Abstract
Aims
We evaluated whether specific clusters of metabolic syndrome (MetS) components differentially impact on arterial structure and function, and whether the impact is similar in men and in women.
Methods and results
Components of the MetS and arterial properties were assessed in 6148 subjects, aged 14–102 in a cluster of four towns in Sardinia, Italy. MetS was defined in accordance with the ATP III criteria. Age groups were classified as: <35, 35–49, 50–64, and ≥65 years. Systolic blood pressure (BP), diastolic BP, pulse pressure, common carotid artery (CCA) diameter, intima–media thickness, distensibility, strain, stiffness index, wall stress, and aortic pulse wave velocity were measured. Common carotid artery plaque was defined as focal encroachment of the arterial wall and CCA calcification as acoustic shadowing. In any age group, subjects with MetS presented thicker, stiffer or less distensible, and wider large arteries than controls. The arterial burden of MetS increased as the number of altered MetS components increased. However, not all MetS components were associated with the same changes in arterial properties. In fact, specific clusters of MetS components, i.e. any combination of altered glucose tolerance, elevated BP, and elevated triglycerides (with or without abdominal obesity), dramatically increased age-associated arterial changes. The impact of MetS on arterial function was similar in men and women.
Conclusion
MetS accelerates age-associated arterial changes, even in older persons. However, not all the clusters of MetS components render the same burden on arterial structure and function.
Keywords: Metabolic syndrome, Ageing, Gender, Arterial stiffness, Carotid IMT
Introduction
Along with the alarming increase in obesity,1 the metabolic syndrome (MetS), with its nexus of metabolic and cardiovascular (CV) features, has mounted to epidemic proportion.2 We have previously reported that the MetS was associated with accelerated central arterial ageing3 and was highly prevalent in older subjects, in whom it is an independent predictor of CV events.4 One major, unresolved issue relates to whether specific clusters of the components of the MetS portend differing effects on CV risk.5 They could, in particular, differentially affect arterial structure and function and, thus, the risk of CV events.
Because elevated triglycerides and reduced HDL cholesterol, two components of the MetS, have been reported as stronger predictors of CV outcomes in women than in men,6,7 it is reasonable to hypothesize that the MetS may differentially impact central arteries structure and function in men and women. Thus, a study to investigate potential gender-specific impact of MetS on subclinical arterial lesions is warranted.
The aims of the present study were to investigate whether specific clusters of MetS components have differential impact on various aspects of central arterial structure and function, including aortic and common carotid artery (CCA) stiffening and thickening, and whether the impact differs between men and women.
Subjects and methods
Study population
The SardiNIA Study was conceived as a study investigating the genetics of complex traits/phenotypes, including CV risk factors and arterial properties, in a Sardinian founder population.8 Over a 3-year period, from November 2001 to December 2004, all residents aged 14 years and older in four towns of Sardinia Region, Italy, were invited to participate to the Study. The response rate was 60%, resulting in 6148 participants aged 14–102 residents in that area. Participants came to the clinic after fasting overnight, participated in an informed consent process, and after donating a blood sample, underwent a detailed medical history and full medical examination, including blood pressure (BP) and anthropometric measurements, a 12-lead resting EKG, measurements of arterial structure and function, and personality testing.
For the purpose of the present study, age groups were classified as: <35, 35–49, 50–64, and ≥65 years.
Variables measured
Blood pressure
Blood pressure determinations were performed in the morning, with subjects in the seated position, and following a 5 min quiet resting period. Blood pressure was measured in both arms with a mercury sphygmomanometer using an appropriately sized cuff. Values for systolic BP (SBP) and diastolic BP (DBP) were defined by Korotkoff phase I and V, respectively. The average of second and third measurements on both the right and left arms was used in the analysis. Pulse pressure (PP) was computed as PP = (SBP − DBP); mean BP (MBP) was computed as MBP = DBP + (PP/3).
Anthropometry
Height, weight, and waist circumference were determined for all participants. Body mass index was calculated as body weight (kg)/height (m)2.
Fasting plasma lipids and glucose
Blood samples were drawn from the antecubital vein between 7 and 8 a.m. after an overnight fast. Subjects were not allowed to smoke, engage in significant physical activity, or take medications prior to the collection of the samples. Plasma triglycerides and total cholesterol were determined by an enzymatic method (Abbott Laboratories ABA-200 ATC Biochromatic Analyzer, Irving, TX, USA). High-density lipoprotein cholesterol was determined by a dextran sulphate–magnesium precipitation. Low-density lipoprotein cholesterol concentrations were estimated by the Friedewald formula. Fasting plasma glucose concentration was measured by the glucose oxidase method (Beckman Instruments Inc., Fullerton, CA, USA).
Definition of the metabolic syndrome
The Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III)9 defined the MetS as an alteration in three or more of the following five components: abdominal obesity (W), high triglycerides (T), low HDL cholesterol (H), elevated BP (systolic or diastolic) (B), and elevated fasting glucose (G). The following cut-off values are used to define each altered component: waist circumference >102 cm for men or >88 cm for women, triglycerides ≥150 mg/dL, HDL cholesterol <40 mg/dL for men or <50 mg/dL for women, BP ≥130/≥85 mmHg, and fasting glucose ≥110 mg/dL.
Because MetS is defined by the presence of three or more altered components, subjects with MetS may have different combinations of the above-mentioned individual components of MetS. We calculated the sex-specific occurrence of individual clusters of components in subjects with MetS. To calculate the expected occurrence of individual clusters of MetS components, we assumed the five alterations occur independently of each other. For instance, the expected occurrence of T–B–W is: n* P(T+)* P(B+)* P(W+)* (1 − P(H+))* (1 − P(G+)), where P(X+) is the prevalence of the altered component in men or women and n the total number of subjects to get the combination of components.
Arterial structure and function
Aortic pulse wave velocity (APWV) was measured as described previously.6 A minimum of 10 arterial flow waves from the right CCA and the right femoral artery were recorded simultaneously using non-directional transcutaneous Doppler probes (Model 810A, 9–10 MHz probes, Parks Medical Electronics, Inc., Aloha, OR, USA) and averaged using the QRS for synchronization. The foot of the flow, i.e. the point of systolic flow onset, was identified off-line by a custom-designed computer algorithm, and verified or manually adjusted by the reader after visual review. The time differential between the feet of simultaneously recorded carotid and femoral flow waves was then measured. The distance travelled by the flow wave was measured with an external tape measure over the body surface, as the distance from the right carotid sampling site to the manubrium subtracted from the distance from the manubrium to the right femoral sampling site. Aortic pulse wave velocity was calculated as the distance travelled by the flow wave divided by the time differential.
