SUMMARY
Background
The Drosophila bHLH gene dimmed promotes a neurosecretory/neuroendocrine phenotype in cells but is not associated with specific neuropeptides or neurohormones. Rather, it is expressed by those peptidergic neurons that project long axons and appear to produce large amounts of secretory peptides. Here we genetically transform non-peptidergic neurons in Drosophila to study DIMM’s action mechanisms.
Results
Non-peptidergic neurons normally fail to accumulate ectopic neuropeptides. We now show they will do so when they are also forced to express ectopic DIMM. Furthermore, mass spectrometry shows that photoreceptors, which are normally non-peptidergic, fail to process an ectopic neuropeptide precursor to make bioactive peptides, but will do so efficiently when DIMM is co-misexpressed. Likewise photoreceptors, which normally package the fast neurotransmitter histamine within small clear synaptic vesicles, now produce numerous large dense-core vesicles (LDCVs) when they misexpress DIMM. These novel LDCVs accumulate ectopic neuropeptide when photoreceptors co-misexpress a neuropeptide transgene. Thus, DIMM-expressing photoreceptors no longer accumulate histamine and lose synaptic organelles critical to their normal physiology.
Conclusions
These findings indicate that DIMM suppresses conventional fast neurotransmission and promotes peptidergic neurosecretory properties. We conclude that DIMM normally provides a comprehensive transcriptional control to direct the differentiation of dedicated neuroendocrine neurons.
Keywords: Drosophila, neuropeptide, neurosecretory, dimmed, bHLH, photoreceptor neuron, mass spectrometry, large dense-core vesicle, Regulated Secretory Pathway, ultrastructure
INTRODUCTION
After translation, neuropeptide precursors and peptide hormones enter the Golgi and are then routed to the Regulated Secretory Pathway [1]. Within that pathway, they are packaged into, and ultimately released from, large dense-core vesicles (LDCVs), similar to ones that package exocrine secretory proteins [2]. LDCVs possess an osmiophilic dense core and are large (>60 nm in diameter), being thus distinguished in appearance and size from small synaptic vesicles (SSVs - 30–50 nm) that contain fast-acting neurotransmitters like acetylcholine, glutamate, or GABA [3]. SSVs and LDCVs co-populate single neurons and are released by Ca2+-triggered exocytosis [4]. While in the Regulated Secretory Pathway, neuropeptide precursors are enzymatically-processed to intermediate and final forms, via cleavage and other post-translational modifications. Despite several mechanistic studies on the Regulated Secretory Pathway and the proteins that control it [5], [6] and [7], very few have addressed the underlying genetic mechanisms: mechanisms that direct the maturation of peptidergic cellular properties, and that provide proper scaling and modulation of the pathways by which peptides and peptide hormones are packaged and released.
In fact, such dedicated genetic regulation is strongly indicated in several systems. Perinatal pancreatic acinar cells produce secretory proteins, but secrete them for several weeks constitutively, not in a regulated manner [8]. PC-12 and AtT-20 cell variants are deficient in the display of the Regulated Secretory Pathway but are otherwise normal [9] and [10]. Finally, two recent genetic studies in Drosophila that reveal intrinsic differences in the capacity of neurons to accumulate neuropeptides ectopically [11] and [12] have concluded that peptidergic neurons have an enhanced ability to accumulate and/or release neuropeptides compared with neurons that primarily release classical neurotransmitters. Here, we examine the hypothesis that, in Drosophila, a specific basic helix-loop-helix (bHLH) transcription factor gene called dimmed (dimm) is fundamental to that intrinsic mechanism and that DIMM underlies the multi-level organization of a major cellular phenotype – the peptidergic neurosecretory cell.
In Drosophila, DIMM expression is highly restricted to neurosecretory cells, but these cells are not homogeneous, and do not exclusively express any particular neuropeptide [13], [14] and [15]. In loss-of-function mutants, dimm-expressing cells survive but fail to accumulate neuropeptides or dedicated processing enzymes [13], [15] and [16]. DIMM is a transcription factor, and to date its only defined gene target is peptidylglycine-α –hydroxylating monooxygenase (PHM) which encodes a neuropeptide amidating enzyme [17]. Here we adapt a gain-of-function strategy to enumerate DIMM’s further actions at the cellular and sub-cellular levels. We first confirm the inability of non-peptidergic neurons in Drosophila to accumulate appreciable amounts of ectopic neuropeptides, and then show that that failure is overcome by supplying ectopic DIMM. We demonstrate that DIMM confers upon normally non-peptidergic photoreceptor neurons each of several critical cellular properties characteristic of dedicated peptidergic neurons. Together our observations support the hypothesis that DIMM organizes the specialized features of the peptidergic neurosecretory cell fate.
RESULTS
Misexpression of DIMM and/or Neuropeptide Precursors in the Larval Drosophila CNS
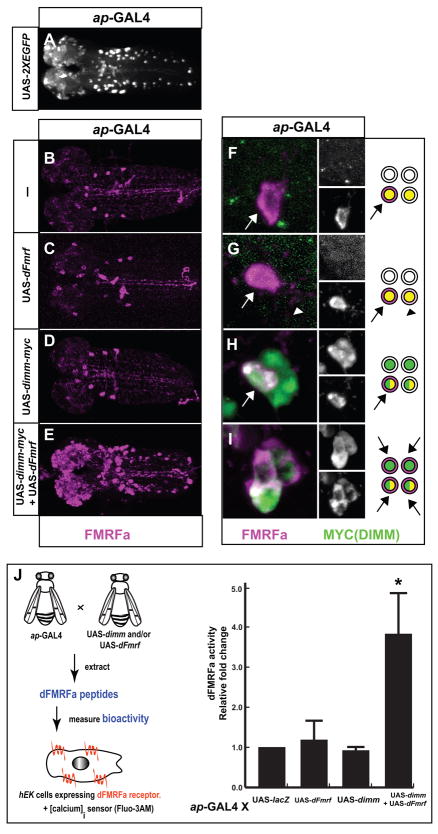
We first used the apterous-GAL4 driver (ap-GAL4: [18]) in the larval CNS to drive either a UAS-neuropeptide transgene, or a UAS-dimm transgene, or both (Figure 1). ap-GAL4 was used because it expresses in a small number of identified DIMM-positive peptidergic neurons [15] and [19] together with a much larger number of DIMM-negative neurons (Figure 1A). We studied two neuropeptides -, either dFMRFamide (dFMRFa) or Pigment-Dispersing Factor (PDF). Misexpression of neither a single UAS-transgene encoding either precursor, UAS-dFmrf (Figure 1C – ap > dFmrf), nor UAS-Pdf (Figure S1B – ap > Pdf), nor a single UAS-transgene encoding dimm alone (ap > dimm: Figures 1D and S1C), produced a clear difference in the overall intensity of immunolabeling for the cognate neuropeptide. The number of novel dFMRFa- or PDF-positive cells (produced, respectively, by UAS-dFmrf or by UAS-Pdf) was typically less than 20. However, co-misexpression of UAS-dimm with either UAS-dFmrf or UAS-Pdf produced greatly enhanced peptide expression in several hundreds of novel neurons (Figures 1E and S1D).
