Abstract
With the increase in sentinel lymph node biopsies in melanoma patients, pathologists are frequently confronted with small deposits of morphologically bland melanocytes in the node, which occasionally cannot be readily classified as benign nodal nevi or melanoma. As most melanomas harbor characteristic chromosomal aberrations which can be used to distinguish it from benign nevi, we used fluorescence in-situ hybridization (FISH) with markers for three regions on chromosome 6 and one on chromosome 11 to determine the presence of chromosomal aberrations in sentinel lymph node specimens with small foci of melanocytes that had been diagnosed as metastatic melanoma or nodal nevi by histopathology. 59 tissue samples from 41 patients (24 lymph node metastases, 17 with nodal nevi, and 18 of the available corresponding primary melanomas) were analyzed by FISH. 20 of 24 (83%) cases diagnosed as metastatic melanoma showed aberrations by FISH. Of the four negative cases, three were unequivocal melanoma metastases, while one on re-review was histopathologically equivocal. Of the 17 nodal nevi one (6%) also showed aberrations by FISH, while the remainder were negative. Multiple aberrations were present in the positive case, some of which were also found in the corresponding primary tumor, identifying this case as a deceptively bland melanoma metastasis that had been misclassified by histomorphology. Our data indicate that FISH is a useful adjunct tool to traditional methods in the diagnostic workup of deposits of melanocytes in the lymph node that are histopathologically ambiguous.
INTRODUCTION
With the increasing use of sentinel lymph node biopsy in melanoma, pathologists are confronted with an increasing load of lymph nodes to be evaluated for microscopic melanoma deposits. As the presence of melanoma in the lymph node is an unequivocal adverse prognostic sign, many physicians carry out a subsequent completion lymphadenectomy in patients with histopathologically confirmed lymph node involvement, although the procedure has been shown to lack a positive effect on overall survival compared to patients who underwent lymph node dissection only if metastases became evident clinically.12 Considering the morbidities associated with lymph adenectomy and adjuvant therapy regimens such as high-dose interferon-alpha 2b, the accurate diagnostic work-up of lymph node biopsies in melanoma patients is of great importance. The histopathologic diagnosis of melanoma metastasis is unequivocal in most cases with bulky involvement of the lymph node parenchyma. The presence of pleomorphic, mitotically active cells on biopsy that are positive for melanocyte markers, and full effacement of lymph node architecture are undisputable criteria for melanoma metastasis. However, diagnostic challenges can arise, when the involvement is restricted to small collections of cells, which do not express marked cytological alterations. This is because not all melanocytes in the lymph nodes indicate metastasis, even if found in the draining basin of a patient with unequivocal melanoma. Incidental nevus cell aggregates in the capsule, connective tissue trabecula or lymphatic spaces of nodes can be frequently found in lymph nodes removed in patients without melanoma. Nodal nevi typically have comparatively small collections of monomorphic melanocytes situated in the fibrous capsule of the lymph node. However, the location of the deposit is not a reliable diagnostic parameter, as nevus aggregates can also be found within the parenchyma of the lymph node and in some cases the involvement can be quite substantial.3 Several studies have described reproducible criteria for small melanoma metastases in the lymph node.1,4,6–7,11,13, 15–16 However, the distinction from nodal nevi can be difficult in cases where the cells lack significant cytologic atypia.
Previous studies have shown that melanomas differ from nevi by the presence of frequent gains or losses of particular chromosomal regions.2 By contrast, the majority of benign melanocytic nevi do not show these chromosomal aberrations. Recently, we have described a fluorescence in situ hybridization (FISH)-based method to determine the most common of these aberrations in tissue sections, even with small amounts of neoplastic cells with high specificity.8 In the present study, we used this to determine the presence of chromosomal aberrations in small lymph node deposits of neoplastic melanocytes that had been classified histopathologically as either metastatic melanoma or nodal nevus.
