Abstract
Mediterranean populations have low incidence rates of cardiovascular disease and hypertension that may be due, in part, to dietary factors, particularly a relatively high intake of monounsaturated fat as olive oil. In this study, nutritional components (as grams per 4200 kJ) (1 kcal = 4.2 kJ) from three-day food records were examined in association with resting blood pressure in a cross-sectional survey of 76 sedentary middle-aged American men, aged 30 to 55 years, with resting blood pressures below 160/100 mm Hg. Systolic and diastolic blood pressures correlated significantly and inversely with monounsaturated fat consumption. Polyunsaturated fat consumption also correlated inversely with diastolic blood pressure; however, this relationship became nonsignificant when adjusted for an index of regional adiposity that characterizes the male-type obesity pattern. Detailed analyses of specific fatty acids showed that the correlations with monounsaturates were specific to oleic acid, and the correlation with polyunsaturates was specific to linoleic acid. Multiple regression analysis suggested that 18.2% of the variance in systolic blood pressure and 23.2% of the variance in diastolic blood pressure were related to monounsaturated and polyunsaturated fat consumption and regional adiposity. Thus, increased consumption of monounsaturated fat is related inversely to resting blood pressure, although causality remains to be determined.
Introduction
Blood pressure control even among nonhypertensive men may promote reduced cardiovascular risk. The final report of the Pooling Project Research Group [1], representing the combined results of five longitudinal investigations on the incidence of coronary heart disease in middle-aged white men, concluded that much of the excess in coronary heart disease associated with blood pressure occurs at levels below clinically diagnosed hypertension (ie, 160/100 mm Hg). Furthermore, pharmacologic management of “mild hypertension” (140 to 160/90 to 100 mm Hg) carries considerable economic costs and potential for long-term toxic effects. Alterations in diet, weight loss, and other blood pressure-related behaviors offer at an acceptable cost the potential for addressing the excess cardiovascular risk associated with elevated blood pressure. Weight loss, reduced intakes of sodium and potassium, and stress reduction have been studied most extensively in this context.
Animal studies, cross-cultural comparisons, and human experiments suggest that polyunsaturated fat may also have favorable hypotensive effects [2], possibly through enhanced synthesis of vasodilating prostaglandins [3,4]. Less is known about the effects of monounsaturated fat on cardiovascular risk factors than of either saturated or polyunsaturated fat, despite the predominance of monounsaturated fat in the Western diet. It is speculated that the low incidence of coronary heart disease and the low blood pressure levels in the Mediterranean region relative to central and northern Europe may be due in part to the greater use of olive oil and monounsaturated fats in general [56]. Monounsaturates may exert no adverse effect on [7], or may favorably reduce, atherogenic lipoproteins [8,9]. Monounsaturated fat is reported to have reduced blood pressure when added to the low-fat diets of Indians in one study [10], however, its effect on blood pressure in the context of Western diets has not been reported.
Cross-sectional blood pressure studies can complement experimental studies in several respects. They provide a basis for generalizing the blood pressure-nutrient relationships established experimentally under restricted, and perhaps unrepresentative, conditions to the typical diets of free-living individuals. Moreover, by contrasting blood pressures over a continuum of nutrient intake levels as typically consumed within a population, graphical analysis of cross-sectional observations may be used to gain insight into the functional relationship between blood pressure and nutrient intake. Cross-sectional studies also provide an effective approach for examining a variety of nutrients for their potentially hypotensive effects, and thereby provide direction for future experimentation.
In this report, we present evidence of a significant relationship between intake of monounsaturated fat and both systolic and diastolic blood pressure when nutrient intakes from three-day food records were compared with resting blood pressure levels in a cross-sectional survey of 76 middle-aged American men. Polyunsaturated fat consumption also correlated with diastolic blood pressure. This relationship became nonsignificant, however, when adjusted for the waist-to-hip girth ratio, an index of regional adiposity that characterizes the male-type obesity pattern [11,12].
Subjects And Methods
Subjects
Our report focuses on baseline dietary records and blood pressure measurements of 76 sedentary but otherwise healthy men, aged 30 to 55 years, who later participated in a one-year exercise trial (five of the original 81 men who entered the exercise study at baseline are excluded from this report because of incomplete diet or blood pressure measurements) [13]. The subjects were selected to be free of known cardiovascular disease or abnormalities, acute illness or active systemic disease, or medication use likely to interfere with lipid metabolism (including anti-hypertensive medication). We also required all participants to have resting blood pressure below 160/100 mm Hg, body weight less than 140% of Metropolitan Life Insurance Company's “ideal” weight [14], plasma total cholesterol level below 300 mg/dL (< 7.76 mmol/L), and plasma triglyceride concentrations below 500 mg/dL (< 5.65 mmol/L).
Laboratory Measurements
Subjects reported to our laboratory in the morning, having abstained for 12 to 16 hours from all food and from any vigorous activity. Before venipuncture and exercise testing, three blood pressure measurements were taken by trained auscultators after the participants had been seated comfortably for five minutes. The average of the second and third measurements was used in the statistical analyses, with systolic blood pressure taken as the first Korotkoff phase and diastolic as the fifth Korotkoff phase.
Following the procedures described by Wilmore and Behnke [12], waist girth was measured horizontally at the umbilicus, and hip girth was measured as the largest horizontal circumference around the buttocks, with standard cloth tapes. Percent body fat was estimated by hydrostatic weighing, and maximal oxygen uptake (VO2max) was calculated from a graded exercise test [13].
Nutritional Assessment
Three-day food records were completed on consecutive days. The starting day was assigned randomly so as to ensure a proportional number of weekdays and weekend days. A trained technician reviewed the written records and, if unclear, verified them with the participants. Records were coded using the Nutrition Coding Center, Minneapolis, code book and rules. Mean total calorie and nutrient intakes over the three days were determined using the computerized food composition tables (version 9) of the Nutrition Coding Center and an analysis program. In this report, all dietary variables are expressed in grams per 4200 kJ (1 kcal = 4.2 kJ) except dietary sodium, calcium, and potassium, which are expressed in milligrams per day. Sodium levels do not include salt added to food during preparation or discretionary use at the table.
