Abstract
Background
Milk-allergic children who tolerate heat-denatured milk (HM) have less severe reactions and outgrow the condition earlier than those who react to HM, which may be related to differences in IgE-dependent effector cell function.
Objective
To apply a novel assay to test the hypothesis that HM-tolerant children have suppressed IgE-mediated basophil responses.
Methods
Allergic, HM-tolerant, Outgrown or Control subjects were defined by oral food challenges. Whole blood cells were stimulated in vitro with a range of milk allergen doses in the presence or absence of autologous serum or with dilutions of autologous serum. Activated basophils were identified by flow cytometry as CD63bright CD123+ CD203c+ HLA-DR- CD41a-.
Results
HM-tolerant subject basophils were significantly less responsive to milk allergen stimulation at all doses than were basophils from HM reactive (Allergic) individuals. In the absence of autologous serum, HM-tolerant subject basophils were significantly more reactive at low allergen concentrations. To a lesser extent, autologous serum also inhibited IL-3- and anti-IgE-induced, but not fMLP-induced responses. The allergen-specific responsiveness of HM-tolerant subject basophils increased with dilution of autologous serum with normal pooled serum.
Conclusion
Milk-allergic children with a favorable prognosis have evidence of extrinsically suppressed allergen-specific effector cell reactivity.
Keywords: milk allergy, basophils, basophil activation test, oral tolerance, cow’s milk allergy
Introduction
Basophils are among the least abundant populations of circulating leukocytes, but by virtue of their sensitization with allergen-specific IgE, they represent a significant effector population in allergic pathogenesis. Basophils are known to be an early and abundant source of Th2 cytokines and other mediators of Th2 inflammation.1 There is also growing recognition of their capacity to modulate adaptive immunity.2–5
We are interested in better understanding the mechanisms of IgE-mediated hypersensitivity and its regulation in the context of food allergy and oral tolerance and in identifying ‘bio-markers’ of immune tolerance that may be useful for prognosis and immunotherapy monitoring.
Since basophils are readily accessible for study, they have been attractive targets for investigating both basic mechanisms of type I allergy as well as for the development of novel diagnostic tools. Several groups, including our own, have developed flow cytometric approaches for assessing the activation status of these cells.6–9 For pediatric studies, in particular, there is a need for approaches that minimize the amount of patient sample that is required for any particular assay and therefore we have focused on methods that allow us to assay basophil activation without enriching those cells from larger volumes of blood.
Here we report the novel application of a direct basophil activation test to determine whether milk-allergic patients who tolerate heat-denatured milk products also have significantly less milk allergen-induced reactivity in vitro. We demonstrate that the difference in basophil responsiveness is partially due to inhibition by an autologous factor present in serum, which we hypothesize to be allergen-specific IgG.
Materials and Methods
Subjects
Fifty-five subjects were recruited from a larger clinical study on the natural history of milk allergy and were characterized by open food challenges as Allergic (reactive to all forms of milk products; n=13), Heated cow’s Milk [HM] tolerant (n=32), or Outgrown (n=10). Non-milk allergic controls were recruited from a separate study of egg allergy (n=13).10 Blood samples from HM-tolerant subjects were obtained at the time of the initial baseline challenge (9/32) as well as after introduction of HM-containing diet (n=23/32). All research protocols were approved by the Mount Sinai Institutional Review Board and informed consent was obtained for all subjects. Allergen-specific levels and skin test data were obtained as previously described.10
Reagents
Milk antigen was prepared from nonfat dried milk (Upstate/Chemicon) diluted in PBS. RPMI 1640 with glutamine and N-formyl-methionyl-leucyl-phenylalanine (fMLP) were purchased from Fisher Scientific. Recombinant human IL-3 was obtained from R&D Systems. Polyclonal anti-IgE antibody was from Bethyl Laboratories. EDTA was obtained from Promega Corporation. FACS lysing solution was obtained from BD Biosciences.
Antibodies
The following monoclonal antibodies were used: fluorescein isothiocyanate (FITC)-conjugated anti-human CD63 (clone H5C6, mouse IgG1, BD Biosciences), phycoerythrin (PE)-conjugated anti-human CD203c (clone 97A6, mouse IgG1, Serotec), phycoerythrin-cyanin 5 (PC5)-conjugated anti-human CD123 (clone 9F5, mouse IgG1, BD Biosciences), allophycocyanin (APC)-conjugated anti-human CD41a (clone HIP8, mouse IgG1, BD Biosciences), and phycoerythrin-cyanin 7 (PC7)-conjugated anti-human HLA-DR (clone L243, mouse IgG2a, BD Biosciences).
