Abstract
When cells are exposed to hyperosmotic stress, the Dictyostelium STAT orthologue STATc is rapidly tyrosine phosphorylated. Previous observations suggest a non-paradigmatic mode of STAT activation, whereby stress-induced serine phosphorylation of the PTP3 protein tyrosine phosphatase inhibits its activity towards STATc. We show that two serine residues in PTP3, S448 and S747, are rapidly phosphorylated after osmotic stress. cGMP is a second messenger for hyperosmotic stress response and 8-bromo-cGMP, a membrane-permeable form of cGMP, is a known activator of STATc. GbpC, a cGMP-binding Ras guanine nucleotide exchange factor protein, is a founder member of a protein family that includes LRRK2, the gene commonly mutated in familial Parkinson's disease. Genetic ablation of gbpC prevents STATc activation by 8-bromo-cGMP. However, osmotic-stress-induced activation of STATc occurs normally in the gbpC null mutant. Moreover, 8-bromo-cGMP does not stimulate phosphorylation of S448 and S747 of PTP3 in a wild-type strain. These facts imply the occurrence of redundant activation pathways. We present evidence that intracellular Ca2+ is a parallel second messenger, by showing that agents that elevate intracellular Ca2+ levels are potent STATc activators that stimulate phosphorylation of S448 and S747. We propose that stress-induced cGMP signalling exerts its stimulatory effect by potentiating the activity of a semi-constitutive tyrosine kinase that phosphorylates STATc, whereas parallel, stress-induced Ca2+ signalling represses STATc dephosphorylation through its inhibitory effect on PTP3.
Keywords: Dictyostelium, STAT activation mechanism, cGMP, Ca2+, Tyrosine phosphatase, ROCO protein, Hyperosmotic stress
Introduction
STAT proteins are important regulators of metazoan gene expression that function principally, but not exclusively, as mediators of cytokine signalling (Bromberg and Darnell, 2000; Horvath, 2000). They are activated by phosphorylation at a C-terminus-proximal tyrosine; this is most often mediated by a tyrosine kinase of the Janus kinase (JAK) subfamily. STATs contain a Src homology 2 (SH2) domain and activated STAT proteins dimerise through intermolecular SH2 domain-phosphotyrosine interactions. They then translocate to the nucleus and bind to their transcriptional targets. STAT signalling pathways are generally controlled by modulation of the tyrosine kinase activity, so that when the receptor binds its ligand the cognate JAK is activated. Dictyostelium is the only non-metazoan in which STATs have been functionally characterised and at least one of its four STAT proteins, STATc, appears to be regulated quite differently.
STATc is a regulator of prestalk cell differentiation and is activated when cells are exposed to the stalk cell inducer DIF-1, a chlorinated hexaphenone. Like metazoan STAT1 (Gatsios et al., 1998), STATc is also activated when cells are exposed to hyperosmotic stress (Araki et al., 2003). The peak level of tyrosine phosphorylation of STATc is higher after stress than after DIF treatment and tyrosine phosphorylation is much more prolonged. This might explain why stress induces the expression of a STATc-dependent gene set, whereas DIF-1 fails to activate these genes (Araki et al., 2003). This is an important stress-response pathway in Dictyostelium; a recent microarray study indicated that 20% of all hyperosmotic-stress-induced genes are dependent for their activation on the presence of STATc (Na et al., 2007).
Metazoan STAT activation is generally considered to result primarily from an increase in specific tyrosine kinase activity, but recent computational modelling suggests that, for STAT3, dephosphorylation in the nucleus by T-cell protein tyrosine phosphatate (TCPTP) and consequent export from the nucleus are the dominant rate-determining steps (Guerriero et al., 2009). In accord with these predictions, DIF-induced nuclear accumulation of STATc is controlled by regulated tyrosine dephosphorylation and at the level of nuclear export (Fukuzawa et al., 2003; Araki et al., 2008). The tyrosine phosphatase that dephosphorylates STATc, protein tyrosine phosphatase 3 (PTP3), was identified using a substrate-trapping form of the phosphatase (Araki et al., 2008). It is the most divergent of the three Dictyostelium PTPs (Gamper et al., 1996). All are predicted to be non-receptor tyrosine phosphatases. PTP3 is believed to be essential for growth, because it has consistently proven impossible to disrupt the PTP3 gene (Gamper et al., 1996; Araki et al., 2008). PTP3 is present in the cytosol of unstimulated cells and accumulates in endosomes when cells are subjected to hyperosmotic stress (Gamper et al., 1999). Three pieces of evidence suggest that PTP3 mediates the response of STATc to DIF and stress (Araki et al., 2008): after DIF or stress treatment, serine-threonine modification of PTP3 increases rapidly; the same two treatments cause a decrease in the enzymatic activity of PTP3; in untreated cells, expression of a dominant inhibitor of PTP3 causes semi-constitutive tyrosine phosphorylation of STATc.