Statistical Calculations
The relationships between blood pressure and other variables were measured by Pearson's product-moment correlation coefficient and multiple regression analysis and graphically displayed by robust locally weighted regression analysis (a scatterplot smoothing procedure described by Cleveland [15]), using half of the data to smooth each point. The smoothing procedure offers considerable flexibility over linear regression because it does not assume a simple straight-line fit between variables and thus may reveal important nonlinear relationships.
Partial correlation coefficients were used to adjust blood pressure-nutrient relationships for the potentially confounding effects of adiposity. Because the assumption of multivariate normality may not apply to our data, the standard estimates of significance and confidence intervals were verified by permutation tests [16] and bootstrap re-sampling procedures [17], respectively. To determine a nonparametric significance level, we obtained adjusted nutrient intake and adjusted blood pressure levels from the residuals of separate regression equations that predict nutrient intake and blood pressure from the adjustment variables. We then randomly permuted, 1000 times, the adjusted nutrients values among the adjusted blood pressure levels to obtain 1000 partial correlation coefficients under the null hypothesis of zero partial correlation. A two-tailed nonparametric significance level for testing whether the observed partial correlation was different from zero was then obtained by doubling the proportion of times that the correlations for the permuted data exceeded the partial correlation of the original (unpermuted) data.
To determine a nonparametric 95% confidence interval for a partial correlation coefficient, we constructed 1000 “bootstrapped” data sets by sampling 76 vector-valued observations (ie, consisting of nutrient intake, blood pressure, and the adjustment variables) with replacement from the original set of 76 observations. Partial correlations were calculated for the 1000 bootstrapped samples, which were then arranged in ascending order. The 25th and the 975th largest correlations define a nonparametric 95% confidence interval, after adjustment by the percentile method [17].
Results
The selection criteria resulted in a sample of middle-aged (45.9 ± 5.9 [SD] years) normotensive men (systolic blood pressure, 110.8 ± 9.7 mm Hg; diastolic blood pressure, 72.9 ± 8.0 mm Hg), who ate 39.3 ± 6.4 g/4200 kJ of protein, 95.0 ± 20.1 g/4200 kJ of carbohydrates, 16.7 ± 3.5 g/4200 kJ of saturated fat, 17.6 ± 3.8 g/4200 kJ of monounsaturated fat, 8.0 ± 2.6 g/4200 kJ of polyunsaturated fat, and 7.7 ± 8.2 g/4200 kJ of alcohol. These nutrient intakes correspond to typical American diets with 15.7% ± 2.5% of total energy intake from protein, 40.9% ± 6.6% from fat, 38.0% ± 8.0% from carbohydrates, and 5.4% ± 5.7% from alcohol. The men were slightly obese, as reflected by Quetelet index (24.6 ± 3.1 kg/m2), body fat (21.7% ± 5.7% of total weight), and the waist-to-hip girth ratio (0.93 ± 0.05). Sixteen men smoked cigarettes.
Relationship of Blood Pressure to Age, Cigarette Use, Fitness, and Stress
We identified first those covariates that could potentially induce significant correlations between blood pressure and nutrient measurements, before assessing their relationship directly. Spurious blood pressure-nutrient relationships could arise through covariates that correlate with both blood pressure and dietary intake. Age [18], physical activity [19], cigarette use [20], stress [21], and adiposity [22,23] are all reputedly related to blood pressure. However, the systolic and diastolic blood pressures of the participants in our study did not correlate significantly with age (r =0.20 and r =0.18, respectively), aerobic capacity (VO2max: r = -0.16 and r = -0.22, respectively), or the number of cigarettes consumed per day (r =0.06 and r = -0.08, respectively). Systolic and diastolic blood pressure were also unrelated to various measures of stress: type A personality, as assessed by structured interview [24], hostility (Cook-Medley hostility scale: r =0.11 and r =0.11, respectively) [25], anxiety (Thurstone activity scale: r =0.01 and r =0.10; Spielberger trait anxiety inventory: r = -0.18 and r = -0.16) [26,27], or depression (Minnesota Multiple Personality Inventory: r = -0.11 and r = -0.16) [28]. Nonsignificance and low values of these correlations suggested that age, aerobic capacity, cigarette use, and stress were unlikely to induce spurious nutrient-blood pressure relationships (partial correlation analyses are described below).
Relationship of Blood Pressure to Adiposity
We examined three adiposity measures for their relationships to blood pressure. The Quetelet index is an indirect adiposity measure derived from height and weight, percent body fat is estimated from the direct measurement of body density from hydrostatic weighing, and the waist-to-hip girth ratio characterizes the male-type obesity pattern [11]. The less than perfect correlations between waist-to-hip girth and percent body fat (r =0.50), the waist-to-hip girth and Quetelet index (r =0.60), and percent body fat and Quetelet index (r =0.53) showed that the three obesity measures were not redundant in their assessment of adiposity.
Table 1 displays correlation coefficients for blood pressure vs the three adiposity measures, nonparametric 95% confidence intervals derived from the bootstrap re-sampling procedure, and significance levels determined parametrically from normal theory and nonparametrically from permutation analyses. Although all three measures of adiposity showed significant relationships to blood pressure, the correlations were substantially greater for the waist-to-hip girth ratio than for percent body fat or Quetelet index. The partial (adjusted) correlations of Table 1 suggested, in fact, that the correlations between diastolic blood pressure and percent body fat and Quetelet index may primarily reflect the association between diastolic blood pressure and male-type obesity, since these correlations lose their significance when adjusted for waist-to-hip girth. In contrast, adjustment for Quetelet index and percent body fat did not eliminate the significance of the correlation between the waist-to-hip girth and diastolic blood pressure (r =0.25, P ≤ 0.05). The primacy of male-type fat deposition was less certain in the determination of systolic blood pressure because the correlation between waist-to-hip girth and systolic blood pressure was much reduced when adjusted for Quetelet index or percent body fat (from r =0.28 to r =0.14).
