Abstract
Protein kinases AKT and PKBR1 of Dictyostelium belong to the AGC protein kinase superfamily. AKT and PKBR1 are phosphorylated at similar sites by phosphoinositide-dependent kinase 1 (PDK1) and TORC2 kinases; however, they have different subcellular localizing domains. AKT has a phosphoinositide 3-kinase (PI3K)/phosphatidylinositol (3,4,5)-trisphosphate [PtdIns(3,4,5)P3]-regulated PH (pleckstrin homology) domain whereas PKBR1 is myristoylated and persistently membrane localized. Using strains defective for PI3K/PtdIns(3,4,5)P3-, PDK1- and TORC2-signaling or strains that express phospho-site mutants of AKT and PKBR1, we dissect the different roles of PI3K/PtdIns(3,4,5)P3, PDK1 and TORC2. We show that activation of AKT and PKBR1 requires PDK1-site phosphorylation, but that phosphorylation by TORC2 is insufficient for AKT or PKBR1 activation. However, PDK1-site phosphorylation is dependent on phosphorylation by TORC2, which suggests that there is regulatory coordination among PDK1, TORC2 and their phospho-site targets. This defines a separate input for signaling in control of chemotaxis and dependency on PDK1 function. We also demonstrate that PDK1 in Dictyostelium functions independently of PI3K/PtdIns(3,4,5)P3. Finally, we show that AKT and PKBR1 exhibit substrate selectivity and identify two novel lipid-interacting proteins preferentially phosphorylated by AKT. Despite certain similarities, AKT and PKBR1 have distinct regulatory paths that impact activation and effector targeting, with PDK1 serving a central role.
Keywords: PDK1; Chemotaxis; PI3K/PtdIns(3,4,5)P3; BAR domains; PH domains; TORC2
Introduction
AKT protein kinases have consensus kinase, regulatory, and PH (pleckstrin homology) localization domains (Song et al., 2005); the latter allows recruitment to membrane-associated phosphatidylinositol (3,4,5)-trisphosphate [PtdIns(3,4,5)P3], a product of phosphoinositide 3-kinase (PI3K) and related lipid moieties (Park et al., 2008). Full activation of mammalian AKT requires phosphorylation at two sites, a phosphoinositide-dependent kinase 1 (PDK1) site within the kinase activation loop and a PDK2 site within the C-terminal hydrophobic motif (HM) (Alessi et al., 1996a; Chan and Tsichlis, 2001). The former is phosphorylated by phosphoinositide-dependent kinase 1 (PDK1), and the latter by TORC2, a multi-protein complex that includes the TOR (target of rapamycin) kinase (Sarbassov et al., 2005). Once activated, AKT phosphorylates substrates at the consensus motif (R/K)X(R/K)XX(S/T) (Alessi et al., 1996b; Obata et al., 2000).
Dictyostelium discoideum has been proven a unique and powerful model for functional studies of AKT and related AGC [cAMP-dependent protein kinase (PKA), cGMP-dependent protein kinase (PKG) and protein kinase C (PKC)] protein kinases during cellular signaling, chemotaxis, and development (McMains et al., 2008; Meili et al., 2000; Meili et al., 1999). Individual Dictyostelium chemotax toward nutrient signals (e.g. folate) and propagate under nutrient-abundant conditions but, upon starvation, initiate multicellular development; developing cells secrete cAMP, which functions as a primary signaling molecule, a chemoattractant, and a morphogen to direct multicellular development (McMains et al., 2008).
The closely related protein kinases AKT and PKBR1 of Dictyostelium are integrated in multiple pathways to organize cell polarity, chemoattractant sensing, and differentiation (Kamimura et al., 2008; McMains et al., 2008; Meili et al., 2000; Meili et al., 1999). Dictyostelium AKT is a definitive ortholog of metazoan AKT, sharing highly related kinase, regulatory, and N-terminal PH domains (Tanaka et al., 1999). Although PKBR1 has similar kinase and C-terminal regulatory domains, it lacks the defining PH domain (Meili et al., 2000). PKBR1 is myristoylated and associates with membrane compartments independently of PtdIns(3,4,5)P3. The shared kinase and regulatory domains, but different localization motifs, suggest functional overlap and specialized activities of AKT and PKBR1.
Previous data had shown that AKT and PKBR1 are phosphorylated at both PDK1 and PDK2/HM sites during development in response to the chemoattractant cAMP (Kamimura et al., 2008). But the separate functions of these phosphorylations in the regulation of AKT and PKBR1 activity had not been addressed and the specific role of PDK1 is unknown. Using a series of Dictyostelium strains that are defective in PtdIns(3,4,5)P3-, PDK1- and TORC2-signaling or that express phospho-site mutants of AKT and PKBR1, we investigated common and distinct pathways that regulate AKT and PKBR1 in response to stimulation during growth by folate and development by cAMP. We demonstrate that PDK1 in Dictyostelium is activated independently of PI3K/PtdIns(3,4,5)P3 signaling, but that PDK1-site phosphorylation is absolutely required for activation of both AKT and PKBR1; phosphorylation by TORC2 is insufficient to activate either kinase. However, PDK1 and TORC2 show cooperative interactions. Loss of phosphorylation at the PDK2/HM site prevents phosphorylation at the PDK1 site. Thus, previously defined functions of TORC2 are dependent upon PDK1. In addition, loss of PDK1 enzyme may lead to reduced phosphorylation at the PDK2/HM site. PDK1 thus defines a new regulatory input for signaling downstream of the chemoattractant receptors. Finally, we show that AKT and PKBR1 exhibit substrate selectivity and identify two novel lipid-interacting proteins preferentially phosphorylated by AKT. Our data provide unique mechanistic differences for the regulation and function of AKT and PKBR1 and context for the complexity of PDK1 and TORC2 regulation of multiple AGC protein kinases.
Results
AKT and PKBR1 are differentially phosphorylated and activated by stimulation with folate and cAMP
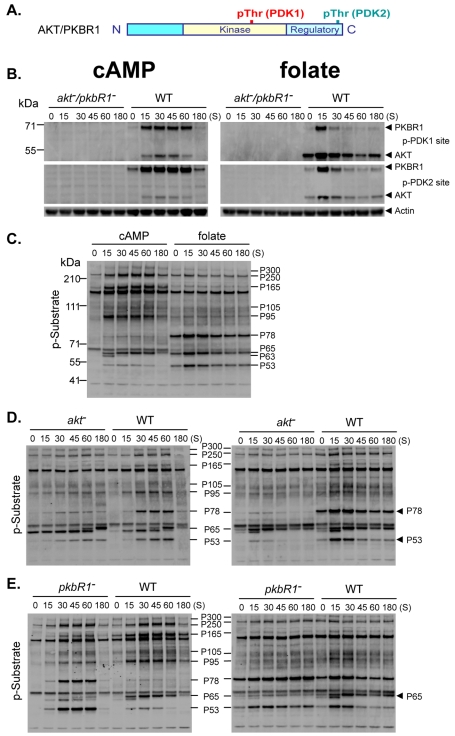
We compared regulation of AKT and PBR1 by chemotactic signaling during growth and development. We chose antibodies that specifically recognize phosphorylated sequences within the kinase [PDK1 site; pTFCGTPEYLAPE (pT278 for AKT and pT309 for PKBR1)] and C-terminal HM regulatory [PDK2/HM site; FEGFpTYVA (pT435 for AKT and pT470 for PKBR1)] domains identical in both AKT and PKBR1 (Fig. 1A). AKT and PKBR1 show low PDK1- and PDK2/HM-site phosphorylations in quiescent cells, but rapid (<15 seconds), transient phosphorylation during growth in response to folate and development in response to cAMP (Fig. 1B). Although the predicted sizes of AKT and PKBR1 are nearly identical, they migrate differently. Mobilities were confirmed using purified proteins, and phosphorylation specificity confirmed with akt-, pkbR1- and akt/pkbR1-null strains (Fig. 1B, supplementary material Fig. S1A,B).
