Abstract
Rationale
Smoking typically begins during adolescence and is largely reinforced by social cues. During adolescence in rats, sensitivity to both social cues and drugs of abuse is enhanced.
Objectives
We have previously demonstrated in adolescent male rats that a low dose of cocaine interacts with social reward to produce an enhanced conditioned place preference (CPP) relative to either reward given alone. The present study further examined the nature of drug:social reward interactions using nicotine.
Methods
Dose-effect functions for nicotine-CPP were established using two different routes of administration (vehicle, 0.1, 0.3, and 0.6 mg/kg, SC and vehicle, 0.01, 0.03, and 0.06 mg/kg, IV). The effects of nicotine on social reward-CPP and social play behavior were next examined using parameters presumed to be sub-threshold for establishing social reward- and nicotine-CPP.
Results
Dose-dependent nicotine-CPP was observed using both routes of administration. Two pairings of the initially non-preferred side of the apparatus with either SC nicotine or another adolescent rat failed to produce CPP when examined alone, but together produced a robust CPP despite nicotine reducing social play. This interaction effect was not observed with the IV nicotine. A final experiment demonstrated that the enhancement of CPP with the combination of rewards was not due to additive effects of weak, sub-threshold conditioning.
Conclusions
These findings suggest that nicotine and social rewards interact synergistically in adolescent rats resulting in a greater, perhaps qualitatively different, reward than either reward given alone. Understanding drug:social reward interactions may provide new directions for development of preventions and interventions of adolescent smoking.
Keywords: Adolescence, Conditioned Place Preference, Place conditioning, Drug Conditioning, Intravenous nicotine
Introduction
Adolescence constitutes a period of increased risk for initiation of smoking (Taioli and Wynder 1991; Breslau et al.1993; Olds and Thombs 2001) and early initiation of smoking increases an individual’s risk of dependence later in life (Nelson et al. 1995; Chen & Miller 1998; Grant 1998; Hanna and Grant 1999; Kandel and Chen 2000; Jefferis et al. 2003). Smokers who begin smoking during adolescence as opposed to adulthood become dependent more quickly and experience more difficulty trying to quit (Breslau and Peterson 1996; Chen and Miller 1998; Colby et al. 2000; Kandel and Chen 2000). Studies also suggest that rodent adolescents, like humans, are particularly sensitive to the rewarding effects of nicotine (Vastola et al. 2002; Belluzzi et al. 2004; Shram et al. 2006; Brielmaier et al. 2007), and exposure to nicotine early in life sensitizes nicotine’s reinforcing effects in adulthood (Adriani et al. 2003; Adriani et al. 2006). Peers are one of the strongest influences on smoking during adolescence (Pierce et al. 1996; Jackson 1997), and teens are more likely to begin smoking if they have friends who also smoke (Glynn 1989; Skara and Sussman 2003; Letherdale et al. 2005). In fact, first-time smoking often has adverse effects, such as nausea and coughing, yet likely persists because the social context is reinforcing (West et al. 1999; Baker et al. 2004; Geckova et al. 2005; Sussman 2005).
Social cues are highly salient to adolescents (Vanderschuren et al. 1997; Spear 2000). Engaging in social interaction during adolescence promotes healthy development and influences development of adult social behavior (Einon et al. 1978; Meaney and Stewart 1979; Smith 1982; Van den Berg et al. 1999a). In rodents, social play is reinforcing and highly rewarding. Rats will learn to lever press for social interaction (Angermeier et al. 1959; Evans et al. 1994), traverse a T-maze to gain access to another rat (Werner 1976; Normansell and Panksepp, 1990), and exhibit conditioned place preference (CPP) for a rat-paired environment (Calcagnetti and Schecter 1992; Crowder and Hutto 1992; Van den Berg et al. 1999b; Douglas et al. 2004).
Social interaction influences drug effects and intake in rodents. For instance, social interaction attenuates ethanol-induced place aversion (Gauvin et al. 1994), and cues paired with social interaction enhance subsequent ethanol intake (Tomie et al. 2004). Social interaction in adolescent rats influences general responsiveness and sensitivity to alcohol (Varlinskaya et al. 2001), and low doses of alcohol facilitate social preferences (Varlinskaya and Spear 2002, 2006). Also, morphine increases resistance to extinction of socially-reinforced choices in a T-maze in adolescent rats (Normansell and Panksepp 1990), and cocaine enhances a social reward-CPP (Thiel et al. 2008).
Nicotine enhances reinforcing effects of other nonpharmacological stimuli (Donny et al. 2003). Using the self-administration paradigm, Palmatier et al. (2006) established an interaction between nicotine and a visual stimulus such that operant responses for a combination of both visual stimulus and nicotine produced synergistic reinforcement compared to that afforded by either stimulus presented alone. The present study extended these findings by examining whether nicotine enhances the rewarding effect of social context using the CPP model. This model is used to measure the rewarding effects of both drug and non-drug stimuli, including social interaction reward (Bardo & Bevins 2000; Tzschentke 2007; Thiel et al. 2008). Bevins (2002) demonstrated that the degree of CPP produced by a combination of cocaine with novel objects was synergistically greater than CPP produced by either stimulus alone. Similarly, our previous research demonstrated that a low dose of cocaine given in combination with a social partner produced synergistic CPP in comparison to the CPP produced by either stimulus given alone (Thiel et al. 2008).
In the present study, we tested the hypothesis that nicotine and social rewards interact synergistically in adolescent rats such that together they are a stronger reward than either stimulus alone. We first examined dose-effect functions for CPP established using either IV or SC administration of nicotine. We then estimated sub-threshold parameters for establishing nicotine-CPP and social reward-CPP in order to examine their interaction. We predicted that experiencing nicotine along with social interaction would produce a robust CPP, whereas either of these stimuli alone would fail to produce CPP. Our final experiment was designed to further test whether nicotine interacts with social reward synergistically.
Methods
Animals
Male Sprague-Dawley rats (Charles River, San Diego, CA) arrived at Arizona State University on post-natal day (PND) 22 (i.e., 22 days old, 55 – 60 g). They were individually housed in a climate-controlled facility with a 12-h light dark cycle (lights on at 6 p.m.) and ad libitum access to food and water. All experiments were conducted within a conservative estimate of rodent adolescence: PNDs 28-42 (Spear 2000). Rats weighed approximately 125 – 145 g at the start of baseline assessment for preferences (i.e., between PNDs 34 – 36) and gained on average about 5 g/day throughout conditioning. On the CPP test day (i.e., PND 40), rats weighed approximately 165 – 175 g. Rats remained isolated except when paired during conditioning. Housing and care were conducted in accordance with the Guide for the Care and Use of Laboratory Rats (Institute of Laboratory Animal Resources on Life Sciences, National Research Council 1996).