High-resolution B-mode carotid ultrasonography was performed by use of a linear-array 5–7.5 MHz transducer (HDI 3500, ATL Ultramark Inc.) as described previously.3 The subject lay in the supine position in a dark, quiet room. The stabilized BP after 15 min from the onset of testing was used for subsequent analyses. The right CCA was examined with the head tilted slightly upward in the midline position. The transducer was manipulated so that the near and far walls of the CCA were parallel to the transducer footprint and the lumen diameter was maximized in the longitudinal plane. A region 1.5 cm proximal to the carotid bifurcation was identified, and the intima–media thickness (IMT) of the far wall was evaluated as the distance between the luminal–intimal and the medial–adventitial interfaces. Intima–media thickness was measured on the frozen frame of a suitable longitudinal image with the image magnified to achieve a higher resolution of detail in areas without plaques or calcification (at least 1 mm distance from the plaque shoulder, if plaque was present). The IMT measurement was obtained from five contiguous sites at 1 mm intervals, and the average of the five measurements was used for analyses. All the measurements were performed by a single reader (A.S.). Common carotid artery systolic and diastolic diameters (d and D, respectively) were identified via ECG gating and measured similarly to IMT. Common carotid artery wall-to-lumen ratio (%) was calculated as:
Common carotid artery cross-sectional area (CSA) was calculated as:
where ρ is the arterial wall density (ρ = 1.06), Re = CCA IMT + CCA D.
CCA distensibility = ΔD/ΔP/D, where ΔD and ΔP are the diameter and pressure changes over the cardiac cycle and D is the carotid diameter at end-diastole.
Common carotid artery stiffness was evaluated by the stiffness index (no unit):
where SBP and DBP are systolic and diastolic BP, Δd the difference between the systolic and diastolic right CCA diameter, and D the diastolic diameter.
where VTI is the time velocity integral (cm/s) and HR the heart rate in b.p.m.
Common carotid artery plaque was defined as focal encroachment of the arterial wall. Common carotid artery calcification was defined as acoustic shadowing.
Statistical analysis
All analyses were performed using the SAS package for Windows (9.1 Version Cary, NC, USA). Data are presented as mean ± SD unless otherwise specified. Differences in mean values for each of the measured variables among groups were compared by ANOVA, followed by Bonferroni's test for multiple comparisons. ANCOVA, including LDL cholesterol levels and current smoking status as covariates, was used to assess three-way interaction between age, sex, and MetS on arterial properties, independently of the possible effects of LDL cholesterol and/or current smoking on the same variables.
Chi-square goodness of fit was adopted to compare differences in observed and expected occurrence of different clusters of MetS in men and women separately.
Univariate and multivariable logistic regression analyses were used to individuate determinants of categorical variables. Specifically, for the multivariate logistic regression models, the model building strategy was to start with a base model that included age at baseline and sex as independent variables, then to evaluate the impact of diabetes mellitus by adding it to the base model. As a next step, we controlled for the individual LDL cholesterol levels and current smoking status by adding them individually to the model. Model fit was verified using the Hosmer and Lemeshow goodness-of-fit test. Secondary analyses were conducted after excluding subjects with prevalent coronary and/or cerebrovascular disease.
A two-sided P-value of <0.05 indicated statistical significance.
Results
Age-associated trends in prevalence of MetS and its components
The prevalence of the MetS was 7.1% in men (n = 185) and 6.1% in women (n = 214) and progressively increased with age in both sexes (in men, from 1.4 to 5.0, to 13.1, and to 15.5% from <35 to 35–49, to 50–64, and to 65 years and older, respectively; in women, from 0.5 to 2.2, to 11.4, and to 20.0%, in the same age groups).
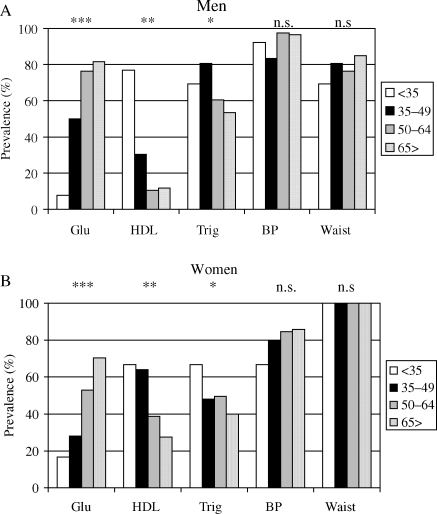
In men with MetS, the prevalence of B and W was similar across all age groups, whereas H was more common in younger, especially in those <35 years, and G significantly increased with age (from 7.7% in <35 years to 81.7 in >65 years); and T decreased in the oldest group (Figure 1A). In women with MetS, the prevalence of W was similar across age groups; the prevalence of B increased from <35 to 35–49 years group and then stabilized; the prevalence of G increased continuously across age groups, with a sharp increase after age 50 (ranging from 16.7 in the youngest to 28.0 to 52.9, and to 70.4 in increasingly older groups), whereas the prevalence of H and T was higher in younger individuals and decreased after age 50 (Figure 1B).
Figure 1.
Age-associated distribution of altered components of the MetS in men (A) and women (B) with MetS. ***P < 0.001; **P < 0.01; *P < 0.05; n.s., not significant.
Effects of MetS on arterial structure and function
Average values for arterial properties in different age groups for subjects with and without MetS are summarized in Table 1. Figures 2–5 illustrate the gender-specific trends of arterial properties across age groups according to the MetS status. Description below is based upon the output of a three-way interaction (age–sex–MetS), after adjustment for LDL cholesterol and current smoking status, summarized in Table 2.
Table 1.