Here, we focus on the stress activation of STATc. We analyse the phosphorylation changes that are induced by stress and identify two sites of regulated phosphorylation. One of the known rapid responses to hyperosmotic stress is the accumulation of intracellular cGMP; the membrane-permeable cGMP analogue 8-bromo-cGMP is an activator of STATc (Kuwayama et al., 1996; Araki et al., 2003). GbpC is a cGMP-binding protein that contains Ras, mitogen-activated protein kinase kinase kinase (MAPKKK) and Ras guanine nucleotide exchange factor (GEF) domains (Goldberg et al., 2002; Goldberg et al., 2006; van Egmond et al., 2008). It was a founder member of the ROCO family of proteins. A human ROCO protein, LRRK2, is commonly mutated in familial forms of Parkinson's disease (reviewed by Marin, 2008). We present evidence for the involvement of GbpC in a STATc activation process that is independent of the PTP3-STATc pathway. We also present evidence to suggest that intracellular Ca2+ might be the second messenger of the PTP3-STATc pathway and propose a mechanism whereby the cGMP- and Ca2+-based pathways might work in concert to activate STATc.
Results and Discussion
Identification of stress-regulated sites of serine-threonine phosphorylation in PTP3
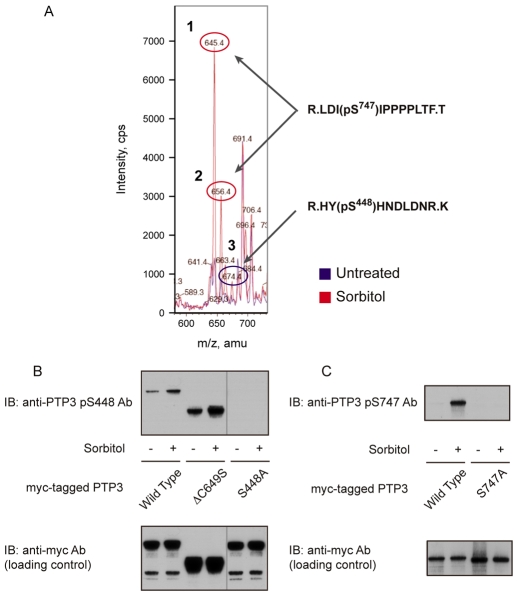
Stress causes an increase in the total phosphoserine and phosphothreonine content of PTP3 (Gamper et al., 1999). We employed mass spectrometry to identify specific phosphorylated sites in an N-terminally deleted, myc-tagged form of PTP3 — myc:PTP3ΔCS (Araki et al., 2008). When the parent-ion scans of the stress-treated and untreated samples were overlaid, species that increased or decreased in apparent relative abundance could be identified (Fig. 1A). The overlay patterns appear complex, but this is a result of different protease miscleavage products, different oxidation states, different bound small ions or different numbers of phosphate groups on peptides derived from the same protein region. Considering only peptides that show a twofold or greater difference in abundance in stress-treated and untreated samples, the pattern resolves to just three phosphorylated peptides. Unique assignment of the phosphorylation site could be made in only two cases, HYSHNDLDNR and LDISIPPPLTF; these two sites are henceforth respectively termed S448 and S747. There could, however, well be other sites of phosphorylation, because we only analysed about 50% of the protein using this technique (see legend to Fig. 1A).
Fig. 1.