Fig. 1.
AKT and PKBR1 are activated by chemoattractant stimulation with cAMP and folate and phosphorylate preferential substrates. (A) Generic diagram of AKT and PKBR1 with phosphorylated threonine at PDK1 and PDK2/HM sites. (B-E) WT, akt−/pkbR1−, akt−, and pkbR1− cells were collected seconds (S) following stimulation with cAMP or folate and assayed by immunoblot with α-phospho-PDK1 site (p-PDK1), α-phospho-PDK2 site (p-PDK2), α-actin, and/or α-AKT substrate motif (p-Substrate). Wildtype (WT) substrate bands are indicated. P78 and P53 are preferentially phosphorylated by AKT. P65 is preferentially phosphorylated by PKBR1.
AKT and PKBR1 exhibit substrate preferences
The AKT and PKBR1 kinase domains are extremely related and phosphorylate the same substrate motif (R/K)X(R/K)XX(pS/pT) as mammalian AKTs (Alessi et al., 1996b; Kamimura et al., 2008; Obata et al., 2000). Following stimulation with folate or cAMP, we detected a series of phospho-proteins (P300, P250, P165, P105, P95, P78, P65 and P53) using the α-AKT phospho-substrate probe (Fig. 1C). Although each protein generally responds to both folate and cAMP, their relative stimulations to each vary. This suggests that there may be differential actions of AKT and PKBR1 during growth compared with development. Partly this relates to the differing relative expression levels of AKT and PKBR1 during growth and development (Meili et al., 2000; Meili et al., 1999). Regardless of chemoattractant, the substrate phosphorylations follow similar induction kinetics for AKT/PKBR1 phosphorylation and are absent in cells lacking both AKT and PKBR1 (supplementary material Fig. S1C). Some of the proteins may have been previously identified (Kamimura et al., 2008); P250 may be Talin B, P105 may be RasGef5 or PI5K, and P65 may be RhoGAP/GacQ. P300, P165 and P95 are not yet characterized; proteins P78 and P53 had not been previously identified (see below).
We focused on the preferential targets for each kinase. Phosphorylation of P78 and P53 is most sensitive to loss of AKT (Fig. 1D), but is enhanced in folate- and cAMP-stimulated pkbR1-null cells, whereas P65 phosphorylation levels are significantly suppressed in pkbR1-null cells (Fig. 1E). Other proteins are less specifically impacted by loss of either AKT or PKBR1. We conclude that P78/P53 and P65 phosphorylations represent specific readouts for the activities of AKT and PKBR1, respectively.
AKT and PKBR1 are differentially sensitive to regulation by TORC2
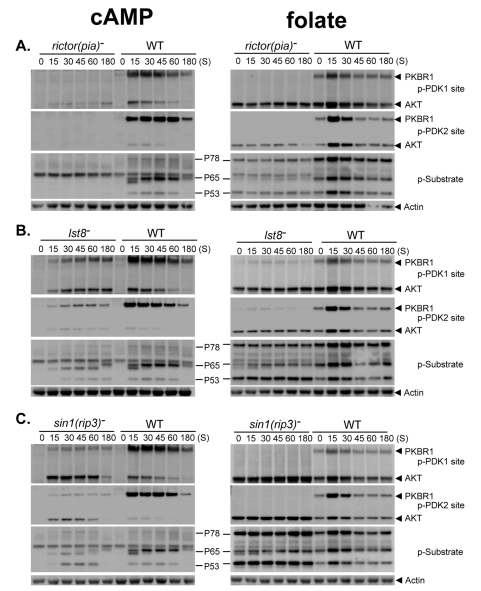
The PDK2/HM site within the C-terminal regulatory domain of AGC kinases is primarily phosphorylated by the TOR kinase, as part of TORC2 (Kamimura et al., 2008; Lee et al., 2005; Sarbassov et al., 2005). Loss of the TORC2 subunit Rictor [rapamycin-insensitive companion of mTOR; Dictyostelium Pia, (pianissimo)] does not alter expression of AKT or PKBR1 (Lee et al., 2005), but blocks cAMP-induced phosphorylation at both PDK1 and PDK2/HM sites of PKBR1 (Kamimura et al., 2008), and mostly blocks phosphorylation of AKT (Fig. 2A); substrate phosphorylations (P78, P65 and P53) by AKT and PKBR1 are also blocked (Fig. 2A). Whereas folate-stimulated phosphorylation of AKT and PKBR1 is inhibited in rictor(pia)-null cells, basal phosphorylation of AKT at both PDK1 and PDK2/HM sites persists and AKT substrates P53 and P78 exhibit constitutive basal phosphorylation (Fig. 2A). Thus, Rictor(Pia) (or perhaps even TORC2) is not required for all PDK2/HM phosphorylations in Dictyostelium.
Fig. 2.
AKT and PKBR1 are differentially sensitive to regulation by TORC2. (A-C) WT, rictor−(pia−), and SIN1−(rip3−) cells were collected following stimulation with cAMP or folate and assayed by immunoblot with α-phospho-PDK1 site (p-PDK1), α-phospho-PDK2 site (p-PDK2), α-actin and α-AKT substrate motif (p-Substrate).
Because the PDK1 site of PKBR1 is not phosphorylated following cAMP stimulation of rictor(pia)-null cells, PDK1 phosphorylation must be dependent upon phosphorylation at the PDK2/HM site. A similar observation has been described in certain mammalian cells in which TORC2 activity has been biochemically inhibited (Garcia-Martinez et al., 2009). Although TORC2 is required to activate PKBR1 (Kamimura et al., 2008; Lee et al., 2005), it may not directly regulate enzymatic activity, but could function primarily to facilitate phosphorylation by PDK1, a principal regulatory pathway for activity of metazoan AKTs (Balendran et al., 1999; Yang et al., 2002).
We also examined TORC2 regulation of AKT and PKBR1 in cells deficient in TORC2 components Lst8 (lethal with sec-thirteen protein 8) and SIN1 [SAPK (stress-activated MAP kinase) interacting protein 1; Dictyostelium RIP3 (Ras-interacting protein 3)]. lst8-null cells resemble a weaker phenotype of rictor(pia)-nulls, with reduced PDK1- and PDK2/HM-site phosphorylations, and delayed and suppressed AKT/PKBR1 substrate phosphorylation (Fig. 2B). More dramatic differences between PDK2/HM phospho-regulation of AKT and PKBR1 are observed in cells lacking SIN1(RIP3) (Fig. 2C). sin1(rip3)-null cells show weak phosphorylation of PKBR1, but phosphorylation of AKT is significantly upregulated by both folate and cAMP when compared with that in wildtype (WT) cells. sin1(rip3)-null cells also exhibit a significant increase in the phosphorylation of the AKT substrates P78 and P53. These data partially explain the comparatively weak developmental phenotype of sin1(rip3)-null cells compared with rictor(pia)-nulls (Lee et al., 2005) and suggest that the subunit composition of TORC2 can influence its substrate targeting.