Figure 1. Ectopic dimm Enables Non-Peptidergic Neurons to Accumulate Ectopic Neuropeptide.
(A) The expression pattern of ap-GAL4 (visualized by driving UAS-EGFP) in the larval central nervous system (CNS - ap >EGFP). (B–E) Whole mount single-immunolabeling with anti-FMRFa of the larval CNS of different genotypes. (F–I) Whole mount double-immunolabeling with anti-FMRFa (magenta) and anti-MYC (DIMM, green) in the four-cell larval Tv cluster in the same series of genotypes as indicated in the left column. The anti-FMRFa labeling was performed with anti-FMRFa (anti-PT2-5); comparable results were found with anti-proFMRFa (anti-CT–data not shown). All four cells are ap-GAL4 positive. (B and F) Isoparental control, ap-GAL4. (C and G) ap > dFmrf. (D and H) ap > dimm-myc. (E and I) ap > dimm-myc, dFmrf. The diagrams represent the expression of dFMRFa (magenta), endogenous DIMM (yellow), and DIMM-MYC (green) among the four cells in the Tv cluster in each of these genotypes. In ap > dFmrf larvae, the two peptidergic cells in the Tv cluster accumulate dFMRFa, either strongly (Tv cell - arrow) or weakly (Tvb cell - arrowhead). (H) ap > dimm-myc misexpression in the Tv cluster increases dFMRFa immunolabeling only in the peptidergic neuron (arrow); whereas co-misexpression (ap > dimm-myc, dFmrf) gives dFMRFa expression in all four Tv neurons (I). Immunolabeling with anti-MYC represents ectopic DIMM expression. The confocal images were acquired as stacks of 130 images (1 m intervals). (J) Quantification of dFMRFa-like bioactivity by dFMRFa receptor activation assays. Scale bars: 50 μm.
We improved cellular resolution by focusing on the identified four-cell Tv cluster present in each thoracic hemi-segment. In the third-larval instar, two Tv cluster cells are peptidergic and DIMM-positive [19]: (i) the Tv neuron expresses PHM and dFMRFa neuropeptides (Figure 1F); and (ii) the Tvb neuron expresses PHM and neuropeptides derived from Neuropeptide-Like Precursor 1 (NPLP1) [15]. By contrast the other Tv-cluster neurons, Tva and Tvc, lack peptidergic character. Misexpression of UAS-dFmrf throughout the four-cell cluster produced strong dFMRFa immunolabeling in Tv (as is normal); weak, ectopic dFMRFa labeling in Tvb neurons (Figure 1G); but no ectopic dFMRFa labeling in either Tva or Tvc (Figure 1G). Misexpressing UAS-dimm alone throughout the cluster only increased dFMRFa immunolabeling in the Tv neuron (arrow in Figure 1H), but gave no ectopic expression in either of the two non-peptidergic neurons. However, co-misexpression of UAS-dFmrf with UAS-dimm promoted strong dFMRFa immunolabeling in each of the four Tv cluster neurons (Figure 1I). Similar results were obtained using UAS-Pdf (Figure S1). Thus ectopic accumulation in vivo of a neuropeptide precursor within non-peptidergic neurons is not easily accomplished. However, it is greatly promoted by co-misexpression with the transcription factor DIMM.
To quantify these outcomes, we measured ectopic neuropeptide activity using functionally-expressed dFMRFa receptor (encoded by CG2114) in mammalian hEK 293 cells and a calcium-based signaling assay (Figure 1J; see details in Supplemental Data). We found that misexpressing single UAS-dimm or UAS-dFmrf transgenes (driven by ap-GAL4) did not increase the amount of biologically-active dFMRFa peptides present in extracts of second-instar larvae. However co-misexpression of the two UAS-transgenes together increased dFMRFa bioactivity about four-fold (Figure 1J), suggesting that DIMM was required to produce increases in functional neuropeptide at ectopic locations.
DIMM Permits Ectopic Neuropeptide to Accumulate in Non-Peptidergic Adult Photoreceptors
We hypothesized that DIMM-dependent, ectopic neuropeptide accumulation reflects the expression of peptidergic cell properties in non-peptidergic cells. To pursue this possibility with increased cellular resolution, we reproduced the same misexpression design in Figure 1 in a single class of non-peptidergic neurons, the photoreceptors of the adult compound eye. Importantly, the R1-R6 photoreceptors have a well-studied ultrastructure with no evidence of a peptidergic character; specifically there is no recorded photoreceptor cell expression of DIMM, nor of any specific neuropeptide, nor of LDCVs. To drive misexpression of transgenes in R1-R6 photoreceptors, we used either of two GAL4 drivers, Rhodopsin1 (Rh1)-GAL4 or GMR-GAL4. Rhodopsin 1 (Rh1), the opsin protein of R1-R6 photoreceptors is encoded by ninaE, and expressed late in pupal development after photoreceptor fate is fully established [20]. GMR-GAL4 reflects regulation of the glass gene [21] and drives expression of transgenes strongly in all photoreceptors, starting soon after they begin to differentiate. Thus Rh1 is a weak and relatively late-acting promoter, while GMR is strong and acts relatively early (Supplemental Figure S5).