Identification of osmotic-stress-induced phosphorylation sites on PTP3. (A) Comparative mass spectrometry of the ΔCS form of myc-tagged PTP3 isolated from control and sorbitol-treated cells. Cells treated with either KK2 or KK2 containing sorbitol (200 mM) for 5 minutes were fractionated by anti-c-myc immunoprecipitation, followed by SDS gel electrophoresis. The myc:PTP3ΔCS bands were excised from the gel and digested with trypsin and chymotrypsin; because initial experiments showed that, to obtain a reasonable representation of the different parts of the protein, it was necessary to double digest in this way. With this enzyme combination, approximately 50% of residues proved analysable. The digested samples were analyzed with nano-liquid chromatography tandem mass spectrometry and the results were overlaid, with the untreated sample shown as a blue line and the sorbitol-treated sample shown in red. From the MS-MS analysis, peptides 1 and 2 were identified as LDI(pS)IPPPPLTF (phospho-Ser747) and peptide 3 as HY(pS)HNDLDNR (phospho-Ser448). (B) Analysis of phospho-Ser448- and phospho-Ser747-specific antibodies. Because of the low amount of PTP3 protein present in the cell, neither of the two antibodies could detect endogenous protein. Thus, PTP3-overepressing cells were used to analyze phosphorylation levels. Total cell lysate of PTP3-overexpressing cells was used for the phospho-Ser448 antibody and immunoprecipitated PTP3 protein was used for the phospho-Ser448 antibody.
Generation and characterization of phospho-specific antibodies against S448 and S747
To confirm the phosphorylation sites as S448 and S747, and also to quantitate phosphorylation levels at the two sites, phospho-specific antibodies were raised and purified by sequential chromatography on the phosphorylated and unphosphorylated forms of the peptide immunogens. The specificity of the antibody directed against S448 was assessed using S448A transformant cells. These contain a myc-tagged PTP3 construct bearing mutations that convert S448 into alanine (S448A). There is no detectable binding to the S448A fusion protein, but there is binding to both full-length PTP3 and to its N-terminally deleted derivative myc:PTP3ΔCS (Fig. 1B). The level of binding to phosphorylated S448 increases after sorbitol treatment, but there is background binding in the non-induced sample. Mutation of S747 to alanine (S747A) completely blocks binding of the S747 antibody (Fig. 1C). Hence, the S747 antibody is highly specific. Furthermore, the S747 site consistently shows a much larger induction ratio, sorbitol induced to control phosphorylation, than S448.
These results confirm the mass spectrometry data that identified S448 and S747 as stress-inducible phosphorylation sites. The two phospho-regulated peptide regions flank the presumptive PTP catalytic domain and they bear little apparent similarity. When used in a program that scans for consensus kinase phosphorylation (SITESCAN; http://scansite.mit.edu), neither site scored positively at high selection stringency, but at medium stringency S448 scored as a potential CDC-like kinase 2 (CLK2) modification site. CLK2 is a dual-specificity serine-threonine and tyrosine kinase of the ‘LAMMER’ family.
cGMP regulation of STATc
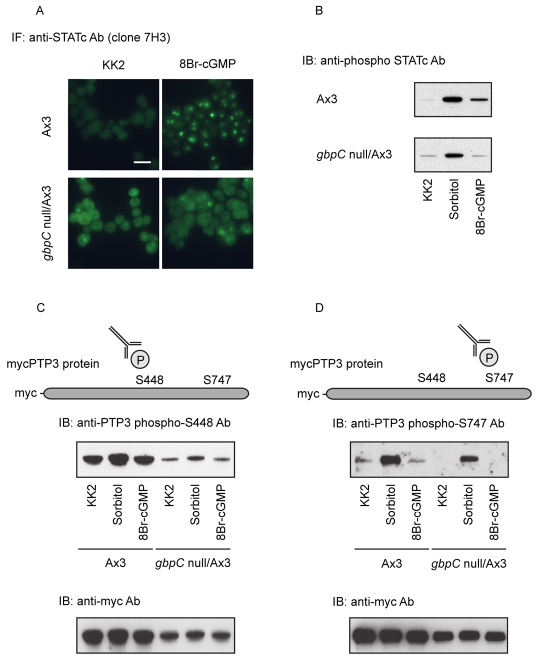
8-bromo-cGMP activates STATc, that is, it induces its tyrosine phosphorylation, with kinetics that closely resemble the kinetics of the hyperosmotic STATc stress response (Araki et al., 2003). Consistent with cGMP playing an important role in the STATc stress response, 8-bromo-cGMP does not induce tyrosine phosphorylation and nuclear accumulation of STATc in a gbpC null strain (Fig. 2A,B). Surprisingly, however, osmotic stress does activate STATc in the gbpC null strain (Fig. 2B). We therefore analysed the phosphorylation of residues S448 and S747 of PTP3 in response to 8-bromo-cGMP in both the parental and the gbpC null strains. There is no 8-bromo-cGMP-induced phosphorylation of S448 or S747 in either strain (Fig. 2C,D). Thus, phosphorylation at S448 and S747 is induced by osmotic stress, but not by 8-bromo-cGMP. These facts suggest the existence of an alternate signalling pathway that is in some way redundant with the cGMP-GbpC pathway and that stimulates the phosphorylation of PTP3 at S448 and S747 upon osmotic shock.