Surgery
Surgical implantation of an intravenous catheter occurred for all rats in Experiments 1 and 2. Rats in subsequent experiments did not undergo surgery. Our acclimation procedure consisted of handling each rat for approximately 2 min/day for 4 days prior to surgery. Prior to surgery (PND 26 or 27), the rats were initially anesthetized with 4% isoflurane gas (MWI VetOne, Meridian, ID) and subsequently maintained at 2-3%. Catheter construction and surgery were similar to that previously described by Belluzzi et al. (2005). One modification was that a small ball of 100% silicone aquarium sealant (Dow Corning, Baltimore, MD) was added 1.5 cm from the free end of the catheter to mark the depth of catheter insertion during surgery and to secure the catheter in place with sutures around the vein on either side of the ball. Post-surgery, the skin incisions were treated with a topical antibiotic to prevent infection and the rats were placed into paper-lined cages on top of heating pads. The rats were also given buprenorphine hydrocholoride analgesia (0.05 mg/kg, IP; Reckitt Benckiser Pharmaceuticals, Richmond, VA). Rats were given 5-8 days of recovery following surgery. Throughout the experiments catheters were flushed daily with a solution of 0.1 ml of bacteriostatic saline containing heparin sodium (70 U/ml; Hospira Inc., Lake Forest, IL), streptokinase (0.67 mg/ml; Astra Pharmaceutical Products, Westerborough, MA), and ticarcillin disodium (66.67 mg/ml; SmithKline Beecham Pharmaceuticals, West Chester, PA) to maintain patency. Catheter patency was verified periodically throughout the experiment by administering 0.03 ml Brevital (16.6 mg/ml; Jones Pharma Inc., St. Louis, MO) through the IV catheter and watching for brief loss of motor reflexes.
Drug preparation
(−)Nicotine hydrogen tartrate (Sigma, St. Louis, MO) was dissolved in sterile saline and the pH was adjusted to approximately 7.2. All IV injections were infused at an injection volume of 0.5 ml/kg. All SC injections were given at a volume of 1 ml/kg. The doses are reported as nicotine base.
Apparatus
In the CPP paradigm, the rewarding effects of an unconditioned stimulus (US) become associated with distinct environmental stimuli (i.e., conditioned stimuli, CS) such that the environment itself acquires secondary rewarding effects and alone can elicit incentive motivation to approach and maintain contact within it (Schneirla 1959). Conditioning in the present study took place in Plexiglas chambers containing two equal-sized compartments divided by a solid removable partition. Each compartment measured 36 × 24 × 30 cm high. One compartment had pine bedding beneath a wire mesh floor and all but the front wall were white. The other compartment had cedar-scented bedding beneath a bar grid floor and all but the front wall were black. The front wall of both compartments was transparent to allow direct observation of the rats’ behavior. The conditioning room had an overhead fluorescent light. In addition, there were small fluorescent lights suspended 32 cm above the black compartments, such that light intensity measured from the floor of the black and white sides was equal. During the 10-min preference tests, the solid partition was replaced by one containing an opening in the center (8 × 8 cm high), allowing the rat free-access to both compartments. Across all experiments, 42% of the rats preferred the black side and 58% preferred the white side prior to conditioning; however, the higher percentage preferring white was not a strong bias as rats spent on average (±SEM) 287 ± 4.7 s on the black side and 313 ± 4.7 s on the white side. A third clear plastic chamber placed in a room separate from the CPP room was used as an alternate environment for control procedures described below. It measured 34 × 22 × 26 cm, and contained corncob bedding placed on top of a plastic bottom.
General CPP Procedure
On the first day rats were transported to the CPP room, placed into the CPP apparatus, and allowed to explore for 10 min. Across the next 2 consecutive days, initial baseline preference was assessed by allowing each rat free-access to the entire apparatus during 10-min tests. The starting compartment was counterbalanced and entry into a compartment was operationally defined as the rats’ two forepaws in contact with the floor/walls of that compartment and continued to be recorded as such until the rats’ two forepaws contacted the floor of the other compartment. Total time that rats spent in each compartment was averaged across the two baseline days to determine initial side preference. Rats that failed to demonstrate at least five compartment crossovers during either baseline day were excluded from analyses due to inadequate expression of choice behavior, but were still used as playmates for other rats during conditioning.
Next, conditioning sessions were conducted twice/day. Each rat was confined to one side of the CPP apparatus for 10 min during a morning session, and confined to the opposite side of the apparatus for 10 min during the afternoon session. A biased CPP design [i.e., pairing the unconditioned stimulus (US) with the initially non-preferred side of the apparatus] was utilized based on previous research demonstrating that social reward-CPP is established regardless of whether a biased or unbiased design is used (Thiel et al. 2008), and an advantage of the biased design is that it allows greater sensitivity for detecting varying degrees of preference shifts. Starting side for the first conditioning session was counterbalanced such that half of the rats in each group were exposed first to their initially non-preferred side immediately following drug injection, and half were exposed to their initially preferred side immediately following saline injection. The rats received the opposite of these conditions during the afternoon session. Conditioning sessions were conducted at the same time each day. Morning and afternoon sessions were separated by 6 h to allow for sufficient nicotine clearance from blood.
CPP was assessed 24 h after the last conditioning day (i.e., PND 39 or 40). Time spent in each side was recorded for 10 min by an observer unaware of group assignments.
Specific Experiments
The timeline and procedural details of Experiments 1- 4 are outlined in Table 1 and Experiment 5 is outlined in Table 2.
Table 1.
Timeline of procedures across post-natal days (PND) and the stimuli paired with each context during the conditioning phase; stimuli included injections of vehicle (Veh) or nicotine (Nic) just prior to context pairing in isolation (Iso) versus with a playmate (Soc).
| Exp. | Group | n | Timeline of Procedures Across PNDs | Stimulus Conditions Paired With Each Context During Conditioning Sessions1 |
||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Conditioning | Test | Preferred | Non-preferred | ALT Environment | |||
| 1 | Veh, IV | 8 | 34-35 | 36-39 | 40 | Veh | Veh | N.A. |
| 0.01 mg/kg, IV | 8 | 34-35 | 36-39 | 40 | Veh | Nic | N.A. | |
| 0.03 mg/kg, IV | 8 | 34-35 | 36-39 | 40 | Veh | Nic | N.A. | |
| 0.06 mg/kg, IV | 9 | 34-35 | 36-39 | 40 | Veh | Nic | N.A. | |
| 2 | Veh/Iso | 12 | 36-37 | 38-39 | 40 | Iso + Veh | Iso + Veh | Soc + Nic |
| Veh/Soc | 12 | 36-37 | 38-39 | 40 | Iso + Veh | Soc + Veh | Iso + Nic | |
| 0.01 N/Iso | 11 | 36-37 | 38-39 | 40 | Iso + Veh | Iso + Nic | Soc + Veh | |
| 0.01 N/Soc | 12 | 36-37 | 38-39 | 40 | Iso + Veh | Soc + Nic | Iso + Veh | |
| 0.03 N/Iso | 12 | 36-37 | 38-39 | 40 | Iso + Veh | Iso + Nic | Soc + Veh | |
| 0.03 N/Soc | 10 | 36-37 | 38-39 | 40 | Iso + Veh | Soc + Nic | Iso + Veh | |
| 3 | Veh, SC | 9 | 34-35 | 36-39 | 40 | Veh | Veh | N.A. |
| 0.1 mg/kg, SC | 9 | 34-35 | 36-39 | 40 | Veh | Nic | N.A. | |
| 0.3 mg/kg, SC | 9 | 34-35 | 36-39 | 40 | Veh | Nic | N.A. | |
| 0.6 mg/kg, SC | 9 | 34-35 | 36-39 | 40 | Veh | Nic | N.A. | |
| 4 | Veh/Iso | 9 | 36-37 | 38-39 | 40 | Iso + Veh | Iso + Veh | Soc + Nic |
| Veh/Soc | 10 | 36-37 | 38-39 | 40 | Iso + Veh | Soc + Veh | Iso + Nic | |
| 0.1 N/Iso | 9 | 36-37 | 38-39 | 40 | Iso + Veh | Iso + Nic | Soc + Veh | |
| 0.1 N/Soc | 10 | 36-37 | 38-39 | 40 | Iso + Veh | Soc + Nic | Iso + Veh | |
On a given day, rats received pairings in each side of the CPP apparatus with 6 h intervening. Where applicable, rats also received pairings in the alternate (ALT) environment at least 2 h after the last conditioning session.