Effects of the metabolic syndrome on arterial structure and function by age groups (means ± SD)
| <35 years |
35–49 years |
50–64 years |
>65 years |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | MetS | P-value | C | MetS | P-value | C | MetS | P-value | C | MetS |
P-value |
|
| 2142 | 19 | 1699 | 61 | 1164 | 161 | 719 | 158 | |||||
| APWV (cm/s) | 520 | 644 | 0.0001 | 632 | 753 | 0.0001 | 768 | 886 | 0.0001 | 944 | 1095 | 0.0001 |
| 109 | 241 | 132 | 162 | 193 | 191 | 247 | 348 | |||||
| APWV/MBP (cm/s) | 611 | 633 | 0.47 | 689 | 714 | 0.15 | 777 | 827 | 0.01 | 933 | 1037 | 0.0001 |
| 129 | 205 | 135 | 164 | 200 | 173 | 246 | 333 | |||||
| CCA stiffness | 4.32 | 5.03 | 0.05 | 5.63 | 6.97 | 0.0001 | 7.09 | 8.11 | 0.01 | 9.36 | 9.66 | 0.52 |
| 1.92 | 1.12 | 2.54 | 3.29 | 3.56 | 4.86 | 5.69 | 5.16 | |||||
| CCA strain (%) | 12.6 | 9.9 | 0.001 | 8.9 | 7.5 | 0.001 | 7.9 | 7.5 | 0.16 | 7.2 | 6.8 | 0.24 |
| 4.2 | 2.2 | 2.9 | 3.1 | 3.0 | 3.3 | 3.0 | 3.0 | |||||
| CCA distensibility (µm/mm/mmHg) | 289 | 196 | 0.0001 | 211 | 156 | 0.0001 | 159 | 135 | 0.0001 | 124 | 113 | 0.05 |
| 112 | 48 | 82 | 68 | 66 | 59 | 56 | 53 | |||||
| CCA diameter (mm) | 5.10 | 5.60 | 0.0001 | 5.32 | 5.67 | 0.0001 | 5.57 | 5.94 | 0.0001 | 6.00 | 6.13 | 0.05 |
| 0.49 | 0.58 | 0.56 | 0.60 | 0.67 | 0.81 | 0.81 | 0.75 | |||||
| CCA IMT (mm) | 0.48 | 0.53 | 0.0001 | 0.53 | 0.57 | 0.0001 | 0.60 | 0.64 | 0.0001 | 0.69 | 0.71 | 0.25 |
| 0.05 | 0.08 | 0.07 | 0.11 | 0.10 | 0.12 | 0.13 | 0.15 | |||||
| CCA CSA | 17.0 | 20.5 | 0.0001 | 19.6 | 22.5 | 0.0001 | 23.5 | 27.0 | 0.0001 | 29.6 | 30.9 | 0.06 |
| 2.5 | 3.6 | 3.77 | 5.39 | 5.7 | 7.5 | 8.3 | 8.8 | |||||
| CCA W/L ratio | 0.189 | 0.190 | 0.90 | 0.199 | 0.201 | 0.64 | 0.216 | 0.218 | 0.64 | 0.234 | 0.233 | 0.79 |
| 0.026 | 0.036 | 0.030 | 0.039 | 0.037 | 0.042 | 0.046 | 0.048 | |||||
| CCA wall stress | 16.2 | 19.1 | 0.0001 | 18.4 | 21.4 | 0.0001 | 21.5 | 23.4 | 0.0001 | 23.7 | 24.7 | 0.05 |
| 2.5 | 4.2 | 3.5 | 4.9 | 4.3 | 5.02 | 5.2 | 5.7 | |||||
| CCA flow (mL/min) | 1354 | 1308 | 0.47 | 1276 | 1157 | 0.001 | 1202 | 1138 | 0.01 | 1091 | 1072 | 0.46 |
| 273 | 276 | 259 | 292 | 277 | 260 | 273 | 301 | |||||
| CCA plaque, % (n) | — | — | 0.9 (15) | 1.6 (1) | 0.54 | 7.6 (88) | 8.7 (14) | 0.61 | 20.6 (148) | 29.1 (46) | 0.05 | |
| CCA calcification, % (n) | — | — | — | — | 2.3 (27) | 3.7 (6) | 0.28 | 8.1 (58) | 14.6 (23) | 0.01 | ||
| CCA stenosis, % (n) | — | — | — | — | 1.3 (15) | 3.1 (5) | 0.08 | 5.0 (36) | 9.5 (15) | 0.05 | ||
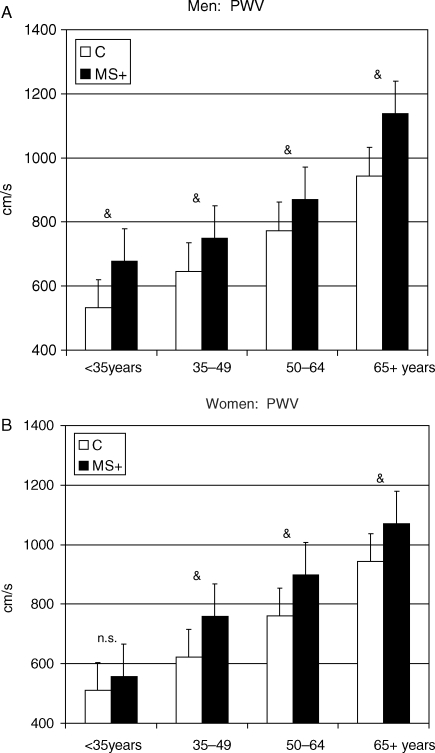
Figure 2.
Gender-specific effect of MetS on aorta stiffness (aortic pulse wave velocity) across age groups. *P < 0.05; **P < 0.01; &P < 0.001 vs. control of the same age group.
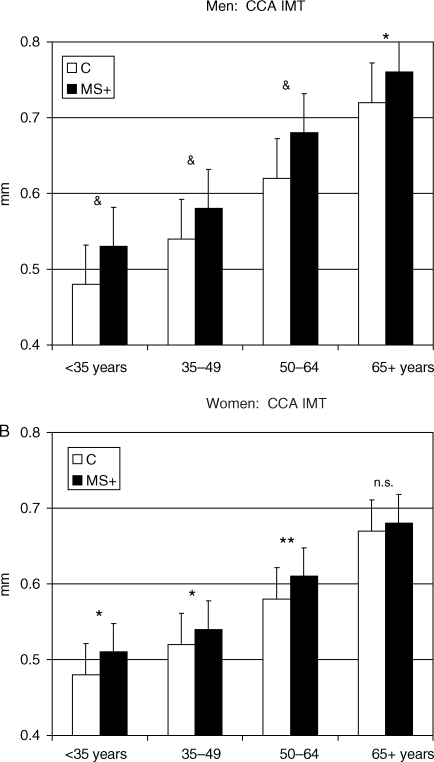
Figure 3.
Gender-specific effect of MetS on arterial thickness (common carotid artery intima–media thickness) across age groups. *P < 0.05; **P < 0.01; &P < 0.001 vs. control of the same age group.
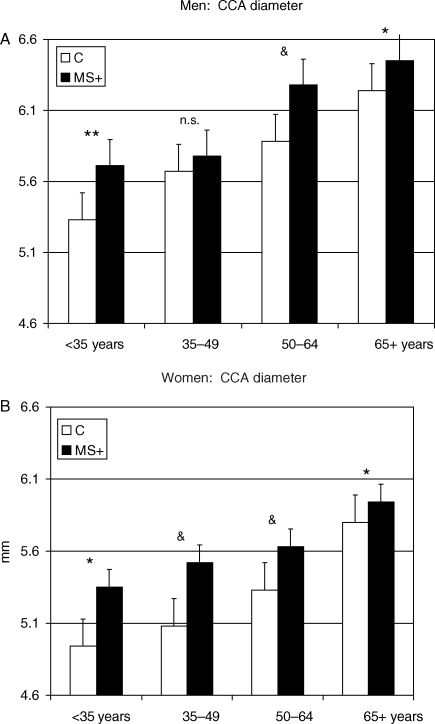
Figure 4.
Gender-specific effect of MetS on arterial lumen (common carotid artery diameter) across age groups. *P < 0.05; **P < 0.01; &P < 0.001 vs. control of the same age group.