AKT and PKBR1 are differentially sensitive to PI3K signaling
Class I PI3Ks are the primary kinases that generate PtdIns(3,4,5)P3. Dictyostelium has at least five class I PI3Ks. pi3k1-5-null strain has low levels of PtdIns(3,4,5)P3 during development. Whereas AKT kinase activity is unresponsive to cAMP stimulation in this strain, PKBR1 is membrane-associated independently of PtdIns(3,4,5)P3 levels and is activated independently (Kamimura et al., 2008; Lee et al., 2005; Meili et al., 2000; Takeda et al., 2007). To understand mechanistic differences, we monitored effects of varying levels of PtdIns(3,4,5)P3 in response to folate and cAMP.
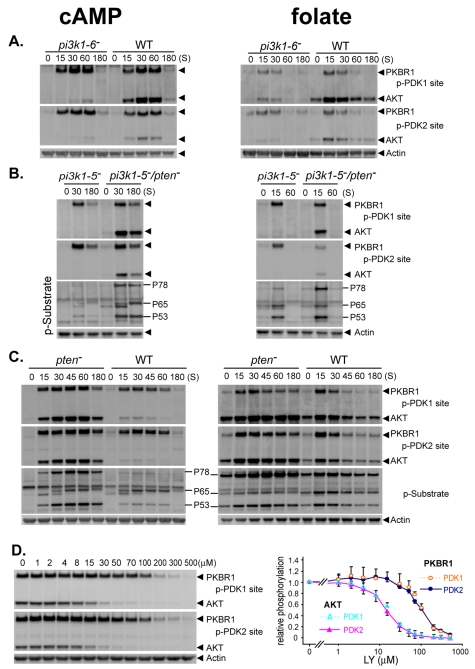
pi3k1-6-null cells are mutated for all five PI3K class I genes plus a more distantly related PI3K gene, pikH (Hoeller and Kay, 2007). Basal and stimulated phosphorylation of AKT at PDK1 and PDK2/HM sites are significantly inhibited in pi3k1-6-null and pi3k1-5-null strains (Fig. 3A,B). By contrast, phosphorylation of PKBR1 was unchanged.
Fig. 3.
AKT and PKBR1 are differentially regulated by PI3K signaling. (A-C) WT, pi3k1—6−, pi3k1-5−, pi3k1-5−/pten− and pten− cells were collected following stimulation with cAMP or folate and assayed by immunoblot with α-phospho-PDK1 site (p-PDK1), α-phospho-PDK2 site (p-PDK2), α-actin and α-AKT substrate motif (p-Substrate). (D) Differential effects of LY on PDK1/2 phosphorylation of AKT and PKBR1. WT cells were pre-treated with various doses of LY and collected 15 seconds following stimulation with folate; they were assayed by immunoblot with α-phospho-PDK1 site (p-PDK1), α-phospho-PDK2 site (p-PDK2) and α-actin. Band phosphorylations were quantified and normalized to 1 for 0 μM LY.
We reasoned that loss of the PtdIns(3,4,5)P3 phosphatase PTEN might stabilize the minimal levels of PtdIns(3,4,5)P3 synthesized in cells lacking PI3K1-5 and rescue AKT response, without impacting PKBR1 (Fig. 3B). Secondary loss of PTEN in cells lacking PI3K1-5 showed rescue of AKT phosphorylation in response to both folate and cAMP, and increased phosphorylation of AKT targets P78 and P53. P78 and P53 responses were also enhanced in pten-null cells compared with those in WT cells; phosphorylation of PKBR1 was unchanged in both PTEN-deficient strains (Fig. 3B,C).
To follow PtdIns(3,4,5)P3 signaling further we studied dose-dependent effects of LY294002 (LY), a phosphoinositide kinase-related kinase (PIKK)-family inhibitor, on phosphorylation at PDK1 and PDK2/HM sites. LY is often presumed to have ‘specificity’ for PI3K, but at high concentrations can target mammalian TOR kinase (Brachmann et al., 2009; Toral-Barza et al., 2005). LY effects on AKT and PKBR1 were markedly distinct (Fig. 3D). AKT phosphorylation at both PDK1 and PDK2/HM sites was identically inhibited with an EC50 of ~15 μM, whereas ~100 μM of LY was required to inhibit both PKBR1-site phosphorylations to 50%.
At low concentrations (<30 μM), LY is relatively specific for PI3K/AKT and inhibits recruitment of AKT to membrane compartments and availability for phosphorylation by PDK1 and TORC2. Thus, the inhibitory effects of LY on PDK1- and PDK2/HM-site phosphorylations are perfectly superimposable for AKT.
At higher concentrations (>100 μM) of LY, PDK2/HM-site phosphorylation of PKBR1 is inhibited. Because phosphorylation at the PDK2/HM site is required for PDK1 phosphorylation, the inhibitory effects of LY on PDK1- and PDK2/HM-site phosphorylations are also perfectly superimposable. These conclusions differ from previous assumptions about the effects of LY in Dictyostelium, which have uniformly viewed LY as specific for PI3K and AKT, regardless of the concentrations used. Furthermore, since both PDK1- and PDK2/HM-site phosphorylations of PKBR1 are resistant to the inhibitory effects of LY on PI3K, PDK1 kinase activity in Dictyostelium must function independently of PtdIns(3,4,5)P3 signaling.
Dictyostelium PDK1 regulates AKT and PKBR1, chemotaxis and development independently of PtdIns(3,4,5)P3
As genetic and biochemical inactivation of TORC2 simultaneously blocks phosphorylation at both PDK2/HM and PDK1 sites, significance of PDK1 phosphorylation in the regulation of AKT and PKBR1 is unknown. To separate the PDK1 and TORC2 kinase functions, we identified two Dictyostelium PDK1 orthologs, PdkA and PdkB, with conserved PDK1-type kinase domains as well as C-terminal PH domains characteristic of all PDK1 kinases. We also generated strains deficient in either gene or in both.
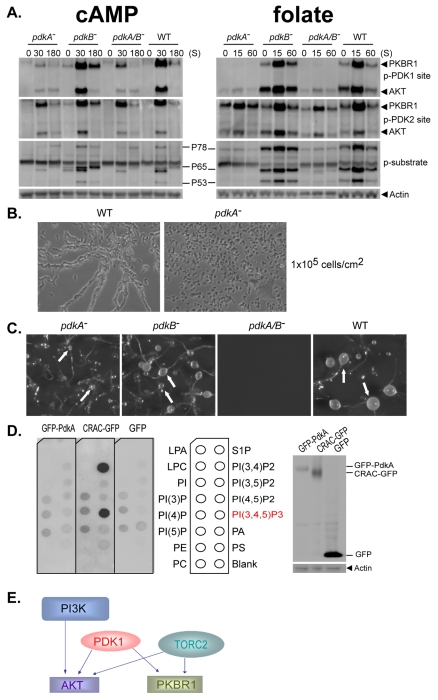
pdkA- and pdkA/B-null cells showed no detectable PDK1 phosphorylation of PKBR1 when stimulated with folate, and only minimal levels with cAMP (Fig. 4A). Loss of PdkB alone had only minimal impact on the phosphorylations and activities of AKT and PKBR1. Thus, PdkA appears to be the predominant regulatory kinase for both PKBR1 and AKT. PDK2/HM phosphorylation of PKBR1 was observed in folate- and cAMP-stimulated pdkA- and pdkA/B-null cells but, compared with WT and pdkB-null cells, P65 phosphorylation was undetected in folate-treated cells, and highly reduced in cAMP-treated cells. Thus, PDK2/HM-site phosphorylation, in the absence of PDK1-site phosphorylation, is not sufficient for activation of PKBR1. AKT regulation was similarly impacted. In folate-treated pdkA- and pdkA/B-null cells, AKT has basal PDK1 phosphorylation and activated PDK2/HM-site phosphorylation, but only poor phosphorylation of the AKT substrates P78 and P53. In cAMP-treated pdkA- and pdkA/B-null cells, PDK2/HM-site phosphorylation of AKT was reduced slightly.