Table 2.
Stimuli conditions used for Experiment 5: vehicle (Veh) or nicotine (N) was injected immediately prior to sessions in the preferred side alternating every other day with Veh or N injected immediately prior to sessions with a playmate (Soc) in the initially non-preferred side. Alternate (ALT) environment sessions controlled for total N exposure across groups.
| Group (n=16) | PND of Each Conditioning Day |
Stimulus Conditions Paired With Each Context During Conditioning1 |
||
|---|---|---|---|---|
| Preferred | Non-preferred | ALT environment | ||
| Veh vs. Veh/Soc | 35 | Iso + Veh | -- | Iso + Nic |
| 36 | -- | Soc + Veh | Iso + Nic | |
| 37 | Iso + Veh | -- | Iso + Nic | |
| 38 | -- | Soc + Veh | Iso + Nic | |
| N vs. N/Soc | ||||
| 35 | Iso + Nic | -- | Iso + Veh | |
| 36 | -- | Soc + Nic | Iso + Veh | |
| 37 | Iso + Nic | -- | Iso + Veh | |
| 38 | -- | Soc + Nic | Iso + Veh | |
CPP and Alternate (ALT) Environment sessions were separated by 6 h. Actual start side of the CPP apparatus was counterbalanced across groups.
Experiment 1: IV nicotine CPP dose-effect function
Rats were divided into 4 groups (final n = 8-9/group), counterbalanced for magnitude of preference for their initially preferred side. The groups received either vehicle, 0.01, 0.03, or 0.06 mg/kg nicotine, IV immediately upon placement into their initially non-preferred (i.e., nicotine-paired) side; all rats received vehicle immediately upon placement into the initially preferred side. Rats received 4 of each session type across 4 consecutive days. For the nicotine and vehicle infusions, rats were placed into their assigned compartment and immediately infused with their assigned dose manually via Tygon tubing (Saint-Gobain, Akron, OH) over 3 s. The vehicle/drug solution was back-filled into the tubing, which already contained enough heparin solution to flush the dose through the length of the catheter into the bloodstream; the two solutions were separated by a tiny air bubble. The tubing was removed from the catheter after the infusion was completed.
Experiment 2: IV nicotine and social reward interaction
Rats were assigned to pairs matched for initial compartment preference and body weight (within 10 g). Rat pairs were assigned to one of the following 6 groups (n = 10-12/group) that received either vehicle (Veh), 0.01 mg/kg, IV nicotine (0.01 N), or 0.03 mg/kg, IV nicotine (0.03 N) immediately upon placement into their initially non-preferred side either in isolation (Iso) or with the other rat of the pair (Soc): Veh/Iso, Veh/Soc, 0.01 N/Iso, 0.01 N/Soc, 0.03 N/Iso, or 0.03 N/Soc. All rats received Veh/Iso conditions in their initially preferred side. Rats received 2 of each session type across 2 consecutive days. Two, rather than 4 pairings, were given in an attempt to produce sub-threshold or weak CPP in this experiment when either social or nicotine reward was given alone. Furthermore, we purposefully selected only the lower doses of nicotine to examine its interactions with social context. We predicted that these parameters would allow sensitivity for detecting a synergistic interaction between nicotine and social rewards. Drug experience and exposure to playmate were equated by including a third session 2 h after the afternoon conditioning session during which Iso groups were paired with a playmate in the alternate environment and Veh groups received 0.01 mg/kg or 0.03 mg/kg, IV nicotine immediately upon placement into the alternate environment (i.e., exposure to both USs was equal across groups; only location of the US varied).
Experiment 3: SC nicotine CPP dose-effect function
Rats were divided into 4 groups (final n = 9/group) counterbalanced for magnitude of initial preference, that received either vehicle, 0.1, 0.3, or 0.6 mg/kg nicotine, SC immediately prior to placement into the initially non-preferred side of the CPP apparatus; all rats received vehicle immediately prior to placement into the initially preferred side. Rats received 4 of each session type across 4 consecutive days.
Experiment 4: SC nicotine and social reward interaction
Rats pairs were assigned to one of the following 4 groups (final n = 9-10/group) that received either vehicle (Veh) or 0.1 mg/kg nicotine SC (0.1 N) immediately prior to placement into their initially non-preferred side either in isolation (Iso) or with their playmate (Soc): Veh/Iso, Veh/Soc, 0.1 N/Iso, or 0.1 N/Soc. Assignment to groups and all other conditioning parameters were identical to that used in Experiment 2.
Experiment 5: Nature of the interaction between nicotine and social rewards
For Experiments 2 and 4, CPP in the N/Soc group, but not in the N/Iso and Veh/Soc groups, is consistent with a synergistic interaction between nicotine and social reward. This experiment was conducted to provide further support for synergism and to rule out the possibility that the interaction was due to additive effects of small, non-significant shifts resulting from weak associations between the individual USs (i.e., nicotine and social rewards) and the CS (initially non-preferred side). The design of this experiment eliminated the associative strength of nicotine alone as a contributing factor to CPP by pairing nicotine with both sides of the apparatus, thereby preventing either side (potential CSs) from acquiring a predictive relationship with nicotine reward alone. Social reward, on the other hand, was paired only with the initially non-preferred side and its conditioning strength was compared among groups receiving either nicotine or vehicle paired with both sides of the apparatus. A difference between these two groups would support the hypothesis that the nicotine/social rewards in combination interact synergistically to produce a qualitatively stronger US.
Rats were assigned to pairs matched for initial compartment preference and body weight (within 10 g), and the pairs were then assigned to 2 groups (final n = 16/group) that each received only 1 session/day in the CPP apparatus along with 1 session/day in the alternate environment. Rats in the Veh vs. Veh/Soc group were injected with vehicle and placed alone on their initially preferred side, and on alternating days they were injected with vehicle then paired with a playmate on their initially non-preferred side. In the alternate environment, they were injected with nicotine (0.1 mg/kg, SC) and placed alone. Rats in the N vs. N/Soc group were injected with nicotine (0.1 mg/kg, SC) and placed alone on their initially preferred side, and on alternating days they were injected with nicotine (0.1 mg/kg, SC) and paired with a playmate on their initially non-preferred side. In the alternate environment, they were injected with vehicle and placed alone. Thus, conditioning took place over a total of 4 days rather than the 2 days used in previous experiments (see Table 2). Order of starting side and session type were counterbalanced and US exposure was equated. Each day, conditioning and alternate environment sessions were separated by 6 h.
Play Behavior
In Experiments 2 and 4, play behavior was videotaped during the last conditioning session for rats in the 0.03 N/Soc and 0.1 N/Soc conditions, respectively, as well as all rats in Veh/Soc groups in both experiments. The videos were later scored for nape attacks and pins by an observer blind to the conditions of the rat pairs. A pin was operationally defined as standing above the pinned rat with the latter lying on his dorsal surface with his ventral surface exposed; this measure assesses play fighting. A nape attack was operationally defined as a rat lunging forward and directing the tip of its snout toward the nape of his playmate; this measure is associated with play initiation (Pellis and Pellis 1987).