MATERIALS AND METHODS
A search was preformed for sentinel lymph nodes containing deposits of metastatic melanoma and nodal nevi from the files of UCSF and Wilford Hall Medical Center from 1990–2009. One H&E and adjacent unstained sections were prepared from the blocks of cases with small aggregates of melanoma (less than 10mm in diameter) or cases of lymph nodes that contained a nodal nevus. 115 cases were reviewed of which 41 SLN (24 with melanoma and 17 with nevus deposits) had adequate material in the newly prepared sections for histopathologic evaluation and FISH analysis. 14 of the 17 nodal nevi were from melanoma patients, two from breast cancer patients and one from a patient who underwent neck dissection for squamous cell carcinoma.
FISH
Sections of 5 μm thickness were mounted onto positively charged slides (SuperFrost Plus, ThermoShandon, Pittsburgh, PA). All slides were baked at 56°C overnight to fix the tissue onto the slides, and were then stored at room temperature. Tissue sections were de-paraffinized by soaking in 3 changes of Hemo-De™ Solvent and Clearing Agent (Scientific Safety Solvents, Keller, TX) for 5 minutes each, followed by two 1-minute rinses in absolute ethanol, treatment in 1XSSC pH 6.3 at 80°C for 35 minutes, and a final rinse in water for 3 minutes. Sections were immersed in a solution of Protease (4 mg protease/ml 0.2 N HCl) at 37° C for 15 minutes, rinsed in water for 3 minutes, dehydrated in 70%, 85%, and 100% ethanol for 1 minute each, and allowed to dry. Hybridizations were carried out in an automated co-denaturation oven (HYBrite™, Abbott Molecular Inc, Des Plaines, IL) in a volume of 10 to 30 μl. Slides were coverslipped, sealed with rubber cement, denatured for 5 minutes at 73°C and hybridized at 37°C for 16–18 h. Hybridized slides were soaked in 2X SSC (SSC = 0.3 M NaCl, 15 mM sodium citrate)/0.3% Nonidet P40 (NP40; Abbott Molecular Inc., Des Plaines, IL) at room-temperature for 2 to 10 minutes to remove the coverslips and immersed in 73° C 2X SSC/0.3% NP40 for 2 minutes for removal of nonspecifically bound probe, and allowed to dry in the dark. DAPI I antifade solution (Abbott Molecular Inc., Des Plaines, IL) was applied to the specimen to allow visualization of the nuclei.
Enumeration of FISH Signals
Slides were analyzed with an epi-fluorescence microscope equipped with DAPI single band-pass, Aqua single band-pass, Gold single band-pass, GreenV2 single bandpass, and Red single band-pass filter sets (Abbott Molecular Inc., Des Plaines, IL). Tumor-bearing areas were identified using the DAPI filter at low magnification 10x lens) with the aid of the H&E. Wherever possible, 30 cells were analyzed for each specimen representing three different regions of neoplastic cells. All areas involved by tumor cells were scanned and areas in which cursory inspection of probe signals indicated possibly abnormal copy numbers of any of the four probes were preferentially selected for probe enumeration. Within these areas, cells with nuclei that did not overlap with those of neighboring cells and had bright hybridization signals were randomly selected for enumeration. Nuclei that had complete absence of signals for any two or more of the probes were excluded. Based on the analysis the following thresholds were defined: Counting 30 cell nuclei, a sample was called melanoma if the average gain of 11q signal count was greater than 38%; or the relative loss of 6q signals to CEP 6 signals was less than 40%; or the relative gain of 6p signals to CEP6 signals was greater than 55%; or the average 6p gain was greater than 29% according to the previously determined cut-offs.8
RESULTS
A total of 41 sentinel lymph nodes (24 with melanoma and 17 with nevus deposits) were analyzed by FISH (table 1). The corresponding primary melanomas of the melanoma metastases were available for FISH analysis in 18 of the cases. Of these, 14 (78%) showed aberrations by FISH, consistent with the sensitivity of 85% found in previous studies.8 No aberrations were detected in four primaries, one of which was diagnostically challenging and could not unequivocally be diagnosed by histomorphology. The nodal deposits of these cases, two interpreted as nodal nevi and two as melanoma metastasis also did not show any aberrations detectable by FISH (cases 4, 6, 25, and 37)
TABLE 1.