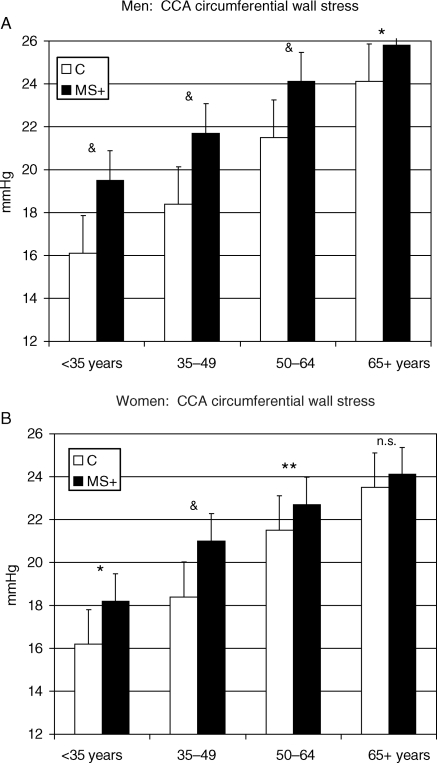
Figure 5.
Gender-specific effect of MetS on arterial wall stress (common carotid artery circumferential stress) across age groups. *P < 0.05; **P < 0.01; &P < 0.001 vs. control of the same age group.
Table 2.
P-values from three-way interaction (age–sex–MetS), after adjustment for low-density lipoprotein cholesterol and current smoking status, for selected arterial properties
| Age | Sex | Age * sex | MetS | Age * MetS | Sex * MetS | Age * sex * MetS | |
|---|---|---|---|---|---|---|---|
| APWV | <0.0001 | 0.02 | 0.04 | 0.0008 | <0.0001 | 0.12 | 0.12 |
| CCA strain | <0.0001 | 0.15 | 0.12 | <0.0001 | <0.0001 | 0.90 | 0.96 |
| CCA IMT | <0.0001 | 0.67 | 0.03 | 0.27 | 0.004 | 0.24 | 0.72 |
| CCA CSA | <0.0001 | 0.19 | 0.005 | 0.07 | <0.0001 | 0.20 | 0.55 |
| CCA diameter | <0.0001 | 0.003 | 0.34 | 0.49 | 0.19 | 0.78 | 0.74 |
Aortic pulse wave velocity significantly increased with age (Pearson's correlation coefficient = 0.694, P < 0.0001) and was higher in men than in women. MetS accelerated the age-associated increase in APWV levels, at any age, similarly in men and women. This trend was similar when APWV was normalized for MBP, although the effect of MetS on the age-associated changes appeared larger at older age.
Common carotid artery strain, a distensibility index supposedly independent of BP and more related to ‘intrinsic’ arterial wall properties, dramatically decreased with age, with a similar trend in men and women. MetS accelerated the age-associated decrease in CCA strain, but the detrimental effect of MetS on CCA strain became progressively weaker at older ages.
Common carotid artery distensibility decreased with advancing age and was further impaired by the presence of MetS at any age.
Common carotid artery IMT and CCA CSA (a measure of CCA intima–media layers somehow accounting for CCA lumen diameter) progressively increased with age; MetS accelerated their age-associated changes, and this effect did not differ in men and women.
Common carotid artery diameter progressively enlarged with age and was larger in men than in women. No significant effect of MetS on CCA diameter was observed. The CCA wall-to-lumen ratio increased with age but was not significantly impacted by the presence of MetS.
Common carotid artery circumferential wall stress significantly increased with age and was further increased by MetS in any age group.
Focal alteration in CCA structure (the presence of plaque or calcification or stenosis) significantly increased with advancing age, with no calcification or stenosis observed in subjects younger than 50 years. MetS was accompanied by a significantly higher occurrence of focal CCA alterations only in those subjects aged 65 years or older.
Secondary analyses, run after excluding subjects with prevalent CV and cerebrovascular disease, showed similar results to those presented above.
Specific clusters of components in subjects with the MetS
We calculated the sex-specific expected occurrence of individual clusters of components in subjects with MetS (see the Methods section).
The difference between expected and observed occurrence of specific clusters of MetS components was highly significant in both men (chi-square = 131.5, P < 0.0001) and women (χ2 = 364.0, P < 0.0001). Table 3 shows the expected and observed prevalence of each of the 16 possible combinations of MetS components. The deviations from ‘independently’ clustering are extreme. Among all the possible combinations of MetS components, some clusters (e.g. G–H–W and G–H–T–W in men; H–T–B and G–H–T in women) were not observed at all in the population. Approximately 20% of the subjects with the MetS presented clustering of T–B–W; and 29%, of G–B–W. This prevalence was similar in both men and women. The clustering of G–T–B(–W) was more frequent in men than in women (27 vs. 6%). Conversely, G–H–W(–B) (9 vs. 2%) and H–B–W (16 vs. 4%) were more frequent in women than in men.
Table 3.
Occurrence of specific clustering of the components of the MetS in men and women when compared with the expected prevalence calculated on the basis of gender-specific prevalence of each MetS component (see Methods for details)
| Men |
Women |
|||
|---|---|---|---|---|
| Expected | Observed | Expected | Observed | |
| T–B–W | 29.2 | 20.0 | 18.4 | 21.0 |
| H–B–W | 7.9 | 4.3 | 6.4 | 15.9 |
| H–T–W | 1.6 | 1.1 | 4.9 | 2.3 |
| H–T–B | 7.9 | 4.3 | 5.0 | 0 |
| H–T–B–W | 1.4 | 2.2 | 1.9 | 3.7 |
| G–B–W | 18.1 | 28.7 | 17.3 | 29.0 |
| G–T–W | 3.6 | 4.3 | 2.7 | 6.5 |
| G–T–B | 18.1 | 12.4 | 2.7 | 0.5 |
| G–T–B–W | 3.2 | 15.1 | 1.0 | 5.6 |
| G–H–B | 4.9 | 2.2 | 4.7 | 0.5 |
| G–H–W | 1.0 | 0 | 4.6 | 4.7 |
| G–H–B–W | 0.9 | 2.2 | 1.8 | 4.7 |
| G–H–T | 1.0 | 0.5 | 0.7 | 0 |
| G–H–T–B | 0.9 | 1.1 | 0.3 | 0.5 |
| G–H–T–W | 0.2 | 0 | 27.4 | 2.3 |
| G–H–T–B–W | 0.2 | 1.6 | 0.1 | 2.8 |
G, elevated glucose component; H, low HDL cholesterol component; T, elevated triglycerides component; B, elevated blood pressure component; W, abdominal obesity.
In men, the occurrence of any observed combination differed from what would be predicted; this difference was most prominent for G–B–W, and G–T–B(–W), which together accounted for 56.2% of MetS cases vs. an expected prevalence of 39.4%. Similarly, in women, all the observed combinations were distributed differently from what would be predicted. The gap between observed and predicted combinations was more impressive for all the observed combinations sharing altered B–W components.
Effects of specific clusters of MetS components on arterial structure and function
In both men and women, any cluster of MetS components was accompanied by alteration in all the measures of arterial structure and function when compared with controls (Tables 4 and 5).
Table 4.