Table 1.
Cross-sectional Correlations Between Blood Pressure and Three Adiposity Measures in 76 Middle-aged Men
| Systolic Blood Pressure | Diastolic Blood Pressure | |||
|---|---|---|---|---|
| Unadjusted | Adjusted for Waist-to-Hip Girth Ratio | Unadjusted | Adjusted for Waist-to-Hip Girth Ratio | |
| Quetelet index, kg/m2 | 0.21 | 0.06 | 0.24* | 0.02 |
| 95% Confidence interval† | (0.03, 0.40) | (-0.13, 0.27) | (0.05, 0.42) | (-0.15, 0.20) |
| Significance‡ | 0.08 | 0.58 | 0.04 | 0.85 |
| Body fat, % of total mass | 0.18 | 0.05 | 0.29§ | 0.13 |
| 95% Confidence interval† | (-0.04, 0.37) | (-0.17, 0.30) | (0.08, 0.49) | (-0.07, 0.35) |
| Significance‡ | 0.13 | 0.61 | 0.006 | 0.22 |
| Waist-to-hip girth ratio | 0.28§ | … | 0.38§ | … |
| 95% Confidence interval† | (0.04, 0.48) | … | (0.17, 0.54) | … |
| Significance‡ | 0.024 | … | 0.002 | … |
P ≤ 0.05
Distribution-free 95% confidence intervals were determined from 1000 bootstrapped samples.
Nonparametric significance levels were determined from 1000 permutations.
P ≤ 0.01
Robust locally weighted regression analysis was applied to assess the deterministic relationship between blood pressure and the degree of male-type obesity. Diastolic blood pressure (Fig 1) and systolic blood pressure (not displayed) were found to increase linearly throughout the range of waist-to-hip girth ratios represented in these men.
Fig 1.
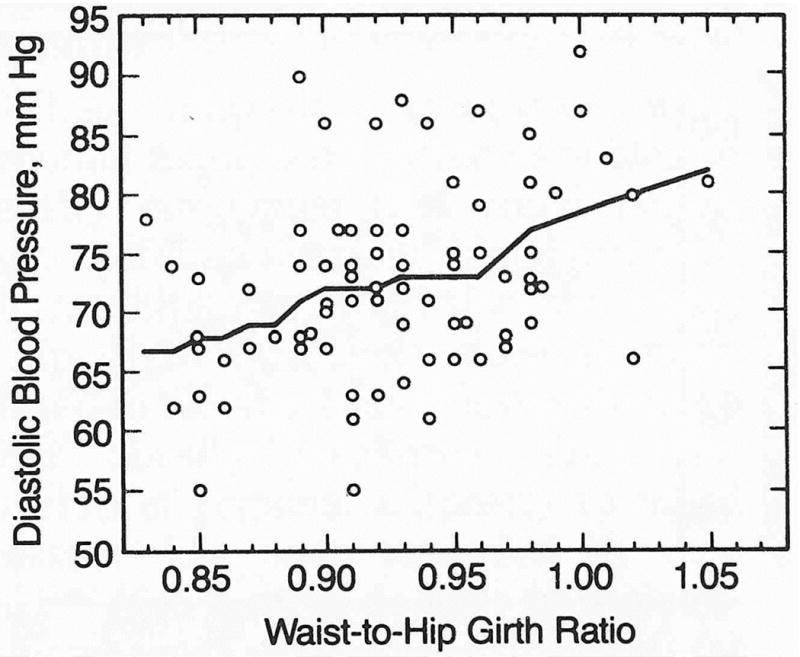
Robust locally weighted regression of diastolic blood pressure vs waist-to-hip girth ratio (male-type obesity index) in 76 middle-aged men. Plot shows that diastolic blood pressure increased linearly with increasing waist girth relative to hip girth.
In the analyses of nutrient-blood pressure relationships that follow, we selected waist-to-hip girth over Quetelet index and percent body fat as a covariate for adjustment because (1) the correlations between blood pressures and waist-to-hip girth dominated the other blood pressure-adiposity correlations, and (2) the percentage of total energy obtained from polyunsaturated fat correlated significantly with waist-to-hip girth (r = -0.27, P ≤ 0.02), but not percent body fat (r = -0.17) or Quetelet index (r = -0.13). Saturated and monounsaturated fat intakes were not related significantly to any obesity measure.
Relationship of Blood Pressure to Diet
Table 2 displays the correlational analyses for blood pressure vs nutrient intake. Systolic and diastolic blood pressure correlated significantly with monounsaturated fat intake, and diastolic blood pressure correlated significantly with polyunsaturated fat consumption. The correlations between monounsaturated fat consumption and blood pressure were not affected when adjusted for waist-to-hip girth (Table 2) or other potentially confounding variables (age, VO2max, cigarette use, stress, Quetelet index, and percent body fat; results not displayed). The correlation between polyunsaturated fat and diastolic blood pressure, however, was made nonsignificant when adjusted for waist-to-hip girth. The smooth scatterplot of Fig 2 suggests that the inverse correlation between systolic blood pressure and monounsaturated fat consumption was due primarily to lower blood pressure levels in men who consumed monounsaturated fat in excess of 19 g/4200 kJ (equivalent to 12.6% of total energy intake). The relationships of diastolic blood pressure to monounsaturated and polyunsaturated fat consumption were primarily linear (regression curves not displayed).
Table 2.