Fig. 2.
The role of cGMP in STATc activation. (A) A comparison of the nuclear localization of STATc in gbpC null cells and parental Ax-3 cells after exposure to 8-bromo (8Br)-cGMP. Cells were fixed and stained for STATc after 5-minutes treatment with 8Br-cGMP, a membrane-permeable form of cGMP, at 20 mM. Scale bar: 20 μm. (B) Activation of STATc in gbpC null cells and parental Ax-3 cells after exposure to 8Br-cGMP. Shown is a comparison of STATc activation, monitored using a phospho-specific antibody, after 5-minutes exposure to sorbitol (200 mM) or 8Br-cGMP (20 mM), in gbpC null cells or parental Ax-3 cells. (C,D) Phosphorylation of PTP3 on S448 and S747 in parental Ax-3 and gbpC null cells exposed to 8Br-cGMP. The level of phosphorylation at the two sites was monitored after 5 minutes of sorbitol (200 mM) or 8Br-cGMP (20 mM) treatment using phospho-specific antibodies as described in Fig. 1B.
Regulation of STATc activation by Ca2+ signalling
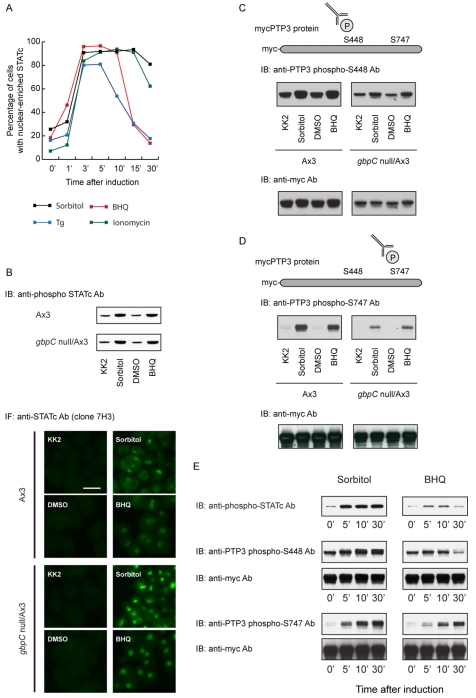
STATc is a regulator of prestalk cell differentiation. There is evidence that alterations of the intracellular Ca2+ concentration directly regulate prestalk-specific gene expression (Gross, 2009). Hence, in searching for an alternative second messenger that might direct STATc activation, we tested the effects of agents that elevate intracellular Ca2+: BHQ [2,5,-di(tert-butyl)-1-4-benzohydroquinone] and a structurally dissimilar sesquiterpene lactone, thapsigargin (Tg). Both agents elevate intracellular Ca2+ levels by inhibiting the pumps that cause Ca2+ to accumulate in the endoplasmic reticulum and acid stores. Ionomycin is a Ca2+ ionophore that causes an increase in intracellular Ca2+ concentration when present in a Ca2+-containing medium. If a cellular process responds to all three drugs, it is likely to reflect a Ca2+-mediated effect and not some side effect of the drug. All three agents induce rapid and robust nuclear translocation of STATc (Fig. 3A).
Fig. 3.