Effects of specific clusters of MetS components on central arterial structure and function: men
| C | T–B–W | H–B–W | G–B–W | G–T–B(–W) | G–H–B(–W) | P-value& | |
|---|---|---|---|---|---|---|---|
| 2452 | 37 | 8 | 53 | 51 | 4 | ||
| APWV (cm/s) | 670 ± 217 | 884 ± 307 | 838 ± 359 | 948 ± 291 | 958 ± 274 | 1016 ± 402 | 0.65 |
| APWV/MBP (cm/s) | 701 ± 204 | 809 ± 247 | 722 ± 257 | 866 ± 292 | 875 ± 250 | 922 ± 415 | 0.50 |
| CCA diameter (mm) | 5.7 ± 0.7 | 5.8 ± 0.6 | 6.4 ± 1.2 | 6.4 ± 0.9 | 6.2 ± 0.7 | 6.9 ± 0.6 | 0.01 |
| CCA IMT (mm) | 0.56 ± 0.12 | 0.64 ± 0.12 | 0.68 ± 0.13 | 0.71 ± 0.16 | 0.70 ± 0.16 | 0.64 ± 0.11 | 0.24 |
| CCA W/L | 19.7 ± 3.9 | 22.1 ± 3.8 | 21.4 ± 3.8 | 22.0 ± 5.0 | 22.9 ± 5.4 | 18.6 ± 3.1 | 0.47 |
| CCA CSA | 22.3 ± 7.0 | 26.5 ± 7.3 | 30.9 ± 10.2 | 32.0 ± 9.4 | 30.9 ± 8.1 | 30.5 ± 7.5 | 0.06 |
| CCA circum. stress | 19.0 ± 4.9 | 24.0 ± 4.3 | 24.4 ± 4.5 | 24.1 ± 5.7 | 24.9 ± 5.8 | 20.5 ± 4.9 | 0.60 |
| CCA distensibility (µm/mm/mmHg) | 207 ± 111 | 146 ± 66 | 124 ± 61 | 127 ± 68 | 124 ± 55 | 63 ± 36 | 0.12 |
| CCA strain (%) | 9.9 ± 4.6 | 8.4 ± 4.0 | 7.0 ± 3.1 | 7.6 ± 4.0 | 7.1 ± 2.9 | 4.2 ± 4.0 | 0.19 |
| CCA stiffness | 6.06 ± 3.74 | 7.08 ± 3.01 | 8.98 ± 6.44 | 9.55 ± 7.23 | 8.45 ± 3.95 | 18.76 ± 13.19 | 0.01 |
| CCA plaque, % (n) | 6.7 (164) | 16.2 (6) | 0 | 28.3 (15) | 21.6 (11) | 25.0 (1) | 0.38 |
| CCA calcification, % (n) | 2.6 (64) | 10.8 (4) | 0 | 13.2 (7) | 5.9 (3) | 25.0 (1) | 0.48 |
| CCA stenosis, % (n) | 1.3 (32) | 0 | 0 | 11.3 (6) | 11.3 (6) | 0 | 0.20 |
| CCA flow (mL/min) | 1315 ± 306 | 1146 ± 215 | 1274 ± 149 | 1103 ± 286 | 1143 ± 294 | 1397 ± 408 | 0.17 |
| Prevalent CVD, % (n) | 2.4 (59) | 8.1 (3) | 0 | 11.3 (6) | 0 | 75.0 (3) | 0.01 |
| Prevalent diabetes, % (n) | 3.9 (95) | 0 | 12.5 (1) | 62.3 (33) | 49.0 (25) | 50.0 (2) | 0.0001 |
C, control; G, elevated glucose component; H, low HDL cholesterol component; T, elevated triglycerides component; B, elevated blood pressure component; W, abdominal obesity.
&ANOVA excluding those without the MetS.
Table 5.
Effects of specific clusters of MetS components on central arterial structure and function: women
| C | T–B–W | H–B–W | G–B–W | G–T–B(–W) | G–H–B(–W) | P-value& | |
|---|---|---|---|---|---|---|---|
| 3344 | 45 | 34 | 62 | 13 | 20 | ||
| APWV (cm/s) | 648 ± 208 | 895 ± 240 | 849 ± 203 | 1053 ± 383 | 1064 ± 325 | 951 ± 225 | 0.01 |
| APWV/MBP (cm/s) | 718 ± 192 | 840 ± 219 | 793 ± 194 | 975 ± 380 | 953 ± 262 | 990 ± 230 | 0.05 |
| CCA diameter (mm) | 5.2 ± 0.6 | 5.7 ± 0.7 | 5.6 ± 0.5 | 5.9 ± 0.8 | 5.8 ± 0.9 | 5.8 ± 0.6 | 0.36 |
| CCA IMT (mm) | 0.53 ± 0.10 | 0.62 ± 0.11 | 0.62 ± 0.15 | 0.65 ± 0.13 | 0.59 ± 0.09 | 0.66 ± 0.12 | 0.35 |
| CCA W/L | 20.7 ± 3.4 | 22.1 ± 4.1 | 21.9 ± 4.3 | 22.3 ± 4.3 | 20.7 ± 4.3 | 22.7 ± 3.4 | 0.72 |
| CCA CSA | 19.5 ± 5.3 | 25.1 ± 6.5 | 24.8 ± 8.2 | 27.4 ± 7.8 | 23.9 ± 5.6 | 27.0 ± 7.8 | 0.28 |
| CCA circum. stress | 18.9 ± 4.3 | 23.4 ± 3.9 | 23.6 ± 5.1 | 24.4 ± 5.7 | 23.0 ± 5.0 | 22.0 ± 4.3 | 0.41 |
| CCA distensibility (µm/mm/mmHg) | 224 ± 105 | 132 ± 52 | 132 ± 64 | 125 ± 57 | 138 ± 72 | 154 ± 62 | 0.44 |
| CCA strain (%) | 9.8 ± 3.8 | 7.3 ± 2.9 | 6.7 ± 2.7 | 7.6 ± 2.5 | 8.1 ± 4.7 | 7.6 ± 2.5 | 0.53 |
| CCA stiffness | 5.87 ± 3.45 | 8.08 ± 4.31 | 8.38 ± 4.7 | 8.08 ± 3.26 | 7.09 ± 2.35 | 7.62 ± 4.34 | 0.87 |
| CCA plaque, % (n) | 2.9 (97) | 11.1 (5) | 0 | 16.1 (10) | 0 | 20.0 (4) | 0.06 |
| CCA calcification, % (n) | 0.9 (30) | 6.5 (3) | 0 | 11.2 (7) | 0 | 10.0 (2) | 0.23 |
| CCA stenosis, % (n) | 0.7 (23) | 2.2 (1) | 0 | 6.5 (4) | 0 | 0 | 0.30 |
| CCA flow (mL/min) | 1230 ± 261 | 1092 ± 273 | 1125 ± 310 | 1131 ± 340 | 1044 ± 228 | 1066 ± 219 | 0.82 |
| Prevalent CVD, % (n) | 0.8 (27) | 2.2 (1) | 0 | 6.5 (4) | 7.6 (1) | 15.0 (3) | 0.28 |
| Prevalent diabetes, % (n) | 2.2 (74) | 2.2 (1) | 2.9 (1) | 66.1 (41) | 76.9 (10) | 75.0 (15) | 0.0001 |
C, control; G, elevated glucose component; H, low HDL cholesterol component; T, elevated triglycerides component; B, elevated blood pressure component; W, abdominal obesity.
