Abstract
Epithelial polarization modulates gene expression. The transcription factor zonula occludens 1 (ZO-1)–associated nucleic acid binding protein (ZONAB) can shuttle between tight junctions and nuclei, promoting cell proliferation and expression of cyclin D1 and proliferating cell nuclear antigen (PCNA), but whether it also represses epithelial differentiation is unknown. Here, during mouse kidney ontogeny and polarization of proximal tubular cells (OK cells), ZONAB and PCNA levels decreased in parallel and inversely correlated with increasing apical differentiation, reflected by expression of megalin/cubilin, maturation of the brush border, and extension of the primary cilium. Conversely, ZONAB reexpression and loss of apical differentiation markers provided a signature for renal clear cell carcinoma. In confluent OK cells, ZONAB overexpression increased proliferation and PCNA while repressing megalin/cubilin expression and impairing differentiation of the brush border and primary cilium. Reporter and chromatin immunoprecipitation assays demonstrated that megalin and cubilin are ZONAB target genes. Sparsely plated OK cells formed small islands composed of distinct populations: Cells on the periphery, which lacked external tight junctions, strongly expressed nuclear ZONAB, proliferated, and failed to differentiate; central cells, surrounded by continuous junctions, lost nuclear ZONAB, stopped proliferating, and engaged in apical differentiation. Taken together, these data suggest that ZONAB is an important component of the mechanisms that sense epithelial density and participates in the complex transcriptional networks that regulate the switch between proliferation and differentiation.
During ontogeny, epithelial cells undergo a regulated transition from proliferation to differentiation. This switch is recapitulated during polarization of epithelial monolayers in vitro and tissue repair and is reversed during carcinogenesis. Kidney proximal tubular cells (PTCs) are an exemplary model to study this switch and its perturbations. Apical PTC differentiation features include the primary cilium, the brush border, and the tandem endocytic receptors megalin/cubilin.1 Genetic defects of the primary cilium lead to various familial cystic kidney lesions, including polycystic disease, the most prevalent autosomal dominant disease in human.2 Impaired apical endocytic trafficking is associated with X-linked nephrolithiasis.3,4 Malignant transformation of PTCs leads to renal clear cell carcinomas, one of the 10 most frequent cancers and its most aggressive form in kidneys.
During cortical expansion, proliferation is synchronous among individual neph-rons but asynchronous between adjacent nephrons, arguing against paracrine control and pointing to communication within epithelial monolayers, likely via junctional complexes.5 Epithelial polarization involves three steps: Primordial homotypic E-cadherin interactions generate adherens junctions, which induce formation of tight junctions, a prerequisite to differentiation of the apical domain.6,7 Both junctions and the apical domain affect gene expression. First, β-catenin can be recruited on adherens junctions, degraded by the proteasome, or triggered by Wnt signaling to shuttle into nuclei and promote gene expression and proliferation via the T cell-specific transcription factor/lymphoid enhancer binding factor.8 Deregulated Wnt/β-catenin signaling leads to carcinogenesis.8 Second, zonula occludens 1 (ZO-1)–associated nucleic acid binding protein (ZONAB) can be sequestered at tight junctions upon binding to the SH3 domain of ZO-1 or shuttle into nuclei to promote cell proliferation genes directly.9,10 Third, at the apical pole, the primary cilium sequesters the mother centriole as basal body, thereby preventing mitotic spindle formation, and acts as a sensory organelle repressing proliferation. It thus provides a negative feedback whereby apical differentiation inhibits proliferation.11 Conversely, defective extension or signaling in ciliopathies is associated with unchecked epithelial expansion.2,12,13 Fourth, megalin was recently reported to undergo intramembrane proteolysis, releasing a transcriptionnally active C-terminal domain.14
We speculated that transcription factors expressed by growing epithelia could simultaneously promote proliferation and repress polarization/differentiation programs, then become silenced upon maturation of junctional complexes as part of the machinery sensing epithelial density. Accordingly, these transcription factors would be turned on during early embryogenesis, tissue repair, and cancer and turned off for terminal differentiation. We focused on ZONAB, a transcription factor also known as YB-3, MSY4 (in mice), and DNA binding protein A or Cold shock domain protein A in human, with two isoforms of undistinguishable functional properties generated by alternative splicing.10,15–20 ZONAB is directly controlled by Myc21 and E2F, two key gatekeepers of the cell division cycle,22 and, in turn, promotes expression of cyclin D1 and proliferating cell nuclear antigen (PCNA) and, thus, cell proliferation.18 ZONAB is overexpressed in hepatocarcinomas and favors their progression.22–24 No relation between ZONAB and kidney cancer has been reported so far. Whether ZONAB can regulate the expression of essential apical differentiation genes, such as those controlling the primary cilium, is also unknown.
Here we investigated the expression and effects of ZONAB in three complementary PTC systems: (1) Mouse kidney ontogeny; (2) human renal clear cell carcinomas; and (3) opossum kidney (OK) cells, a highly differentiating PTC line. We found that ZONAB was abundantly expressed in proliferative states and inversely correlated with apical differentiation. ZONAB mRNA was downregulated during OK cell polarization. Its opposite causal role on proliferation and apical differentiation markers was confirmed by overexpression and reporter assays. Multiplex immunolabeling of small colonies revealed predictable opposite choices at the level of single adjacent cells. Taken together, our data suggest that ZONAB is an important component of the sensory mechanisms of epithelial density and complex transcriptional networks regulating the switch between proliferation and differentiation.
Results
Inverse Relation between ZONAB Expression and Epithelial Differentiation during Kidney Ontogeny
The relationship between ZONAB and epithelial proliferation versus differentiation was first analyzed during mice kidney ontogeny. In situ hybridization at embryonic day 14.5 disclosed strong ZONAB expression in the kidney cortex (comma- and S-shape bodies), surpassed only by the liver, another major epithelial organ (Figure 1, A through C). ZONAB-expressing kidney structures were highly proliferative (PCNA labeling) and poorly differentiated (megalin absence; Supplemental Figure S1, a and d). Thereafter, ZONAB expression declined as the nonproliferating, megalin-expressing population steadily increased (Supplemental Figure S1, b, c, e, and f). The parallel decline of ZONAB and PCNA mRNAs was inversely mirrored by increasing expression of megalin/cubilin (Figure 1D). Western blotting confirmed the inverse relation between decreasing abundance of the two ZONAB isoforms and PCNA versus increasing abundance of E-cadherin and β-catenin (epithelial markers), as well as megalin, cubilin, and villin (apical differentiation markers; Figure 1E). Altogether, the study of kidney ontogeny evidenced an inverse relation between declining ZONAB level and increasing apical epithelial differentiation.
Figure 1.