DIMM Confers the Biochemical Properties of a Peptidergic Cell upon Non-Peptidergic Neurons
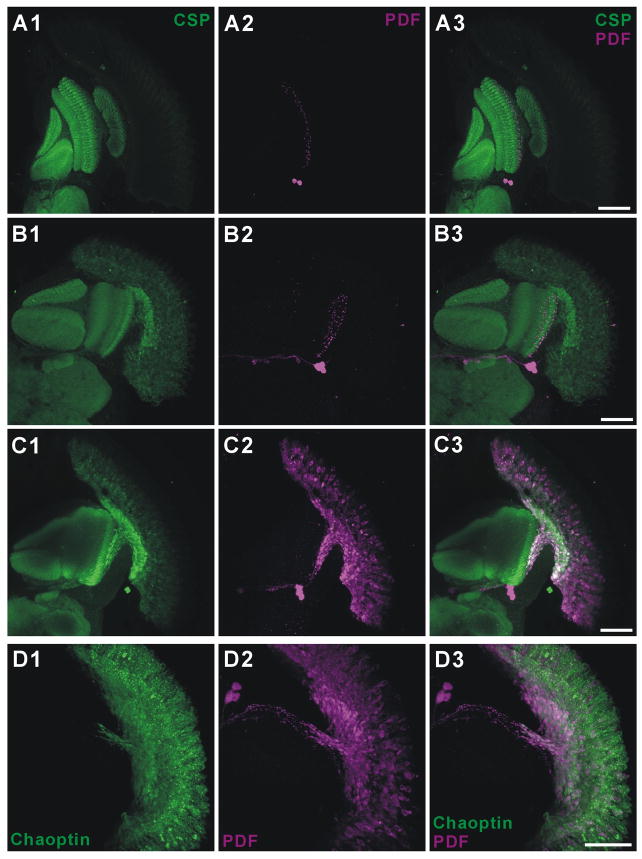
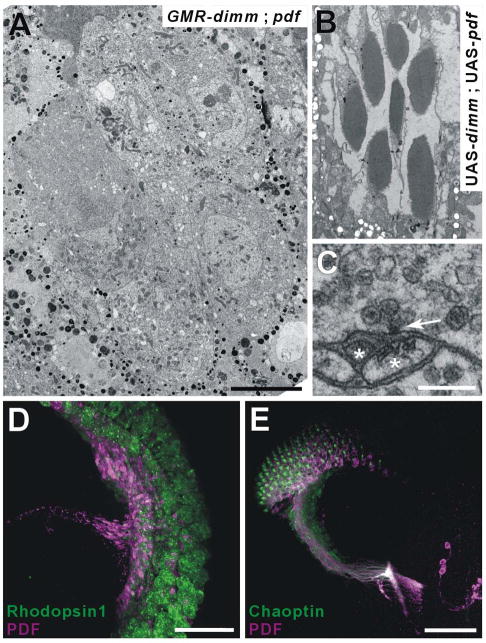
When photoreceptors misexpressed either Pdf (Figure 2A – GMR > Pdf) or dimm alone (Figure 2B - GMR > dimm), immunoreactivity to PDF was lacking in R1-R6 and restricted to normal varicosities that originate from PDF-expressing central brain neurons (cf. [12]; visible in Figures 2A2 and 2B2). In isoparental control GMR-GAL4 flies, PDF immunoreactivity was likewise not detected in photoreceptors (data not shown). In contrast, with double UAS misexpression (GMR > dimm, Pdf), photoreceptors now displayed ectopic PDF immunoreactivity (Figure 2C). PDF distribution was evaluated relative to the expression of a synaptic vesicle marker, cysteine string protein (CSP), and occurred throughout the entire photoreceptor neuron, relative to the expression pattern of photoreceptor membrane-specific protein, Chaoptin (Figure 2D). CSP expression showed an altered distribution in all DIMM misexpressing photoreceptors, extending into the cell bodies as well as their terminals (Figures 2B–D). The lamina was also distorted in flies with GMR > dimm, reduced to a thin, disordered layer that lay somewhere beneath the compound eye basement membrane (Figure 2C and 2D, see later). We could not detect ectopic PDF peptide accumulation with the later-onset Rh1-GAL4 driver however (data not shown). In summary, the accumulation of ectopic neuropeptide by photoreceptor neurons was completely dependent on dimm co-misexpression.
Figure 2. Ectopic dimm Enables Non-Peptidergic Photoreceptors to Accumulate Ectopic Neuropeptide.
GMR-driven co-misexpression of dimm and Pdf genes transforms photoreceptor cells to a peptidergic phenotype. (A–D) Stacks of 20–30 (43 in D) confocal images at 1 μm intervals from horizontal slices of the adult Drosophila optic lobe. (A) GMR >Pdf. (B) GMR > dimm. (C and D) GMR > dimm, Pdf. Preparations double-labeled either with anti-CSP (green; A1, B1, and C1) or photoreceptor-specific anti-Chaoptin (green; D1), and anti-PDF (magenta; A2, B2, C2, and D2). (A3, B3, C3, and D3) Corresponding merged images. Photoreceptor cells express PDF immunoreactivity only in flies doubly transformed by GMR >dimm, Pdf (C and D). Also note that CSP expresses in the retinae of flies when these misexpress dimm alone (B) or together with Pdf (C), whereas it expresses just in synaptic terminals of control GMR > Pdf flies (A). (D) GMR >dimm, Pdf head double-labeled with anti-PDF and anti-Chaoptin reveals that immunoreactivity to PDF fills the entire length of the photoreceptor neurons, including their somata. Scale bars: 50 μm.