Basophil activation
Whole blood aliquots (250 μL) were incubated with equal volumes of basophil stimulation buffer alone (RPMI + IL-3 at 2 ng/mL; IL-3 alone control), or with the addition of milk antigens at serial 10-fold dilutions (from 3 × 101 to 3 × 10−4 μg/mL total protein), anti-IgE antibody (0.5 μg/mL; positive control), or N-fMLP (1 μM; IgE-independent positive control) or RPMI alone (negative control) at 37°C for 30 minutes. The reaction was stopped with 50 μl of cold PBS + 20 mM EDTA. Cells were then stained for expression of CD63, CD123, CD203c, CD41a, and HLA-DR at 4°C in the dark for 30 minutes. Basophil activation was assessed by flow cytometry7 (see Figure E1, Online Repository for gating example). Following incubation, cells were washed with PBS + 0.5% bovine serum albumin + 2 mM EDTA. Red cells were then lysed by adding 4 mL of FACS Lysing Solution to each sample for 15 minutes.
IgG depletion
Plasma (1mL) was removed from whole blood aliquots (2mL), with 650 μL used for IgG depletion, and 350 μL for mock treatment. Protein A beads (650 μL; Fisher Scientific) were washed with RPMI and subsequently incubated with plasma at room temperature on a rocker for an hour. The sample was then spun down and the supernatant removed. Cells (100 μL) were then incubated with 100 μL of serum (mock-treated or IgG-depleted) and 200 μL of either basophil stimulation buffer (RPMI + IL-3 at 2 ng/mL; negative control), anti-IgE antibody (0.5 μg/mL; positive control), or milk antigen (30 μg/mL total protein) at 37°C for 30 minutes. Reactions were stopped and processed for flow cytometry as above.
Flow cytometry
Samples were analyzed on a BD LSRII flow cytometer. Single-color compensation samples were prepared using anti-mouse Ig beads (Bangs Laboratories). Fluorescence data were acquired and auto-compensated on a modified LSR-II configured for seven-color parameters using FACS Diva software (version 4.0; BD Biosciences). As shown in Figure E1, basophils were identified as CD123+ CD203c+ HLA-DR- CD41a- (Online Repository). A minimum of 500 CD123+ CD203c+ HLA-DR- CD41a- events (i.e. basophils) were recorded for each condition or the sample was excluded.
Statistical analyses
Offline analysis of cytometry data was performed using FlowJo version 8.1 (Tree Star). Graphical display and statistical analyses were performed using R analysis 2.5.0 (www.R-project.org). For determining the significance of differences in %CD63 between groups across various antigen stimulation concentrations (Figure 1), two-way ANOVA was used. For other between-group and pre/post serum-depletion comparisons, the unpaired or paired Wilcoxon rank sum test was used, as noted in the figure legends. Complete analysis script and raw data tables are available on the Online Repository. Non-responders were defined as individuals with <10% CD63 up-regulation in response to either allergen or anti-IgE control.
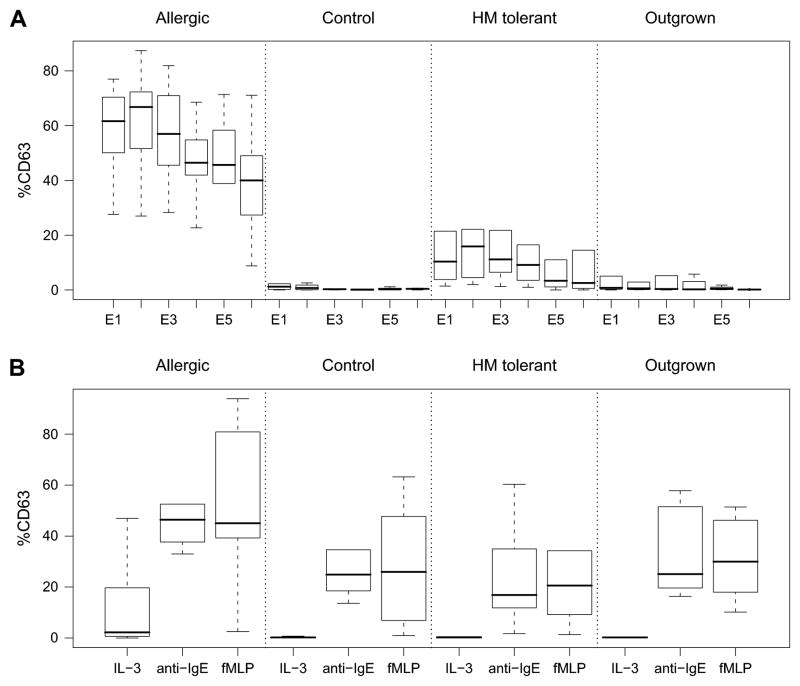
Figure 1.