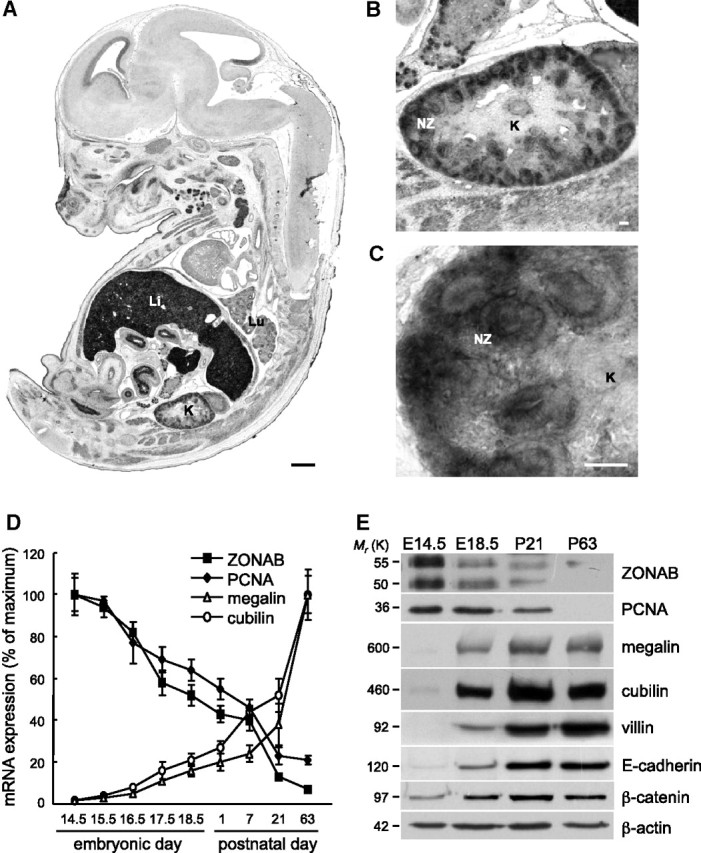
ZONAB/MSY4 expression declines during mouse kidney ontogeny. (A through C) In situ hybridization of ZONAB in an embryo at day 14.5 (antisense probe, bright field). (A) A sagittal section shows highest expression in epithelial tissues such as liver (Li), kidney (K), and respiratory tree (Lu). Bar = 500 μm. The kidney is enlarged at B and its cortex further enlarged at C (bars = 50 μm) to show high expression in the induced cortical primary epithelia (nephrogenic zone [NZ]), contrasting with no detectable expression in medulla. For hybridization between embryonic day 17.5 (E17.5) and postnatal day 63 (P63), see Supplemental Figure S1, e and f. (D) Relative mRNA levels were measured by quantitative RT-PCR (qRT-PCR) in four pools of 14 kidney embryos (E14.5 to E18.5) or four pairs of kidneys (P1 to P63) and expressed as percentage of maximal expression (P21, full differentiation; P63, adult size). (E) Representative Western blots of total lysates from three different pools of kidney embryos collected at E14.5 and E18.5 or pairs at P21 and P23. Note that the kinetics of ZONAB disappearance during kidney ontogeny correlates with the loss of the late proliferation marker, PCNA, and the appearance of epithelial (E-cadherin) and specific apical differentiation markers (megalin, cubilin, and villin).
Reversed Relation between ZONAB Expression and Epithelial Differentiation in Kidney Carcinomas
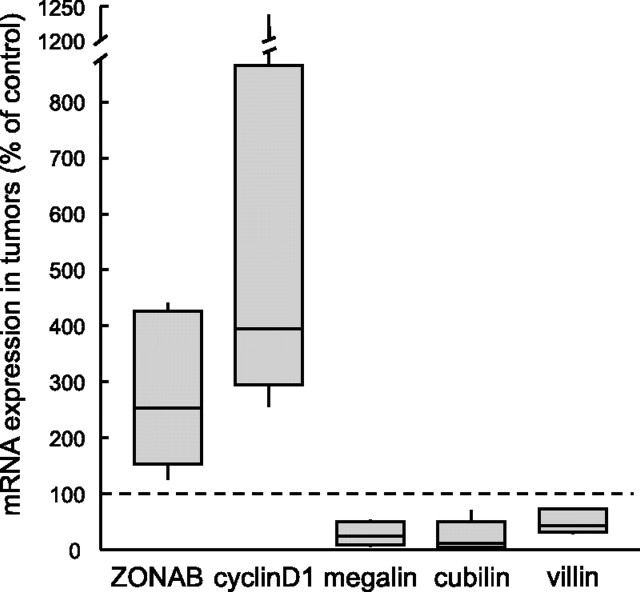
We next analyzed ZONAB expression in human renal clear cell carcinomas (RCCs), in which differentiated PTCs resume proliferation and lose several normal characteristics, such as single-layered tubule and lumen formation. In silico meta-analysis of available expression profiles indicated (1) systematic correlation between ZONAB and proliferation markers (cyclin D1, Ki-67) and (2) an inverse relationship with differentiation markers (megalin, cubilin, and villin; Supplemental Figure S2).25 We fully confirmed these results by quantitative reverse transcriptase–PCR (RT-PCR) comparison of six new cases of RCC and control samples (Figure 2). Because it is not found in other kidney cancer types, this pattern could serve as RCC signature (data not shown). These investigations thus indicated that ZONAB reexpression is part of the genetic program associated with a reverse switch of epithelial cells from differentiation to proliferation.
Figure 2.
ZONAB/Cold shock domain protein A is reexpressed in human kidney cancer. Relative mRNA levels were measured in six pairs of RCCs and corresponding normal tissue from the same kidney (n = 3) or matched normal kidney (n = 3) using qRT-PCR by reference to β-actin, then normalized to the corresponding ratio in nontumoral kidney, set as 100% (dotted line). Results are presented as plots showing 25th and 75th percentiles, medians (horizontal lines), and minimal and maximal values (vertical bars). All values in cancer samples are statistically different from controls by the nonpaired Wilcoxon ranking test, except for cubilin. Note that Cold shock domain protein A overexpression in carcinomas is associated with increased expression of the early proliferation regulator cyclin D1 and decreased expression of the apical differentiation markers megalin, villin, and presumably cubilin. For a meta-analysis of published microarray data, see Supplemental Figure S2.
ZONAB Is Downregulated during PTC Polarization/Differentiation In Vitro
To test a causal relationship between ZONAB disappearance and epithelial polarization, we first characterized apical differentiation in the paradigmatic OK cell line. When plated at high density to reach immediate confluence, OK cells rapidly established monolayers with irregular nuclei positioning, immature cell junctions (Supplemental Figure S3b, day 1), and poorly structured cytoskeleton (Figure 3A, top). When monolayers reached maximal transepithelial resistance (day 4; Supplemental Figure S3a), nuclei were aligned; tight junctions formed a polygonal pattern (Supplemental Figure S3b); actin cytoskeleton was reorganized into lateral arrays, bottom stress fibers, and apical microvilli clusters; and a primary cilium was developing (Figure 3A, bottom; Supplemental Figure S4). By Western blotting, polarization of confluent OK cells led to an approximately two-fold decreased abundance of the two ZONAB isoforms from day 1 to day 4, contrasting with a strong increase of megalin, cubilin, and villin (Figure 3B). Undistinguishable acetylated tubulin combined with decreased α-tubulin levels suggested a relative increase in tubulin acetylation. This was supported by elongation of the primary cilium (Figure 3A, x-z optical sections). Increasing abundance of megalin/cubilin suggested that receptor-mediated endocytosis could serve as a quantitative functional assay for apical differ-entiation. Increasing efficiency of apical receptor–mediated endocytosis, suggested by confocal microscopy (Figure 3C), was confirmed by accelerated clearance of radioiodinated ligands, in particular cathepsin B (Figure 3D).26 Altogether, the study of polarization in confluent OK cells revealed a strong decrease in ZONAB expression, concomitant with upregulation of apical endocytic receptors together with brush border and primary cilium maturation, reflecting global apical differentiation.