The role of intracellular Ca2+ in STATc activation and PTP3 phosphorylation. (A) The kinetics of STATc nuclear accumulation induced by various agents. Ax-2 cells were treated with sorbitol at 200 mM, BHQ at 30 μM, Tg at 5 μM or ionomycin at 10 μM for the indicated times, and analysed for STATc nuclear accumulation as in Fig. 2A. (B) Analysis of STATc tyrosine phosphorylation and nuclear accumulation induced by BHQ in gbpC null cells. STATc tyrosine phosphorylation and nuclear accumulation, induced by 5-minutes exposure to either sorbitol (200 mM) or BHQ (30 μM), in parental Ax-3 cells or gbpC null cells, were monitored as in Fig. 2. DMSO was included as a control inducer because it was the vehicle used to dissolve BHQ. Scale bar: 10 μm. (C,D) Analysis of phosphorylation at S448 and S747 of PTP3 induced by BHQ. After 5-minutes exposure to either sorbitol (200 mM) or BHQ (30 μM), parental Ax-3 cells and gbpC null cells were analysed as in Fig. 1B,C. (E) The kinetics of STATc activation and phosphorylation of S448 and S747. After exposure to either sorbitol (200 mM) or BHQ (30 μM), parental Ax-2 cells (upper panel) or PTP3-overexpressing cells (all lower panels) were harvested at the indicated times and analysed for STATc activation as in Fig. 2B or for phosphorylation of S448 and S747 of PTP3 as in Fig. 1B.
In both control and gbpC null cells, 5-minutes exposure to BHQ causes an increase in tyrosine phosphorylation of STATc and its nuclear accumulation (Fig. 3B). This is just as would be predicted if there are two separate pathways. We next analysed PTP3 phosphorylation at S448 and S747, again after 5-minutes exposure to BHQ. There is a small but reproducible increase in phosphorylation of S448 and a much larger increase in phosphorylation of S747 (Fig. 3C,D). Thus, mobilization of intracellular Ca2+ causes phosphorylation of the same serine residues in PTP3 that are modified under stress conditions. Again, as would be predicted if there are two separate pathways, phosphorylation at S448 and S747 remains inducible by BHQ in the gbpC null mutant (Fig. 3C,D).
Finally, we compared the longer-term kinetics of STATc activation and phosphorylation at S448 and S747 of PTP3 after treatment with either sorbitol or BHQ (Fig. 3E). STATc is activated after 5-minutes exposure to either inducing agent and, as usual, there is an increase in phosphorylation at S448 and S747 after only 5 minutes of treatment. As expected from the nuclear translocation kinetics (Fig. 3A), sorbitol treatment maintains STATc in an activated state for at least 30 minutes. The phosphorylation of PTP3 at S747 and S448 is similarly long lived. BHQ treatment results in the more transient nuclear accumulation of STATc (Fig. 3A). Concordantly, STATc tyrosine phosphorylation and phosphorylation of PTP3 at S448 decline after 30 minutes of BHQ treatment. This is not true for the phosphorylation of S747, which continues to increase for up to 30 minutes. This might indicate that S448 phosphorylation is more tightly linked to the inhibition of PTP3 activity and the consequent increase in tyrosine phosphorylation of STATc than phosphorylation at S747.
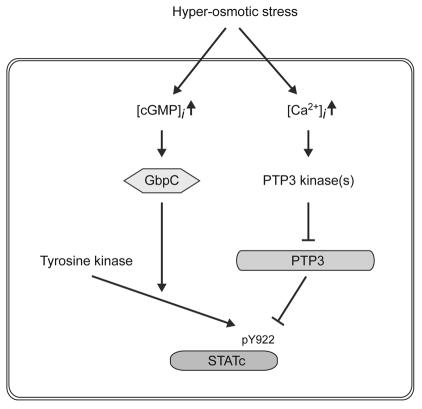
In summary, although 8-bromo-cGMP is a potent activator of STATc, it does not induce phosphorylation of S448 and S747 of PTP3. Moreover, GbpC is required for 8-bromo-cGMP-induced activation of STATc, but not for its activation by hyperosmotic stress. Thus, cGMP does not form part of the stress-regulated STATc dephosphorylation pathway that mediates STATc activation. Based on these results, we propose a two-pronged pathway for STATc activation: one arm regulating STATc phosphorylation and the other regulating its dephosphorylation (Fig. 4). We suggest that cGMP functions as a positive regulator of the, as-yet-unidentified, semi-constitutive tyrosine kinase that phosphorylates STATc. The other second messenger that activates STATc, Ca2+, does induce phosphorylation of S448 and S447; we therefore propose that Ca2+ is the regulator that directs inhibition of PTP3 activity when cells are subjected to hyperosmotic stress. By coordinately and oppositely regulating the activation and deactivation of PTP3, Dictyostelium cells presumably ensure a rapid and efficient response to hyperosmotic stress.