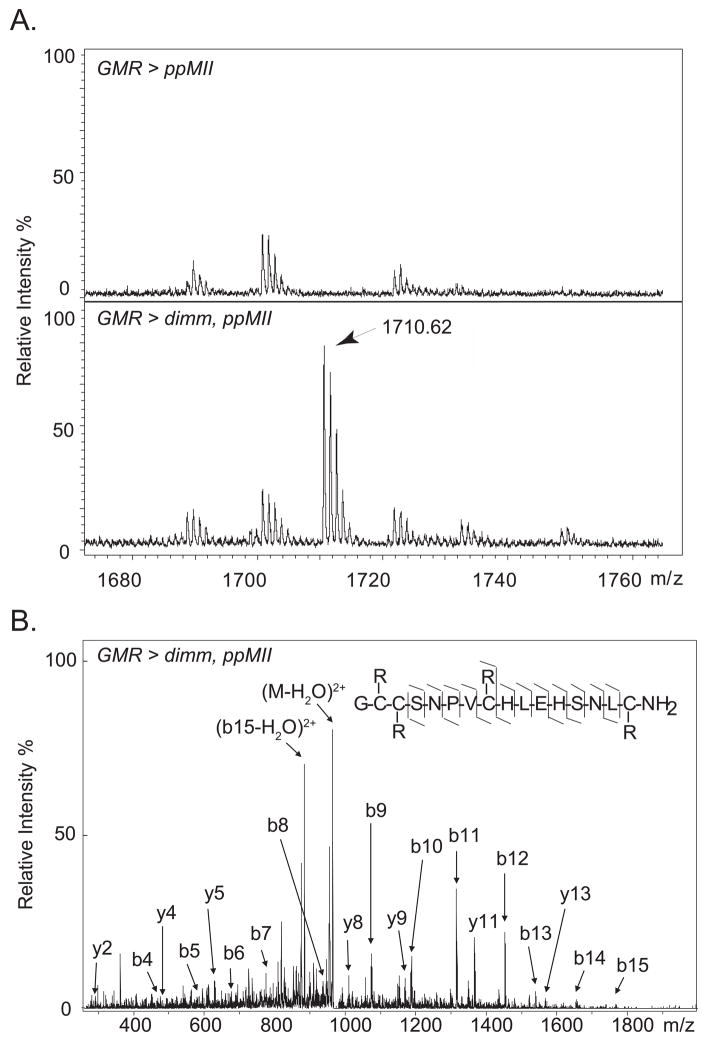
We next asked whether DIMM-dependent ectopic neuropeptide accumulation is accompanied by normal post-translational processing. Since mass spectrometry (MS) has characterized neuropeptides in insects including Drosophila [22], we employed mass spectrometric methods to investigate DIMM-dependent peptide processing. We constructed a novel neuropeptide precursor (called ppMII) consisting of the 16 amino acid MII secretory peptide from the poisonous snail Conus [23] fused to the Drosophila neuropeptide PDF precursor at a position that substitutes for the PDF peptide (Supplemental Data). If this precursor was fully processed, we predicted detection of MII peptide at m/z 1710.69, assuming C-terminal amidation and two separate disulfide bonds. We analyzed the peptide content of head extracts from the single and double transgenic lines (GMR > ppMII and GMR > ppMII; dimm) using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). The mass of the predicted MII peptide was observed in the double transgenic line but not in the single transgenic line (Figure 3A). To confirm the assignment of this peak, we reduced the disulfide-bonds with DTT and then alkylated the thiols with iodoacetamide. The mass spectrum of the extracts after the reaction showed a peak consistent with the theoretical mass shift from the MII peptide (m/z 1942.93) (Figure S2). After reduction/alkylation, the identity of this fully processed MII peptide was confirmed using electrospray ionization ion trap tandem mass spectrometry (Figure 3B). Additionally, no evidence, in either single or double transgenic lines, for any of 31 possible ppMII processing intermediates was found with liquid chromatography (LC) coupled to MS, a technique well suited to resolving individual peptides in complex biological samples. Together, these data strongly support the hypothesis that non-peptidergic cells can not normally process neuropeptide precursors. However, ectopic DIMM confers upon such cells a complete and efficient post-translational processing pathway for precursors of amidated secretory peptides.
Figure 3. MS and MS/MS Spectra of MII Peptide in Drosophila Head Extracts.
(A) MALDI-TOF mass spectra of fresh head extracts from the single transgenic line GMR > ppMII (top) and the double transgenic line GMR > dimm, ppMII (bottom). The peak at m/z 1710.62 (z=1) matches the expected MII peptide within 40 ppm. (B) ESI tandem mass spectrum of MII peptide after reduction/alkylation (m/z = 972.0, z=2). The assignment of b- and y-ions matches expected fragments within 0.07 Da. R=CH2CONH2.