Figure 3.
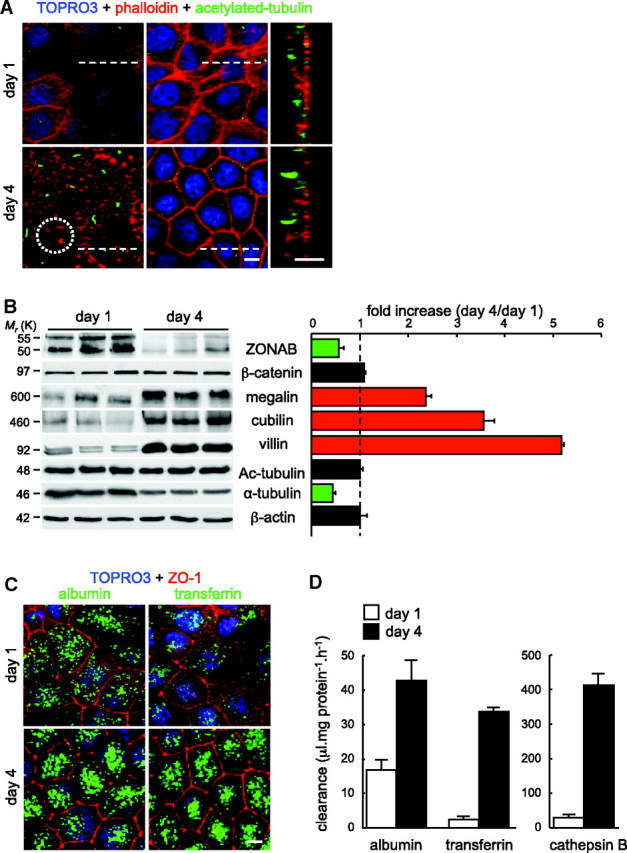
Polarization of OK PTC monolayers inversely affects expression of ZONAB and differentiation markers. OK cells were plated at high density to reach immediate confluence, then compared after 1 or 4 days (maximal transepithelial resistance). (A) Effect of time in culture on polarity and apical membrane differentiation by confocal microscopy. Coverslips collected after 1 or 4 days were labeled for nuclei (TOPRO-3; blue), actin microfilaments (Alexa 568 phalloidin; red) and primary cilia (acetylated tubulin; green); left, horizontal (x-y) apical sections; middle, horizontal nuclear sections; right, vertical sections (x-z at the position of dotted lines). At day 4, besides epithelial cell thickening and regular positioning of nuclei, note at the apical membrane patchy labeling of microvilli (dotted circle at bottom left) and increased extension of sensory cilia (vertical sections). (B) Western blotting. ZONAB was compared with markers of apical differentiation in three pools of Petri dishes collected after 1 or 4 days; Ac-tubulin, acetylated tubulin. For quantification shown at right, densitometry values were normalized to β-actin and expressed as fold increase at day 4 by reference to day 1 (dotted vertical line). (C and D) Effect of polarity on the efficiency of apical receptor–mediated endocytosis. (C) Confocal microscopy. Coverslips collected after 1 or 4 days were incubated with Alexa 488 (green) albumin (a megalin ligand) or transferrin (a cubilin ligand) for 10 minutes, then fixed and immunolabeled for ZO-1 (red) with TOPRO-3 blue staining. Full projections are shown. (D) Biochemical assay. Petri dishes collected after 1 or 4 days were incubated with 150 nM 125I-albumin, 150 nM 125I-transferrin, or 1.8 nM 125I-cathepsin B for 1 hour (n = 3). Endocytosis is expressed in clearance units. Bars = 10 μm.
Assay of Proliferation/Differentiation at the Single-Cell Level
To analyze further on a cell basis the inverse relationship between ZONAB and apical differentiation markers disclosed by population-based assays, we plated OK cells at very low density. We had noticed that sparsely plated OK cells migrate until they aggregate to form small, independent colonies. These colonies, referred to hereafter as “islands,” further differentiate in their center and expand by peripheral cell division. After 4 days, ZO-1 immunolabeling revealed polygonal junctional belts around all central cells; in the peripheral cell rim, junctions were interrupted by necessity at their outer margin and ZO-1 partially remained in the perinuclear cytoplasm. Apical megalin expression was strong in central cells but poor in peripheral cells. Conversely, ZONAB showed strong nuclear labeling specifically in peripheral cells (Figure 4, A and B). By scanning electron microscopy, peripheral cells showed flat ruffles at their outer margin and random individual microvilli everywhere else (Figure 4, C and D). In contrast, adjacent central cells extensively clustered microvilli tips to form asters (resembling edelweiss), presumably a first step of brush border maturation (see next section). To test whether ZONAB correlated with cell proliferation in islands, we visualized DNA synthesis by 5-ethynyl-2′-deoxyuridine (EdU) incorporation and compared it with ZO-1 and ZONAB. As shown by Figure 4F, only peripheral cells with strong nuclear ZONAB labeling detectably incorporated EdU. A similar pattern was observed when OK cell islands were studied on a porous membrane, indicating that the opposite behavior of proliferating peripheral cells and differentiating central cells could not be trivially explained by impaired access of growth factors to basolateral domains, as a result of impermeable junctions (data not shown). Thus, morphologic analysis of individual adjacent cells in islands at day 4 nicely complemented the population-based comparison between confluent monolayers at day 1 and day 4 by providing a cell-specific assay confirming the tight correlation between ZONAB and proliferation (parallel) or apical differentiation (opposite).
Figure 4.

ZONAB is expressed in peripheral proliferating cells but not in differentiated central cells. Sparsely plated OK cells were cultured for 4 days, allowing individual cell clustering and reorganization into small colonies, with opposite status between peripheral and central cells. Islands were examined by confocal microscopy (A, B, and F; bars = 10 μm) and scanning electron microscopy (C through E; bars = 2 μm). (A) Double immunolabeling for ZO-1 (red) and megalin (green); boxed area at top left is enlarged at bottom. Whereas central cells are equipped by continuous polygonal junctional belts (ZO-1), with little or no detectable ZO-1 in the cytoplasm, junctions of peripheral cells are by necessity interrupted at their outer margin: Their ZO-1 belt seems to be reinforced at the border of the island (arrowheads), then abruptly disappears. Peripheral cells show an additional diffuse cytoplasmic and perinuclear ZO-1 labeling. Note the strong expression of megalin in all central cells, contrasting with its poor labeling in the peripheral cell rim (arrows). (B) Double immunolabeling for megalin and either β-catenin (top) or ZONAB (bottom). Note the strong nuclear labeling in peripheral cells by ZONAB but not by β-catenin (similar pattern to that of ZO-1). The opposite immunolabeling pattern for ZONAB and megalin between adjacent cells is obvious at the merge shown at bottom right. (C through E) Scanning electron microscopy. The low-power image in C shows a peripheral cell with a ruffled membrane at its outer margin (arrowhead) and scattered individual microvilli elsewhere (better seen in D), contrasting with the adjacent polarized cell shown below, where tips of microvilli cluster as asters (better seen in E); emerging primary cilia were not detected in islands after 4 d. (F) Relation between ZONAB and proliferation. OK cells incorporated EdU for 1 hour for monitoring of entry into the S phase, then were immunolabeled for ZONAB. Selective peripheral nuclear ZONAB labeling coincides with EdU incorporation (arrowheads).