Partial Correlation Analyses of Blood Pressure vs Nutrient Intake (g/1000 kcal) in 76 Middle-aged Men
| Systolic Blood Pressure | Diastolic Blood Pressure | |||
|---|---|---|---|---|
| Unadjusted | Adjusted for Waist-to-Hip Girth Ratio | Unadjusted | Adjusted for Waist-to-Hip Girth Ratio | |
| Total protein | 0.04 | 0.03 | 0.02 | 0.01 |
| 95% Confidence interval* | (-0.18, 0.26) | (-0.20, 0.25) | (-0.24, 0.29) | (-0.24, 0.25) |
| Significance† | 0.78 | 0.80 | 0.80 | 0.89 |
| Total carbohydrates | 0.13 | 0.14 | 0.15 | 0.18 |
| 95% Confidence interval* | (-0.12, 0.35) | (-0.17, 0.38) | (-0.06, 0.37) | (-0.05, 0.39) |
| Significance† | 0.31 | 0.22 | 0.20 | 0.13 |
| Saturated fat | 0.01 | -0.03 | -0.03 | -0.09 |
| 95% Confidence interval* | (-0.18, 0.21) | (-0.23, 0.16) | (-0.25, 0.17) | (-0.29, 0.12) |
| Significance† | 0.89 | 0.59 | 0.78 | 0.39 |
| Monounsaturated fat | -0.33‡ | -0.33‡ | -0.28‡ | -0.28‡ |
| 95% Confidence interval* | (-0.51, -0.12) | (-0.52, -0.14) | (-0.46, -0.08) | (-0.49, -0.06) |
| Significance† | 0.004 | 0.004 | 0.008 | 0.02 |
| Polyunsaturated fat | -0.18 | -0.11 | -0.29‡ | -0.21 |
| 95% Confidence interval* | (-0.42, 0.08) | (-0.36, 0.12) | (-0.53, -0.01) | (-0.45, 0.10) |
| Significance† | 0.11 | 0.32 | 0.006 | 0.08 |
| Alcohol | 0.09 | 0.07 | 0.09 | 0.06 |
| 95% Confidence interval* | (-0.12, 0.30) | (-0.17, 0.30) | (-0.11, 0.27) | (-0.13, 0.24) |
| Significance† | 0.42 | 0.55 | 0.45 | 0.60 |
Distribution-free 95% confidence intervals were determined from 1000 bootstrapped samples.
Nonparametric significance levels were determined from 1000 permutations.
P < 0.01.
Fig 2.
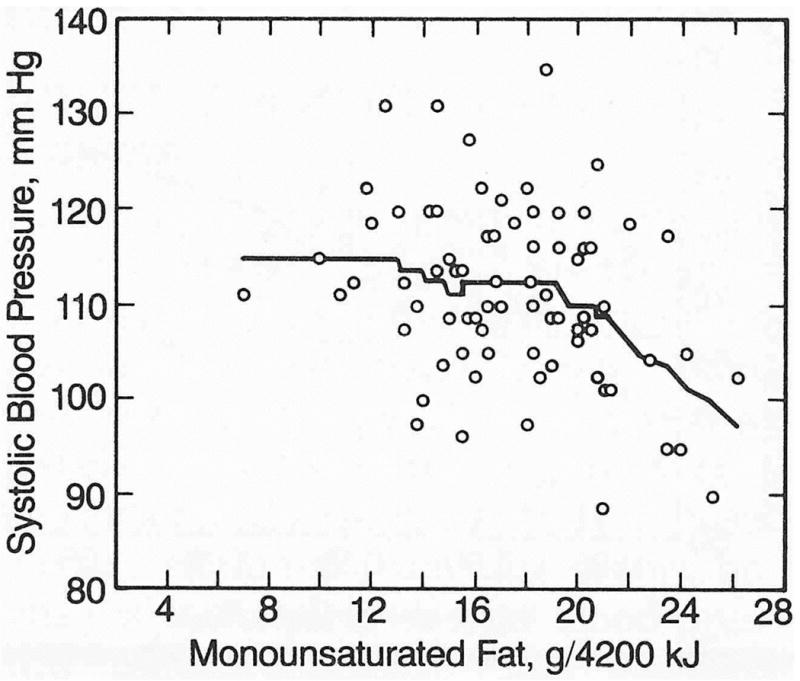
Smoothed scatterplot of systolic blood pressure vs monounsaturated fat intake after adjustment for waist-to-hip girth ratio in 76 middle-aged men. Plot suggests that inverse correlation between monounsaturated fat intake and blood pressure largely reflected lower blood pressure levels in men who consumed monounsaturated fat in excess of 19 g/4200 kJ.
Coffee intake (cups per day), total energy intake, and percentage of total energy derived from alcohol, protein (total, plant, and animal), carbohydrates (total, sucrose, starch, and other carbohydrates), and crude fiber were each unrelated to blood pressure levels. Intakes of sodium, potassium, and calcium correlated weakly and nonsignificantly with blood pressure (Table 3). These findings do not necessarily negate the relationships of these minerals to blood pressure since the 95% confidence intervals for the correlation coefficients spanned a wide range of possible values.
Table 3.
Cross-sectional Correlations of Blood Pressure vs Intakes of Sodium, Potassium, and Calcium in 76 Middle-aged Men
| Correlations With Blood Pressure | |||
|---|---|---|---|
| Mean ± SD | Systolic | Diastolic | |
| Sodium, mg | 3214.9 ± 1141.0 | 0.06 | 0.04 |
| 95% Confidence interval* | … | (-.12, .27) | (-.18, .26) |
| Significance† | … | .56 | .77 |
| Calcium, mg | 832.4 ± 383.1 | 0.10 | 0.01 |
| 95% Confidence interval* | … | (-.15, .31) | (-.17, .20) |
| Significance† | … | .47 | .96 |
| Potassium, mg | 2986.9 ± 788.7 | 0.03 | -0.07 |
| 95% Confidence interval* | … | (-.20, .24) | (-.28, .14) |
| Significance† | … | .82 | .55 |
Distribution-free 95% confidence intervals were determined from 1000 bootstrapped samples.