Comparison of FISH results for the 24 lymph node metastases of melanoma, available corresponding primary tumors and 17 cases of nodal nevus
| FISH results* | |||||
|---|---|---|---|---|---|
| Case | Pathology | 11q gain | 6p>Cep6 | 6q<Cep6 | 6pgain |
| 1 | Nodal metastasis | 96.77 | 9.68 | 29.03 | 6.45 |
| Primary melanoma | 100.00 | 20.00 | 23.33 | 3.33 | |
| 2 | Nodal metastasis | 50.00 | 87.50 | 0.00 | 62.50 |
| Primarymelanoma | 13.33 | 46.67 | 70.00 | 46.67 | |
| 3 | Nodal metastasis | 0.00 | 23.33 | 33.33 | 7.07 |
| 4 | Nodal metastasis | 10.00 | 26.67 | 26.67 | 23.33 |
| Primary melanoma | 0.00 | 26.67 | 13.33 | 22.36 | |
| 5 | Nodal metastasis | 0.00 | 56.67 | 33.33 | 70.00 |
| 6 | Nodalmetastasis | 0.00 | 13.33 | 6.67 | 3.33 |
| Primarymelanoma | 13.33 | 30.00 | 16.67 | 13.33 | |
| 7 | Nodal metastasis | 10.53 | 73.68 | 36.84 | 47.37 |
| 8 | Nodalmetastasis | 0.00 | 20.00 | 13.33 | 3.33 |
| 9 | Nodal metastasis | 40.00 | 63.33 | 26.67 | 70.00 |
| 10 | Nodal metastasis | 3.33 | 56.67 | 15.38 | 26.37 |
| 11 | Nodal metastasis | 66.67 | 40.00 | 36.67 | 30.00 |
| 12 | Nodal metastasis | 100.00 | 73.33 | 33.33 | 63.33 |
| 13 | Nodal metastasis | 66.67 | 46.67 | 60.00 | 43.33 |
| 14 | Nodal metastasis | 50.00 | 33.33 | 30.00 | 36.67 |
| 15 | Nodal metastasis | 30.00 | 50.00 | 66.67 | 26.67 |
| 16 | Nodal metastasis | 27.27 | 90.91 | 18.18 | 63.64 |
| 17 | Nodal metastasis | 33.33 | 80.00 | 10.00 | 13.33 |
| 18 | Nodal metastasis | 33.33 | 53.34 | 40.00 | 56.67 |
| 19 | Nodal metastasis | 83.33 | 40.00 | 20.00 | 46.67 |
| Primary melanoma | 76.67 | 60.00 | 16.67 | 30.00 | |
| 20 | Nodal metastasis | 63.33 | 6.67 | 96.67 | 16.67 |
| Primary melanoma | 96.67 | 30.00 | 70.00 | 66.67 | |
| 21 | Nodal metastasis | 9.00 | 19.00 | 44.00 | 13.33 |
| Primary melanoma | 5.39 | 23.04 | 46.08 | 14.22 | |
| 22 | Nodal metastasis | 30.00 | 56.66 | 33.33 | 43.33 |
| Primary melanoma | 36.66 | 73.33 | 16.66 | 60.00 | |
| 23 | Nodal metastasis | 46.66 | 60.00 | 23.33 | 50.00 |
| Nodal nevus | 0.00 | 26.66 | 6.66 | 0.00 | |
| Primary melanoma | 46.66 | 56.66 | 10.00 | 53.33 | |
| 24 | Nodal metastasis | 0.00 | 10.00 | 43.33 | 3.33 |
| Primary melanoma | 20.00 | 83.33 | 30.00 | 63.33 | |
| 25 | Nodal nevus | 0.00 | 26.67 | 16.67 | 23.33 |
| Primary melanoma | 0.00 | 20.00 | 10.00 | 0.00 | |
| 26 | Nodal nevus | 0.00 | 30.00 | 10.00 | 0.00 |