Fig. 4.
Dictyostelium PDK1 regulates AKT and PKBR1, and chemotaxis and development independently of PtdIns(3,4,5)P3. (A) WT, pdkA−, pdkB− and pdkA/B− cells were collected following stimulation with cAMP or folate and assayed by immunoblot with α-phospho-PDK1 (p-PDK1), α-phospho-PDK2 site (p-PDK2), α-actin and α-AKT substrate motif (p-Substrate). (B) WT and pdkA− cells were plated in submerged culture at 1×105 cells/cm2 and imaged after 8 hours. (C) WT, pdkA−, pdkB− and pdkA/B− cells were plated on solid substrata and developed for 24 hours; arrows indicate sori of terminally differentiated organisms. (D) Lipid binding assay for PdkA. Cell extracts from pdkA-nulls expressing functional GFP-PdkA were incubated with a PIP lipid strip, as indicated. Bound proteins were detected with α-GFP. WT cells expressing GFP or GFP fused to CRAC were controls. Relative expression was determined by immunoblot with α-GFP. (E) PI3K, PDK1 and TORC2 regulation of AKT and PKBR1. PI3K regulates PtdIns(3,4,5)P3 accumulation and recruitment of AKT to membranes. Membrane-bound AKT and PKBR1 are phosphorylated by PDK1 and TORC2 independently of PI3K.
As expected of cells that poorly activate AKT and PKBR1, pdkA-null cells do not aggregate at low density in submerged culture (Fig. 4B), whereas aggregation of pdkB-null cells is similar to that in WT cells (data not shown). When plated on solid substrata at high cell densities, pdkA-null cells formed much smaller aggregates and terminal developmental structures compared with those of WT cells (Fig. 4C), whereas pdkB-null cells formed smaller aggregates than did the WT. However, the pdkA/B-null cells have more severe developmental defects and fail to develop during the 24 hour time-course, which suggests there are developmental regulatory functions for PDK1 beyond AKT and PKBR1.
Although phosphorylation at the PDK1 site is critical for activation of AKT and PKBR1, PKBR1 is activated independently of PI3K. The LY and pi3k-null data reconcile these observations and show that PDK1 phosphorylation in Dictyostelium occurs independently of PtdIns(3,4,5)P3-signaling. To examine this more directly, we expressed a GFP-PdkA fusion in pdkA-null cells and monitored lipid interaction specificity (Fig. 4D). Although GFP-PdkA can rescue the mutant phenotype, it exhibits no PtdIns(3,4,5)P3-binding specificity when compared with a control, the PH domain of CRAC. Further, structural predictions of the PdkA and PdkB PH domain are not consistent with PtdIns(3,4,5)P3 interaction (Park et al., 2008). In contrast to mammalian PDK1, Dictyostelium PDK1 functions independently of PtdIns(3,4,5)P3 signaling (Fig. 4D).
PDK2/HM-site phosphorylation of PKBR1 and AKT is not sufficient for kinase activation by folate or cAMP
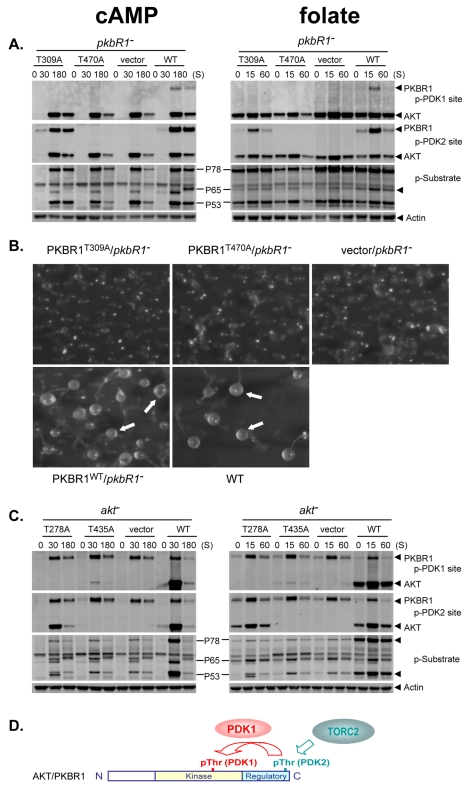
The TORC2-inactivation studies indicate that PDK1-site phosphorylation of AKT and PKBR1 is dependent upon phosphorylation at the PDK2/HM site, but data from pdkA- and pdkA/B-null cells indicate that PDK2/HM-site phosphorylations alone are insufficient for activation of AKT and PKBR1. To address inter-dependency of PDK1 and PDK2/HM phosphorylation more directly, we studied cells expressing PDK1- or PDK2/HM-site-specific mutants of AKT and PKBR1, respectively; T309A and T470A for PKBR1, and T278A and T435A for AKT.
Re-expression of PKBR1WT in pkbR1-null cells fully rescued folate- and cAMP-regulated phosphorylation of PKBR1, substrate phosphorylation of P65, and development (Fig. 5A,B). pkbR1-null cells expressing the PDK2/HM mutant PKBR1T470A was similar to the pkbR1-null parental. There was no rescue of PDK1 phosphorylation, consistent with PDK1-site phosphorylation requiring phosphorylation at the PDK2/HM site, and no rescue of p65 substrate phosphorylation or of development.
Fig. 5.
PDK2/HM phosphorylation of PKBR1 and AKT is not sufficient for activation by cAMP. (A) pkbR1− cells transfected with control vector or vectors engineered to express PKBR1WT, PKBR1T309A, or PKBR1T470A and collected following stimulation with cAMP or folate and assayed by immunoblot with α-phospho-PDK1 (p-PDK1), α-phospho-PDK2 site (p-PDK2), α-actin, and α-AKT substrate motif (p-Substrate). (B) The various pkbR1− strains (see Fig. 5A) were plated on solid substrata and developed for 24 hours; arrows indicate terminally differentiated organisms. (C) akt− cells transfected with control vector or vectors engineered to express AKTWT, AKTT278A, or AKTT435A and collected following stimulation with either cAMP or folate and assayed by immunoblot with α-phospho-PDK1 (p-PDK1), α-phospho-PDK2 site (p-PDK2), α-actin and α-AKT substrate motif (p-Substrate). (D) A generic diagram of AGC kinases AKT and PKBR1 indicating the coordination of kinases PDK1 and TORC2 to phosphorylate the threonine residues in the kinase and C-terminal domains, respectively, at PDK1 and PDK2/HM sites.