ZONAB Overexpression Promotes Proliferation and Represses Differentiation
To test for a causal role of ZONAB in the repression of differentiation, we stably transfected OK cells with an expression vector for canine ZONAB-B (cZONAB). Three clones (cZ1, cZ2, and cZ3) were compared with wild-type OK cells and a mock-transfected clone. In agreement with a previous report on MCF10A cells,18 stable cZONAB transfection of OK cells promoted proliferation in confluent monolayers at day 4, as evidenced in flow cytometry (Figure 5A), consistent with increased PCNA abundance (Figure 5B). In contrast, stable cZONAB transfection strongly decreased megalin/cubilin expression at mRNA and protein levels (Figure 5, B and C), as reflected by impaired endocytic uptake of cognate ligands (Figure 5D). In addition, ZONAB overexpression led to decreased villin content and α-tubulin acetylation (Figure 5B), reflected by impaired clustering of microvilli and shorter primary cilia (Figure 5, E and F). By scanning electron microscopy after 8 days, wild-type and mock-transfected cells had further tilted clustered microvilli more perpendicular to the apical membrane, reminiscent of the maturation of brush border foci in vivo, and generally extended full-length sensory cilia with typical terminal bulbs. In contrast, fewer and looser aggregates of microvilli, as well as rarer, shorter, and sometimes twin sensory cilia, were found in cZONAB transfectants (data not shown). Thus, ZONAB overexpression prevented the natural progression of epithelial cells toward a polarized and differentiated phenotype (compare Figures 3 and 5).
Figure 5.

Stable ZONAB transfection promotes proliferation and represses differentiation in OK cells monolayers. (A) Flow cytometry. Representative histograms of DNA profiles for confluent monolayers of mock and cZ1 clone after 4 days of culture, including synchronization during the last 2 days. Combined S and G2/M phases represented 37.8 ± 0.6% in cZ1 versus 20.8 ± 3.5% in mock clones (P = 0.005 by t test). A qualitatively comparable difference was observed with clone cZ2 (Supplemental Figure 5). (B) Western blotting. cZONAB overexpression increases abundance of PCNA and decreases abundance of megalin, cubilin, and villin, as well as tubulin acetylation level. (c) qRT-PCR. cZONAB represses megalin and cubilin by approximately five-fold. (d) Endocytosis. The indicated clones endocytosed Alexa 488 (green) albumin or transferrin for 10 minutes, then were immunolabeled for megalin or β-catenin, as indicated. Apical confocal sections are shown. ZONAB overexpression decreases megalin expression and albumin or transferrin uptake, without detectable effect on junction organization. Bars = 10 μm. (E) Actin cytoskeleton and extension of the sensory cilium. (Top) Apical confocal microscopy sections at day 4 stained with Alexa 568 phalloidin (red) and immunolabeled for acetylated tubulin (green). ZONAB overexpression prevents the formation of apical clusters of microvilli and impairs the development of the sensory cilium. For a comprehensive analysis of full cell thickness, see Supplemental Figure 4. (Bottom) Scanning electron microscopy at day 8. In the mock clone, prolonged culture favors regular clustering of tightly packed microvilli and their tilting perpendicular to the cell surface; the white arrow indicates a fully extended sensory cilium with dilated terminal bulb. Both processes are delayed by ZONAB overexpression, with a red arrowhead pointing to the normal terminal bulb of a typically shorter cilium. Bar = 1 μm. (F) Quantification of sensory cilium extension. The length of individual sensory cilia was measured in 150 mock and ZONAB-overexpressing cells (pooled from three independent assays; P < 0.00001 by t test).
ZONAB Is a Direct Repressor of Megalin and Cubilin Genes
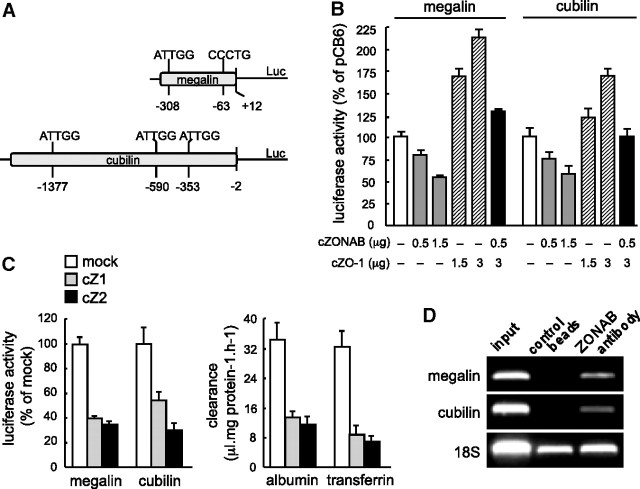
To address whether ZONAB could directly repress genes required for apical differentiation, we investigated megalin and cubilin expression. In silico, the sequence of their promoters in the opossum genome indicated the presence of respectively two and three putative ZONAB binding sites (Figure 6A). The promoter regions encompassing these sites were cloned upstream of Firefly luciferase, and resulting plasmids were transiently transfected into wild-type OK cells together with a cZONAB expression plasmid to test for repressor function, and/or cZO-1 plasmid as cZONAB decoy. Co-transfection with cZONAB alone induced a dosage-dependent repression of megalin and cubilin promoters (Figure 6B). An opposite effect was observed with cZO-1 plasmid alone, and simultaneous co-transfection with ZO-1 apparently neutralized the effect of cZONAB, indicating titration of ZONAB protein (Figure 6B). In two stable cZONAB transfectants, luciferase activities driven by megalin or cubilin promoters were lower as compared with a mock clone (Figure 6C) and were perfectly mirrored by decreased clearance of cognate ligands. Finally, chromatin immunoprecipitation performed at day 1 confirmed in vivo the association of endogenous ZONAB with megalin and cubilin promoter regions (Figure 6D). These data demonstrated that ZONAB could repress two important epithelial differentiation genes.
Figure 6.