Data Analysis
CPP was operationally defined as a significant increase in time spent in the initially non-preferred side (i.e., US-paired side) post-conditioning relative to pre-conditioning baseline. Mixed factor ANOVAs with Day (baseline vs. test day) as a repeated measures factor and nicotine dose and social condition as between subjects factors were used to analyze time spent in the initially non-preferred side. Significant interactions were further probed using Tukey’s HSD tests for between-group comparisons and paired t-tests with Bonferonni correction for within-group comparisons. Crossovers between compartments on the test day were also analyzed with ANOVAs. Pins and nape attacks were analyzed using independent sample t-tests.
Results
CPP
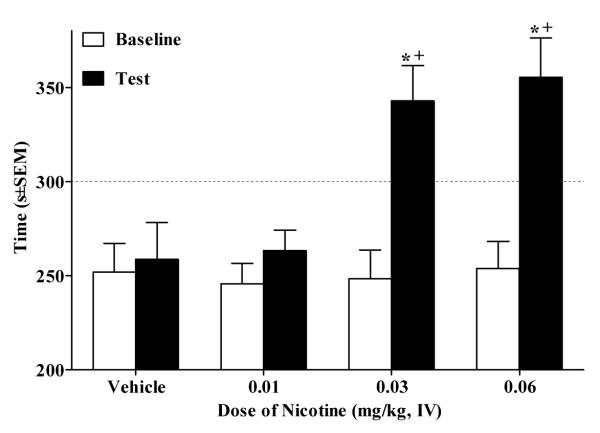
In Experiment 1, the ANOVA of time in the nicotine-paired side revealed a Day × Dose interaction (F(3,29) = 5.02, p<.01). Paired sample t-tests revealed that rats that received the 0.03 mg/kg (t(7) = 3.3, p<.0125, Bonferroni correction) and 0.06 mg/kg (t(8) = 4.9, p<.0125, Bonferroni correction) IV nicotine exhibited significantly more time spent in the nicotine-paired side on test day relative to baseline. On test day, rats in the 0.03 and 0.06 mg/kg groups spent more time in the nicotine-paired side relative to the vehicle controls (p<.05, Tukey’s HSD test; Fig. 1).
Figure 1.
Dose-dependent nicotine-CPP using IV administration shown as time (mean s±SEM) spent in the nicotine-paired (i.e., initially non-preferred) side pre-conditioning (i.e., Baseline, white bars) vs. post-conditioning (i.e., Test, black bars). Asterisk (*) indicates an increase in amount of time spent in the nicotine-paired side on Test day relative to Baseline (p<.0125, Bonferroni correction). Cross (+) indicates a greater amount of time spent in nicotine-paired side relative to Vehicle group (p<.05, Tukey’s HSD). The dotted line represents 50% of the total test period (i.e., 300 s).
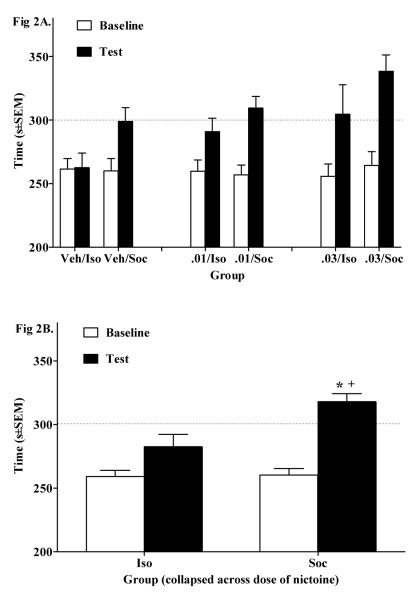
In Experiment 2, the ANOVA of time spent in the nicotine- and/or playmate-paired side failed to reveal a significant Day × Nicotine Dose × Social interaction (see Fig 2A). However, there was a significant Day × Social interaction (F(1,63) = 5.78, p<.05, Fig. 2B). A paired sample t-test on the data collapsed across Nicotine Dose revealed that rats in the Soc group spent more time in their playmate-paired side on test day relative to baseline (t(33) = 9.5, p<.025, Bonferroni correction). In addition, rats in the Soc group spent more time in their initially non-preferred compartment on test day relative to the Iso group (p<.05, Tukey’s HSD test).
Figure 2.
(A) Nicotine (0.01 mg/kg & 0.03 mg/kg, IV) and/or social reward-CPP shown as time (mean s±SEM) spent in the playmate and/or nicotine-paired side pre-conditioning (i.e., Baseline, white bars) vs. post-conditioning (i.e., Test, black bars) across groups. Although there was no Day × Nicotine Dose × Social interaction, there was a Social × Day interaction. (B) Social × Day interaction collapsed across Nicotine Dose. Asterisk (*) indicates an increase in time spent in the playmate-paired side on Test day relative to Baseline (p<.025, Bonferroni correction). Cross (+) indicates a greater amount of time spent in the initially non-preferred side on Test day for the socially conditioned rats relative to the isolated conditioned rats (p<.05, ANOVA main effect). The dotted line represents 50% of the total test period (i.e., 300 s).
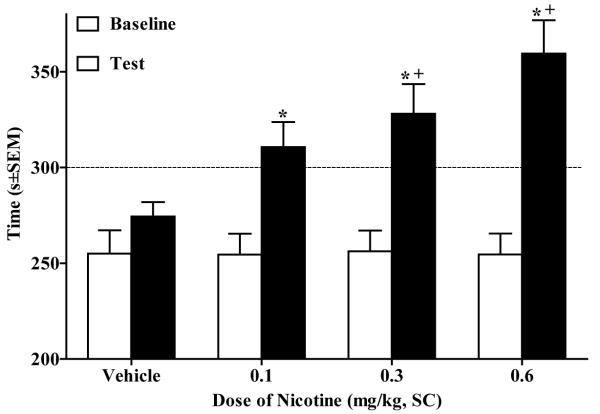
In Experiment 3, the ANOVA of time in the nicotine-paired side revealed a Day × Dose interaction (F(3,32) = 4.36, p<.01). Paired sample t-tests revealed that rats that received 0.1 mg/kg (t(8) = 3.9, p<.0125, Bonferroni correction), 0.3 mg/kg (t(8) = 3.8, p<.0125, Bonferroni correction), or 0.06 mg/kg (t(8) = 5.2, p<.0125, Bonferroni correction) SC nicotine exhibited significantly more time spent in the nicotine-paired side on test day relative to baseline. On test day, rats in the 0.3 and 0.6 mg/kg SC groups spent more time in the nicotine-paired side relative to the vehicle controls (p<.05, Tukey’s HSD test; Fig. 1). A trend analysis of preference shift (time spent in the nicotine-paired side post-conditioning minus pre-conditioning) as a function of dose revealed a significant linear trend (F(1,32) = 12.82, p<.001), indicating greater preference shift with increasing nicotine dose.
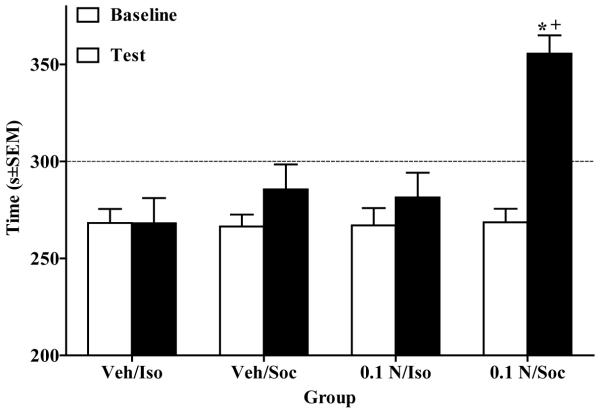
In Experiment 4, the ANOVA of time spent in the nicotine- and/or playmate-paired side revealed a Day × Nicotine × Social interaction (F(1,34) = 4.24, p<.05). A paired sample t-test revealed that rats in the 0.1 N/Soc group exhibited significantly more time spent in the nicotine/social-paired side on test day relative to baseline(t(9) = 7.4, p<.0125, Bonferroni correction). In addition, this group demonstrated significantly more time spent in the nicotine/social-paired side on test day relative to all other groups (p<.05, Tukey’s HSD test; Fig. 4).