Focus on the PDK1 mutant PKBR1T309A is more revealing (Fig. 5A,B). Expressed PDK1 mutant PKBR1T309A in pkbR1-null cells fails to phenotypically rescue P65 substrate phosphorylation or development; despite the high levels of PDK2/HM phosphorylation, the PKBR1T309A-expressing cells are phenocopies of the pkbR1-null parent.
Similar functional conclusions can be derived from studies of mutated AKT. Expression of AKTWT rescues phosphorylation of AKT, P78 and P53 (Fig. 5C). Expression of the PDK1 mutant AKTT278A in akt-null cells shows PDK2/HM-site phosphorylation, no PDK1-site phosphorylation, and no rescue of P78 and P53 phosphorylation above vector controls. Only weak PDK1 phosphorylation of AKT is observed with the PDK2/HM mutant AKTT435A.
We conclude that PDK1-site phosphorylation is an obligatory activation step for both PKBR1 and AKT, but phosphorylation by TORC2 is wholly insufficient for their activations. Although TORC2 is essential for the activation of AKT and PKBR1, it may primarily function to make AKT and PKBR1 permissive for targeting by PDK1 (Fig. 5D). Potentially, TORC2 has only minimal impact on the overall enzymatic activity of AKT and PKBR1.
Possibly other interacting and coordinating processes regulate PDK1 and TORC2 phosphorylation of AKT and PKBR1. Although PDK2/HM-site phosphorylation of AKT and PKBR1 is unaffected if the PDK1 is unphosphorylated, PDK2/HM-site phosphorylation may be impacted by loss of the PDK1 enzymes (Fig. 4A,D).
The two preferential AKT substrates possess lipid-interacting motifs
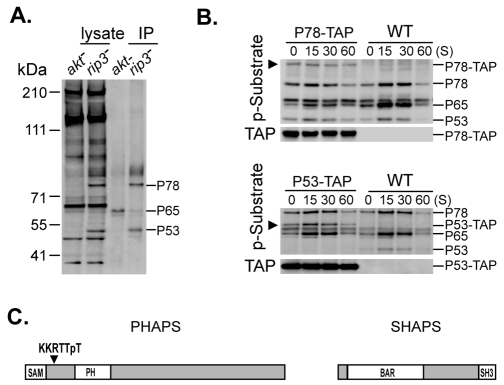
To identify substrates preferentially targeted by AKT, we used folate to stimulate strains fully deficient (i.e. akt-null) or hyper-activated (e.g. rip3- or pten-null) for AKT (see Fig. 1D, Fig. 2C, Fig. 3C). Cell extracts from rip3-null cells that had been stimulated with folate for 15 seconds show very high levels of P78 and P53 phosphorylation, whereas these phosphorylations are highly suppressed in folate-treated akt-null cells (Fig. 6A). By contrast, phosphorylation of the PKBR1 substrate P65 is observed in akt-null cells. We purified the substrates using immobilized antibodies to the AKT phospho-substrate site. Immunoblot confirmed enriched recovery of AKT substrates P78 and P53 from the rip3- and pten-null cells (Fig. 6A).
Fig. 6.
Purification and identification of AKT preferential substrates PHAPS and SHAPS. (A) akt− and rip3− cells were lysed 15 seconds after folate stimulation. Samples from cell lysates and AKT-substrate immunoprecipitations (IP) were immunoblotted with α-phospho-AKT substrate. (B) Confirmation of P78 and P53 as AKT substrates. The TAP epitope was fused to the endogenous P78 and P53 gene loci by targeted homologous recombination; there are two P78 genes in Dictyostelium (supplementary material Fig. S2) and only 1 P53 gene. Equivalent cell sample volumes of WT, P78-TAP or P53-TAP cells were collected at various times following stimulation with folate and assayed by immunoblot with α-phospho-AKT substrate and α-TAP. A new band detected by both antibodies is indicated by an arrow in each TAP cell and is absent in WT cells. P53 is detected only in WT cells. (C) Structure of PHAPS and SHAPS. PHAPS (P78) contains SAM and PH domains and the phosphorylated AKT motif KKRTTpT. SHAPS (P53) contains BAR and SH3 domains.
Immunopurified proteins from akt- and rip3-null cells were separated by electrophoresis, extracted from the 78 and 53 kDa gel regions, and analyzed by LC/MS/MS peptide sequencing. For P78, we obtained 25 unique peptides/25 unique spectra that identified (100% probability) a novel 86 kDa protein from Dictyostelium. No Dictyostelium peptides were identified in the 78 kDa region from the akt-null control. For the 53 kDa region, we also identified a single Dictyostelium protein (53 kDa) (99% probability). To confirm that both P78 and P53 were AKT targets, we fused a TAP-coding sequence with the endogenous genomic loci and demonstrated that both P78 and P53 exhibited a gel mobility shift to a new protein band that was co-detected with α-TAP (Fig. 6B).
The amino acid sequence of P78 offers several interesting characteristics (Fig. 6C). Its N-terminus is defined by a protein-protein interacting SAM (sterile alpha motif) module and a downstream PH-domain; we refer to P78 as PHAPS, PH/AKT-preferential substrate. Structural considerations suggest that the PH domain of PHAPS would not interact strongly with PtdIns(3,4,5)P3 (Park et al., 2008) and so it would not be simultaneously recruited to membrane compartments with AKT. Re-examination of the LC/MS/MS peptide sequencing data confirms the phospho-peptide TTpTISIGK, within a consensus AKT recognition motif, KKRTTpTISIGK (Fig. 6C). During characterization studies, we detected two PHAPS genes (supplementary material Fig. S2).
P53 has an N-terminal BAR (Bin/Amphiphysin/Rvs) domain and a C-terminal SH3 domain and is termed SHAPS, SH3/AKT-preferential substrate (Fig. 6C). The SH3 motif is a well characterized protein-protein interaction domain and the BAR motif is implicated in lipid binding and membrane curvature sensing (Frost et al., 2009; Itoh and Takenawa, 2009; Peter et al., 2004). SHAPS may be a Dictyostelium equivalent of the bridging integrator (or amphiphysin) proteins implicated in cell polarization and phagocytosis in several species (Prendergast et al., 2009).
Discussion
Dictyostelium AGC kinases AKT and PKBR1 are phosphorylated at PDK1 and PDK2/HM sites, have similar kinase domains, and phosphorylate the same substrate motif (Fig. 1A). However, they have distinct regulatory inputs. Dictyostelium AKT activation requires PI3K signaling; inhibition of PtdIns(3,4,5)P3 signaling blocks AKT recruitment to membrane compartments and consequent phosphorylation by both PDK1 and TORC2. PKBR1 lacks a defining AKT regulatory PH-domain and is activated independently of PI3K/PtdIns(3,4,5)P3 (Kamimura et al., 2008; Lee et al., 2005; Meili et al., 2000; Meili et al., 1999). We now demonstrate an essential function for PtdIns(3,4,5)P3-independent regulation of PDK1 in the activation of both AKT and PKBR1 (Fig. 4E). We also describe unique interactions and dependencies of PDK1 and TORC2 in AKT and PKBR1 regulations and differential specificity toward downstream targets (Fig. 5D). These data emphasize a new regulatory input in the pathway through PDK1 that is separate from PI3K or TORC2 (Fig. 4E).