Nonparametric significance levels were determined from 1000 permutations.
Correlation and regression analyses of specific fatty acids in relation to blood pressure did not provide further resolution of the blood pressure-nutrient associations beyond that achieved for the less specific classifications of fats by saturation level. The correlation of diastolic blood pressure with the proportion of total energy from polyunsaturated fat (r = -0.29, P ≤0.01) reflected primarily its correlation with linoleic acid (r = -0.29, P ≤ 0.01), its major component (87.5% of total polyunsaturated fat intake). Diastolic blood pressure did not correlate significantly with minor contributors to the total polyunsaturated fat intake, including linolenic (9.3%), arachidonic (1.0%), and eicosapentaenoic (0.4%) acids (the correlations were < 0.10 in each case). Similarly, the correlations of systolic and diastolic blood pressure with the proportion of total energy from monounsaturated fat (r = -0.33 and r = -0.28, respectively; P ≤ 0.01 for both) and oleic acid (r = -0.34 and r = -0.30, respectively; P ≤ 0.01) were comparable, an expected result since oleic acid represented 91% of all monounsaturated fat consumed. Blood pressure failed to correlate significantly with other monounsaturated fatty acids (P ≥ 0.20) or with any specific saturated fatty acid (P ≥ 0.08). Since this more refined classification of fats offered no further improvement in defining the nutrient-blood pressure relationships, further analyses and discussion focus on the simpler classification of fats by saturation level.
Multivariate Analysis of Blood Pressure and Fat Consumption
Table 4 displays four multiple regression models that fit separate coefficients relating monounsaturated fat intake, polyunsaturated fat intake, and waist-to- hip girth ratio to blood pressure levels. When the waist-to-hip girth ratio was excluded from the model, the relationship of systolic blood pressure to monounsaturated fat intake was significant, independent of the effects of polyunsaturated fat consumption (column 1). The relationships of diastolic blood pressure to both monounsaturated and polyunsaturated fat were independently significant (column 3). The independent relationships of these fats to blood pressure in the multivariate model agreed with their unadjusted relationships in the univariate analysis (Table 2) because monounsaturated and polyunsaturated fat intake were only weakly correlated with each other (r = 0.20, P ≤ 0.09).
Table 4.
Multiple Regression Analyses of Blood Pressure vs Intakes of Monounsaturated and Polyunsaturated Fat and Waist-to-Hip Girth Ratio in 76 Middle-aged Men
| Systolic Blood Pressure | Diastolic Blood Pressure | |||
|---|---|---|---|---|
| Waist-to-Hip Girth Ratio: | Excluded | Included | Excluded | Included |
| Coefficients | ||||
| Intercept | 128.18* | 78.85* | 87.68* | 34.76 |
| Monounsaturated fat | -0.79† | -0.79† | -0.50‡ | -0.50‡ |
| Polyunsaturated fat | -0.44 | -0.19 | -0.75‡ | -0.48 |
| Waist-to-hip girth ratio | … | 51.08‡ | … | 54.79† |
| % of Blood Pressure explained | 12.4‡ | 18.2† | 13.5† | 23.2* |
P ≤ 0.001.
P ≤ 0.01.
P < 0.05.
When the waist-to-hip girth ratio was included in the model, monounsaturated fat intake and the ratio of waist-to-hip girth both showed significant independent relationships to blood pressure (columns 2 and 4). Polyunsaturated fat did not relate significantly to diastolic blood pressure, however, when adjusted for male-type adiposity. The addition of the waist-to-hip girth ratio to monounsaturated and polyunsaturated fat increased the percentage variance explained by the regression model from 12.4% to 18.2% for systolic blood pressure and from 13.5% to 23.2% for diastolic blood pressure.
Comment
Dietary Fat and Blood Pressure
The correlations presented in this report reveal a significant, and unexplained, association between increasing levels of monounsaturated fat intake and decreasing levels of both systolic and diastolic blood pressure. Monounsaturates are not recognized as precursors of prostaglandins; a physiological explanation for lower blood pressure with higher monounsaturated fat consumption is not immediately forthcoming. To our knowledge, this inverse association has been reported previously only when monounsaturated fat supplemented the low fat diet in Indians [10]. Our findings suggest that this isolated observation may indeed apply in the context of the higher fat diets of American and other Western societies.
We also found that polyunsaturated fat correlated inversely with diastolic blood pressure. This observation is consistent with much experimental evidence and many cross-cultural comparisons. In rats, feeding high polyunsaturated fat diets reportedly inhibits the elevation of mean systolic blood pressure [29] and the development of salt-induced hypertension [30,31]. Trappist monks consume a greater proportion of their total fat from vegetables than Benedictine monks, and this dietary difference may account for less hypertension among the Trappists (12%) than the Benedictines (51%) [32]. Similarly, vegetarians are reported to have lower blood pressure than nonvegetarian controls [33-34].
Puska and his colleagues [35] achieved significant decreases in both systolic and diastolic blood pressure in Finnish couples who were prescribed low-fat diets with a high polyunsaturated-to-saturated fat (P/S) ratio; significant increases in systolic and diastolic blood pressure levels were achieved when these couples returned to their usual diets. The intervention diet reduced both saturated and monounsaturated fat intake to approximately one third of their baseline levels and increased polyunsaturated fat consumption slightly. Margetts et al [36], however, failed to achieve significant blood pressure differences in a double-blind, randomized control trial when total fat was kept constant, saturated fat reduced by about 25%, and polyunsaturated fat increased by twofold to threefold, producing a P/S ratio above 1. Margetts and colleagues attributed the differences between their results and those achieved by Puska et al to (1) nutritional changes in the Finnish trial other than alterations in the P/S ratio (cholesterol consumption and total energy intake were also reduced during the intervention period and increased during the switchback period, and alcohol consumption varied over time), (2) different methods of achieving a P/S ratio near 1 (ie, decreased saturated and total fat intake in the Finnish study vs increased polyunsaturated fat intake with total fat held constant), and (3) bias in the Finnish study accruing from the awareness by the observer of the group assignment of the participant. The discrepancy may also be due in part to the greater statistical power of the Finnish study to detect blood pressure differences, since it included twice as many subjects as the study by Margetts et al.