| Primary melanoma | 13.33 | 80.00 | 16.67 | 56.67 | |
| 27 | Nodal nevus | 0.00 | 15.00 | 20 | 0.00 |
| Primary melanoma | 86.67 | 96.67 | 83.33 | 96.67 | |
| 28 | Nodal nevus | 0.00 | 20.00 | 30 | 3.33 |
| Primary melanoma | 43.33 | 86.67 | 6.67 | 73.33 | |
| 29 | Nodal nevus | 0.00 | 30.00 | 26.67 | 0.00 |
| 30 | Nodal nevus | 62.07 | 93.10 | 55.17 | 89.66 |
| Primary melanoma | 55.40 | 51.35 | 36.48 | 63.51 | |
| 31 | Nodal nevus | 10.00 | 53.33 | 6.66 | 26.66 |
| 32 | Nodal nevus | 0.00 | 30.00 | 16.67 | 13.33 |
| 33 | Nodal nevus | 0.00 | 33.33 | 36.67 | 13.33 |
| 34 | Nodal nevus | 3.33 | 20.00 | 23.33 | 6.67 |
| 35 | Nodal nevus | 3.33 | 26.66 | 10.00 | 10.00 |
| 36 | Nodal nevus | 0.00 | 16.66 | 10.00 | 0.00 |
| 37 | Nodal nevus | 30.76 | 38.46 | 10.76 | 16.92 |
| Primary melanoma | 23.25 | 32.55 | 23.25 | 20.93 | |
| 38 | Nodal nevus | 0.00 | 16.66 | 3.33 | 0.00 |
| 39 | Nodal nevus | 20.00 | 0.00 | 20.00 | 0.00 |
| Primary melanoma | 36.66 | 43.33 | 36.66 | 50.00 | |
| 40 | Nodal nevus | 0.00 | 33.33 | 16.67 | 6.66 |
| Primary melanoma | 10.00 | 76.66 | 26.66 | 43.33 | |
Bold lettering indicates values that exceed the threshold established for melanoma
Of the 24 cases diagnosed as metastatic melanoma by histopathology, 20 (83%) showed aberrations by FISH (figure 1a–d), while the remaining four did not. In two of the four negative cases, the nuclear size and nucleoli size of the melanocytes in the lymph node was increased and mitoses and pleomorphism were present, so that there was no doubt that they represented metastatic melanomas that tested falsely negative by FISH (cases 3 and 4); one patient (case 4) developed brain metastases. However, of the two remaining cases upon re-review, case 8 showed more bland appearing melanocytes in the node, raising the possibility that it represented a nodal nevus (figure 2a–b).
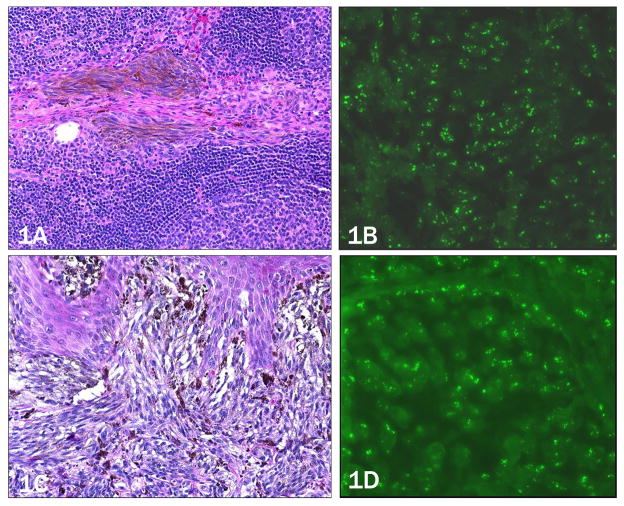
Figure 1. Case 1, 64 year-old white male with lymph node involvement (a,b) originating from a primary melanoma, 1.0 mm in thickness on the right upper arm (c,d).
a) Pigmented, spindled melanocytes within trabecula of the sentinel lymph node (10× objective). b) Increased copy number of 11q13 (CCND1) by FISH as evidence by more than two green signals per nucleus (40x objective). c) Primary melanoma (20× objective). d) Primary melanoma with similarly increased copy number of 11q13 (40× objective).