ZONAB is a direct repressor of megalin and cubilin genes. (A) Promoter regions in OK cells for megalin (372 bp) and cubilin (1593 bp) genes encompassing the potential cZONAB binding sites (sequences above, central position below) were cloned in front of the luciferase reporter (Luc) into PGL3 expression vector. (B) Luciferase reporter assay in transient transfectants. Wild-type OK cells were co-transfected with megalin (left) or cubilin (right) Firefly luciferase reporter, Renilla luciferase vector, and the indicated amounts of cZONAB (▩) or cZO-1 plasmid (a decoy for ZONAB; ▨) either alone or combined (■) or an empty pCB6 vector (□). Note the dosage-dependent repression of the two promoters by cZONAB and their apparent induction by ZO-1, with mutual neutralization. (C) Luciferase reporter assays in stable cZONAB clones. Mock and the two indicated clones stably overexpressing cZONAB were transiently transfected with the luciferase reporter under the megalin or cubilin promoters (as indicated), then assayed for luciferase activities (left). In parallel, the same clones were analyzed for radioligand receptor–mediated endocytosis as a functional assay (right). Values in B and C are means ± SEM of six, pooled from three separate experiments. (D) Chromatin immunoprecipitation assay on OK cells at day 1 using immobilized ZONAB antibodies or control beads. Physical association with megalin and cubilin genes were tested by PCR, and amplification was normalized by reference to that of the 18S rRNA gene.
Downregulation of ZONAB during Epithelial Polarization
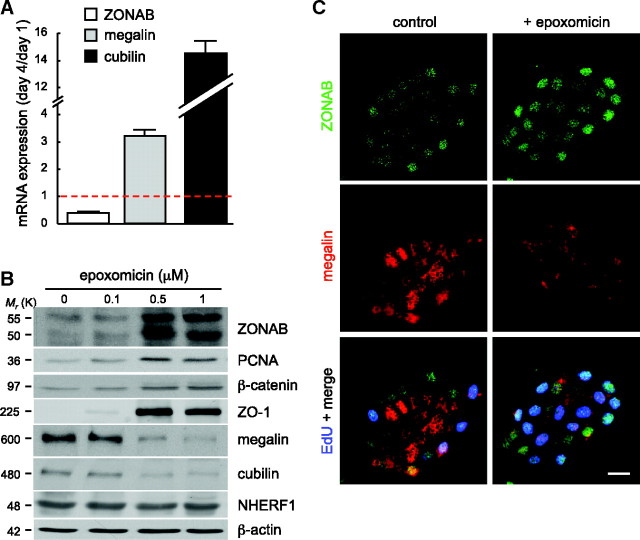
We finally addressed the regulation of ZONAB itself, because it was clearly downregulated during polarization of confluent OK cells (Figure 3B) and barely detected in the differentiated central islands cells (Figure 4, B and F). To understand better how polarization caused ZONAB disappearance, we first looked for transcriptional downregulation. Comparison of confluent wild-type OK cells between day 1 and day 4 showed an approximately three-fold decrease of endogenous ZONAB mRNA, whereas megalin and cubilin mRNA levels increased two- and 14-fold, respectively (Figure 7A). Surprisingly, despite control by the strong constitutive cytomegalovirus (CMV) promoter, cZONAB mRNA and protein were also downregulated by polarization (Supplemental Figure 6a). The dominance of polarity over the CMV promoter unraveled the power of (unknown) polarity signals on transcriptional regulation.
Figure 7.
ZONAB stabilization in differentiated cells reverses their fate to proliferation. (a) qRT-PCR analysis of confluent wild-type OK cells at days 1 and 4 after plating. Expression level at day 4 was analyzed by reference to day 1 (dotted line). (B) Effect of proteasome inhibition. Confluent monolayers of wild-type OK cells were polarized for 4 days, then treated for 24 hours with the indicated concentrations of epoxomicin. Proteasome inhibition causes a concentration-dependent increase in the abundance of both ZONAB isoforms ZO-1 and PCNA, together with decreased abundance of megalin and cubilin. The modest increase of β-catenin serves as a positive control. The apical scaffold NHERF-1 and β-actin are negative controls. (C) Confocal microscopy. OK cell islands were either kept untreated (left) or treated with 30 nM epoxomicin for 24 hours (right; OK cells are more sensitive to the drug in small islands than in confluent monolayers) and allowed to incorporate EdU for 1 hour under the continued presence of epoxomicin and immunolabeled for ZONAB (top and bottom), megalin (middle and bottom), and EdU (bottom). Note that proteasome inhibition extends nuclear ZONAB labeling and EdU incorporation toward the center of the island, where megalin is repressed. Bar = 20 μm.
Because several transcriptional regulators are downregulated by the ubiquitin-proteasome system, confluent monolayers and OK cell islands were treated by proteasome inhibitors. As shown by Figure 7B and Supplemental Figure S6c, proteasome inhibition in polarized wild-type monolayers increased the abundance of both ZONAB isoforms in a concentration-dependent manner, together with increased PCNA and strongly decreased megalin and cubilin (both insensitive to the proteasome), compatible with enhanced ZONAB transcriptional activity. Interestingly, epoxomicin also strongly increased ZO-1 level. Similar results were obtained with lactacystin. Because proteasome effects are pleiotropic, we questioned whether epoxomicin treatment could, as is the case for polarity, affect ZONAB mRNA. As shown by Supplemental Figure 6b, epoxomicin upregulated endogenous ZONAB mRNA and protein to a comparable extent. From an operational perspective, epoxomicin thus appeared as a convenient tool to manipulate ZONAB expression by overcoming cell polarity effects. Confocal microscopy analysis of wild-type OK cell islands indeed revealed that extension of nuclear ZONAB labeling to central cells by the epoxomicin treatment triggered cells to reenter S phase, as demonstrated by EdU incorporation, and to dedifferentiate, as demonstrated by loss of megalin expression (Figure 7C). Taken together, our data demonstrate that apical differentiation of polarizing epithelial cells involves ZONAB downregulation, at least at the mRNA level, and that stabilization of ZONAB in differentiated cells reverses their fate to proliferation.
Discussion
This multifaceted study first evidenced an inverse correlation between spontaneous ZONAB disappearance and three hallmarks of apical differentiation in PTC: (1) Receptor-mediated endocytosis, (2) brush border, and (3) primary cilium. Analysis of small islands suggested that these changes resulted from individual cell choices resulting from polarity sensing, which could be related to the junctional belt. Conversely, stable ZONAB overexpression promoted OK cell proliferation while repressing (1) megalin/cubilin expression and function, (2) villin and thus brush border maturation,27,28 and (3) α-tubulin acetylation and thus primary cilium elongation.29 We conclude that ZONAB is simultaneously and causally associated with stimulation of proliferation and repression of epithelial differentiation in PTCs. A recent report on enterocyte differentiation indicated that ZONAB interaction with symplekin, another tight junction partner, also promotes proliferation and represses differentiation.30 Our conclusions in PTC terminal differentiation and the control by ZONAB of the permanently renewing intestinal epithelium are convergent.