Allergen specific and non-specific basophil reactivity by patient group. A. Percentage of basophils that are CD63 bright after 30 minutes stimulation with 10-fold serial dilutions of milk protein (E1 to E6 corresponds to 3 × 101 to 3 × 10−4 μg/mL). B. Percentage of basophils that are CD63 bright after 30 minutes stimulation with IL-3 alone, polyclonal anti-IgE (1 μg/mL) or fMLP (1 μM). Allergic (n=13); Control (n=13); HM-tolerant (n=32); Outgrown (n=10). Box plots represent median, 25th and 75th percentile and range.
Results
Allergic donor basophils are more reactive to milk allergen than HM-tolerant donor basophils. In total, 68 subjects were studied: Allergic (n=13), Outgrown (n=10), and HM-tolerant (n=32), and non-milk allergic Controls (n=13). The ages of each group were as follows (median and range in years): Allergic, 9 [3.6–16.5]; HM-tolerant, 7.7 [2.8–16.3]; Outgrown, 6.9 [4.8–10.4]; Control, 5.7 [1.8–13.4]. Twenty-three percent (16/68) of these subjects exhibited the previously described ‘non-responder’ phenotype to IgE cross-linking11 and were eliminated from subsequent analyses. There was no significant difference in the frequency of non-responders between groups (Table E1).
Basophils from Allergic subjects who were reactive to extensively heated cow’s milk protein (HM) were significantly more reactive than basophils from the HM-tolerant group across the range of allergen dilutions (Figure 1; p=2.2e−16). Peak responses were observed for both Allergic (66.8 [51.6 – 71.3]; median [25–75%]) and HM-tolerant groups (15.9 [4.5 – 22.1]) at the 1 × 102 fold dilution (E2; ~1 μg/ml). Control subject basophils were not reactive and only a few Outgrown subject basophils were weakly reactive. HM-tolerant basophils were significantly more reactive to milk allergen than either Control (p=6.6e−08) or Outgrown (p=1.7e−05) subjects (Figure 1). Consistent with the findings for the entire study population,10 the same trend between these clinical groups existed for milk-specific serum IgE and prick skin test (PST) wheal size; however, there is less overlap between groups as measured by basophil response than by skin test wheal size (Figure E2, Online Repository). In fact, basophil activation appears to correspond more strongly with allergen-specific IgE levels, particularly within the Outgrown group who were more likely to have persistent skin test reactivity (Figure E3).
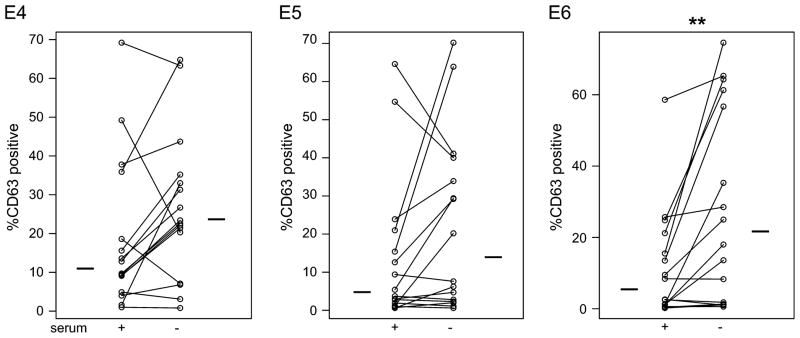
Autologous serum from HM-tolerant subjects inhibited allergen-specific responses. In order to evaluate whether the observed differences in basophil responsiveness between study subject groups reflected intrinsic or extrinsic features of those cells, whole blood cells from each subject were divided and washed and then resuspended in either autologous plasma or RPMI prior to stimulation. HM-tolerant basophil responses across the antigen dose range were enhanced in the absence of serum and that trend reached statistical significance at lower allergen concentrations (Figures 2; p= 0.0060 at 300 pg/ml). A similar trend was observed in the Outgrown subjects (Figure 2), although it did not reach statistical significance. Representative examples of individual allergen dose-response curves in the absence or presence of autologous serum are shown in the Online Repository (Figure E4). In contrast, Allergic donor basophils were not more reactive to milk allergen in the absence of autologous serum protein (Figure 2), suggesting either the specific presence of an inhibitory factor in the serum of HM-tolerant and Outgrown subjects, or specific sensitivity to suppression by a non-specific serum factor.