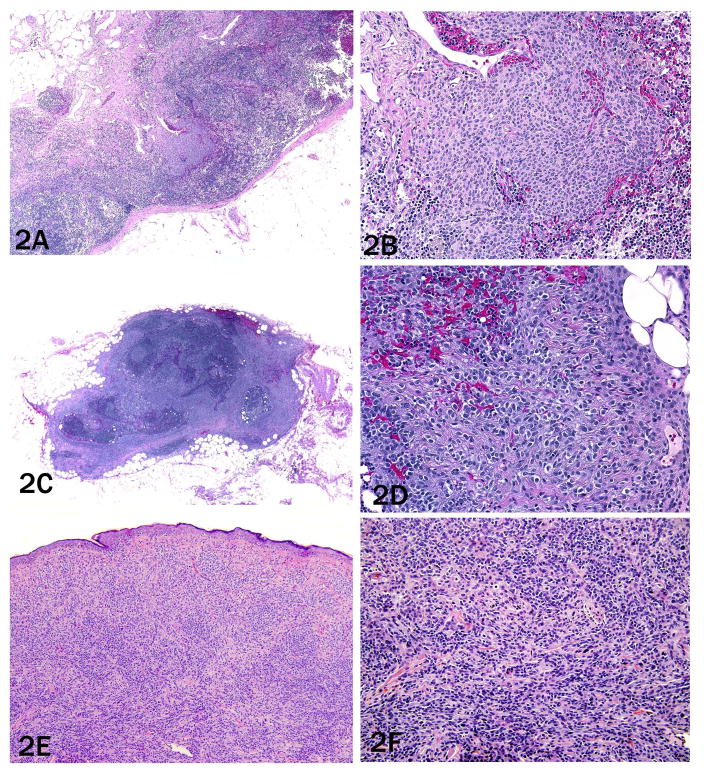
Figure 2. Case 8, need to put in description as in 1 and 4.
a) Trabecular melanocytes with bland, monomorphic morphology (4× objective) that b) are only one to two times the size of the surrounding lymphocytes (20× objective).
Unfortunately, the primary melanoma of this case could not be obtained for comparison. The presence of clear aberrations in the primary, but none in the lymph node deposit would have supported the notion that the nodal deposit was in fact benign. Case 6, the other lymph node deposit that was FISH negative, also had no aberrations in the primary tumor (figure 2c–d). The primary lesion was a nevoid melanoma with a monomorphic population of melanocytes with large sheet-like nests in the superficial dermis with some dispersion and slight maturation with descent (figure 2e–f). Deep dermal mitoses within melanocytes were also identified. Neither the primary nor lymph node metastasis showed aberrations, precluding any assessment of clonal relationship between the two lesions that would have helped establish whether the lymph node deposit was in fact benign.
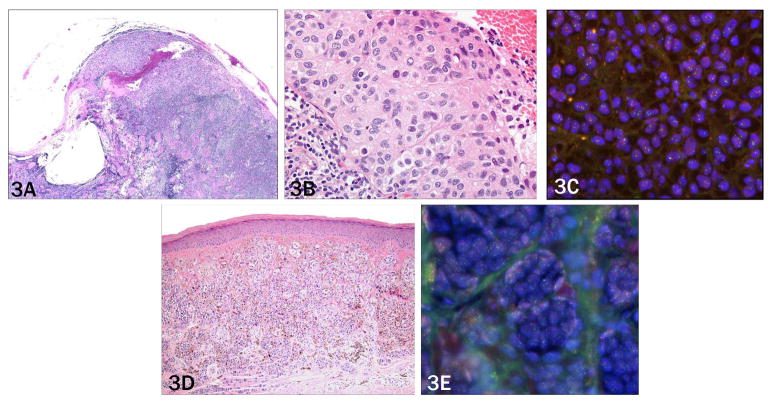
Only one of 17 cases (6%) diagnosed as nodal nevus by histology showed aberrations by FISH (case 30, figure 3a–c). In the positive case, all four parameters were significantly above the threshold, making a false positive FISH result highly unlikely (table 1). The corresponding primary melanoma also showed multiple aberrations, two of which were identical as in the lymph node deposit (11q and 6p gain). This indicates that during the metastatic progression the number of aberrations increased from two to four and that obviously the nodal deposit had been misclassified on morphological grounds (figure 3d–e). Interestingly, one case with a primary melanoma had lymph nodes that contained two morphologically different populations of melanocytes; one diagnosed as melanoma and the other as nodal nevus. The primary melanoma and area representing apparent lymph node metastasis morphologically showed a gain in 11q and 6p and a gain in 6p relative to centromere 6. No aberrations were found in the separate focus of nodal nevus (case 23), confirming the histopathological interpretation.