Phosphorylation of the PDK2/HM site by Dictyostelium TORC2 has been well described. However, its contribution to AKT and PKBR1 activity has not been clear and the function of PDK1 phosphorylation has been entirely unknown. We now show that phosphorylation of the PDK2/HM site is completely insufficient for activation of either PKBR1 or AKT. Neither is activated in the absence of PDK1-site phosphorylation, data and conclusions consistent with observations in other systems, where activation of AKT absolutely requires phosphorylation by PDK1 (Bellacosa et al., 1998; Mora et al., 2003; Williams et al., 2000). In contrast to mammalian PDK1, Dictyostelium PDK1 lacks PtdIns(3,4,5)P3-binding specificity and functions independently of PI3K/PtdIns(3,4,5)P3 signaling (Fig. 4E). These conclusions are further supported by structural analysis of the PH domains of PdkA and PdkB (Park et al., 2008).
While activation of both PKBR1 and AKT requires phosphorylation by PDK1, phosphorylation by PDK1 is mediated by PDK2/HM-site phosphorylation (Fig. 5D); PKBR1 (or AKT) is inactive in cells that lack functional TORC2 or that express PKBR1 (or AKT) carrying a PDK2/HM phospho-site mutation. PDK2/HM-site phosphorylation by TORC2 in the regulation of AKT activity is complex and has differing functions. PDK2/HM-site phosphorylation can allosterically enhance AKT activity (Biondi et al., 2000), can slightly broaden substrate specificity of activated AKT (Jacinto et al., 2006), but can also promote interactions with PDK1 to promote AKT phosphorylation at the PDK1 site within its kinase activation loop (Balendran et al., 1999; Yang et al., 2002). This latter mechanism may be the significant regulatory role of TORC2 in the activation of AKT and PKBR1 in Dictyostelium. However, PDK1 and PDK2/HM-site phosphorylations may be too rapid to demonstrate that PDK2/HM-site phosphorylation precedes that of PDK1 (Fig. 4E, Fig. 5D). Alternatively, in the absence of PDK2/HM-site phosphorylation, phosphorylation of the PDK1 site may occur normally, but become rapidly de-phosphorylated.
Although PDK1 phosphorylation of AKT and PKBR1 requires phosphorylation at the PDK2/HM site, the opposite is not the case. PDK2/HM-site phosphorylation persists in AKT and PKBR1 protein kinases that carry PDK1 phospho-site mutations. Nonetheless, data from cells lacking PdkA suggest the potential for functional interaction between PDK1 and TORC2 that mediates efficient phosphorylation of AKT and PKBR1 at both PDK1 and PDK2/HM sites (Fig. 5D). Dictyostelium may provide a unique approach to study interactions among PDK1 and PDK2/HM sites and their upstream kinases, PDK1 and TORC2. It should be noted that, although absolute cross-dependency of PDK1- and PDK2/HM-site phosphorylations is not universal, similar mechanisms may occur in other systems (Garcia-Martinez et al., 2009; Jacinto et al., 2006; Williams et al., 2000).
Despite the differences in their regulation and localization (Fig. 4E), AKT and PKBR1 have been viewed as functionally identical and inferred to exhibit minimal substrate preference (Kamimura et al., 2008). However, in addition to the distinct regulatory paths for activation of AKT and PKBR1, we now demonstrate that these kinases have substrate preferences and identify two novel AKT substrates, PHAPS and SHAPS. Both PHAPS and SHAPS have lipid-binding motifs and protein interaction domains. No equivalent PHAPS protein is identified in other systems. SHAPS, however, is part of the BIN/amphiphysin family. Consistent with the targeted phosphorylation of SHAPS following chemotactic stimulation, BIN/amphiphysin proteins are implicated in a wide array of membrane processes, including actin function, interaction with small GTPases and other signaling pathways, and clathrin-mediated endocytosis (Frost et al., 2009; Peter et al., 2004; Yamada et al., 2007). PHAPS and SHAPS may function as adapters to recruit components to membrane compartments and are potentially regulated by differential states of phosphorylation.
Materials and Methods
Strains and genes
Dictyostelium strain ID names are: akt− (DBS0236784), pkbR1− (DBS0236785), akt−/pkbR1− (DBS0236785), pi3k1-6− (DBS0252653), pi3k1-5− (DBS0252652), pi3k1-5−/pten− (DBS0252654), pten− (DBS0252655), rip3− and pia− strains were courtesy of Richard Firtel (UCSD, La Jolla, CA). akt−/pkbR1− and pi3k1-6− strains were grown in association with Klebsiella aerogenes bacteria.
Gene ID names are SHAPS (DDB_G0288895), PHAPS (DDB_G0271552), PdkA (DDB_G0281471), PdkB (DDB_G0284489), AKT (pkbA DDB_G0268620) and PKBR1 (pkbG DDB_G0290157).
Dictyostelium development
Growing cells were washed and spread evenly on pre-boiled black filters on wet paper pads. For aggregation, growing cells were washed and incubated in microtitre wells. After 8 hours, cells in the center of each well were imaged.
AKT and PKBR1 phosphorylation
cAMP and folate stimulations are modifications (Liao and Kimmel, 2009). Immunoblots used α-phospho-PDK1 site (Cell Signaling), α-phospho-PDK2/HM site (Cell signaling), α-phospho-AKT Substrate (Cell Signaling), and α-Actin (Santa Cruz Biotechnology).
AKT, PKBR1, PdkA, SHAPS and PHAPS
AKT and PKBR1 were mutated using QuikChange II (Stratagene). pGEX was used for bacterial expression and PDXA was used for Dictyostelium expression.
We used primer pairs 5′-GGATCCATGTCAACAGCACCAATTAAACATG-3′ and 5′-CTCGAGTTATCTTAAATGTTCAGATTCAGCGAC-3′ to amplify AKT and primers pairs 5′-GGATCCATGGGAAAAGGACAAAGTAAAATAAAG-3′ and 5′-CTCGAGTTAATCCTTTAAGATTGAATCAGCTACA-3′ to amplify PKBR1 protein coding regions from mRNA. Point mutations at PDK1 and PDK2/HM sites were made in the TA vector using QuikChange II site-Directed Mutagenesis Kit (Stratagene). pGEX was used for bacterial expression and PXDA was used for expression in Dictyostelium.
pGEX-6p-1-AKT or pGEX-6p-1-PKBR1 cells were grown overnight, diluted to OD ~0.1, and shaken at 23°C to OD ~0.4. 0.3 mM IPTG was added to induce expression. After 6 hours, bacteria were pelleted and resupended in 1× PBS plus protease inhibitor cocktail. Lysozyme was added to 100 ng/ml for 10 minutes and samples were frozen in a dry ice-isopropanol bath and thawed at 4°C overnight. The thawed samples were centrifuged at 50,000 g for 1 hour. Supernatant was added to 500 μl of equilibrated sepharose 4B beads and rotated gently for 1 hour. After 3 rounds of wash, beads were added with cleavage buffer and precession protease (GE healthcare). After rotating gently at 4°C for 4 hours, the AKT or PKBR1 was eluted from beads.
The PdkA locus was targeted using pLPBPP. pLPBLP-TAP contained the TAP-coding sequence fused upstream of BlastR in pLPBLP and was used for TAP tag fusions to PHAPS and SHAPS.