Figure 4.
Nicotine (0.1 mg/kg, SC) and/or social reward-CPP shown as time (mean s±SEM) spent in the playmate and/or nicotine-paired side pre-conditioning (i.e., Baseline, white bars) vs. postconditioning (i.e., Test, black bars) across groups. Asterisk (*) indicates an increase in time spent in initially non-preferred side on Test day relative to Baseline (p<.0125, Bonferroni correction). Cross (+) indicates a greater amount of time spent in the initially non-preferred side on Test day for the 0.1 N/Soc group relative to all other groups (p<.05, Tukey’s HSD). The dotted line represents 50% of the total test period (i.e., 300 s).
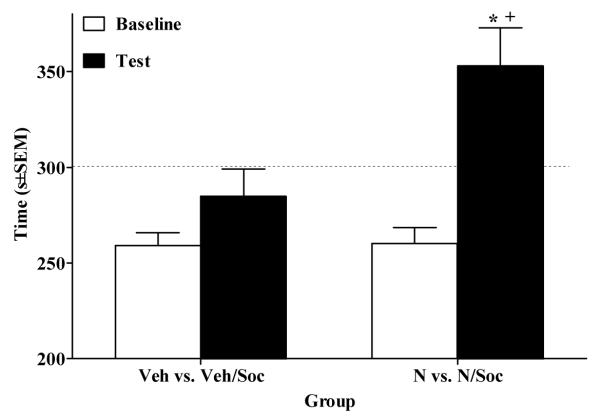
In Experiment 5, the ANOVA of time spent on the playmate-paired side revealed a Day × Nicotine interaction (F(1,30) = 10.63, p<.01). A paired sample t-test revealed that rats in the N vs. N/Soc group exhibited significantly more time in the playmate-paired side on test day relative to baseline (t(15) = 5.6, p<.025, Bonferroni correction). In addition, this group exhibited significantly more time spent in their playmate-paired side on test day relative to the Veh vs. Veh/Soc group (p<.05, Tukey’s HSD test; Fig. 5).
Figure 5.
Nicotine (0.1 mg/kg, SC) enhanced social reward shown as time (mean s±SEM) spent in playmate-paired (i.e., initially non-preferred) side preconditioning (i.e., Baseline, open bars) vs. post-conditioning (i.e., Test, closed bars). Note that in both groups, drug pretreatment was held constant across sides of the apparatus in order to eliminate drug conditioning, whereas social reward conditioning was maintained by pairing the playmate with the initially non-preferred side. Asterisk (*) indicates an increase in time spent in initially non-preferred side on Test day relative to Baseline (p<.025, Bonferroni correction). Cross (+) indicates a greater amount of time spent in the initially non-preferred side on Test day for the N vs. N/Soc group relative to the Veh vs. Veh/Soc group (p<.05, Tukey’s HSD). The dotted line represents 50% of the total test period (i.e., 300 s).
In each of the above experiments, ANOVAs of crossovers on test day revealed no differences between groups (see Table 3).
Table 3.
Crossovers (mean ± SEM) on the test day
| Experiment | Group (n) | Crossovers |
|---|---|---|
| 1 | Veh (8) | 21.8 ± 3.1 |
| 0.01 mg/kg, IV (8) | 24.9 ± 1.6 | |
| 0.03 mg/kg, IV (8) | 21.0 ± 4.3 | |
| 0.06 mg/kg, IV (9) | 18.0 ± 3.1 | |
| 2 | Veh/Iso (12) | 23.8 ± 2.5 |
| Veh/Soc (12) | 26.8 ± 2.4 | |
| 0.01 N/Iso (11) | 28.5 ± 2.6 | |
| 0.01 N/Soc (12) | 28.9 ± 2.9 | |
| 0.03 N/Iso (12) | 25.1 ± 2.8 | |
| 0.03 N/Sol (10) | 25.4 ± 2.1 | |
| 3 | Veh (9) | 30.8 ± 3.0 |
| 0.1 mg/kg, SC (9) | 29.1 ± 4.0 | |
| 0.3 mg/kg, SC (9) | 26.1 ± 4.3 | |
| 0.6 mg/kg, SC (9) | 25.7 ± 2.9 | |
| 4 | Veh/Iso (9) | 26.3 ± 2.2 |
| Veh/Soc (10) | 22.4 ± 2.2 | |
| 0.1 N/Iso (9) | 26.1 ± 3.4 | |
| 0.1 N/Soc (10) | 23.6 ± 2.4 | |
| 5 | Veh vs. Veh/Soc (16) | 23.2 ± 1.0 |
| N vs. N/Soc (16) | 25.0 ± 1.5 |
Play Behavior
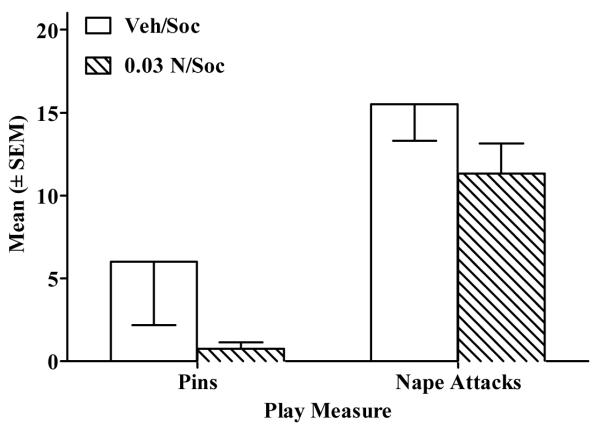
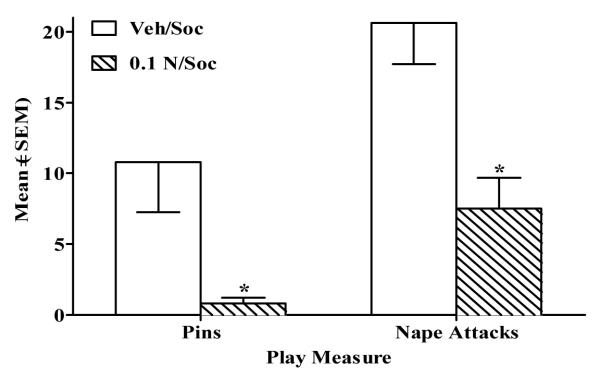
In Experiment 2, there were no significant differences in pins or nape attacks between rats receiving IV vehicle (i.e., Veh/Soc) vs. nicotine (0.03 N/Soc; Fig. 6). By contrast in Experiment 4, rats given SC vehicle (i.e., Veh/Soc) exhibited more pins (t(18) = 2.87, p<.01) and more nape attacks (t(18) = 3.6, p<.01) than rats given nicotine (i.e., 0.1 N/Soc; Fig. 7).
Figure 6.
Nicotine administered IV did not alter pins or nape attacks relative to vehicle on the last day of conditioning in the 0.03 N/Soc group (diagonal stripe bar) vs. the Veh/Soc group (open bar).
Figure 7.