That polyunsaturated fat intake showed an inverse association with diastolic blood pressure, but no apparent relationship with systolic pressure, is consistent with the observation by Rao and colleagues [10] that supplementing polyunsaturated fat in the diets of Indians decreased diastolic blood pressure without effecting a corresponding decrease in systolic blood pressure. These results lend some support to the hypothesis that prostaglandins mediate the hypotensive effects of polyunsaturated fat through reduced vasoconstriction or increased vasodilation, since peripheral resistance is thought to be more directly associated with diastolic than systolic blood pressure [37-39].
Previous epidemiologic surveys have generally not found associations between fat intake and blood pressure. In Finns, mean arterial pressure was reported to be correlated significantly cross-sectionally with the intake of saturated fat but not other fats [40], while other studies did not reveal significant associations between blood pressure and any fats [41]. The questionnaires regarding usual intake [40] or the one-day diet-recall questionnaires [41] used in these studies may not assess nutrient intake with the precision required to discern their relationships with blood pressure.
Regional Adiposity and Blood Pressure
When adiposity measures were screened along with other variables to identify covariates that could potentially confound nutrient-blood pressure relationships, we found that the waist-to-hip girth ratio was more strongly related to blood pressure levels than the other obesity measures. The relationship of regional adiposity to blood pressure has been reported by others [23,42]. Men tend to deposit fat abdominally whereas women tend to deposit fat in gluteal and femoral regions, so that a high waist-to-hip girth ratio characterizes a male-type obesity pattern [11]. In our study, approximately 14% of the variance in diastolic blood pressure was associated with this ratio. Hypertension may be more prevalent in men than in women in part because men have a greater tendency to gain abdominal fat, which appears to be metabolically distinct from gluteal or femoral fat [11,43]. Experimental studies of polyunsaturated fat intake and blood pressure usually rule out an obesity effect when total weight remains constant throughout the intervention. Further control for change in regional adiposity may be required to substantiate an effect due to polyunsaturated fat intake and blood pressure that is independent of adiposity effects.
Other Nutrients and Blood Pressure
Hypertensive effects have been ascribed to sodium [44] and simple sugars [45], whereas potassium [46], calcium [41,47], magnesium [47], and vegetable fiber [48] are reputedly hypotensive. Alcohol has also been reported to be associated with elevated blood pressure [49,50]. However, this relationship was most pronounced in older individuals [49], and the oldest individuals in the present study were aged 55 years. In our data, neither systolic nor diastolic pressures correlated significantly with the proportion of total energy from alcohol (Table 2), sucrose (r =0.09 and r =0.06 for systolic and diastolic blood pressure, respectively), or crude fiber (r = -0.12 and r = -0.16). Low intakes of fish (< 1% of total energy intake) and eicosapentaenoic acid (< 1% of polyunsaturated fat intake) limited our ability to assess their effects on blood pressure. The correlations we observed for blood pressure vs calcium, potassium, and sodium intake were also not statistically significant (Table 3), but it is not possible to negate their postulated relationships from our data, given the width of the confidence intervals. The low-order correlation between sodium consumption and blood pressure levels may reflect in part the fact that volitional salt use was not assessed (estimated to provide 20% to 40% of all sodium consumed [51]). Substantially greater correlations for systolic blood pressure vs calcium (r =-0.60), potassium (r = -0.46), and sodium intake (r = -0.28) were presented by McCarron et al [41] for the First National Health and Nutrition Examination Survey nutritional data. They divided 10 419 participants into 25 to 30 strata and then correlated the mean systolic level with mineral intake across the strata. Averaging blood pressure levels within each category of mineral consumption produces a substantially smaller blood pressure variance, so that the correlations obtained for the averages are necessarily larger than correlations that contrast blood pressure and mineral intake among individuals.
Limitations and Caveats
Our analyses suggest that monounsaturated fat intake is inversely related to both systolic and diastolic blood pressures. Polyunsaturated fat consumption also correlated with diastolic blood pressure; however, this relationship became nonsignificant when adjusted for regional adiposity. We emphasize caution in extrapolating these nutrient-blood pressure associations to the general population, given the more selected nature and modest size of our sample. While the statistical tests demonstrated that chance was an unlikely explanation of our findings, even with this sample size, it is possible that the selection procedure produced a unique group of individuals. The causality of the monounsaturated fat-blood pressure relationships in the content of Western dietary patterns is not proved by these cross-sectional associations and remains to be determined in controlled clinical trials.
Recent experimental results suggest that monounsaturates and polyunsaturates may be equally effective for reducing plasma levels of total cholesterol and low-density lipoprotein cholesterol [8]. Hypotensive properties of monounsaturated fat, if verified, could provide greater flexibility in attaining more desirable cardiovascular risk factor profiles through diet.
Acknowledgments
This study was supported in part by grants HL-24462 and HL-30856 from the National Heart, Lung, and Blood Institute and a grant from Best Foods, Union, NJ, a unit of CPC North America.
Ping Ho, Stephen Blair, PEd, and Barbara Frey-Hewitt, MS, assisted with this study.