Figure 3. Case 6 need to put in description as in 1 and 4.
a) Collections of epithelioid melanocytes in the lymph node capsule, extensively involving the lymph node circumferentially (2× objective). b) Melanocyte nuclei show only mild variation in shape and are approximately three times the size of adjacent lymphocytes (40 × objective). c) Primary tumor with sheets of monomophic melanocytes with moderate cytologic atypia (10× objective), and d) scattered deep mitoses (20× objective).
Histopathologic analysis
The histomorphologic analyses of the cases studied is summarized in table 2. Only nodal nevi, not metastases, were found exclusively in the lymph node capsule. The majority of lymph node metastases and all of the nevi demonstrated an epithelioid cellular morphology. By contrast nuclear pleomorphism was more commonly observed in the metastases (63% vs 12%). In contrast to the nodal nevi which had nuclei that were small (less than two times a lymphocyte nucleus) or medium sized (two to three times the size of a lymphocyte), 42% of the metastases demonstrated melanocytes with large nuclear size (at least four times the size of a lymphocyte) and mitoses (50% of cases). Metastases also more frequently showed larger nucleoli (50% vs 18%). Almost half of the 24 metastases showed melanocytic deposits confined to the lymph node parenchyma. The diameter of the lymph nodes deposits was also larger in the metastases (table 2).
Table 2.
Histopathologic features of lymph nodes with metastatic melanoma or nodal nevus
| Histologic criteria | Nodal melanoma | Nodal nevus |
|---|---|---|
| Localization | ||
| Capsule only | 0% | 59% |
| Sinus only | 29% | 0% |
| Parenchyma only | 46% | 6% |
| Capsule andsinus | 4% | 35% |
| Sinus and parenchyma | 21% | 0% |
| Cell shape | ||
| Epithelioid | 79% | 100% |
| Spindled | 17% | 0% |
| Rhabdoid | 4% | 0% |
| Nuclear size | ||
| Small | 25% | 65% |
| Medium | 33% | 35% |
| Large | 42% | 0% |
| Nucleolar size | ||
| Small | 25% | 59% |
| Medium | 25% | 24% |
| Large | 50% | 18% |
| Mitoses detected | 50% | 0% |
| Nuclear pleomorphism | 63% | 12% |
| Melanin present | 21% | 0% |
| Cellular dishesion | 21% | 0% |
| Deposit diameter | 0.05–10mm | 0.05–1.6mm |
Immunohistochemistry analysis
Strong HMB-45 positivity has been repeatedly reported as a helpful criterion in distinguishing melanoma metastasis from nodal nevus.3,5,9–10 HMB-45 immunohistochemistry had been performed on eight of the 24 melanoma metastases at the time of diagnosis, five of which showed strong HMB-45 immunoreactivity, and three were weakly positive. Twelve of the 17 nodal nevi had HMB-45 stain available for review, nine of which were negative and three that were weakly positive.
DISCUSSION
Our data indicate that FISH may be a useful adjunctive tool to histopathology in the diagnostic workup for deposits of melanocytes in the lymph node that are histopathologically ambiguous. In primary melanomas, the sensitivity of the FISH procedure used in the present study has been found to be 85%.8 In the current series of 24 sentinel lymph node biopsies diagnosed as melanoma metastasis by histopathology,20 were positive by FISH, corresponding to a sensitivity of 83%. While one case tested FISH-positive that was previously diagnosed as nodal nevus by histopathology, the presence of multiple aberrations, partially overlapping with the corresponding primary melanoma demonstrate that the case is not a false-positive FISH result but a misclassification due to deceptively bland cytomorphologic features. Our study thus has identified at least one case, in which the diagnostic accuracy could have improved by FISH. While our study confirms that strong HMB45 expression has high specificity for melanoma metastases, the misclassified case only expressed low levels of HMB45, demonstrating that FISH adds additional information to immunohistochemistry.