Nicotine administered SC decreased mean ±SEM pins and nape attacks on the last day of conditioning in the 0.1 N/Soc group (diagonal stripe bar) vs. the Veh/Soc group (open bar). Asterisk (*) represents a decrease relative to Veh/Soc group (p<.01).
Discussion
The results from the present study are consistent with the notion that nicotine and social interaction have synergistic rewarding effects. Presented together, both stimuli have stronger reinforcing effects than would be expected from either stimulus alone. Nicotine-CPP in adolescent rats was established using the SC route of administration, consistent with previous reports (Vastola et al. 2002; Belluzzi et al. 2004; Torres et al. 2008). Furthermore, Experiment 1 extends upon previous findings in demonstrating nicotine-CPP in adolescent rats using IV administration. Sub-threshold parameters were then specifically chosen for establishing CPP with the individual stimuli in order to allow sensitivity to detect an interaction (i.e., reduced number of CS-US pairings to 2 and use of the lowest doses of nicotine that supported CPP with 4 CS-US pairings). Importantly, CPP was observed only when SC nicotine and a playmate were experienced together in the CS environment, but not when these same stimuli were individually paired with the CS environment, suggesting that nicotine and social rewards interact synergistically. We have previously demonstrated a similar interaction between cocaine and social rewards (Thiel et al. 2008). To rule out the possibility that the interaction may instead be additive effects of 2 individual weak rewards, Experiment 5 demonstrated that even when the conditioned rewarding effects of nicotine were negated by pairing it with both sides of the CPP apparatus, nicotine still interacted with social reward resulting in more robust CPP relative to social reward controls receiving vehicle paired with both sides.
An important methodological concern to address is that nicotine was paired exclusively with the initially non-preferred compartment of the CPP apparatus (i.e., biased design). This design is thought to have interpretational problems given that increased time spent in the non-preferred compartment following conditioning could reflect the US’s aversion-reduction properties rather than its rewarding properties (Bardo & Bevins 2000). The issue is particularly pertinent when studying nicotine given that its anxiolytic effects could reduce the initial aversion to the CS context (Picciotto et al. 2002). Importantly, our use of an unbiased CPP apparatus (in which there is no strong initial aversion to a given side) helps to mitigate the reduction of aversion issue. Without a strong initial aversion to the CS compartment, it is unlikely that nicotine’s anxiolytic effects are contributing to preference shifts. Furthermore, if nicotine only reduced aversion, then preference shifts should result in approximately equal amounts of time spent in the nicotine- and the neutral saline-paired compartments. Nevertheless, it is not possible to fully determine how much of the observed preference shifts is due to reward versus anxiety reduction. However, the CPP produced in the present study, along with others (see Le Foll & Goldberg 2005; Thiel et al. 2008), was evidenced by a preference switch: rats reversed their preference and spent more than 50% of their time on test day in the nicotine-paired (i.e., previously non-preferred compartment), suggesting that the preference shift was in part a result of conditioned rewarding effects. Finally, Brielmaier et al. (2008) reported that nicotine paired with both compartments of a CPP apparatus failed to alter preferences while nicotine paired with only the non-preferred compartment produced a preference shift toward that side, suggesting that nicotine-CPP could not be explained solely as an unconditioned reduction in aversion to a non-preferred compartment (Brielmaier et al. 2008).
Another potential confound is that nicotine can produce conditioned activity (Bevins et al. 2001, 2005), which could compete with expression of the initial preference for the saline-paired, thereby increasing the amount of time spent in the initially non-preferred side on the test day. However, it is unlikely that conditioned activity influenced CPP measures as groups did not differ in crossovers between compartments on test days.
It is notable that the nicotine:social reward interaction was only detected when nicotine was administered SC. With IV nicotine, the 3-way interaction of Social × Nicotine Dose × Day was not significant, although there was a Social × Day interaction, suggesting greater preference shifts in the Social condition relative to the isolated condition regardless of Nicotine Dose. We intended to select sub-threshold parameters for producing CPP with nicotine alone in order to maintain sensitivity for detecting enhanced CPP by the combination of social and nicotine rewards. Although CPP was not observed with nicotine alone, the 2 nicotine doses produced a non-significant trend toward a preference shift, resulting in enough variability to obscure the expected 3-way interaction. Further parametric considerations are needed before drawing any firm conclusions as to whether IV nicotine and social rewards interact synergistically similar to that observed with SC nicotine. For instance, it is possible that the nicotine reward produced with IV administration in the present study was too short-lived to interact with social reward compared to that produced with SC nicotine, with the latter likely sustained across the entire conditioning session. Perhaps an interaction could be detected using shorter conditioning sessions or by giving multiple IV injections during the social conditioning sessions.
To our knowledge, this is the first study to demonstrate nicotine-CPP using the IV route of administration in adolescent rats. Although the IV route of administration has apparent disadvantages for investigating drug interaction with social reward, it is possible that IV administration may prove to be more reliable in supporting CPP than the SC route given that IV administration better approximates the absorption of smoked nicotine (Rose and Corrigall 1997). Indeed, with SC administration of nicotine, both dose-dependent CPP and conditioned place aversion (CPA) have been observed over a range of overlapping doses (Jorenby et al. 1990; Laviolette and Van der Kooy 2003; Fudala et al. 1985; Le Foll and Goldberg 2005). The range of doses selected for IV administration in the present study was similar to those traditionally used to examine nicotine self-administration in both adolescent and adult rats (Donny et al. 1998; Adriani et al. 2003; Chen et al. 2007; Shram et al. 2008). This dose range is also effective in the runway self-administration paradigm that combines instrumental and place preference learning (Cohen and Ettenberg 2007). Furthermore, Shoaib and Stolerman (1999) found that nicotine levels attained following IV administration in rats at doses ranging from 0.015 to 0.06 mg/kg/infusion is similar to levels following inhalation of a cigarette in humans (e.g., Benowitz et al. 1983). Thus, IV administration provides for a closer approximation to the pharmacokinetics of smoking in humans compared to the SC route, and allows for comparison of IV nicotine reward and reinforcement established with CPP and self-administration models, respectively.
SC nicotine produced a linear dose-response function such that nicotine-CPP increased as dose increased, whereas IV nicotine produced a stair-step dose-response function. We were surprised to observe CPP with the lowest dose of SC nicotine given that previous studies examining a similar dose range in adolescent rats failed to demonstrate CPP at doses below 0.5 mg/kg (see Belluzzi et al. 2004; Shram et al. 2006). There are several procedural differences across studies that might explain the discrepancy, most notable of which are differences in number of conditioning sessions (i.e., single trial conditioning in Belluzi et al. 2004) and CPP design (i.e., unbiased design in Belluzzi et al. 2004 and Shram et al. 2006 vs. the biased design in the present study). Consistent with our findings, Torres et al. (2008) recently reported CPP at 0.2 – 0.6 mg/kg, SC doses of nicotine utilizing the biased design. Based on a literature review, Le Foll and Goldberg (2005) suggests that nicotine-CPP is most reliably produced using the biased design, and therefore, the use of the biased design in the present study likely afforded the sensitivity needed to detect an effect at low doses.
Nicotine-CPP has recently been demonstrated in adult male rats using the IV route of administration (Wilkinson and Bevins 2008), although stronger conditioning parameters were needed to detect the effect than those used in the present study (i.e., 4 versus 8 CS-US pairings). This difference across studies may again be due to the use of a biased versus unbiased design, or may reflect developmental differences in sensitivity to IV nicotine as demonstrated using the nicotine self-administration model (Adriani et al. 2003). Future research is needed to directly assess developmental, as well as sex differences in nicotine-CPP using the IV route of administration.