References
- 1.The Pooling Project Research Group. Relationship of Blood Pressure, Serum Cholesterol, Smoking Habit, Relative Weight and ECG Abnormalities to Incidence of Major Coronary Events: Final Report of the Pooling Project, monograph 60. Dallas: American Heart Association; 1978. [DOI] [PubMed] [Google Scholar]
- 2.Smith-Barbaro PA, Pucak GJ. Dietary fat and blood pressure. Ann Intern Med. 1983;98:828–831. doi: 10.7326/0003-4819-98-5-828. [DOI] [PubMed] [Google Scholar]
- 3.Comberg HU, Heyden S, Hames CG, et al. Hypotensive effect of dietary prostaglandin precursor in hypertensive man. Prostaglandins. 1978;15:193–197. doi: 10.1016/s0090-6980(78)80018-5. [DOI] [PubMed] [Google Scholar]
- 4.Mathias MM, Dupont J. The relationship of dietary fats to prostaglandin biosynthesis. Lipids. 1979;14:247–252. doi: 10.1007/BF02533877. [DOI] [PubMed] [Google Scholar]
- 5.Iacono JM, Dougherty RM. The role of dietary polyunsaturated fatty acids and prostaglandins on reducing blood pressure and improving thrombogenic indices. Prev Med. 1983;12:60–69. doi: 10.1016/0091-7435(83)90172-x. [DOI] [PubMed] [Google Scholar]
- 6.Keys A. Coronary heart disease in seven countries. Circulation. 1970;41(suppl 1):1211. [PubMed] [Google Scholar]
- 7.Williams PT, Krauss RM, Kindel S, et al. Relationship of dietary fat and protein intake to atherogenic lipoproteins in men. Am J Clin Nutr. 1986;44:788–797. doi: 10.1093/ajcn/44.6.788. [DOI] [PubMed] [Google Scholar]
- 8.Mattson FH, Grundy SM. Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. J Lipid Res. 1985;26:194–202. [PubMed] [Google Scholar]
- 9.Becker N, Illingworth DR, Alaupovic P, et al. Effects of saturated, monounsaturated, and w-6 polyunsaturated fatty acids on plasma lipids, lipoproteins, and apolipoproteins in humans. Am J Clin Nutr. 1983;37:355–360. doi: 10.1093/ajcn/37.3.355. [DOI] [PubMed] [Google Scholar]
- 10.Rao RH, Rao UB, Srikantia SG. Effect of polyunsaturate-rich vegetable oils on blood pressure in essential hypertension. Clin Exp Hypertens. 1981;3:27–38. doi: 10.3109/10641968109037166. [DOI] [PubMed] [Google Scholar]
- 11.Smith U. Regional differences and effect of cell size on lipolysis in human adipocytes. In: Angel A, editor. The Adipocyte and Obesity: Cellular and Molecular Mechanisms. New York: Raven Press; 1983. pp. 245–250. [Google Scholar]
- 12.Wilmore JH, Behnke AR. An anthropometric estimation of body density and lean body weight in young men. J Appl Physiol. 1969;27:25–31. doi: 10.1152/jappl.1969.27.1.25. [DOI] [PubMed] [Google Scholar]
- 13.Wood PD, Haskell WL, Blair SN, et al. Increased exercise level and plasma lipoprotein concentrations: A one-year randomized, controlled study in sedentary middle aged men. Metabolism. 1983;32:31–39. doi: 10.1016/0026-0495(83)90152-x. [DOI] [PubMed] [Google Scholar]
- 14.Metropolitan Life Insurance Company, New York. New weight standards for men and women. Stat Bull. 1959 Nov-Dec [Google Scholar]
- 15.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–836. [Google Scholar]
- 16.Edgington ES. Randomization Tests. New York: Marcel Dekker Inc; 1980. [Google Scholar]
- 17.Efron B. The Jackknife, The Bootstrap, and Other Resampling Plans. Philadelphia: Society for Industrial and Applied Mathematics; 1982. [Google Scholar]
- 18.Blood Pressure Levels of Persons 6-74 years, US 1971-1974, US Dept of Health, Education, and Welfare publication (HRA) 78-1648. National Center for Health Statistics; 1977. [PubMed] [Google Scholar]
- 19.Boyer JL, Kasch FW. Exercise therapy in hypertensive men. JAMA. 1970;211:1668–1671. [PubMed] [Google Scholar]
- 20.Greene SB, Aavedal MJ, Tyroler HA, et al. Smoking habits and blood pressure change: A seven year follow up. J Chronic Dis. 1977;30:401–413. doi: 10.1016/0021-9681(77)90034-0. [DOI] [PubMed] [Google Scholar]
- 21.Taylor CB. Comprehensive Handbook of Behavioral Medicine. Vol. 1. New York: Spectrum Books; 1980. Behavioral approaches to hypertension; pp. 55–88. [Google Scholar]
- 22.Stamler R, Stamler J, Riedlinger WF, et al. Weight and blood pressure. JAMA. 1978;240:1607–1610. doi: 10.1001/jama.240.15.1607. [DOI] [PubMed] [Google Scholar]
- 23.Blair D, Habicht JP, Sims EAH, et al. Evidence for an increased risk for hypertension with centrally located body fat and the effect of race and sex on this risk. Am J Epidemiol. 1984;119:526–540. doi: 10.1093/oxfordjournals.aje.a113770. [DOI] [PubMed] [Google Scholar]
- 24.Rosenman RH. The interview method of assessment of the coronary-prone behavior pattern. In: Dembroski TM, Weiss SM, Shields JL, et al., editors. Coronary Prone Behavior. New York: Springer-Verlag NY Inc; 1978. pp. 62–64. [Google Scholar]
- 25.Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales of the MMPI. J Appl Psychol. 1954;38:414–418. [Google Scholar]
- 26.Thurstone LL. Thurstone Temperment Scale. Chicago: Science Research Association; 1953. [Google Scholar]