Upon epithelial polarization, ZONAB is downregulated by signals that are dominant over a strong constitutive viral promoter, suggesting that ZONAB could act as a gatekeeper of differentiation, like the core regulators of embryonic stem cells Oct4/Sox2.31 Little is known on the control of ZONAB gene expression. E2F1, a key transcription factor for entry into the cell division cycle, binds to ZONAB promoter and enhances its expression.22 In Burkitt's lymphoma, ZONAB is a target for c-Myc,21 also involved in RCCs.32 Although no repressor of ZONAB is known, an attractive candidate is p53, which represses proliferation genes (PCNA, cdc2) while inducing cell-cycle arrest genes (p21) and renal function genes.33 Regulation by proteasomal degradation has been reported for multiple transcription factors such as the ZONAB-related, YB-1, and hypoxia-inducible factor but without direct link with epithelial polarization. Interestingly, von Hippel-Lindau factor (VHF), frequently defective in RCCs, is a ubiquitin-ligase of hypoxia-inducible factor, thereby normally acting as anti-oncogene regulating multiple targets. In view of the impact of VHF on the primary cilium,34 ZONAB could be another VHF substrate.35
Among multiple ZONAB downstream effects, this study identified two new target genes, megalin and cubilin; these tandem multiligand endocytic receptors are essential for PTC function1 and involved in genetic disease.3,4,36 Most interesting, ZONAB represses the primary cilium, both the target and the origin of multifaceted signaling.37 Which (and how) of the multiple components of the cilium proteome is affected by ZONAB is totally unknown. The possibility that ZONAB transactivates HDAC6, a tubulin deacetylase activated upon cell-cycle reentry to allow primary cilium shrinking, or its activators HEF1 and Aurora A kinase29 deserves to be examined. Because the primary cilium is an antiproliferative organelle,38 a reciprocal antagonistic loop whereby mitogenic ZONAB in immature epithelia represses the extension of the primary cilium emerges. Conversely, well-differentiated epithelia would be stabilized by the combined effect of ZONAB loss (transcriptional repression and proteasomal degradation) and the mature cilium (antimitogenic signaling and sequestration of the mother centriole as basal body).
The island model could provide an important clue for focal epithelial repair: Proliferating peripheral cells that expressed ZONAB stayed attached to adjacent differentiated central cells via extensive (yet discontinuous) junctions and thus remained not only epithelial in nature but also partially polarized. This differential choice between neighbors is probably most relevant to the repair of individual cell death in compact parenchymas such as kidneys and liver,39,40 which does not involve epithelial-to-mesenchymal transition. We suggest that, in these organs, any of the polarized epithelial cells adjacent to an apoptotic body could react via ZONAB reexpression to replace the expelled apoptotic body by cell division and then redifferentiate. This hypothesis is currently being tested.
In conclusion, this study identified two new ZONAB target genes (megalin and cubilin) and two new regulated subcellular structures (brush border and primary cilium), all relevant for kidney PTC development, function, genetic diseases, and cancer. More general, our data support a new role of ZONAB in the reversible switch between epithelial cell proliferation and differentiation, thereby opening a new avenue for the study of kidney PTC regeneration. Finally, deciphering this role revealed a novel mechanism for ZONAB action. In OK cells, transcriptional control by ZONAB could not be explained by shuttle of a stable protein pool between junctional complexes and nuclei but involved a primary control of ZONAB mRNA in relation with polarity, with resulting changes in nuclear ZONAB abundance and opposite transcriptional effects on proliferation and apical differentiation. We therefore suggest a mechanism whereby a soluble transcription factor (ZONAB) that can interact with junctional complexes but also with other unidentified partners elsewhere is an important component of a sensory mechanism regulated by polarity, affecting the proliferation/differentiation switch. The exact role of ZONAB in transcriptional networks controlling longitudinal nephron expansion, apical differentiation, and repair deserves extensive further studies.
Concise Methods
Reagents
Antibodies are described in Supplemental Table 1. Immunofluorescence was performed as described previously,41 after conventional fixation with 4% formaldehyde in 0.1 M phosphate buffer (pH 7.4). Epoxomicin (Calbiochem) was dissolved in DMSO to 1 mM (stock solution). Alexa-labeled BSA and human transferrin were from Molecular Probes/Invitrogen. Human liver cathepsin B (Calbiochem), transferrin (Sigma), and albumin (Sigma) were radioiodinated in Pierce® Pre-coated Iodination tubes (Pierce).4 Information on primers (sequence and application) and antibodies is provided in Supplemental Tables 1 and 2. Available anti-cubilin antibodies failed to recognize this receptor in OK cells by immunofluorescence.
Developmental Studies
Mice (C57BL/6J and CD1) were raised and treated according to the principles of laboratory animal care of the University Animal Welfare Committee. For immunoblotting and RT-PCR, pools of five to seven embryonic and individual postnatal kidneys were used. Tissues were frozen in liquid nitrogen and stored at −80°C before protein or RNA extraction. For in situ hybridization, whole embryos or postnatal kidneys were fixed overnight at 4°C in 60% ethanol, 30% formaldehyde, and 10% acetic acid before paraffin embedding.
In Situ Hybridization
A fragment (nt 215 to nt 524 from the ATG) of the MSY4/ZONAB-coding region was generated by PCR using reverse-transcribed total RNA derived from mouse kidney as template and cloned into TOPO-TA vector. DIG-labeled antisense RNA probe was produced by in vitro transcription of the MSY4/ZONAB cDNA using the T7 polymerase, and hybridization was performed on 16-μm-thick paraffin sections as described previously.42
Real-Time RT-PCR
Total RNA was extracted from mouse and human tissues and cultured OK cells using Trizol reagents (IVT), and contaminating DNA was digested with 20 U of DNase I for 30 minutes at room temperature. RNA (0.5 to 1.0 μg) was reverse-transcribed with oligo-dT primers or random hexamers using SuperScript II RNase H (Invitrogen). PCR was performed with platinum TaqDNA polymerase (Invitrogen) for 32 cycles, and amplicons were visualized on a 1.5% agarose gel. Real-time quantitative PCR was performed in duplicate by using iQ SYBR Green Supermix (Bio-Rad). Primer sequences are provided in Supplemental Table 1. The relative changes in target gene/β-actin mRNA ratio were determined by the formula 2ΔΔct or by transformation of threshold cycles to absolute mRNA numbers.43
Western Blotting
Whole kidneys were homogenized with 10 strokes of a glass/Teflon Potter homogenizer in 250 mM sucrose, 3 mM imidazole, 1 mM PMSF, 10 mM NaF, and 2 mM sodium orthovanadate and cleared at low speed. OK cells were washed twice with PBS; lysed on ice in 25 mM Tris-HCl (pH 8), 50 mM NaCl, and 1% IGEPAL (Sigma) with complete protease inhibitor cocktail (Roche); scraped with a rubber policeman; and sonicated. Protein content in lysates was determined using BCA protein assay. Equal protein amounts (50 μg) were solubilized by boiling in Laemmli buffer under reducing conditions, resolved by SDS-PAGE, and blotted onto Hybond P membrane (Amersham). After saturation, the membrane was incubated overnight with first antibodies (see Supplemental Table 2), then with peroxidase-conjugated secondary antibodies for 1 hour (Biosource), followed by enhanced chemiluminescence detection (PerkinElmer). Signals were quantified using Scion IMAGE 4.0.2 and normalized to β-actin signal.