The SC nicotine-induced reduction of play behavior in Experiment 4 is in line with previous reports (Irvine et al. 1999; Panksepp et al. 1984). Although IV nicotine did not significantly reduce play behaviors relative to vehicle, there was a trend towards a reduction. The lack of effect on play behavior with IV nicotine may be due to the relatively short duration of the drug effect with this route of administration. Although previous studies suggest that the opportunity to engage in social play is crucial for the rats to find a social context rewarding (e.g., Humphreys and Einon 1981; Calcagnetti and Schechter 1992; Pellis and McKenna 1995; Douglas et al. 2004), our own previous findings suggest that the amount of specific play behaviors (i.e., pins and nape attacks) during the social interactions is not related to the magnitude of social reward-CPP (Thiel at al. 2008). The present findings are consistent with the notion that pinning and nape attacks do not completely predict the degree to which social context is rewarding given that the 0.1 N/Soc group demonstrated a robust CPP relative to the Veh/Soc group, despite the 0.1 N/Soc group demonstrating significantly less play behavior than the Veh/Soc group. Clearly, further research will be necessary to examine what other aspects of social interaction can be used to explain the preference shift. For example, rat pairs in the present study were observed sniffing and maintaining contact with each other. Precise and explicit measurement of these types of non-playful, investigative behaviors is warranted in future studies. In addition, it would be interesting to examine the degree/quality of auditory communication (e.g., ultrasonic vocalization) among playmates as it relates to social reward-CPP. Such an approach may gauge deeper into the affective and motivational aspects of rat social play, especially in terms of how drugs modulate this experience (Knutson, Burgdorf, & Panksepp, 1998, 2002).
In conclusion, the present findings provide strong evidence that nicotine interacts synergistically with the rewarding effects of social interaction in adolescent rats. These findings underscore the significant influence of social context on the rewarding effects of nicotine in adolescents. Future studies are needed to examine the neural mechanisms involved in these social:drug interaction effects. This line of research may provide for new preventions or interventions for nicotine dependence.
Figure 3.
Dose-dependent nicotine-CPP using SC administration shown as time (mean s±SEM) spent in the nicotine-paired (i.e., initially non-preferred) side pre-conditioning (i.e., Baseline, white bars) vs. post-conditioning (i.e., Test, black bars). Asterisk (*) indicates an increase in amount of time spent in the nicotine-paired side on Test day relative to Baseline (p<.0125, Bonferroni correction). Cross (+) indicates a greater amount of time spent in nicotine-paired side relative to Vehicle group (p<.05, Tukey’s HSD). There was also a significant linear trend (p<.001) across groups on test day. The dotted line represents 50% of the total test period (i.e., 300 s).
Acknowledgements
The project described was supported by grants DA011064 and F31DA02746 from the National Institute on Drug Abuse. The authors wish to thank Jenny Browning, Valeria Routt, Sarah Thiel, Michael Painter, Suzanne Weber, and Erin Dickey for their contributions during data collection. The authors also wish to thank Glenn Guerin for advice with catheter construction.
Funding sources: The research presented here was supported by grants DA11064 and F31DA02746 from the National Institute on Drug Abuse.
Abbreviations
- CS
Conditioned Stimulus
- US
Unconditioned Stimulus
- IV
Intravenous
- SC
Subcutaneous
- IP
Intraperiotneal
- CPP
Conditioned place preference
- Veh
Vehicle
- N
Nicotine
- Soc
Social Playmate
- Iso
Isolated
References
- Adriani W, Deroche-Gamonet V, Le Moal M, Laviola G, Piazza PV. Preexposure during or following adolescence differently affects nicotine-rewarding properties in adult rats. Psychopharmacology (Berl) 2006;184:382–90. doi: 10.1007/s00213-005-0125-1. [DOI] [PubMed] [Google Scholar]
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–6. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angermeier WF, Schaul LT, James WT. Social conditioning in rats. J Comp Physiol Psychol. 1959;52:370–2. doi: 10.1037/h0042566. [DOI] [PubMed] [Google Scholar]
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annu Rev Psychol. 2004;55:463–91. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 2004;174:389–9. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–12. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Kuyt F, Jacob P, 3rd, Jones RT, Osman AL. Cotinine disposition and effects. Clin Pharmacol Ther. 1983;34:604–11. doi: 10.1038/clpt.1983.222. [DOI] [PubMed] [Google Scholar]
- Bevins RA. Novelty seeking and reward: Implications for the study of high-risk behaviors. Curr Dir Psychol Sci. 2002;10:189–193. [Google Scholar]
- Bevins RA, Besheer J, Pickett KS. Nicotine-conditioned locomotor activity in rats: dopaminergic and GABAergic influences on conditioned expression. Pharmacol Biochem Behav. 2001;68:135–45. doi: 10.1016/s0091-3057(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Eurek S, Besheer J. Timing of conditioned responding in a nicotine locomotor conditioning preparation: manipulations of the temporal arrangement between context cues and drug administration. Behav Brain Res. 2005;159:135–43. doi: 10.1016/j.bbr.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Breslau N, Fenn N, Peterson EL. Early smoking initiation and nicotine dependence in a cohort of young adults. Drug Alcohol Depend. 1993;33:129–37. doi: 10.1016/0376-8716(93)90054-t. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL. Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am J Public Health. 1996;86:214–20. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG, Smith RF. Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicol Teratol. 2007;29:74–80. doi: 10.1016/j.ntt.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG, Smith RF. Nicotine place preference in a biased conditioned place preference design. Pharmacol Biochem Behav. 2008;89:94–100. doi: 10.1016/j.pbb.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav. 1992;51:667–72. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- Carr GD, Fibiger HC, Phillips AG. Conditioned place preference as a measure of drug reward. In: Liebman JM, Cooper SJ, editors. The neuropharmacological basis of reward. Oxford University Press; New York: 1989. pp. 264–319. [Google Scholar]
- Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32:700–9. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- Chen J, Millar WJ. Age of smoking initiation: implications for quitting. Health Rep. 1998;9:39–46. 39–48. (Eng) (Fre) [PubMed] [Google Scholar]
- Cohen A, Ettenberg A. Motivational effects of nicotine as measured in a runway model of drug self-administration. Behav Pharmacol. 2007;18:265–71. doi: 10.1097/FBP.0b013e3281f19b3c. [DOI] [PubMed] [Google Scholar]
- Colby SM, Tiffany ST, Shiffman S, Niaura RS. Are adolescent smokers dependent on nicotine? A review of the evidence. Drug Alcohol Depend. 2000;59(Suppl 1):S83–95. doi: 10.1016/s0376-8716(99)00166-0. [DOI] [PubMed] [Google Scholar]
