetochores. EMBO J. 28, 2511-2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J. V., Botvinick E., Bonday Z., Furnari F., Berns M., Cleveland D. W. (2004). Dynamics of centromere and kinetochore proteins; implications for checkpoint signaling and silencing. Curr. Biol. 14, 942-952 [DOI] [PubMed] [Google Scholar]
- Skoufias D. A., Andreassen P. R., Lacroix F. B., Wilson L., Margolis R. L. (2001). Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints. Proc. Natl. Acad. Sci. USA 98, 4492-4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern B. M., Murray A. W. (2001). Lack of tension at kinetochores activates the spindle checkpoint in budding yeast. Curr. Biol. 11, 1462-1467 [DOI] [PubMed] [Google Scholar]
- Sudakin V., Ganoth D., Dahan A., Heller H., Hershko J., Luca F. C., Ruderman J. V., Hershko A. (1995). The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol. Biol. Cell 6, 185-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin V., Chan G. K., Yen T. J. (2001). Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 154, 925-936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. U., Rachidi N., Janke C., Pereira G., Galova M., Schiebel E., Stark M. J., Nasmyth K. (2002). Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell 108, 317-329 [DOI] [PubMed] [Google Scholar]
- Uchida K. S., Takagaki K., Kumada K., Hirayama Y., Noda T., Hirota T. (2009). Kinetochore stretching inactivates the spindle assembly checkpoint. J. Cell Biol. 184, 383-390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandenBeldt K. J., Barnard R. M., Hergert P. J., Meng X., Maiato H., McEwen B. F. (2006). Kinetochores use a novel mechanism for coordinating the dynamics of individual microtubules. Curr. Biol. 16, 1217-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoosthuyse V., Hardwick K. G. (2009). A novel protein phosphatase 1-dependent spindle checkpoint silencing mechanism. Curr. Biol. 19, 1176-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma D., Monzo P., Stehman S. A., Vallee R. B. (2008). Direct role of dynein motor in stable kinetochore-microtubule attachment, orientation, and alignment. J. Cell Biol. 182, 1045-1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X., O'Quinn R. P., Pierce H. L., Joglekar A. P., Gall W. E., DeLuca J. G., Carroll C. W., Liu S. T., Yen T. J., McEwen B. F., et al. (2009). Protein architecture of the human kinetochore microtubule attachment site. Cell 137, 672-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. W., Long S., Ciferri C., Westermann S., Drubin D., Barnes G., Nogales E. (2008). Architecture and flexibility of the yeast Ndc80 kinetochore complex. J. Mol. Biol. 383, 894-903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J. C., Chen R. H., Murray A. W., Salmon E. D. (1998). Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol. 141, 1181-1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R. R., Al-Bassam J., Harrison S. C. (2007). The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nat. Struct. Mol. Biol. 14, 54-59 [DOI] [PubMed] [Google Scholar]
- Wong O. K., Fang G. (2005). Plx1 is the 3F3/2 kinase responsible for targeting spindle checkpoint proteins to kinetochores. J. Cell Biol. 170, 709-719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong O. K., Fang G. (2007). Cdk1 phosphorylation of BubR1 controls spindle checkpoint arrest and Plk1-mediated formation of the 3F3/2 epitope. J. Cell Biol. 179, 611-617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T. G., Watanabe S., Essex A., Kitagawa R. (2008). SPDL-1 functions as a kinetochore receptor for MDF-1 in Caenorhabditis elegans. J. Cell Biol. 183, 187-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Tulu U. S., Wadsworth P., Rieder C. L. (2007). Kinetochore dynein is required for chromosome motion and congression independent of the spindle checkpoint. Curr. Biol. 17, 973-980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Kenny A. E., Brito D. A., Rieder C. L. (2009). Cells satisfy the mitotic checkpoint in Taxol, and do so faster in concentrations that stabilize syntelic attachments. J. Cell Biol. 186, 675-684 [DOI] [PMC free article] [PubMed] [Google Scholar]