DIMM Misexpression Alters the Vesicle Phenotype in Drosophila Photoreceptor Terminals
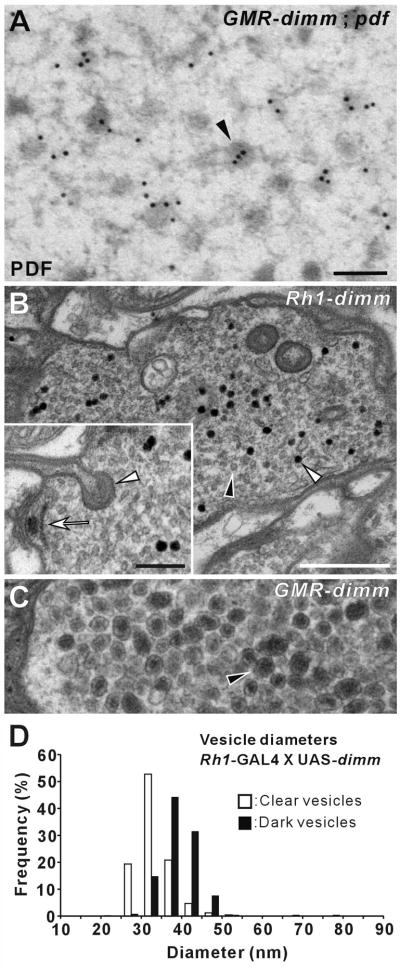
Using conventional EM, we examined vesicle phenotypes in the synaptic terminals of photoreceptors of three genotypes that misexpressed dimm and/or Pdf driven by the GMR-GAL4 driver. In GMR > dimm and GMR > dimm, Pdf, we observed completely atypical vesicles that resembled peptidergic LDCVs. In GMR >dimm flies, LDCVs had a diameter of 60.1 ± 1.2 nm (n = 2196 vesicles) (Figure 4B). In GMR > dimm, Pdf flies, LDCVs had a diameter of 60.2 ± 3.9 nm (n = 2589 vesicles) but typically a smaller dense core (Figure 4C). In both cases, the LDCVs were significantly larger than the clear SSVs in R1-R6 of an isoparental control, UAS-dimm (Figure 4D) which had a diameter of 33.1 ± 1.6 nm (n = 1754) (p < 0.001; t-test). The latter were the same size and appearance as those in wild-type flies, which contain histamine [24].
Figure 4. dimm Misexpression Transforms the Vesicle Phenotype of R1-R6 Photoreceptor Terminals.
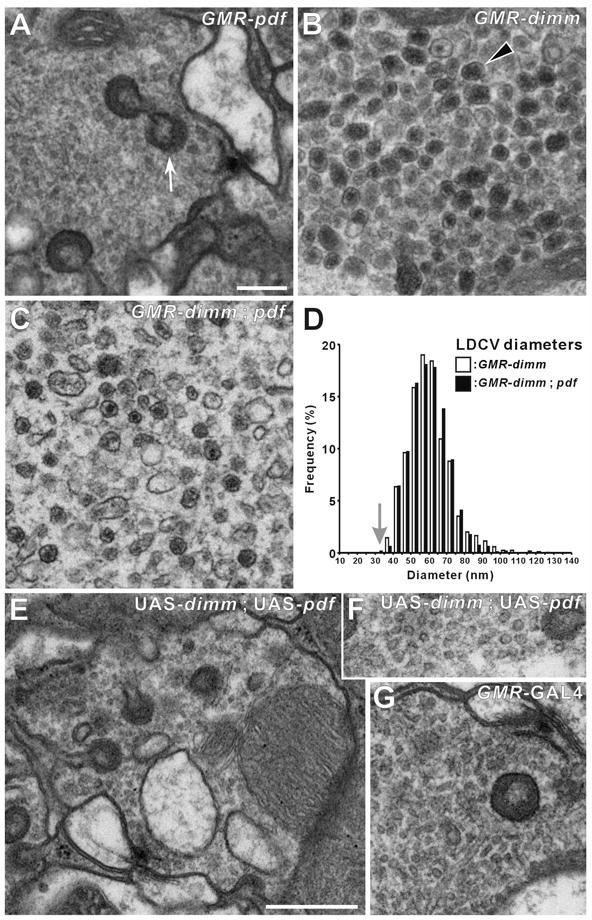
(A–C and E–G) EMs of R1-R6 terminals. (A) GMR > Pdf. (B) GMR > dimm. (C) GMR > dimm, Pdf. Ectopic large dense-core vesicles (LDCVs: arrowhead in B) appear in the terminals of single GMR > dimm (B) and double GMR > dimm, Pdf (C) UAS transgenic lines, but are lacking in the control GMR > Pdf (A). Capitate projection profiles (arrow, in A) identify profiles of R1-R6 terminals. (D) Distribution of the diameters of these ectopic LDCVs in genetically-transformed photoreceptor cells, either without (as in B) or with (as in C) Pdf. LDCV profile diameters in GMR > dimm and in GMR > dimm, Pdf flies have similar distributions that peak at about 60 nm, far larger than the mean of the small clear synaptic vesicles in an isoparental control, UAS-dimm fly (arrow). (E–G) Isoparental controls (UAS-dimm, UAS-Pdf) (E and F) and GMR-GAL4 (G), have small, clear synaptic vesicles, presynaptic T-bar ribbons, and capitate projections. Such normal synaptic phenotypes were also seen in terminals of photoreceptors misexpressing Pdf alone (A). Scale bars: 200 nm in A (also applies to B, C, F, and G); 500 nm in E.
When either Pdf or dFmrfa neuropeptide gene was misexpressed alone by GMR-GAL4, transformation of the vesicle phenotype did not occur and the photoreceptor terminals showed normal vesicle and synaptic phenotypes (Figures 4A and S3D). Hence dimm rather than the peptide gene is essential to transform the vesicles from a synaptic to a peptidergic phenotype. Small vesicles were either lacking or very few in the terminals of GMR > dimm, Pdf flies, but were retained among the LDCVs when misexpression was driven by the weaker Rh1-GAL4 driver (Figures S3B and S3C). LDCVs have not previously been reported in R1-R6 photoreceptor terminals of the fly, neither in the wild-type nor in any mutant so far described.
Ectopic neuropeptide is packaged within the ectopic LDCVs
DIMM permits photoreceptors to accumulate ectopic neuropeptide (Figure 2C), and also produces ectopic LDCVs within these cells (Figures 4B and 4C). We next asked whether the first result was explained by the second. Using immuno-gold EM, label to PDF was indeed localized to the ectopic LDCVs in transformed photoreceptor terminals (Figure 5A). We found 35.9 ± 29.5 (mean ± S.D.) particles per μm2 over the area of the LDCVs, including a 20-nm surrounding corridor (see Experimental Procedures), whereas only 8.2 ± 4.7 (mean ± S.D.) particles per μm2 fell over all other regions in the lamina (p < 0.001; t-test).
Figure 5. GMR- and Rh1-GAL4 Driver-Dependent DIMM-Transformed Vesicle Phenotypes.