- 27.Spielberger CD, Gorsuch RL, Lukshene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, Calif: Consulting Psychological Press; 1970. [Google Scholar]
- 28.Dempsey PA. A unidimensional scale for the MMPI. J Consult Clin Psychol. 1964;281:364–370. doi: 10.1037/h0045123. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald MC, Kline RL, Mogenson GJ. Dietary linoleic acid and salt-induced hypertension. Can J Physiol Pharmacol. 1981;59:872–875. doi: 10.1139/y81-130. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman P, Taube H, Forester K, et al. Influence of linoleic acid content on arterial blood pressure of salt loaded rats. Acta Biol Med Ger. 1978;37:863–867. [PubMed] [Google Scholar]
- 31.Ten Hoor F, Van De Graff HM. The influence of a linoleic acid-rich diet and acetyl salicyclic acid on NaCl induced hypertension, Na+-H2O-balanced and urinary prostaglandin excretion in rats. Acta Biol Med Ger. 1978;37:875–877. [PubMed] [Google Scholar]
- 32.Greon JJ, Tijong KB, Koster M, et al. The influence of nutrition and ways of life on blood cholesterol and the prevalence of hypertension and coronary heart disease among Trappist and Benedictine monks. Am J Clin Nutr. 1962;10:456–470. doi: 10.1093/ajcn/10.6.456. [DOI] [PubMed] [Google Scholar]
- 33.Sacks FM, Rosner B, Kass EH. Blood pressure in vegetarians. Am J Epidemiol. 1974;100:390–398. doi: 10.1093/oxfordjournals.aje.a112050. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong B, Van Merwyk AJ, Coates H. Blood pressure in Seventh Day Adventist. Am J Epidemiol. 1977;105:444–449. doi: 10.1093/oxfordjournals.aje.a112403. [DOI] [PubMed] [Google Scholar]
- 35.Puska P, Nissinen A, Vartiainen E, et al. Controlled, randomized trial of the effect of dietary fat on blood pressure. Lancet. 1983;1:1–5. doi: 10.1016/s0140-6736(83)91556-8. [DOI] [PubMed] [Google Scholar]
- 36.Margetts BM, Beilin LJ, Armstrong BK, et al. Dietary fats and blood pressure. Aust NZ J Med. 1984;14:444–447. doi: 10.1111/j.1445-5994.1984.tb03612.x. [DOI] [PubMed] [Google Scholar]
- 37.Jacob SW, Francone CA, Lossow WJ. Structure and Function in Man. 5. Philadelphia: WB Saunders Co; 1982. pp. 385–386. [Google Scholar]
- 38.Ganong WF. Review of Medical Physiology. Los Altos, Calif: Lange Medical Publications; 1963. pp. 479–480. [Google Scholar]
- 39.Berne RM, Levy MN. Physiology. St Louis: CV Mosby Co; 1983. pp. 512–513. [Google Scholar]
- 40.Salonen JT, Toumilehto J, Tanskanen A. Relation of blood pressure to reported intake of salt, saturated fats, and alcohol in healthy middle-aged populations. J Epidemiol Commun Health. 1983;37:32–37. doi: 10.1136/jech.37.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarron DA, Morris CD, Henry HJ, et al. Blood pressure and nutrient intake in the United States. Science. 1984;224:1392–1398. doi: 10.1126/science.6729459. [DOI] [PubMed] [Google Scholar]
- 42.Vague J. The degree of masculine differentiation of obesities: A factor determining predisposition to diabetes, arteriosclerosis, gout, and uric calculus disease. Am J Clin Nutr. 1956;4:20–34. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]
- 43.Evans DJ, Hoffmann RG, Kalkhoff RK, et al. Relationship of androgenic activity to body fat topography, fat cell morphology, and metabolic aberrations in premenopausal women. J Clin Endocrinol Metab. 1983;57:304–310. doi: 10.1210/jcem-57-2-304. [DOI] [PubMed] [Google Scholar]
- 44.Porter GA. Chronology of the sodium hypothesis and hypertension. Ann Intern Med. 1983;98:720–723. doi: 10.7326/0003-4819-98-5-720. [DOI] [PubMed] [Google Scholar]
- 45.Hodges RE, Rebello T. Carbohydrates and blood pressure. Ann Intern Med. 1983;98:838–841. doi: 10.7326/0003-4819-98-5-838. [DOI] [PubMed] [Google Scholar]
- 46.Langford HG. Dietary potassium and hypertension: Epidemiologic data. Ann Intern Med. 1983;98:770–772. doi: 10.7326/0003-4819-98-5-770. [DOI] [PubMed] [Google Scholar]
- 47.McCarron DA. Calcium and magnesium nutrition in human hypertension. Ann Intern Med. 1983;98:800–805. doi: 10.7326/0003-4819-98-5-800. [DOI] [PubMed] [Google Scholar]
- 48.Anderson JW. Plant fiber and blood pressure. Ann Intern Med. 1983;98:842–845. doi: 10.7326/0003-4819-98-5-842. [DOI] [PubMed] [Google Scholar]
- 49.Friedman GD, Klatsky AL, Siegelaub AB. Alcohol intake and hypertension. Ann Intern Med. 1983;98:846–848. doi: 10.7326/0003-4819-98-5-846. [DOI] [PubMed] [Google Scholar]
- 50.Fortmann SP, Haskell WL, Vranizan KM, et al. The association of blood pressure and dietary alcohol: Differences by age, sex, and estrogen use. Am J Epidemiol. 1983;118:497–507. doi: 10.1093/oxfordjournals.aje.a113655. [DOI] [PubMed] [Google Scholar]
- 51.Engstrom AM, Tobelman RC. Nutritional consequences of reducing sodium intake. Ann Intern Med. 1983;98:870–871. doi: 10.7326/0003-4819-98-5-870. [DOI] [PubMed] [Google Scholar]