&ANOVA excluding those without the MetS.
In men, specific clusters of MetS components were associated with differences in CCA diameter and CCA stiffness (Table 4). Specifically, men with the MetS having altered G–B–W or G–H–B(–W) had carotid arteries that were larger in diameter and stiffer than those having MetS composed of other component clusters. There is a very high prevalence of CV disease (CVD) and diabetes in any glucose component group, and prevalence differs among clusters. Thus, whether these differences are attributable to the specific components of the MetS or are related to the higher prevalence of CVD observed in these two specific groups (Table 4) cannot be ascertained, given the size of the two groups. The trend described remained significant after adjustment for age.
In women, specific clusters of MetS components were associated with differences in aortic arterial stiffness, as measured by APWV, but not in CCA stiffness, and in the occurrence of CCA plaques. Specifically, the presence of an altered glucose component was accompanied by a significantly higher APWV (even after normalization for MBP), and with a considerably higher occurrence of CCA plaques (16–20%) (Table 5). The trend described remained significant after adjustment for age.
Higher large artery thickness and stiffness and components MetS
We next determined the association of specific components of the MetS with the occurrence of extreme values of CCA thickness and of APWV, i.e. with values >2 SD from the mean of CCA IMT and APWV. After controlling for age and gender, all altered components of the MetS were associated with significantly higher odds of having extreme values in CCA IMT (Table 6). Specifically, low HDL cholesterol and abdominal obesity showed the strongest association with extreme CCA IMT (approximately a 70% higher likelihood in those with than in those without reduced HDL or abdominal obesity). Reduced HDL cholesterol was not associated with higher likelihood of having extremely stiff arteries as reflected in APWV. Elevated BP and abdominal obesity were both associated with a two-fold increase in the likelihood of having an extremely stiff aorta (Table 6). The odds of the association between each MetS components and extreme values in CCA IMT or APWV were similar in men and women.
Table 6.
Extreme (>95% of population distribution) alteration in central arterial structure and function by metabolic syndrome components
| % of population | n | CCA IMT >95th percentile, n = 329 |
APWV >95th percentile, n = 305 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | Age- and gender-adjusted OR |
% | Age- and gender- adjusted OR |
|||||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | |||||
| Fasting glucose | Normal | 5693 | 4.3 | 4.0 | ||||||
| 7.3% | Elevated | 445 | 18.4 | 1.54 | 1.13–2.10 | 0.01 | 17.5 | 1.87 | 1.38–2.53 | <0.001 |
| Blood pressure | Normal | 3930 | 2.0 | 1.6 | ||||||
| 36.0% | Elevated | 2208 | 11.4 | 1.57 | 1.17–2.10 | 0.01 | 11.0 | 2.44 | 1.80–3.33 | <0.001 |
| HDL cholesterol | Normal | 5690 | 5.3 | 5.0 | ||||||
| 7.3% | Elevated | 448 | 5.8 | 1.73 | 1.05–2.84 | 0.05 | 5.1 | 1.24 | 0.75–2.03 | <0.40 |
| Triglycerides | Normal | 5547 | 4.8 | 4.5 | ||||||
| 9.6% | Elevated | 591 | 10.7 | 1.59 | 1.14–2.22 | 0.01 | 9.0 | 1.50 | 1.07–2.11 | <0.05 |
| Waist circumference | Normal | 4780 | 3.5 | 2.6 | ||||||
| 22.1% | Elevated | 1358 | 11.9 | 1.67 | 1.28–2.18 | 0.01 | 13.3 | 2.29 | 1.76–2.99 | <0.001 |
As expected, an increasing number of altered components of the MetS were associated with higher occurrence of extremely thick or stiff arteries (Table 7). No significant gender-specific difference in the association of MetS altered components with extremely thick or stiff arteries was observed.
Table 7.
Extreme alterations in central artery structure and function by number of altered metabolic syndrome components
| # of MetS components | CCA IMT >95th percentile, n = 329 |
APWV >95th percentile, n = 305 |
||||||
|---|---|---|---|---|---|---|---|---|
| % | Age- and gender-adjusted OR |
% | Age- and gender-adjusted OR |
|||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | |||
| 0 | 1.3 | 1.0 | ||||||
| 1 | 6.2 | 1.41 | 0.92–2.16 | 5.6 | 2.34 | 1.44–3.81 | ||
| 2 | 11.5 | 1.67 | 1.08–2.58 | 14.4 | 3.76 | 2.32–6.08 | ||
| 3 | 18.7 | 2.84 | 1.74–4.64 | 21.6 | 5.62 | 3.30–9.57 | ||
| 4 | 31.4 | 4.97 | 2.50–9.90 | 30.8 | 8.05 | 3.95–16.43 | ||
| 5 | 30.9 | 5.24 | 0.0001 | 26.6 | 7.65 | 1.33–44.12 | 0.0001 | |
We further evaluated the association of specific clusters of altered MetS components with extremely thick or stiff arteries. Given the altered glucose and arterial alterations in both men and women, and the higher prevalence of diabetes mellitus in specific clusters of MetS components, we adjusted these analyses for the presence of type 2 diabetes mellitus. As shown in Table 8, G–T–B(–W) remained significantly associated with a 2.5-fold higher likelihood of thicker artery even after adjusting for age, gender, and diabetes mellitus. Both G–T–B(–W) and G–B–W remained significant determinants of an extremely stiff aorta, independently of age, gender, and diabetes mellitus.
Table 8.