To construct the pdkA-null strain, PDKA genomic DNA was amplified using primer pairs 5′-CCTTAATGCTAAAATTTCGGTGTC-3′ and 5′-TTTTTGAGGATTATGAGCAACATCTTCT-3′ and cloned into pCR4-TOPO (Invitrogen). The resulting plasmid was linearized within the genomic sequence with BglII and blunt-end ligated to the SmaI sites flanking the Blasticidin resistant cassette in pLPBPP (Faix et al., 2004).
To construct the pdkB-null strain, we prepared left and right arm recombination fragments. Primer pairs 5′-GCGGCCGCAACAATGGCTAGATGATCAAGTTATTC-3′ and 5′-ACTAGTCTTCTTTTTGGTAACGACCATTTATC-3′ were used to amplify the left arm from genomic DNA; the right arm was amplified using primer pairs 5′-AAGCTTTCCAAGATATATAAATCCATTACCAACTC-3′ and 5′-GTCGACGAAATGTCTTGATCTTCCTTTTGAG-3′. The left arm and right arms were then inserted into pLPBLP.
For PHAPS mutagenesis, we prepared left and right arm recombination fragments. Primer pairs 5′-GCGGCCGCGAGTTGTGTTGTGGTTTACACGTC-3′ and 5′-ACTAGTGCAACCAAATCATCTTCTGTTAATTCTG-3′ were used to amplify the left arm from genomic DNA; the right arm was amplified using primer pairs 5′-AAGCTTCAACCTATGCTCTTCAAGATAAAG-3′ and 5′-GTCGACGATTTCCCAGAAAGTTGAACGG-3′. The left arm and right arms were then inserted into pLPBLP.
pLPBLP-TAP contained the TAP coding sequence fused upstream of BlastR in pLPBLP. The TAP sequence was amplified from plasmid PKK4 (kindly provided by Ralf Graph, Ludwig-Maximilians University, Munich, Germany) using primer pairs 5′-AAGCTTGCATGCTCAATGGAAAAGAGAAGATGGAAAAAG-3′ and 5′-CCATGGTCAGGTTGACTTCCCCGCGGAAT-3′. The TAP fragment was excised with HindIII and NcoI and inserted into pLPBLP, generating pLPBLP-TAP. For PHAPS-TAP fusion mutagenesis we prepared left and right arm recombination fragments. The PHAPS left recombination arm was amplified using primer pairs 5′-GTC GACGAATGGAATATTAATGGTAAAGAACC and ′5-AAGCTTACCACCACCATTAGTTTTTTCATCTTTTTTAGCAGTC-3′; the left arm terminated just upstream of the UAA stop codon in the genomic DNA. The PHAPS right recombination arm was amplified from genomic DNA using primer pairs 5′-ACTAGTTAAAAATAGGCATTTTGCTCAAAC-3′ and 5′-GCGGCCGCATACACTTAATGGTACCATTAAAGTGTG-3′. The left arm was then inserted in-frame with TAP into pLPBLP-TAP; the right arm was ligated downstream.
For SHAPS-TAP fusion mutagenesis we prepared left and right arm recombination fragments. The SHAPS left recombination arm was amplified using primer pairs 5′-GTCGACCAATTCAGAGATATTAGATCTCGTCTTG-3′ and 5′-AAGCTTACCACCACCACCACTTGTATGATTACATGGAACTAAAC-3′; the left arm terminated just upstream of the UAA stop codon in the genomic DNA. The SHAPS right recombination arm was amplified from genomic DNA using primer pairs 5′-ACTAGTTTATCAACATTCACTCTTTTTAACTTTATC-3′ and 5′-GCGGCCGCGTTGATTTGACACCAGTCTACCC-3′. The left arm was then inserted in-frame with TAP into pLPBLP-TAP; the right arm was ligated downstream.
The knock-out or TAP knock-in constructs were linearized and electroporated into AX3 strain and transformants were selected for growth under blasticidin selection pressure. Homologous recombinants were identified by genomic PCR and RT-PCR. TAP knock-in strains were also confirmed by immunoblot with TAP antibody (Open Biosystems CAB1001).
PIP-strip binding assay
The protocol for the PIP-strip binding assay has been described previously (Comer et al., 2005). Briefly, 2×108 cells were collected, differentiated for 2 hours with cAMP, and resuspended in 2.5 ml of lysis buffer (10 mM Tris, pH 7.5, 0.2 μM EGTA, 0.2 M sucrose and protease inhibitor). Cells were lysed through a 5 μm filter membrane, samples were centrifuged at 9500 g for 15 minutes, and the supernatant diluted into 12 ml of TBST containing 1% nonfat dry milk and protease inhibitor. Extracts were incubated overnight at 4°C with PIP strips (Echelon Bioscience) that had been pre-blocked with 1% nonfat dry milk in TBST. Strips were washed and assayed by binding to α-GFP (COVANCE).
Immunopurification of AKT preferential substrates
Cells were resuspended in PB (11.4 mM sodium phosphate, pH 6.5) at a density of 5×107 cells/ml and starved for 30 minutes. Cells were stimulated at 50 μM folic acid and lysed with an equal volume 2× NP-40 lysis buffer [2× PB, 1% NP-40, 100 mM NaF, 4 mM Na3VO4, 50 mM sodium pyrophosphate, 400 μM PMSF, 1 complete mini, EDTA-free, protease inhibitor cocktail tablet (Roche)]. Cell lysates were incubated on ice for 5 minutes and then centrifuged at 4°C for 30 minutes at 20,000 g. Immobilized α-phospho-AKT substrate resin [Cell Signaling Immobilized Phospho-(Ser/Thr) Akt substrate antibody] was added to the supernatant in a 1:10 ratio and allowed to incubate at 4°C overnight with gentle rotating. The resin was washed twice with 1× NP-40 lysis buffer and twice with 1× RIPA buffer [150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl (pH 8.0)]. The proteins were eluted by gentle shaking with 8 M urea. The protein samples were concentrated and the buffer was replaced with 100 mM NaCl with sequential washes using a Microcon YM-30 column (Millipore). The samples were resolved on a 3-8% gel, with prior reduction and alkylation, and visualized by silver staining (SilverQuest Silver Staining Kit, Invitrogen). Experimental and control gel bands were excised, de-stained and washed according to SilverQuest, and subjected to in-gel trypsin digestion and peptide extraction (Shevchenko et al., 2006) using a MassPrep robot (Micromass/Waters modified Packard Multiprobe II). The resulting peptide extracts were analyzed by LC/MS/MS (NanoAquity, Waters). Data were automatically searched using the Mascot Daeomon and the NIH-CIT Mascot Server (Perkins et al., 1999). Flat files from these searches were used to generate Scaffold files (Proteome Software).