(A–C) EMs of the lamina terminals of R1-R6. (A) Immunogold labeling with anti-PDF on a photoreceptor terminal from a GMR > dimm, Pdf fly. Large dense-core vesicles (LDCVs: arrowhead) in photoreceptor terminals transformed by dimm and Pdf co-misexpression are immunoreactive to PDF peptide. (B) Rh1 >dimm alone caused a population of slightly larger, dark vesicles (open arrowhead) to appear among normal clear synaptic vesicles (filled arrowhead). Compare the latter, shown at higher magnification in the inset, with the size of ectopic LDCVs (arrowhead) in GMR > dimm R1-R6 (C), at the same magnification. Note that Panel C is a portion of the same image shown in Figure 4B. Although a different type of vesicle formed in Rh1 > dimm R1-R6, the terminals had presynaptic T-bar ribbons (arrow: inset in B) and capitate projections (arrowhead: inset in B), just as in controls, but largely absent in GMR > dimm R1-R6. (D) Distribution of profile diameters of small, clear (open bars) and dark (closed bars) vesicles in R1-R6 genetically transformed by dimm misexpression under regulation of Rh1-GAL4 driver (as in B). The distribution was calculated from measurements of 1,432 clear and 251 dark vesicle profiles. Scale bars: 100 nm in A; 500 nm in B; 200 nm in inset of B (also applies to C).
The choice of GAL4 driver affects the extent and severity of the vesicle transformation
In R1-R6 terminals of Rh1 > dimm flies, we observed a different type of vesicle than when misexpression was under control of the stronger GMR-GAL4 driver: small dark vesicles formed in addition to the normal clear SSVs (Figure 5B). Clear SSVs were 33.0 ± 0.7 nm in diameter (n = 1432 vesicles, similar to those in the wild-type [24]); dark vesicles were slightly larger - 41.9 ± 6.1 nm (n = 251 vesicles), although that difference was not significant (Figure 5D; p > 0.05; t-test). The dark vesicles did not accumulate in large numbers (as did LDCVs with GMR > dimm, Figure 5C), but were numerous and easily distinguished from normal clear vesicles. The R1-R6 terminals with small dark vesicles had typical presynaptic T-bar ribbons (inset of Figure 5B), thus resembling wild-type synapses.
DIMM-expressing photoreceptor terminals lack normal presynaptic release sites
R1-R6 terminals in both GMR > dimm and GMR > dimm, Pdf also lacked normal presynaptic T-bar ribbons (Table 1). Normal sites were clearly revealed in photoreceptor terminals of the other genotypes (Table 1, Figures 4A, 4E, 4G, 5B inset, and S3). Photoreceptors in GMR >dimm, Pdf flies revealed, on the other hand, profiles of an alternative and abnormal synapse-like structure (Figure 6C, arrow): the presynaptic density was smaller than normal, resembling the pedestal of a T-bar ribbon, contacted by profiles of postsynaptic elements of normal appearance. The numbers of abnormal sites were smaller than those of normal R1-R6 tetrads in control flies (Table 1). R1-R6 terminals mis-expressing dimm via the Rh1-GAL4 driver did not show these effects, indicating additional dose- or onset-dependent differences between the GMR- and Rh1-driven transformations of R1-R6.
Table 1.
Organelle counts
| Genotype | CP/perimeter (μm−1) | Tetrad AZ/perimeter (μm−1) | * Abnormal presynaptic T-bar/perimeter (μm−1) | PR profile area (μm2) | PR profile perimeter (μm) |
|---|---|---|---|---|---|
| Rh1-Gal4 | 0.37 ± 0.087ab (a) | 0.17 ± 0.025a (a) | – | 1.59 ± 0.40a (a) | 5.30 ± 0.51a (a) |
| UAS-Dimm | 0.69 ± 0.20a (b) | 0.080 ± 0.058ab (b) | – | 1.77 ± 0.29a (a) | 6.23 ± 0.41a (ab) |
| Rh1-Gal4 X UAS-Dimm | 0.43 ± 0.063a (ab) | 0.086 ± 0.0055ab (b) | – | 2.05 ± 0.18a (a) | 6.60 ± 0.49a (ab) |
| long GMR-Gal4 X UAS-Dimm | 0.019 ± 0.027b (c) | 0b (c) | 0.030 ± 0.051a | 5.70 ± 2.81ab (ab) | 9.13 ± 2.33ab (bc) |
| long GMR-Gal4 X UAS-Dimm ; UAS-PDF | 0.031 ± 0.034b (c) | 0b (c) | 0.024 ± 0.013a | 10.24 ± 2.75b (b) | 12.58 ± 1.65b (c) |
All values: mean ± standard deviation. AZ, active zone; CP, capitate projection; PR, photoreceptor.
Abnormal presynaptic T-bar ribbons were presynaptic densities that lacked a platform, opposite which lay normal postsynaptic profiles.
Statistical analysis performed by Tukey test except for abnormal active zone organelles, in which a t-test was performed (p>0.05).
Significant differences exist between values with superscripts of different letters (those without parentheses, p=0.01; those in parentheses, p=0.05).
Figure 6. Misexpressing dimm and Pdf Causes a Radical Conversion of Membrane Trafficking Within Photoreceptor Cells.
(A–C) EMs of the retina (A and B) and lamina (C). (D and E) Confocal stacks (D: adult optic lobe, horizontal plane; E: larval optic disc wholemount). (A) Loss of the rhabdomeres in ommatidia in eyes of GMR >dimm, Pdf flies. (B) Emphasizing the loss in A, the isoparental control, UAS-dimm, UAS-Pdf, has normal rhabdomeres. Also note the size compared with the larger size in dimm and Pdf-double mutant ommatidia (A). (C) An abnormal synapse-like structure in the transformed lamina R1-R6 terminals of GMR >dimm, Pdf flies, comprising a presynaptic density (arrow), which lacks the bipartite composition of a control T-bar ribbon and resembles just its pedestal, and postsynaptic profiles (asterisks). (D) A rhabdomeric protein, Rhodopsin 1 (green) expresses even in the absence of membranous rhabdomeres in GMR >dimm, Pdf flies. Stack of 26 confocal images at 1 μm intervals. (E) Expression of photoreceptor-specific membrane protein, Chaoptin in GMR > dimm, Pdf flies at the larval stage. Stack of 29 confocal images at 4 μm intervals. Immunoreactivity to PDF (magenta) and that to Chaoptin (green) colocalize in the photoreceptor cell bodies and their axons. Scale bars: 5 μm in A (also applies to B); 200 nm in C; 50 μm in D and E.