Association of specific clusters of MetS components with extreme alterations in arterial structure and function
| T–B–W |
H–B–W |
G–B–W |
G–T–B(–W) |
G–H–B(–W) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| CCA IMT >95th percentile | ||||||||||
| Univariate | 2.79 | 1.47–5.33 | 2.98 | 1.25–7.12 | 5.52 | 3.51–8.67 | 9.14 | 5.36–15.6 | 6.39 | 2.29–17.8 |
| Age, sex adjust. | 1.60 | 0.87–3.52 | 2.73 | 0.99–7.70 | 1.64 | 1.00–2.71 | 3.11 | 1.68–5.78 | 1.64 | 0.51–2.24 |
| Age, sex, DM | 1.92 | 0.96–3.86 | 2.78 | 0.26–1.57 | 1.17 | 0.68–2.03 | 2.47 | 1.31–4.68 | 1.14 | 0.35–3.78 |
| Age, sex, DM, LDL-cholesterol, current smoking | 1.87 | 0.93–3.78 | 2.96 | 1.05–8.35 | 1.20 | 0.70–2.08 | 2.39 | 1.26–4.57 | 1.18 | 0.36–3.96 |
| Age, sex, DM, LDL-Cholesterol, Current smoking excluding preva-lent CV disease | 2.04 | 1.01–4.14 | 3.03 | 1.07–8.62 | 1.05 | 0.59–1.89 | 2.37 | 1.21–4.63 | 0.75 | 0.17–3.22 |
| APWV > 95th percentile | ||||||||||
| Univariate | 3.72 | 2.03–6.81 | 3.23 | 1.35–7.73 | 6.68 | 4.29–10.4 | 6.11 | 3.38–11.0 | 8.99 | 3.39–23.8 |
| Age, sex adjust. | 2.72 | 1.17–4.21 | 2.13 | 0.81–5.64 | 2.06 | 1.28–3.32 | 2.57 | 1.35–4.92 | 2.64 | 0.91–7.68 |
| Age, sex, DM | 2.38 | 1.25–4.52 | 2.17 | 0.82–5.73 | 1.60 | 0.98–2.81 | 2.11 | 1.08–4.12 | 1.98 | 0.67–5.84 |
| Age, sex, DM, LDL-cholesterol, current smoking | 2.31 | 1.21–4.40 | 2.22 | 0.84–5.86 | 1.58 | 0.93–2.69 | 2.10 | 1.07–4.10 | 2.08 | 0.70–6.19 |
| Age, sex, DM, LDL-cholesterol, current smoking excluding prevalent CV disease | 2.39 | 1.25–4.59 | 2.19 | 0.83–5.79 | 1.47 | 0.84–2.57 | 2.03 | 1.01–4.07 | 1.46 | 0.41–5.21 |
DM, diabetes mellitus; C, control; G, elevated glucose component; H, low HDL cholesterol component; T, elevated triglycerides component; B, elevated blood pressure component; W, abdominal obesity. Bold OR denotes a significant association.
Results remained virtually unchanged when adjusting for LDL cholesterol and current smoking status and when subjects with prevalent CV and cerebrovascular disease were excluded.
Discussion
Extensive literature has discussed the role of MetS as a risk factor for CVD. However, as outlined in a recent joint statement of the American Diabetes Association and the European Association for the Study of Diabetes,5 there are data supporting the notion that MetS does not add further information in addition to the sum of its components and that it does not provide an exhaustive index for the prediction of CV events.5 In marked contrast to this opinion, we have previously described that MetS accelerates arterial ageing3 and that MetS, defined by ATP III criteria as in the present report, predicted CV events in older subjects over and above the predictive power of its individual components.4 The present study sought evidence regarding the specific occurrence of different clusters of MetS components in arterial alterations, alterations known to confer a higher risk of CV events; see Stern et al.7 Additionally, we tested whether this phenomenon was gender-specific.
We observed that MetS was associated with accelerated arterial ageing at any age. In other words, subjects with the MetS presented more abnormal arterial structure and function patterns than controls, regardless of their age. Whether the effects of MetS on accelerated vascular ageing is a gender-specific phenomenon is unclear. Studies that investigated the potential gender-specific impact of MetS or its individual component on the risk of subclinical CVD10–14 indicated a tendency towards a stronger impact of MetS on arterial structure and function in women than in men. However, no study described that MetS accelerated arterial ageing at any age and in a similar manner in men and women, as we are reporting in this study.
A remarkable finding of the present study is that the specific arterial abnormality exacerbated by MetS differed across age groups: specifically, with ageing, the MetS showed a continuum from local arterial functional alteration to systemic functional alteration and to structural alteration of the arterial wall with a higher occurrence of CCA plaques, calcification, and/or stenosis in older groups. In other terms, MetS affects large arteries so that functional defects appear at younger ages than structural changes, the latter being evident at older ages. This finding is new and may provide a novel perspective on arterial ageing.
Within functional arterial alterations, we observed that MetS accelerated aortic stiffness, evaluated as APWV, in all age groups except carotid stiffness in younger ages only. This interesting observation is consistent with a previous report by Bussy et al.15 who described that at a given vascular parietal stress, the ‘intrinsic’ stiffness of arterial wall material was higher in hypertensive than in normotensive subjects at younger age, but was similar in middle-aged and older subjects. These findings indicated that wall material elastic properties differed at younger but not older ages, when adaptive mechanisms to the prolonged exposure to CV risk factors, such as the components of the MetS, produce structural changes in arterial wall. The structural changes in large arteries include an increase in collagen content and calcification of the media, elastic lamellae creasing and breakage, and accumulation and migration of vascular smooth cells in the arterial walls.16 In the presence of additional CV risk factors, such as MetS, these modifications occur earlier in the aorta than in other arterial territories.16,17 Several mechanisms may explain the ‘accelerated ageing’ of the aortic wall compared with the carotid wall in patients with MetS. MetS is characterized by activation of the renin–angiotensin system,18 an important humoral factor involved in regulating the turnover of extracellular matrix proteins and a strong regulator of matrix metalloproteinase and tissue inhibitor of metalloproteinases,19 as well as of cytokines released by adipose tissue.20 Glycation of matrix proteins also exerts an adverse effect on the structure and function of large arteries.21
The arterial burden of MetS increased with increasing number of altered components of the MetS. Of note, extremely thick CCAs showed a stronger association with lipid components of MetS, whereas extremely stiff aorta showed a stronger association with elevated BP and abdominal obesity and no significant association with lipid components of the MetS.
Another remarkable finding in our study is that among the 16 possible combinations of diagnostic criteria for the ATP III definition of MetS, not all occurred with the same frequency and some did not occur at all. Additionally, the distribution of specific clusters of MetS components differed in men and women. This is the first study that characterizes clusters of aggregation in the MetS criteria and supports the conjecture that specific patterns of positive clusters tend to emerge in men and in women.
Notably, clusters of altered components in subjects with MetS apparently differ also with respect to the associated arterial abnormalities. In fact, the combination of altered glucose tolerance, elevated blood pressure, and elevated triglycerides (with or without abdominal obesity) doubled the likelihood of extremely thick carotid arteries and extremely stiff aorta, known markers of subclinical vascular lesion and of increased risk of CV events, even after controlling for age, sex, and occurrence of diabetes mellitus. The novelty of this finding may have substantial implications, given that many studies suggested that the predictive role of MetS in CV risk was entirely attributable to the inclusion of diabetic subjects.7
Our findings may have several potentially relevant clinical implications. We confirmed in a Southern European, i.e. at lower CV risk, our previous observation in US population that MetS accelerated vascular ageing.3 Additionally, if the early arterial damage associated with MetS differed in younger and older subjects, and specific components of the MetS appeared to have a different load on functional or structural arterial alterations, this would imply that treatment of the MetS is not just the treatment of its individual components,5 but it will require a more tailored strategy. This concept is strengthened by our observations that not all the clusters of MetS components carry the same arterial burden and that this burden is not attributable only to the presence of diabetes mellitus.