Figure 2.
Enhancement of HM-tolerant basophil responses to milk allergen in the absence of autologous serum. Panels E4-E6 represent the percentage of activated basophils (CD63 bright) after 30 minutes stimulation with 10-fold serial dilutions of milk protein (E4 to E6 corresponds to 3 × 10−2 to 3 × 10−4 μg/mL) in the presence (‘+’) or absence (‘−’) of autologous serum. Bars indicate median. ** p < 0.01.
There was also enhancement of non-specific basophil reactivity in the HM-tolerant group in the absence of autologous serum; stimulation with either IL-3 alone (p= 0.0025) or anti-IgE (p= 0.032) induced stronger responses in the absence of serum (Figure E5). This effect was specific to IL-3 and anti-IgE; there was no enhanced responsiveness measured in the RPMI (not shown) or fMLP stimulation (Figure E5).
To specifically address the role of IgG, in a subset of subjects we resuspended washed cells in 10% autologous serum that had been either mock-treated (beads alone) or affinity depleted of IgG utilizing protein A beads. These cells were then stimulated with IL-3 alone, milk antigen (0.1 μg/ml; ‘E3’), or anti-IgE. Total serum IgG was depleted by approximately 60–80% as determined by SDS-PAGE (not shown). Basophil responsiveness to milk allergen showed a trend toward enhancement in IgG-depleted serum (n=6; p=0.078), however, the effect was non-specific as both IL-3-induced (p=0.047) and anti-IgE-induced (p=0.078) responses also tended to be enhanced (Figure E8).
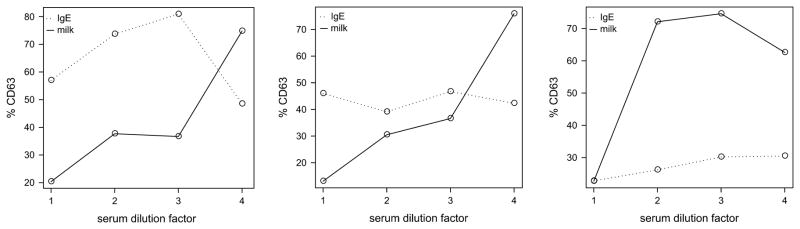
Based on these findings, we hypothesized that polyclonal IgG had a generally inhibitory effect on PI3K-dependent signaling in basophils (see Discussion).12,13 Therefore, to further investigate whether there was an allergen-specific or a general inhibition factor of basophil activation present in the sera from HM-tolerant individuals, we conducted dilution experiments comparing allergen-induced activation to anti-IgE activation of whole blood samples serially diluted using normal human serum to maintain physiological concentrations of total IgG. Basophil responses to the same concentration of allergen (3 × 10−1 μg/mL) increased dramatically with increasing dilution of autologous serum, while there was no consistent change in the anti-IgE response (Figure 3). However, heterologous serum from HM tolerant donors was unable to suppress basophil responses of Allergic donors (not shown).
Figure 3.
Serum inhibition of allergen-specific basophil activation is dose-dependent. Panels show three representative individual donor basophil activation to stimulation with milk allergen (3 × 10−2 μg/mL; solid line) over a 4-fold range of autologous serum diluted with normal human AB serum. Broken line indicates the basophil activation to polyclonal anti-IgE control.