DIMM suppresses the histamine transmitter phenotype of photoreceptors
We next asked whether the vesicle conversion imposed by DIMM indicated parallel conversion of the normal transmitter (histamine) phenotype of R1-R6. In wild-type flies, an antibody against histamine normally immunolabels photoreceptors, both their somata and, more strongly, their terminals (Figure S4A) [25]. DIMM-transformed photoreceptors in GMR > dimm, Pdf flies showed no detectable histamine labeling, despite the normal bright labeling of histamine immunoreactive neurons in the central brain (Figure S4B).
DIMM-expressing photoreceptors lack rhabdomeres
Examination of the retina in GMR > dimm, Pdf flies revealed that the photoreceptors lacked light-absorbing rhabdomeres (Figure 6A). Rhabdomeres were also absent in R1-R6 of GMR > dimm flies (data not shown). Rhabdomeres in isoparental control flies, UAS-dimm, UAS-Pdf (Figure 6B) and GMR-GAL4 (data not shown) appeared normal. When transformed by GMR > dimm misexpression, photoreceptors nevertheless still expressed a critical rhabdomeric component, the visual pigment Rhodopsin 1 (Figure 6D), and also expressed Chaoptin, at both larval and adult stages (Figures 2D and 6E).
In addition to the loss of normal T-bar ribbons, terminals of photoreceptors transformed by GMR > dimm, Pdf also showed other, non-photoreceptor-like features (Table 1). Photoreceptor somata are elongate, but those of transformed photoreceptors were oval, as revealed by immunolabeling with anti-Chaoptin (24B10, data not shown). Second, the lamina was thin and lacked its normal array of cartridges (data not shown). The medulla also failed to rotate into its normal position concentric with the retina, and instead lay at right angles. Third, photoreceptor terminals were enlarged and varicose (Table 1). Fourth, capitate projections, normal invaginations of photoreceptor terminals from surrounding glial cells that are sites for vesicle endocytosis [26], were essentially absent (Table 1). Importantly, the photoreceptors displaying such cellular conversion did not appear unhealthy and revealed none of the features of photoreceptors undergoing degeneration: (i) their cytoplasm was not dark, (ii) their mitochondria not dilated, and (iii) close apposition to postsynaptic partners was not lost (cf.[27]).
DISCUSSION
Peptide-containing LDCVs normally display several essential properties, including: (i) proper aggregation and sorting of their contents; (ii) proper enzymatic processing of peptide precursors; and (iii) normal trafficking and regulated exocytosis. Because all these processes require precise molecular machinery, it has been suggested that a coordinated program of gene expression - and a master gene to control it - is involved in LDCV biogenesis [28]. While many proteins have been associated with the Regulated Secretory Pathway, candidate regulators of the entire sub-cellular pathway have remained enigmatic. Our observations are consistent with the existence of a coordinated system of gene expression representing a core program of LDCV production and of peptidergic cell differentiation. In Drosophila neurosecretory cells, DIMM appears to be its principal transcriptional regulator.
Promoting Neuropeptide Accumulation by Transcriptional Control of the Regulated Secretory Pathway
Previous studies [11] and [12] have suggested that Drosophila neurons have intrinsically dissimilar abilities to process and/or accumulate neuropeptide substances. We have now shown that misexpression of the DIMM transcription factor can equalize these differences. Our results suggest DIMM plays a permissive role within non-peptidergic neurons by providing the cellular machinery to process peptide precursors, and by generating LDCVs in which ectopic secretory peptides may accumulate. We propose these outcomes may be accomplished by any of several non-exclusive mechanisms: (i) increasing LDCV biosynthesis; (ii) diminishing rates of LDCV exocytosis; or (iii) diminishing rates of LDCV turnover. The mammalian DIMM orthologue, Mist1 plays a role in the differentiation of serous exocrine cells: in Mist1-deficient mice, serous exocrine cells display a disorganized cytoskeleton, fewer and smaller LDCVs and reduced rates of LDCV exocytosis [29] and [30].
Our results indicate the degree to which a dedicated transcription factor can exert large-scale control on a specific sub-cellular function, here Regulated Secretory Cell activity. The literature suggests that DIMM belongs to an emerging group of transcription factors which target specific portions of cellular physiology. For example, the transcriptional co-activator PPAR- co-activator 1 α(PGC-1α) controls several key hepatic metabolic pathways [31]. Gain-of-function studies indicate that PGC-1 α not only activates batteries of gene targets that support mitochondrial gene expression, but also up-regulates mitochondrial biogenesis, often at the expense of other sub-cellular organelles like sarcomeres. Likewise, the bHLH-bZIP protein MiTF controls melanin formation and melanocytic differentiation [32] and the bHLH-bZIP protein TFEB controls lysosome biogenesis [33]. Together these studies illustrate how cells use dedicated and efficient transcriptional cascades to organize and regulate complex functions that require coordinated gene expression and a specialized suite of organelles. TFEB regulates lysosome biogenesis; MiTF regulates melanosome biogenesis; PGC-alpha regulates mitochondrial biogenesis. We have now shown that DIMM regulates LDCV biogenesis. Further analysis will help to define the battery of genes that is regulated by DIMM and which is dedicated to LDCV biogenesis and trafficking.