- Crowder WF, Hutto CW., Jr. Operant place conditioning measures examined using two nondrug reinforcers. Pharmacol Biochem Behav. 1992;41:817–24. doi: 10.1016/0091-3057(92)90233-6. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl) 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45:153–62. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Einon DF, Morgan MJ, Kibbler CC. Brief periods of socialization and later behavior in the rat. Dev Psychobiol. 1978;11:213–25. doi: 10.1002/dev.420110305. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Duvel A, Funk ML, Lehman B, Sparrow J, Watson NT, Neuringer A. Social reinforcement of operant behavior in rats: a methodological note. J Exp Anal Behav. 1994;62:149–56. doi: 10.1901/jeab.1994.62-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudala PJ, Teoh KW, Iwamoto ET. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav. 1985;22:237–41. doi: 10.1016/0091-3057(85)90384-3. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Briscoe RJ, Goulden KL, Holloway FA. Aversive attributes of ethanol can be attenuated by dyadic social interaction in the rat. Alcohol. 1994;11:247–51. doi: 10.1016/0741-8329(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Geckova AM, Stewart R, van Dijk JP, Orosova O, Groothoff JW, Post D. Influence of socio-economic status, parents and peers on smoking behaviour of adolescents. Eur Addict Res. 2005;11:204–9. doi: 10.1159/000086403. [DOI] [PubMed] [Google Scholar]
- Glynn TJ. Essential elements of school-based smoking prevention programs. J Sch Health. 1989;59:181–8. doi: 10.1111/j.1746-1561.1989.tb04698.x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:163–73. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- Hanna EZ, Grant BF. Parallels to early onset alcohol use in the relationship of early onset smoking with drug use and DSM-IV drug and depressive disorders: findings from the National Longitudinal Epidemiologic Survey. Alcohol Clin Exp Res. 1999;23:513–22. [PubMed] [Google Scholar]
- Humphreys AP, Einon DF. Play as a reinforcer for maze learning in juvenile rats. Anim Behav. 1981;29:259–70. [Google Scholar]
- Irvine EE, Cheeta S, File SE. Time-course of changes in the social interaction test of anxiety following acute and chronic administration of nicotine. Behav Pharmacol. 1999;10:691–7. doi: 10.1097/00008877-199911000-00016. [DOI] [PubMed] [Google Scholar]
- Jackson C. Initial and experimental stages of tobacco and alcohol use during late childhood: relation to peer, parent, and personal risk factors. Addict Behav. 1997;22:685–98. doi: 10.1016/s0306-4603(97)00005-1. [DOI] [PubMed] [Google Scholar]
- Jefferis B, Graham H, Manor O, Power C. Cigarette consumption and socio-economic circumstances in adolescence as predictors of adult smoking. Addiction. 2003;98:1765–72. doi: 10.1111/j.1360-0443.2003.00552.x. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Steinpreis RE, Sherman JE, Baker TB. Aversion instead of preference learning indicated by nicotine place conditioning in rats. Psychopharmacology (Berl) 1990;101:533–8. doi: 10.1007/BF02244233. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Chen K. Extent of smoking and nicotine dependence in the United States: 1991-1993. Nicotine Tob Res. 2000;2:263–74. doi: 10.1080/14622200050147538. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol Psychiatry. 2003;8:50–9. 9. doi: 10.1038/sj.mp.4001197. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl) 2005;178:481–92. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- Leatherdale ST, McDonald PW, Cameron R, Brown KS. A multilevel analysis examining the relationship between social influences for smoking and smoking onset. Am J Health Behav. 2005;29:520–30. doi: 10.5555/ajhb.2005.29.6.520. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J. Environmental factors influencing the affiliative behavior of male and female rats (Rattus norvegicus) Anim Learn Behav. 1979;7:397–405. [Google Scholar]
- Nelson DE, Giovino GA, Shopland DR, Mowery PD, Mills SL, Eriksen MP. Trends in cigarette smoking among US adolescents, 1974 through 1991. Am J Public Health. 1995;85:34–40. doi: 10.2105/ajph.85.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normansell L, Panksepp J. Effects of morphine and naloxone on play-rewarded spatial discrimination in juvenile rats. Dev Psychobiol. 1990;23:75–83. doi: 10.1002/dev.420230108. [DOI] [PubMed] [Google Scholar]
- Olds RS, Thombs DL. The relationship of adolescent perceptions of peer norms and parent involvement to cigarette and alcohol use. J Sch Health. 2001;71:223–8. doi: 10.1111/j.1746-1561.2001.tb01322.x. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy S, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8:465–92. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Pellis SM, McKenna M. What do rats find rewarding in play fighting?--an analysis using drug-induced non-playful partners. Behav Brain Res. 1995;68:65–73. doi: 10.1016/0166-4328(94)00161-8. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus norvegicus. Agress Behav. 1987;13:227–42. [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Choi WS, Gilpin EA, Farkas AJ, Merritt RK. Validation of susceptibility as a predictor of which adolescents take up smoking in the United States. Health Psychol. 1996;15:355–61. doi: 10.1037//0278-6133.15.5.355. [DOI] [PubMed] [Google Scholar]
- Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology (Berl) 1997;130:28–40. doi: 10.1007/s002130050209. [DOI] [PubMed] [Google Scholar]
- Schnierla T. An evolutionary and developmental theory of biphasic processes underlying approach and withdrawal. In: Jones M, editor. Nebraska Symposium on Motivation. University of Nebraska Press; Nebraska: 1959. pp. 27–58. [Google Scholar]
- Shoaib M, Stolerman IP. Plasma nicotine and cotinine levels following intravenous nicotine self-administration in rats. Psychopharmacology (Berl) 1999;143:318–21. doi: 10.1007/s002130050954. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006;186:201–8. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Li Z, Le AD. Age differences in the spontaneous acquisition of nicotine self-administration in male Wistar and Long-Evans rats. Psychopharmacology (Berl) 2008;197:45–58. doi: 10.1007/s00213-007-1003-9. [DOI] [PubMed] [Google Scholar]
- Skara S, Sussman S. A review of 25 long-term adolescent tobacco and other drug use prevention program evaluations. Prev Med. 2003;37:451–74. doi: 10.1016/s0091-7435(03)00166-x. [DOI] [PubMed] [Google Scholar]
- Smith PK. Does play matter? Functional and evolutionary aspects of animal and human play. Behav Brain Sci. 1982;5:139–84. [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Sussman S. Risk factors for and prevention of tobacco use. Pediatr Blood Cancer. 2005;44:614–9. doi: 10.1002/pbc.20350. [DOI] [PubMed] [Google Scholar]
- Taioli E, Wynder EL. Effect of the age at which smoking begins on frequency of smoking in adulthood. N Engl J Med. 1991;325:968–9. doi: 10.1056/NEJM199109263251318. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–12. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Uveges JM, Burger KM, Patterson-Buckendahl P, Pohorecky LA. Effects of ethanol sipper and social opportunity on ethanol drinking in rats. Alcohol Alcohol. 2004;39:197–202. doi: 10.1093/alcalc/agh055. [DOI] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O’Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev Psychobiol. 1999a;34:129–38. [PubMed] [Google Scholar]
- Van den Berg CL, Pijlman FT, Koning HA, Diergaarde L, Van Ree JM, Spruijt BM. Isolation changes the incentive value of sucrose and social behaviour in juvenile and adult rats. Behav Brain Res. 1999b;106:133–42. doi: 10.1016/s0166-4328(99)00099-6. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–26. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–11. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48:146–61. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Acute effects of ethanol on behavior of adolescent rats: role of social context. Alcohol Clin Exp Res. 2001;25:377–85. [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–14. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Werner CM, Anderson DF. Opportunity for interaction as reinforcement in a T-maze. Pers Soc Psychol Bull. 1976;2:166–69. [Google Scholar]
- West P, Sweeting H, Ecob R. Family and friends’ influences on the uptake of regular smoking from mid-adolescence to early adulthood. Addiction. 1999;94:1397–411. doi: 10.1046/j.1360-0443.1999.949139711.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Bevins RA. Intravenous nicotine conditions a place preference in rats using an unbiased design. Pharmacol Biochem Behav. 2008;88:256–64. doi: 10.1016/j.pbb.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]