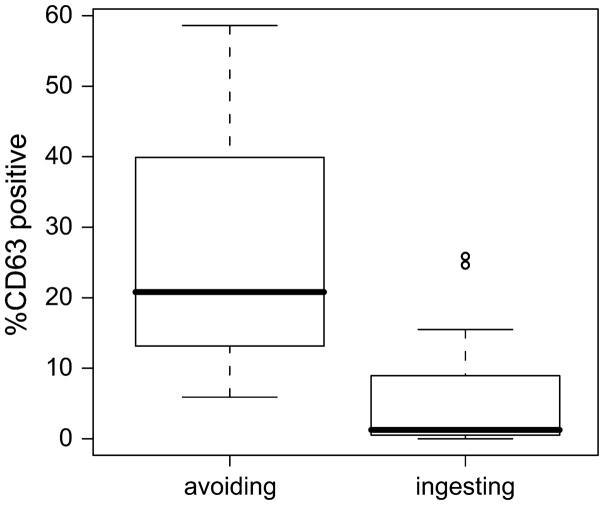
Active ingestion of milk allergen is associated with basophil hyporesponsiveness. Subjects for this study were recruited cross-sectionally from a larger study.10 Some HM-tolerant subjects were evaluated at the time of their initial challenge before the introduction of baked milk products into their diet (n=9), while some were evaluated three or more months following the inclusion of HM products (n=23). Subjects in the parent study who ingested baked milk products for at least 3 months had decreased skin test reactivity and milk-specific IgE levels, and increased casein-specific IgG4 levels over time.10
We compared basophil reactivity within the HM-tolerant subjects with respect to their diet. Basophils from HM-tolerant subjects including milk in their diets were less reactive upon in vitro stimulation with milk protein, especially at low concentrations (~10 ng/ml and lower; see Figure 4). There was also a trend toward lower specific IgE, higher casein-specific IgG4 and lower skin test reactivity in the subjects ingesting milk, although these differences did not reach statistical significance in this small subset (Figure E6). Basophil responses to IL-3 alone and anti-IgE were lower in children actively ingesting milk, suggesting that there may be a degree of intrinsic basophil suppression due to chronic allergen exposure (i.e. desensitization), while fMLP responses were no different between diet groups (Figure E7).
Figure 4.
Ingestion of dietary milk antigen is associated with less milk-induced basophil activation. Percentage of activated basophils (CD63 bright) after 30 minutes stimulation with milk protein at 3 × 10−4 μg/mL. Box plots represent median, 25th and 75th percentile and range, circles represent outliers. p < 0.01
Discussion
IgE-mediated milk allergy is naturally outgrown by most affected children.14–16 The mechanisms of this naturally acquired tolerance are unknown, as is its relationship to the normal oral tolerance of individuals who have no history of milk allergy or to tolerance that may be induced by oral or sublingual immunotherapy.
A recent study found that milk-allergic children who are able to tolerate extensively heated [baked] milk may outgrow their allergy sooner and are less clinically sensitive than those who are reactive to heated milk.10 Here we show that basophils from these individuals, directly stimulated in a whole blood assay, are less reactive to milk allergen, in part due to a serum inhibitory factor.
Allergen-specific IgG has been associated with both natural and immunotherapy-induced tolerance and we hypothesize that it may be associated with inhibition observed in these experiments. We hypothesized that allergen-specific IgG should inhibit allergen-specific, but not general anti-IgE stimulation. Indeed, in serum dilution experiments, we were able to demonstrate a preferential effect on basophil activation by allergen compared to anti-IgE (Figure 3).
Allergen specificity is also suggested by the greater effect of serum inhibition at low antigen doses (Figure 2 and Figure E3), at which we speculate specific IgG may be in excess of antigen. High specific IgG to allergen ratios would favor both competitive inhibition of IgE binding (i.e. ‘blocking’) as well as formation of antibody-antigen complexes with sufficient avidity to interact with inhibitory FcγRIIb. The observation that HM-tolerant individuals ingesting milk have both lower basophil reactivity and higher specific IgG4 appears to support the hypothesis of allergen-specific IgG as the serum inhibitor.