Our data also shows that the interpretation of the FISH results can be enhanced when the analysis is performed in conjunction with FISH analysis of the primary melanoma. Ruling out a melanoma metastasis based on a negative FISH result would not be possible without knowing that detectable aberrations are present in the primary tumor, because 17% of clear-cut metastases tested FISH-negative. Therefore, in cases in which the lymph node deposit shows no aberrations but the accompanying primary melanoma does, the lymph node deposit can be considered a nodal nevus (cases 23, 26, 27, 28, 40). While it is conceivable that rare exceptions may occur in cases in which the aberration patterns changes during progression from primary to metastatic disease, our data (table 1) shows that the in the majority of cases the specific aberrations present in the primary can also be found in the corresponding metastases. We did not encounter a single case in which aberrations were present in the primary and none in the corresponding metastasis.
Limiting factors for FISH analysis in lymph nodes is the presence of adequate material in serial tissue sections and the identification of the melanocytic foci under fluorescent microscopy. Concomitant labeling with a fluorescently-labeled melanocyte antibody could be considered in the future.14 Out of the 115 lymph node deposits reviewed for this study, only 41 cases had sufficient material on the slide for FISH analysis in newly cut sections. To preempt this problem, it could be considered to order additional tissue sections on positively-charged slides required for FISH analysis when leveling the sentinel nodes in the histology laboratory. As illustrated by cases 4, 6, and 25, a minority of cases has no aberrations detectable by the current probe set, resulting in a false-negative FISH-result. Additional studies are underway to determine the utility of probes targeting additional regions found to be aberrant in melanoma.
In summary, our study shows that FISH can serve as an ancillary diagnostic tool in nodal deposits of melanocytes that cannot be unequivocally classified by traditional methods.
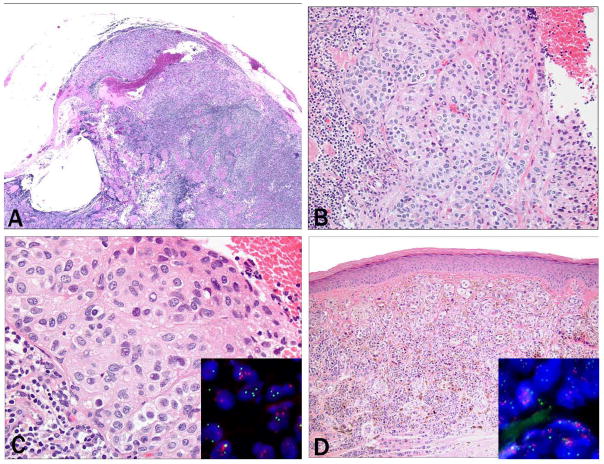
Figure 4. Case 30: femoral sentinel lymph node (a–c) of a 69 year-old female with a 0.9 mm melanoma on the right dorsal foot (d, e).
a) Sentinel lymph node with capsular nevus (a: 4× objective, b: 20× objective) and c) increased copy number of 11q13 (green signals) and 6p25 (red signals) by FISH (40× objective). d) Histopathology of the primary melanoma (10× objective). FISH shows similarly increased copy number of 11q13 (green signals) and 6p25 (red signals) by FISH as the lymph node deposit (40× objective).
Footnotes
Publisher's Disclaimer: The opinions or assertions contained herein are the private views of the authors and not to be construed as official or as reflecting the views of the U.S. Army, U.S. Air Force or the Department of Defense.