Another potentially relevant clinical implication is that MetS is associated with a similar burden on large arteries in men and women. Traditionally, diabetes mellitus is known to confer a disproportionately higher CV risk in women than in men22 as well as the lipid components of the MetS have been reported as stronger risk factors for CV events in women than in men.6,7
Last, but not least, we believe that having explored the association of MetS and specific clusters of its components to extremely stiff or thick artery may have a great impact in clinical practice. In fact, the values for CCA IMT and APWV identifying ‘outliers’ are close to those suggested by the current guidelines as threshold for target organ damage.23 Clinician are more familial and believe more in categorical condition (hypertension/normotension, dyslipidaemia/normolypidaemia, arterial lesion/no arterial damage) than in continuous trait (what is the clinical implication of 0.02 mm changes in CCA IMT, regardless of the P-value associated to this difference?).
In conclusion, at any age, MetS is risky with respect to its association with abnormalities in arterial structure and function, with functional defects appearing at younger ages than structural changes. This further supports the idea that MetS is not just a theoretical construct, but rather can be envisioned as a key component of the early vascular ageing syndrome.24 However, not all the clusters of altered components of the MetS are associated with the same burden on arterial structure and function. This may imply that MetS defined by ATP III criteria is not one entity with a unique underlying common pathophysiological mechanism, but rather we should evolve towards the better characterization of metabolic syndromes.
Funding
The SardiNIA team was supported by Contract NO1-AG-1-2109 from the NIA.
Conflict of interest: none declared.
Acknowledgements
We thank Monsignore Piseddu, Bishop of Ogliastra, the Mayors of Lanusei, Ilbono, Arzana, and Elini, the head of the local Public Health Unit ASL4, and the residents of the towns, for their volunteerism and cooperation. In addition, we are also grateful to the Mayor and the administration in Lanusei for providing and furnishing the clinic site. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging (USA).
References
- 1.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 2.Deedwania PC. Metabolic syndrome and vascular disease: is nature or nurture leading the new epidemic of cardiovascular disease? Circulation. 2004;109:2–4. doi: 10.1161/01.CIR.0000110642.73995.BF. [DOI] [PubMed] [Google Scholar]
- 3.Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter EJ, Lakatta EG. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. JACC. 2004;43:1388–1395. doi: 10.1016/j.jacc.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 4.Scuteri A, Najjar SS, Morrell CH, Lakatta EG. The metabolic syndrome in older individuals: prevalence and prediction of cardiovascular events: the Cardiovascular Health Study. Diabetes Care. 2005;28:882–887. doi: 10.2337/diacare.28.4.882. [DOI] [PubMed] [Google Scholar]
- 5.Kahn R, Buse J, Ferranini E, Stern M. The metabolic syndrome: time for a critical appraisal. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 6.Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 7.Stern MP, Williams K, Hunt KJ. Impact of diabetes/metabolic syndrome in patients with established cardiovascular disease. Atheroscler Suppl. 2005;6:3–6. doi: 10.1016/j.atherosclerosissup.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Pilia G, Chen WM, Scuteri A, Orru M, Albai G, Dei M, Lai S, Usala G, Lai M, Loi P, Mameli C, Vacca L, Deiana M, Olla N, Masala M, Cao A, Najjar SS, Terracciano A, Nedorezov T, Sharov A, Zonderman AB, Abecasis GR, Costa P, Lakatta E, Schlessinger D. Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genet. 2006;2:e132. doi: 10.1371/journal.pgen.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10.Iglseder B, Cip P, Malaimare L, Ladurner G, Paulweber B. The metabolic syndrome is a stronger risk factor for early carotid atherosclerosis in women than in men. Stroke. 2005;36:1212–1217. doi: 10.1161/01.STR.0000166196.31227.91. [DOI] [PubMed] [Google Scholar]
- 11.Kawamoto R, Tomita H, Inoue A, Ohtsuka N, Kamitani A. Metabolic syndrome may be a risk factor for early carotid atherosclerosis in women but not in men. J Atheroscler Thromb. 2007;14:36–43. doi: 10.5551/jat.14.36. [DOI] [PubMed] [Google Scholar]
- 12.Skilton MR, Moulin P, Serusclat A, Nony P, Bonnet F. A comparison of the NCEP-ATPIII, IDF and AHA/NHLBI metabolic syndrome definitions with relation to early carotid atherosclerosis in subjects with hypercholesterolemia or at risk of CVD: evidence for sex-specific differences. Atherosclerosis. 2007;190:416–422. doi: 10.1016/j.atherosclerosis.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Protogerou AD, Blacher J, Aslangul E, Le Jeunne C, Lekakis J, Mavrikakis M, Safar ME. Gender influence on metabolic syndrome's effects on arterial stiffness and pressure wave reflections in treated hypertensive subjects. Atherosclerosis. 2007;193:151–158. doi: 10.1016/j.atherosclerosis.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira I, Boreham CA, Twisk JWR, Gallagher AM, Young ES, Murray LJ, Stehouwer CDA. Clustering of metabolic syndrome risk factors and arterial stiffness in young adults: the Northern Ireland Young Hearts project. J Hypertens. 2007;25:1009–1020. doi: 10.1097/HJH.0b013e3280a94e76. [DOI] [PubMed] [Google Scholar]
- 15.Bussy C, Boutouyrie P, Lacolley P, Challande P, Laurent S. Intrinsic stiffness of the carotid arterial wall material in essential hypertensives. Hypertension. 2000;35:1049–1054. doi: 10.1161/01.hyp.35.5.1049. [DOI] [PubMed] [Google Scholar]
- 16.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 17.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 18.Woodman RJ. Does compensatory nitric oxide and angiotensin II receptor activity reduce arterial stiffness in early-stage insulin resistance? Clin Sci (Lond) 2008;114:119–121. doi: 10.1042/CS20070321. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Lakatta EG. Altered regulation of matrix metalloproteinase-2 in aortic remodeling during aging. Hypertension. 2002;39:865–873. doi: 10.1161/01.hyp.0000014506.13322.66. [DOI] [PubMed] [Google Scholar]
- 20.You T, Nicklas BJ, Ding J, Penninx BW, Goodpaster BH, Bauer DC, Tylavsky FA, Harris TB, Kritchevsky SB. The metabolic syndrome is associated with circulating adipokines in older adults across a wide range of adiposity. J Gerontol A Biol Sci Med Sci. 2008;63:414–419. doi: 10.1093/gerona/63.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, Lakatta EG. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 22.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. American Diabetes Association: clinical practice recommendations 2002. Diabetes Care. 2002;25(Suppl. 1):S1–S147. doi: 10.2337/diacare.25.2007.s1. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson PM, Lurbe E, Laurent S. The early life origins of vascular ageing and cardiovascular risk: the EVA syndrome. J Hypertens. 2008;26:1049–1057. doi: 10.1097/HJH.0b013e3282f82c3e. [DOI] [PubMed] [Google Scholar]
- 24.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]