It is interesting to note, however, that Allergic donor basophils tend to be generally more reactive as well (Figure 1B), and that both serum depletion and IgG depletion of HM-tolerant samples enhances both the IL-3 and anti-IgE-induced basophil response (Figures E5, E8). We found that serum IgG has a broad inhibitory activity, since affinity depletion non-specifically enhanced basophil responsiveness (Figure E8). It is well established that specific IgG by co-aggregation of FcγRIIb and FcεRI, can potently inhibit basophil activation.17 In addition, FcγRIIb signaling and activation of the phosphatase, SHIP-1, can inhibit PI3K-dependent pathways generally even in the absence of direct co-ligation.18 Both IL-3- and FcεRI- basophil activation signal through PI3K,19 while fMLP does not.13 Furthermore, monomeric IgG (specifically monomeric Fc IgG fragments) have been demonstrated to have FcγRIIb-dependent inhibitory function in macrophages.12 We hypothesize that monomeric IgG is signaling in this way in basophils and accounts for the non-specific inhibitory activity observed.
Serum dilution experiments using normal human serum to maintain polyclonal IgG concentration did confirm the presence of an allergen-specific inhibition in HM-tolerant individuals (Figure 3). We hypothesized that this is allergen-specific IgG. We were unable, however, to inhibit Allergic donor basophils using pooled heterologous serum from HM-tolerant subjects despite the presence of allergen-specific IgG (not shown). It may be that distinct epitope specificity accounts for the lack of cross-inhibition. We have previously observed significant heterogeneity of epitope recognition even within restricted epitope-rich regions of a small food allergen.20 If this does account for the lack of heterologous inhibition, this would suggest that competitive blocking, rather than engagement of inhibitory FcγRIIb, predominates in these patients, at least in this in vitro assay. Alternatively, HM-tolerant subject basophils may be more susceptible to the serum inhibitor, whether it is specific IgG or not. Future experiments to affinity deplete allergen-specific IgG, may help to clarify this question.
We did find evidence of intrinsic basophil suppression associated with antigen exposure. Allergen, and anti-IgE, but not fMLP, responses were all reduced in subjects with exposure to dietary antigen (Figure E7). We hypothesize that this may be the result of antigen-induced desensitization, a state of non-antigen specific, but pathway-specific (i.e. FcεRI-mediated) suppression of responsiveness induced by prior stimulation,21–23 occurring due to in vivo antigen exposure. Response to IL-3 alone was also lower with dietary antigen exposure (Figure E7). Activation by IL-3 alone has been documented in a subset of patients (termed hyper-releasable) that are also hyper-responsive to IgE-cross-linking and other secretagogues.24 We are not aware of evidence that FcεRI-mediated desensitization affects IL-3 responsiveness, and the mechanism of desensitization is unclear. However, down-regulation of the protein tyrosine kinase, Syk, has been implicated,25 and this is a known to be upregulated by IL-3.
IgE-sensitized basophils are the largest population of allergen-specific leukocytes in peripheral blood and there is a growing appreciation of the effector role they play as well as their capacity for modulating adaptive immunity.3,4,26 Since basophils have a short half life and the turnover of mast cell-bound IgE is slow, basophil reactivity may correlate more closely with changes in the allergen-specific immune response than mast cell reactivity. Basophil and mast cell reactivity are both suppressed by dietary antigen, which correlates with higher specific IgG4 (Figure E4). However, basophils might be disproportionately affected by intrinsic antigen-induced desensitization owing to their normal compartmentalization the peripheral blood where they are likely to encounter higher levels of antigen than skin mast cells following ingestion. It is not known whether or to what degree basophils participate in the in vivo effector response to food allergens, but it has been found that food allergic patients with chronic exposure to offending antigen have elevated spontaneous histamine release and increased constitutive expression of basophil activation markers7,27 Although elevated plasma histamine is commonly seen during food-induced anaphylactic reactions, tryptase is rarely found to be elevated, suggesting active participation by non-mast cell, histamine-secreting cells, such as the basophil.28
We have shown here that it is possible to monitor allergen-specific basophil responses from pediatric subjects with the use of flow cytometry to identify and phenotype basophils from stimulated, unfractionated peripheral blood cells. Basophil reactivity in this patient population was strikingly distinct between HM-tolerant and reactive subjects, raising the possibility that this assay may be clinically useful to discriminate between these groups. Since reactivity to HM has been associated with a higher risk of reactions requiring epinephrine,10 basophil reactivity may also be a useful prognostic test for identifying individuals with greater clinical sensitivity. We are now using this assay as a research tool to assess both naturally occurring and immunotherapy-induced changes, however, controlled food challenges remain the gold standard for determining clinical reactivity.