Kidney Cancer and Control Adult Human Kidney
In silico analysis was based on primary data accessible at Geo Profiles (National Center for Biotechnology Information, GDS505). For RT-PCR, large samples of six RCCs and normal tissue from the same kidney when available (n = 3) or age-matched normal kidney (n = 3) were obtained from the tissue BioBank of the Cancer Centre of the Cliniques Universitaires Saint-Luc (Brussels, Belgium). Values in normal tissue from patients with cancer and age-matched kidneys were identical. Tissues were fixed and embedded in Tissue-Tek or frozen in liquid nitrogen. All material was reviewed by an expert pathologist who was blind of our results. In one additional case, in which our expression pattern had indicated an obvious outlier, the initial diagnosis of RCC was spontaneously revised. In control sections, the estimated proportion of non-neoplastic cells in the tumor samples was <5%.
Cell Culture Techniques and Transfection
PTCs derived from the opossum kidney were cultured as described previously.44 ZONAB opossum shows 80% overall sequence identity with human and dog, with an even higher conservation in the cold shock domain. Cells were seeded either at high density (5 × 104 cells/cm2) to obtain confluent monolayers (analyzed after 1 or 4 days) or at low density (1 × 103 cells/cm2) to generate two-dimensional small clusters (islands), analyzed after 4 days. Polarized culture of OK cells, measure of transepithelial resistance, immunolabeling, confocal microscopy, and scanning electron microscopy were performed as described previously.41 For monitoring of receptor-mediated endocytosis, OK cells were incubated with freshly radioiodinated tracers for 1 hour at 37°C in DMEM without protein as described previously,26 then cell-associated counts were normalized to cell protein and expressed in clearance values as described previously.41 For identification of the S phase, OK cells were treated with 10 mM EdU at 37°C for 1 hour, then washed with PBS. Incorporation of EdU was detected using Click-iT EdU Alexa Fluor kit (Invitrogen). For transfection, cells were seeded in six- or 12-well plates (3 × 105 or 5 × 105 cells/well) and grown for 24 hours before transfection with Lipofectamine 2000 (Invitrogen) and the expression vectors pCB6-ZONAB-B (for canine ZONAB cloned from MDCK cells) and empty pCB618 to establish cZONAB (cZ1–3) and mock transfectants. Stable transfectants were isolated by limited dilution cloning in the presence of geneticin (350 mg/L; Invitrogen).
Flow Cytometry
Confluent OK cells were synchronized, briefly trypsinized; fixed by 2% formaldehyde; washed twice in 3 ml of PBS with 3 mM EDTA; permeabilized with 0.1% Triton X-100; and stained in a solution containing 4 μg/ml propidium iodide, 3 mM EDTA, and 0.5 mg/ml RNaseA for 30 minutes at room temperature. Samples were studied by FACScalibur (BD), and the cell cycle was analyzed with FlowJo 8.8.4 for Macintosh.
Luciferase Reporter Assay
For generation of luciferase reporter vectors, regions encompassing the putative megalin and cubilin opossum promoters were amplified from genomic DNA of OK cells by PCR (for primers, see Supplemental Table 1) and subcloned in TOPO-TA vectors (Invitrogen) for sequencing. The megalin and cubilin fragments were excised by HindIII/KpnI and HindIII/SacI restriction, respectively, and inserted at the corresponding sites in pGL3-MCS (Promega). The Renilla, pRL-CMV (Promega), vector was used as an internal control. OK cells were transfected overnight with 1 ng of pRL-CMV, 800 ng of megalin- or cubilin-Firefly luciferase construct, and varying concentrations of the expression plasmids pCB6-ZONAB-B and pCB6-ZO-1.18 Total amount of DNA in the transfection was kept constant by addition of pCB6 empty vector. Mock and cZONAB transfectants were transfected with Renilla and Firefly reporter vectors only. Luciferase activity was measured 24 hours after transfection using the Dual-Luciferase reporter assay system (Promega) and a GloMax 96-microplate luminometer (Promega).
Chromatin Immunoprecipitation
Chromatin immunoprecipitation was performed on confluent monolayers at day 1 with anti-ZONAB antibody essentially as described previously.18 Binding of ZONAB to the megalin and cubilin promoters in OK cells was assessed by PCR. One fifteenth of the eluted DNA was used in a 50-μl PCR reaction (containing 50 mM KCl, 10 mM Tris-HCl, 1.5 mM MgCl2, and 1.0 U of TaqDNA polymerase; Promega) and 3 μM of specific primers (see Supplemental Table 1). An 18S amplicon served as a loading control.45 PCR products were resolved by agarose gel electrophoresis, and stained DNA was visualized under ultraviolet illumination. Chromatin immunoprecipitation was performed in triplicate from three independent assays.
Statistical Analyses
The statistical significance of differences between means was analyzed by unpaired Wilcoxon ranking test or t test, as indicated.
Disclosures
C.E.P. is research associate of the Fonds National de la Recherche Scientifique (FNRS).
Supplementary Material
Acknowledgments
This work was supported by the Université catholique de Louvain, Fonds National de la Recherche Scientifique (FNRS), Inter University Attraction Poles, Loterie Nationale, and Région Bruxelloise (Belgium), by the EU VIth and VIIth programs (EuReGene and EuNephron), and by Cystinosis Research Foundation.