Fig. 4.
Two parallel pathways for activation of STATc by osmotic stress. A semi-constitutively acting tyrosine kinase is proposed to phosphorylate STATc. Hyperosmotic stress is suggested to activate two parallel, oppositely acting pathways: one a cGMP-dependent and Ca2+-independent phosphorylation pathway that upregulates tyrosine kinase activity, the other a cGMP-independent and Ca2+-dependent dephosphorylation pathway that acts directly on STATc. We could not detect significant changes in Ca2+ concentration in the cytoplasm after hyperosmotic stress (our unpublished data), but it is possible that a local Ca2+ increase in a particular part of the cell might have an effect on the phosphorylation of PTP3. Because we are unable to disrupt the PTP3 gene, we cannot rule out other possibilities for the cGMP and GbpC pathway. It could activate other, as-yet-unmapped, phosphorylation sites on PTP3 or it could alter the association of PTP3 with STATc.
Materials and Methods
Strains, cell culture and development
Dictyostelium strain Ax-2 (Gerisch isolate), myc-PTP3 (wild type, Ser448 to Ala and Ser747 to Ala)-overexpressing cells, myc:PTP3ΔCS-overexpressing cells, strain Ax-3 and gbpC null strain (Ax-3 background) were grown axenically in HL-5 medium (Watts and Ashworth, 1970) For stimulation, cells were resuspended in KK2 PB buffer at a concentration of 1×107 cells/ml and were shaken for 4 hours at 200 rpm. Serine to alanine point mutations in PTP3 were introduced using a PCR-based method and confirmed by sequencing.
Parent-ion scanning for the identification of phosphorylation sites
Mass spectrometry to identify specific phosphorylation sites was performed using myc:PTP3ΔCS — an N-terminally deleted, myc-tagged form of PTP3 (Araki et al., 2008). The deletion removes long tracts of a simple repeat sequence and myc:PTP3ΔCS accumulates in cells to a higher concentration than the full-length protein. mycPTP3ΔCS proteins were immunopurified from unstimulated or sorbitol-stimulated cells overexpressing myc:PTP3ΔCS. After purification, samples were separated by SDS-PAGE. The selected bands were suspended in 20 mM ammonium bicarbonate, with trypsin added at a concentration of 12.5 μg/ml, and incubated overnight at 30°C. The digested bands were then extracted and dried down. The extracted samples were resuspended in 49 μl of 20 mM ammonium bicarbonate and 1 μl of 1 mg/ml chymotrypsin was added. The samples were incubated overnight at 30°C. Then the samples were injected onto a nano-liquid chromatography tandem mass spectrometry analysis system. UV chromatograms showed that the amounts of protein loaded onto the LC system for each sample were comparable. The samples were subjected to parent-ion scanning, searching for peptides that generate a phosphate ion. Sites of phosphorylation within the parent ions were then deduced by MS-MS analysis, searching for characteristic ions left after loss of a phosphate group.
Generation of phospho-specific antibodies and immunochemical techniques
Phospho-specific PTP3 antibodies were raised by injecting KLH-conjugated phosphorylated peptides [CHHQRHY(pS)HNDLDN for the pSer448 antibody and CMPRLDI(pS)IPPPLT for the pS747 antibody] into goats, followed by purification using sequential phosphopeptide and non-phosphopeptide column chromatography. These and the other antibodies described, those for STATc and c-myc, were used in western transfer and immunochemical staining, just as described previously (Araki et al., 2008).
Acknowledgments
This work was supported by a Wellcome Trust program grant (053640/Z/98β) to J.G.W. and NWO grants to P.J.M.v.H. We also thank Douglas Lamont, from the University of Dundee College of Life Sciences ‘fingerprints’ proteomics facility, and Judith Langenick for very helpful suggestions at various stages of this study. Deposited in PMC for release after 6 months.