Supplementary Material
Acknowledgments
Supported in part by the NIAID [U19 AI-44236] and the NCRR [M01-RR-00071]; WS is supported by a Pediatric NIH-LRP award
Footnotes
Clinical Implications: Assessment of allergen-specific basophil activation may be a useful research tool for identifying patients who will tolerate HM products and for monitoring the acquisition of immune tolerance in patients with a history of milk allergy.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Devouassoux G, Foster B, Scott LM, Metcalfe DD, Prussin C. Frequency and characterization of antigen-specific IL-4- and IL-13- producing basophils and T cells in peripheral blood of healthy and asthmatic subjects. J Allergy Clin Immunol. 1999 Oct;104(4 Pt 1):811–9. doi: 10.1016/s0091-6749(99)70292-7. [DOI] [PubMed] [Google Scholar]
- 2.Lim LHK, Burdick MM, Hudson SA, Mustafa FB, Konstantopoulos K, Bochner BS. Stimulation of human endothelium with IL-3 induces selective basophil accumulation in vitro. J Immunol. 2006 May;176(9):5346–53. doi: 10.4049/jimmunol.176.9.5346. [DOI] [PubMed] [Google Scholar]
- 3.Mack M, Schneider MA, Moll C, Cihak J, Brühl H, Ellwart JW, Hogarth MP, Stangassinger M, Schlöndorff D. Identification of antigen-capturing cells as basophils. J Immunol. 2005 Jan;174(2):735–41. doi: 10.4049/jimmunol.174.2.735. [DOI] [PubMed] [Google Scholar]
- 4.Sokol C, Barton G, Farr A, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2007 Dec; doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denzel A, Maus UA, Gomez MR, Moll C, Niedermeier M, Winter C, Maus R, Hollingshead S, Briles DE, Kunz-Schughart LA, Talke Y, Mack M. Basophils enhance immunological memory responses. Nat Immunol. 2008 Jul;9(7):733–42. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 6.Ebo DG, Hagendorens MM, Schuerwegh AJ, Beirens LMN, Bridts CH, Clerck LSD, Stevens WJ. Flow-assisted quantification of in vitro activated basophils in the diagnosis of wasp venom allergy and follow-up of wasp venom immunotherapy. Cytometry. 2007 May;72B(3):196–203. doi: 10.1002/cyto.b.20142. [DOI] [PubMed] [Google Scholar]
- 7.Shreffler WG. Evaluation of basophil activation in food allergy: present and future applications. Current opinion in allergy and clinical immunology. 2006 Jun;6(3):226–33. doi: 10.1097/01.all.0000225165.83144.2f. [DOI] [PubMed] [Google Scholar]
- 8.de Weck AL, Sanz ML, Gamboa PM, Aberer W, Bienvenu J, Blanca M, Demoly P, Ebo DG, Mayorga L, Monneret G, Sainte-Laudy J. Diagnostic tests based on human basophils: more potentials and perspectives than pitfalls. Int Arch Allergy Immunol. 2008 Jan;146(3):177–89. doi: 10.1159/000115885. [DOI] [PubMed] [Google Scholar]
- 9.Ocmant A, Peignois Y, Mulier S, Hanssens L, Michils A, Schandené L. Flow cytometry for basophil activation markers: the measurement of CD203c up-regulation is as reliable as CD63 expression in the diagnosis of cat allergy. J Immunol Methods. 2007 Mar;320(1–2):40–8. doi: 10.1016/j.jim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, Sampson HA. Tolerance to Extensively Heated Milk in Children with Cow’s Milk Allergy. J Allergy Clin Immunol. 2008;122(2):342–7. doi: 10.1016/j.jaci.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein LM, MacGlashan DW. The concept of basophil releasability. J Allergy Clin Immunol. 1986 Feb;77(2):291–4. doi: 10.1016/s0091-6749(86)80106-3. [DOI] [PubMed] [Google Scholar]
- 12.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001 Jan;291(5503):484–6. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 13.Knol EF, Koenderman L, Mul FP, Verhoeven AJ, Roos D. Differential activation of human basophils by anti-IgE and formyl-methionyl-leucyl-phenylalanine. Indications for protein kinase C-dependent and -independent activation pathways. Eur J Immunol. 1991 Apr;21(4):881–5. doi: 10.1002/eji.1830210404. [DOI] [PubMed] [Google Scholar]