We are grateful to Dr. P. Cornet (UCL-Biobank) for outstanding help in providing and partially processing human kidney samples and to Prof. J.P. Cosyns (Anatomie pathologique spéciale, St.-Luc University Hospital) for reviewing their diagnostic and scoring; Heloïse Gaide-Chevronnay for molecular biology advice; Sabine Cordi and Céline Forez for in situ hybridization; Michelle Leruth for graphic art; Than Lac and Huguette Debaix for technical assistance; Patrick Henriet and Etienne Marbaix for help in statistic analysis; and Drs. S. Robine (Pasteur, Paris, France) and C.H. Yun (Emory University, Atlanta, GA) for providing valuable antibodies.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “ZO-1 and ZONAB Interact to Regulate Proximal Tubular Cell Differentiation,” on pages 388–390.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1. Christensen EI, Birn H: Megalin and cubilin: Multifunctional endocytic receptors. Nat Rev Mol Cell Biol 3: 256–266, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Badano JL, Mitsuma N, Beales PL, Katsanis N: The ciliopathies: An emerging class of human genetic disorders. Annu Rev Genomics Hum Genet 7: 125–148, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Piwon N, Gunther W, Schwake M, Bosl MR, Jentsch TJ: ClC-5 Cl− channel disruption impairs endocytosis in a mouse model for Dent's disease. Nature 408: 369–373, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Christensen EI, Devuyst O, Dom G, Nielsen R, Van der Smissen P, Verroust P, Leruth M, Guggino WB, Courtoy PJ: Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules. Proc Natl Acad Sci U S A 100: 8472–8477, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M: Defective planar cell polarity in polycystic kidney disease. Nat Genet 38: 21–23, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Nelson WJ: Adaptation of core mechanisms to generate cell polarity. Nature 422: 766–774, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shin K, Fogg VC, Margolis B: Tight junctions and cell polarity. Annu Rev Cell Dev Biol 22: 207–235, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Clevers H: Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Matter K, Balda MS: Epithelial tight junctions, gene expression and nucleo-junctional interplay. J Cell Sci 120: 1505–1511, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Balda MS, Matter K: Tight junctions and the regulation of gene expression. Biochim Biophys Acta 1788: 761–767, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Pan J, Snell W: The primary cilium: Keeper of the key to cell division. Cell 129: 1255–1257, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Pan J, Wang Q, Snell WJ: Cilium-generated signaling and cilia-related disorders. Lab Invest 85: 452–463, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Eggenschwiler JT, Anderson KV: Cilia and developmental signaling. Annu Rev Cell Dev Biol 23: 345–373, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Y, Cong R, Biemesderfer D: The COOH terminus of megalin regulates gene expression in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol 295: C529–C537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balda MS, Matter K: The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J 19: 2024–2033, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Balda MS, Garrett MD, Matter K: The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol 160: 423–432, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frankel P, Aronheim A, Kavanagh E, Balda MS, Matter K, Bunney TD, Marshall CJ: RalA interacts with ZONAB in a cell density-dependent manner and regulates its transcriptional activity. EMBO J 24: 54–62, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sourisseau T, Georgiadis A, Tsapara A, Ali RR, Pestell R, Matter K, Balda MS: Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol 26: 2387–2398, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kavanagh E, Buchert M, Tsapara A, Choquet A, Balda MS, Hollande F, Matter K: Functional interaction between the ZO-1-interacting transcription factor ZONAB/DbpA and the RNA processing factor symplekin. J Cell Sci 119: 5098–5105, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Nie M, Aijaz S, Leefa Chong San, Balda M, Matter K: The Y-box factor ZONAB/DbpA associates with GEF-H1/Lfc and mediates Rho-stimulated transcription. EMBO Rep 10: 1125–1131, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Z, Van Calcar S, Qu C, Cavenee WK, Zhang MQ, Ren B: A global transcriptional regulatory role for c-Myc in Burkitt's lymphoma cells. Proc Natl Acad Sci U S A 100: 8164–8169, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arakawa Y, Kajino K, Kano S, Tobita H, Hayashi J, Yasen M, Moriyama M, Arakawa Y, Hino O: Transcription of dbpA, a Y box binding protein, is positively regulated by E2F1: Implications in hepatocarcinogenesis. Biochem Biophys Res Commun 322: 297–302, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Nakatsura T, Senju S, Yamada K, Jotsuka T, Ogawa M, Nishimura Y: Gene cloning of immunogenic antigens overexpressed in pancreatic cancer. Biochem Biophys Res Commun 281: 936–944, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Yasen M, Kajino K, Kano S, Tobita H, Yamamoto J, Uchiumi T, Kon S, Maeda M, Obulhasim G, Arii S, Hino O: The up-regulation of Y-box binding proteins (DNA binding protein A and Y-box binding protein-1) as prognostic markers of hepatocellular carcinoma. Clin Cancer Res 11: 7354–7361, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Lenburg ME, Liou LS, Gerry NP, Frampton GM, Cohen HT, Christman MF: Previously unidentified changes in renal cell carcinoma gene expression identified by parametric analysis of microarray data. BMC Cancer 3: 31, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nielsen R, Courtoy PJ, Jacobsen C, Dom G, Lima WR, Jadot M, Willnow TE, Devuyst O, Christensen EI: Endocytosis provides a major alternative pathway for lysosomal biogenesis in kidney proximal tubular cells. Proc Natl Acad Sci U S A 104: 5407–5412, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Friederich E, Huet C, Arpin M, Louvard D: Villin induces microvilli growth and actin redistribution in transfected fibroblasts. Cell 59: 461–475, 1989 [DOI] [PubMed] [Google Scholar]
- 28. Khurana S, George SP: Regulation of cell structure and function by actin-binding proteins: Villin's perspective. FEBS Lett 582: 2128–2139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA: HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129: 1351–1363, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buchert M, Darido C, Lagerqvist E, Sedello A, Cazevieille C, Buchholz F, Pannequin J, Joubert D, Hollande F: The symplekin/ZONAB complex inhibits intestinal cell differentiation via the repression of AML1/Runx1. Gastroenterology 137: 156–164, 164e.1–164e.3, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA: Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122: 947–956, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang SW, Chang WH, Su YC, Chen YC, Lai YH, Wu PT, Hsu CI, Lin WC, Lai MK, Lin JY: MYC pathway is activated in clear cell renal cell carcinoma and essential for proliferation of clear cell renal cell carcinoma cells. Cancer Lett 273: 35–43, 2009 [DOI] [PubMed] [Google Scholar]
- 33. El-Dahr SS, Aboudehen K, Saifudeen Z: Transcriptional control of terminal nephron differentiation. Am J Physiol Renal Physiol 294: F1273–F1278, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lutz MS, Burk RD: Primary cilium formation requires von Hippel-Lindau gene function in renal-derived cells. Cancer Res 66: 6903–6907, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Harten SK, Shukla D, Barod R, Hergovich A, Balda MS, Matter K, Esteban MA, Maxwell PH: Regulation of renal epithelial tight junctions by the von Hippel-Lindau tumor suppressor gene involves occludin and claudin 1 and is independent of E-cadherin. Mol Biol Cell 20: 1089–1101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gräsbeck R: Imerslund-Gräsbeck syndrome (selective vitamin B(12) malabsorption with proteinuria). Orphanet J Rare Dis 19: 1–17, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singla V, Reiter JF: The primary cilium as the cell's antenna: Signaling at a sensory organelle. Science 313: 629–633, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF: Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol 10: 70–76, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Stamatoglou SC, Enrich C, Manson MM, Hughes RC: Temporal changes in the expression and distribution of adhesion molecules during liver development and regeneration. J Cell Biol 116: 1507–1515, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mettlen M, Platek A, Van Der Smissen P, Carpentier S, Amyere M, Lanzetti L, de Diesbach P, Tyteca D, Courtoy PJ: Src triggers circular ruffling and macropinocytosis at the apical surface of polarized MDCK cells. Traffic 7: 589–603, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Jacquemin P, Pierreux CE, Fierens S, van Eyll JM, Lemaigre FP, Rousseau GG: Cloning and embryonic expression pattern of the mouse Onecut transcription factor OC-2. Gene Expr Patterns 3: 639–644, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Pierreux CE, Poll AV, Kemp CR, Clotman F, Maestro MA, Cordi S, Ferrer J, Leyns L, Rousseau GG, Lemaigre FP: The transcription factor hepatocyte nuclear factor-6 controls the development of pancreatic ducts in the mouse. Gastroenterology 130: 523–541, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Barone R, Van Der Smissen P, Devuyst O, Beaujean V, Pauwels S, Courtoy PJ, Jamar F: Endocytosis of the somatostatin analogue, octreotide, by the proximal tubule-derived opossum kidney (OK) cell line. Kidney Int 67: 969–976, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Pierreux CE, Vanhorenbeeck V, Jacquemin P, Lemaigre FP, Rousseau GG: The transcription factor hepatocyte nuclear factor-6/Onecut-1 controls the expression of its paralog Onecut-3 in developing mouse endoderm. J Biol Chem 279: 51298–51304, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.