References
- 1.Abrahamsen HN, Hamilton-Dutoit SJ, Larsen J, et al. Sentinel lymph nodes in malignant melanoma: extended histopathologic evaluation improves diagnostic precision. Cancer. 2004;100(8):1683–1691. doi: 10.1002/cncr.20179. [DOI] [PubMed] [Google Scholar]
- 2.Bastian BC, Leboit PE, Hamm H, et al. Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hybridization. Cancer Res. 1998;58:2170–5. [PubMed] [Google Scholar]
- 3.Biddle DA, Evans HL, Kemp BL, et al. Intraparenchymal nevus cell aggregates in lymph nodes: a possible diagnostic pitfall with malignant melanoma and carcinoma. Am J Surg Pathol. 2003;27(5):673–681. doi: 10.1097/00000478-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Carson GW, Murray DR, Lyles RH, et al. The amount of metastatic melanoma in a sentinel lymph node: does it have prognostic significance? Ann Surg Oncol. 2003;10(5):575–581. doi: 10.1245/aso.2003.03.054. [DOI] [PubMed] [Google Scholar]
- 5.Carson KF, Wen D, Li P, et al. Nodal nevi and cutaneous melanoma. Am J Surg Pathol. 1996;20(7):834–840. doi: 10.1097/00000478-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Cochran AJ, Robert ME, Wen D. Pathology of the lymph nodes in patients with malignant melanoma. In: LeBoit Philip E., editor. From Pathology: malignant melanoma and melanocytic neoplasms. 1994. pp. 385–400. [PubMed] [Google Scholar]
- 7.Dewar DJ, Newel B, Green MA, et al. The microanatomic location of metastatic melanoma in sentinel lymph nodes predicts nonsentinel lymph node involvement. J Clin Oncol. 2004;22(16):3345–3349. doi: 10.1200/JCO.2004.12.177. [DOI] [PubMed] [Google Scholar]
- 8.Gerami P, Jewell SS, Morrison LE, et al. Fluorescence in situ hybridization (FISH) as an ancillary diagnostic tool in the diagnosis of melanoma. Am J Surg Pathol. 2009;33(8):1146–1156. doi: 10.1097/PAS.0b013e3181a1ef36. [DOI] [PubMed] [Google Scholar]
- 9.Holt JB, Sangueza OP, Levine EA, et al. Nodal melanocytic nevi in sentinel lymph nodes: correlation with melanoma-associated cutaneous nevi. Am J Clin Pathol. 2004;121:58–63. doi: 10.1309/Y5QA-D623-MYA2-1PUY. [DOI] [PubMed] [Google Scholar]
- 10.Lohmann CM, Iversen K, Jungbluth AA, et al. Expression of melanocyte differentiation antigens and Ki-67 in nodal nevi and comparison of Ki-67 expression with metastatic melanoma. Am J Surg Pathol. 2002;26(10):1351–1357. doi: 10.1097/00000478-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Mihic-Pribst D, Saremaslani P, Komminoth P. Immunostaining for the tumour suppressor gene p16 product is a useful marker to differentiate melanoma metastasis from the lymph-node nevus. Virchows Arch. 2003;443:745–751. doi: 10.1007/s00428-003-0897-9. [DOI] [PubMed] [Google Scholar]
- 12.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006 Sep 28;355(13):1307–17. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 13.Murray CA, Leong WL, McCready, et al. Histopathologial patterns of melanoma metastasis in sentinel lymph nodes. J Clin Pathol. 2004;57:64–67. doi: 10.1136/jcp.57.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.North JP, Kageshita T, Pinkel D, et al. Distribution and significance of occult intraepidermal tumor cells surrounding primary melanoma. J Invest Dermatol. 2008 Aug;128(8):2024–30. doi: 10.1038/jid.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scolyer RA, Li LL, McCarthy SW, et al. Micromorphometric features of positive sentinel lymph nodes predicts involvement of nonsentinel nodes in patients with melanoma. Am J Clin Pathol. 2004;122:532–539. doi: 10.1309/TDWJ-TR15-TDM1-TG7Q. [DOI] [PubMed] [Google Scholar]
- 16.Starz H, Balda B, Kramer K, et al. A micromorphometry-based concept for routine classification of sentinel lymph node metastasis and its clinical relevance for patients with melanoma. Cancer. 2001;91(11):2110–2121. [PubMed] [Google Scholar]