- 14.Høst A. Primary and secondary dietary prevention. Pediatric allergy and immunology: official publication of the European Society of Pediatric Allergy and Immunology. 2001 Jan;12 (Suppl 14):78–84. doi: 10.1034/j.1399-3038.2001.121418.x. [DOI] [PubMed] [Google Scholar]
- 15.Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow’s milk allergy. J Allergy Clin Immunol. 2007 Nov;120(5):1172–7. doi: 10.1016/j.jaci.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Wood RA. The natural history of food allergy. Pediatrics. 2003 Jun;111(6 Pt 3):1631–7. [PubMed] [Google Scholar]
- 17.Kepley CL, Cambier JC, Morel PA, Lujan D, Ortega E, Wilson BS, Oliver JM. Negative regulation of FcepsilonRI signaling by FcgammaRII costimulation in human blood basophils. J Allergy Clin Immunol. 2000 Aug;106(2):337–48. doi: 10.1067/mai.2000.107931. [DOI] [PubMed] [Google Scholar]
- 18.Brauweiler AM, Cambier JC. Fc gamma RIIB activation leads to inhibition of signalling by independently ligated receptors. Biochem Soc Trans. 2003 Feb;31(Pt 1):281–5. doi: 10.1042/bst0310281. [DOI] [PubMed] [Google Scholar]
- 19.Hauswirth AW, Sonneck K, Florian S, Krauth MT, Bohm A, Sperr WR, Valenta R, Schernthaner GH, Printz D, Fritsch G, Buhring HJ, Valent P. Interleukin-3 promotes the expression of E-NPP3/CD203C on human blood basophils in healthy subjects and in patients with birch pollen allergy. International journal of immunopathology and pharmacology. 2007 Jan;20(2):267–78. doi: 10.1177/039463200702000207. [DOI] [PubMed] [Google Scholar]
- 20.Shreffler WG, Lencer DA, Bardina L, Sampson HA. IgE and IgG4 epitope mapping by microarray immunoassay reveals the diversity of immune response to the peanut allergen, Ara h 2. J Allergy Clin Immunol. 2005 Oct;116(4):893–9. doi: 10.1016/j.jaci.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 21.Macglashan D, Miura K. Loss of syk kinase during IgE-mediated stimulation of human basophils. J Allergy Clin Immunol. 2004 Dec;114(6):1317–24. doi: 10.1016/j.jaci.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 22.MacGlashan DW, Lichtenstein LM. The transition from specific to nonspecific desensitization in human basophils. J Immunol. 1981 Dec;127(6):2410–4. [PubMed] [Google Scholar]
- 23.Macglashan D, Vilariño N. Nonspecific desensitization, functional memory, and the characteristics of SHIP phosphorylation following IgE-mediated stimulation of human basophils. J Immunol. 2006 Jul;177(2):1040–51. doi: 10.4049/jimmunol.177.2.1040. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald SM, Vonakis BM. Association of the Src homology 2 domain-containing inositol 5′ phosphatase (SHIP) to releasability in human basophils. Mol Immunol. 2002 Sep;38(16–18):1323–7. doi: 10.1016/s0161-5890(02)00082-2. [DOI] [PubMed] [Google Scholar]
- 25.Kepley CL. Antigen-induced reduction in mast cell and basophil functional responses due to reduced Syk protein levels. Int Arch Allergy Immunol. 2005 Sep;138(1):29–39. doi: 10.1159/000087355. [DOI] [PubMed] [Google Scholar]
- 26.Denzel A, Maus UA, Gomez MR, Moll C, Niedermeier M, Winter C, Maus R, Hollingshead S, Briles DE, Kunz-Schughart LA, Talke Y, Mack M. Basophils enhance immunological memory responses. Nat Immunol. 2008 May; doi: 10.1038/ni.1621. aop(current) [DOI] [PubMed] [Google Scholar]
- 27.Sampson HA, Broadbent KR, Bernhisel-Broadbent J. Spontaneous release of histamine from basophils and histamine-releasing factor in patients with atopic dermatitis and food hypersensitivity. N Engl J Med. 1989 Jul;321(4):228–32. doi: 10.1056/NEJM198907273210405. [DOI] [PubMed] [Google Scholar]
- 28.Sampson HA. Food anaphylaxis. Br Med Bull. 2000 Jan;56(4):925–35. doi: 10.1258/0007142001903607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.