References
- Araki T., Tsujioka M., Abe T., Fukuzawa M., Meima M., Schaap P., Morio T., Urushihara H., Katoh M., Maeda M., et al. (2003). A STAT-regulated, stress-induced signalling pathway in Dictyostelium. J. Cell Sci. 116, 2907-2915 [DOI] [PubMed] [Google Scholar]
- Araki T., Langenick J., Gamper M., Firtel R. A., Williams J. G. (2008). Evidence that DIF-1 and hyper-osmotic stress activate a Dictyostelium STAT by inhibiting a specific protein tyrosine phosphatase. Development 135, 1347-1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg J., Darnell J. E., Jr (2000). The role of STATs in transcriptional control and their impact on cellular function. Oncogene 19, 2468-2473 [DOI] [PubMed] [Google Scholar]
- Fukuzawa M., Abe T., Williams J. G. (2003). The Dictyostelium prestalk cell inducer DIF regulates nuclear accumulation of a STAT protein by controlling its rate of export from the nucleus. Development 130, 797-804 [DOI] [PubMed] [Google Scholar]
- Gamper M., Howard P. K., Hunter T., Firtel R. A. (1996). Multiple roles of the novel protein tyrosine phosphatase PTP3 during Dictyostelium growth and development. Mol. Cell. Biol. 16, 2431-2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper M., Kim E., Howard P. K., Ma H., Hunter T., Firtel R. A. (1999). Regulation of Dictyostelium protein-tyrosine phosphatase-3 (PTP3) through osmotic shock and stress stimulation and identification of pp130 as a PTP3 substrate. J. Biol. Chem. 274, 12129-12138 [DOI] [PubMed] [Google Scholar]
- Gatsios P., Terstegen L., Schliess F., Haussinger D., Kerr I. M., Heinrich P. C., Graeve L. (1998). Activation of the Janus kinase/signal transducer and activator of transcription pathway by osmotic shock. J Biol. Chem. 273, 22962-22968 [DOI] [PubMed] [Google Scholar]
- Goldberg J. M., Bosgraaf L., van Haastert P. J. M., Smith J. L. (2002). Identification of four candidate cGMP targets in Dictyostelium. Proc. Natl. Acad. Sci. USA 99, 6749-6754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. M., Wolpin E. S., Bosgraaf L., Clarkson B. K., van Haastert P. J. M., Smith J. L. (2006). Myosin light chain kinase A is activated by cGMP-dependent and cGMP-independent pathways. FEBS Lett. 580, 2059-2064 [DOI] [PubMed] [Google Scholar]
- Gross J. D. (2009). Acidic Ca2+ stores, excitability, and cell patterning in Dictyostelium discoideum. Eukaryot. Cell 8, 696-702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero M. L., Dudka A., Underhill-Day N., Heath J. K., Priami C. (2009). Narrative-based computational modelling of the Gp130/JAK/STAT signalling pathway. BMC Syst Biol. 3, 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath C. M. (2000). STAT proteins and transcriptional responses to extracellular signals. Trends Biochem. Sci. 25, 496-502 [DOI] [PubMed] [Google Scholar]
- Kuwayama H., Ecke M., Gerisch G., van Haastert P. J. M. (1996). Protection against osmotic stress by cGMP-mediated myosin phosphorylation. Science 271, 207-209 [DOI] [PubMed] [Google Scholar]
- Marin I., van Egmond W., van Haastert P. J. M. (2008). The Roco protein family: a functional perspective. FASEB J. 22, 3103-3110 [DOI] [PubMed] [Google Scholar]
- Na J., Tunggal B., Eichinger L. (2007). STATc is a key regulator of the transcriptional response to hyperosmotic shock. BMC Genomics 8, 123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Egmond W. N., Kortholt A., Plak K., Bosgraaf L., Bosgraaf S., Keizer-Gunnink I., van Haastert P. J. (2008). Intramolecular activation mechanism of the Dictyostelium LRRK2 homolog Roco protein GbpC. J. Biol. Chem. 283, 30412-30420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D. J., Ashworth J. M. (1970). Growth of myxamoebae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem. J. 119, 171-174 [DOI] [PMC free article] [PubMed] [Google Scholar]