Supplementary Material
Acknowledgments
We thank Xiuli Huang, Marielle Young and Colette Young for assistance in gene targeting experiments. David Eric Anderson was invaluable for mass spectrometric analyses. Finally, we thank Dictybase and colleagues for various strains. This research was supported by the Intramural Research Program of the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/6/983/DC1
References
- Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. (1996a). Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541-6551 [PMC free article] [PubMed] [Google Scholar]
- Alessi D. R., Caudwell F. B., Andjelkovic M., Hemmings B. A., Cohen P. (1996b). Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 399, 333-338 [DOI] [PubMed] [Google Scholar]
- Balendran A., Casamayor A., Deak M., Paterson A., Gaffney P., Currie R., Downes C. P., Alessi D. R. (1999). PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr. Biol. 9, 393-404 [DOI] [PubMed] [Google Scholar]
- Bellacosa A., Chan T. O., Ahmed N. N., Datta K., Malstrom S., Stokoe D., McCormick F., Feng J., Tsichlis P. (1998). Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene 17, 313-325 [DOI] [PubMed] [Google Scholar]
- Biondi R. M., Cheung P. C., Casamayor A., Deak M., Currie R. A., Alessi D. R. (2000). Identification of a pocket in the PDK1 kinase domain that interacts with PIF and the C-terminal residues of PKA. EMBO J. 19, 979-988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann S., Fritsch C., Maira S. M., Garcia-Echeverria C. (2009). PI3K and mTOR inhibitors: a new generation of targeted anticancer agents. Curr. Opin. Cell Biol. 21, 194-198 [DOI] [PubMed] [Google Scholar]
- Chan T. O., Tsichlis P. N. (2001). PDK2: a complex tail in one Akt. Sci STKE 2001, PE1 [DOI] [PubMed] [Google Scholar]
- Comer F. I., Lippincott C. K., Masbad J. J., Parent C. A. (2005). The PI3K-mediated activation of CRAC independently regulates adenylyl cyclase activation and chemotaxis. Curr. Biol. 15, 134-139 [DOI] [PubMed] [Google Scholar]
- Faix J., Kreppel L., Shaulsky G., Schleicher M., Kimmel A. R. (2004). A rapid and efficient method to generate multiple gene disruptions in Dictyostelium discoideum using a single selectable marker and the Cre-loxP system. Nucleic Acids Res. 32, e143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost A., Unger V. M., De Camilli P. (2009). The BAR domain superfamily: membrane-molding macromolecules. Cell 137, 191-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Martinez J. M., Moran J., Clarke R. G., Gray A., Cosulich S. C., Chresta C. M., Alessi D. R. (2009). Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR). Biochem. J. 421, 29-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeller O., Kay R. R. (2007). Chemotaxis in the absence of PIP3 gradients. Curr. Biol. 17, 813-817 [DOI] [PubMed] [Google Scholar]
- Itoh T., Takenawa T. (2009). Mechanisms of membrane deformation by lipid-binding domains. Prog. Lipid Res. 48, 298-305 [DOI] [PubMed] [Google Scholar]
- Jacinto E., Facchinetti V., Liu D., Soto N., Wei S., Jung S. Y., Huang Q., Qin J., Su B. (2006). SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127, 125-137 [DOI] [PubMed] [Google Scholar]
- Kamimura Y., Xiong Y., Iglesias P. A., Hoeller O., Bolourani P., Devreotes P. N. (2008). PIP3-independent activation of TorC2 and PKB at the cell's leading edge mediates chemotaxis. Curr. Biol. 18, 1034-1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Comer F. I., Sasaki A., McLeod I. X., Duong Y., Okumura K., Yates J. R., 3rd, Parent C. A., Firtel R. A. (2005). TOR complex 2 integrates cell movement during chemotaxis and signal relay in Dictyostelium. Mol. Biol. Cell 16, 4572-4583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X. H., Kimmel A. R. (2009). Biochemical responses to chemoattractants in Dictyostelium: ligand-receptor interactions and downstream kinase activation. Methods Mol. Biol. 571, 271-281 [DOI] [PubMed] [Google Scholar]
- McMains V. C., Liao X. H., Kimmel A. R. (2008). Oscillatory signaling and network responses during the development of Dictyostelium discoideum. Ageing Res. Rev. 7, 234-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meili R., Ellsworth C., Lee S., Reddy T. B., Ma H., Firtel R. A. (1999). Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 18, 2092-2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meili R., Ellsworth C., Firtel R. A. (2000). A novel Akt/PKB-related kinase is essential for morphogenesis in Dictyostelium. Curr. Biol. 10, 708-717 [DOI] [PubMed] [Google Scholar]
- Mora A., Davies A. M., Bertrand L., Sharif I., Budas G. R., Jovanovic S., Mouton V., Kahn C. R., Lucocq J. M., Gray G. A., et al. (2003). Deficiency of PDK1 in cardiac muscle results in heart failure and increased sensitivity to hypoxia. EMBO J. 22, 4666-4676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata T., Yaffe M. B., Leparc G. G., Piro E. T., Maegawa H., Kashiwagi A., Kikkawa R., Cantley L. C. (2000). Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J. Biol. Chem. 275, 36108-36115 [DOI] [PubMed] [Google Scholar]
- Park W. S., Heo W. D., Whalen J. H., O'Rourke N. A., Bryan H. M., Meyer T., Teruel M. N. (2008). Comprehensive identification of PIP3-regulated PH domains from C. elegans to H. sapiens by model prediction and live imaging. Mol. Cell 30, 381-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D. N., Pappin D. J., Creasy D. M., Cottrell J. S. (1999). Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551-3567 [DOI] [PubMed] [Google Scholar]
- Peter B. J., Kent H. M., Mills I. G., Vallis Y., Butler P. J., Evans P. R., McMahon H. T. (2004). BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303, 495-499 [DOI] [PubMed] [Google Scholar]
- Prendergast G. C., Muller A. J., Ramalingam A., Chang M. Y. (2009). BAR the door: cancer suppression by amphiphysin-like genes. Biochim. Biophys. Acta 1795, 25-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005). Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098-1101 [DOI] [PubMed] [Google Scholar]
- Shevchenko A., Tomas H., Havlis J., Olsen J. V., Mann M. (2006). In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856-2860 [DOI] [PubMed] [Google Scholar]
- Song G., Ouyang G., Bao S. (2005). The activation of Akt/PKB signaling pathway and cell survival. J. Cell Mol Med 9, 59-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Sasaki A. T., Ha H., Seung H. A., Firtel R. A. (2007). Role of phosphatidylinositol 3-kinases in chemotaxis in Dictyostelium. J. Biol. Chem. 282, 11874-11884 [DOI] [PubMed] [Google Scholar]
- Tanaka K., Adachi H., Konishi H., Iwamatsu A., Ohkawa K., Shirai T., Nagata S., Kikkawa U., Fukui Y. (1999). Identification of protein kinase B (PKB) as a phosphatidylinositol 3,4,5-trisphosphate binding protein in Dictyostelium discoideum. Biosci. Biotechnol. Biochem. 63, 368-372 [DOI] [PubMed] [Google Scholar]
- Toral-Barza L., Zhang W. G., Lamison C., Larocque J., Gibbons J., Yu K. (2005). Characterization of the cloned full-length and a truncated human target of rapamycin: activity, specificity, and enzyme inhibition as studied by a high capacity assay. Biochem. Biophys. Res. Commun. 332, 304-310 [DOI] [PubMed] [Google Scholar]
- Williams M. R., Arthur J. S., Balendran A., van der Kaay J., Poli V., Cohen P., Alessi D. R. (2000). The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr. Biol. 10, 439-448 [DOI] [PubMed] [Google Scholar]
- Yamada H., Ohashi E., Abe T., Kusumi N., Li S. A., Yoshida Y., Watanabe M., Tomizawa K., Kashiwakura Y., Kumon H., et al. (2007). Amphiphysin 1 is important for actin polymerization during phagocytosis. Mol. Biol. Cell 18, 4669-4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Cron P., Good V. M., Thompson V., Hemmings B. A., Barford D. (2002). Crystal structure of an activated Akt/protein kinase B ternary complex with GSK3-peptide and AMP-PNP. Nat. Struct. Biol. 9, 940-944 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.