DIMM Promotes Neuropeptide Processing
A hallmark of neuropeptide cell biology is the stepwise enzymatic processing of precursor proteins via intermediate forms into one or several biologically-active peptides [34]. Processing enzymes reside within specific compartments of the Regulated Secretory Pathway and are expressed broadly, though at different levels according to region or cell type [35]. Three sets of observations indicate that DIMM-dependent ectopic neuropeptide accumulation involves recruitment of such processing pathways, and not simply a stabilization of the neuropeptide precursor protein. (i) Non-peptidergic neurons that misexpress DIMM and the dFMRFa precursor are labeled by antibodies specific to the processed (amidated) forms of those peptides (cf., [36]). (ii) DIMM-dependent ectopic dFMRFa accumulation produces a four-fold increase in dFMRFa bioactivity, again consistent with normal neuropeptide processing. (iii) Direct measures by mass spectrometry of an ectopic neuropeptide precursor (ppMII) and its predicted peptide products confirm that DIMM-dependent ectopic ppMII accumulation coincides with its efficient processing to a final predicted form. Together these results indicate that within non-peptidergic neurons DIMM can access, stabilize or perhaps de novo activate a collection of biochemical activities required for proper neuropeptide processing.
DIMM Promotes Neuropeptide Packaging into LDCVs
LDCVs derive from a transient vesicle population, the immature secretory granules (ISGs: [5]), that acquire a dark osmiophilic appearance and exclude certain initial proteins to form mature LDCV protein profiles. LDCV biogenesis is regulated independently of other secretory pathways and the machinery for exocytosis [28], but its controlling mechanisms are unknown. We find such dark granules in GMR>dimm transformed photoreceptor terminals, with a dark shell surrounding a darker core. Relative to these, GMR>dimm;pdf terminals contain LDCVs that are more variable in shape, with a lighter shell. Two principal hypotheses propose how cargo is transported from the trans-Golgi to final storage within mature LDCVs [28]. On the one hand, “on/off switches” may gate the process [6]; alternatively, the self-aggregating properties of cargo molecules are considered the fundamental driving force for LDCV biogenesis [7].
Here, we have shown that DIMM may function as a normal master switch for LDCV biogenesis and/or stabilization. It instigates accumulation of ectopic LDCVs that appear comparable to normal LDCVs but are entirely novel in photoreceptors. The weaker Rh1 > dimm combination produces a moderate accumulation of small (~40 nm) dark vesicles. With the stronger GMR > dimm combination, however, individual vesicles are larger (~60 nm) and less dark, but have a typical dense core; collectively these are more numerous and densely-packed, so that the ultrastructure of photoreceptor terminals resembles that of identified peptidergic neurosecretory varicosities. Significantly, DIMM-dependent LDCV accumulation occurs with or without a co-misexpressed neuropeptide transgene. Insofar as misexpression of DIMM alone is not an efficient means to drive ectopic neuropeptide gene expression, we speculate that such LDCVs may lack neuropeptide content. Our results suggest that LDCV biogenesis reflects both self-aggregation of constituent molecules and a higher level of organization by a master switch; the latter feature ensures the quality and stoichiometric blend of LDCV contents. In addition to their large numbers and normal appearance, DIMM-dependent LDCVs display normal function insofar as they contain neuropeptides, when genes for the latter are co-misexpressed with dimm. This critical feature likely explains DIMM’s ability to permit immunohistochemically detectable accumulation of ectopic neuropeptides in non-peptidergic neurons.
Cellular and Molecular Properties of Peptidergic Neurons Controlled by DIMM
Allan et al. [15] have proposed that DIMM serves two distinct roles in promoting peptidergic differentiation. The first is a potential unilateral action by the DIMM homodimer to drive specific gene expression: for example ectopic DIMM activates the neuropeptide biosynthetic enzyme gene PHM in all neurons [15]. In fact such activation is direct [17]. The second potential role for DIMM is to partner with other regulators to activate neuropeptide gene expression in diverse peptidergic neurons via different transcription factor codes. This proposal was based on the observation that ectopic DIMM, when combined with ectopic Apterous and Squeeze, produces many more ectopic neuropeptide FMRFa expressing cells than any of the three factors alone [15]. Our newest findings now suggest an alternative explanation for those results. Thus, apterous and squeeze are responsible for ectopic trans-activation of the neuropeptide gene (dFmrf), but the role of DIMM is not as a combinatorial partner, and remains separate from neuropeptide gene transcription. Instead, we suppose that DIMM produces the intracellular conditions that permit accumulation of ectopic neuropeptides. In this view, provision for neuropeptide gene transcription in the present experiments comes from the UAS-dFmrf or UAS-Pdf transgene, and not from UAS-dimm, which only contributes the cellular machinery to accommodate the ectopic neuropeptides. Further analysis of DIMM’s molecular targets will help decide between the merits of these alternative hypotheses.
DIMM and the Negative Regulation of Presynaptic Machinery
Misexpression of dimm in photoreceptors also produces a constellation of changes in sub-cellular organization. In particular, the alteration and/or loss of presynaptic release site organelles, T-bar ribbons, is striking because these synapses display constant number and composition in both the wild-type [37], and numerous mutants [38]. Possibly as a functional outcome of decreased transmission, there were fewer capitate projections within the terminals. The terminal regions of transformed photoreceptors become extended and greatly increased in volume in the double transgenic (GMR > dimm, Pdf). Defying these changes in R1-R6, lamina cells survive apparently unchanged, indicating their independence from normal histaminergic transmission. While most of the effects were exacerbated by co-misexpression with UAS-Pdf, they were all evident as significant trends in GMR>dimm alone. Rh1 > dimm did not block formation of tetrad presynaptic sites, a difference likely reflecting the earlier and/or stronger action of the GMR-GAL4 driver. Thus, DIMM affects a suite of features, including two that are critical for high-output synaptic neurotransmission at visual synapses: the T-bar ribbons, sites for vesicle exocytosis, and capitate projections, sites for vesicle endocytosis, as well as the normal architecture of the terminal itself.
There is very little correlation between sites of neuropeptide release and sites of synaptic vesicle exocytosis or endocytosis [4] and as a result these differences are compatible with the conversion of photoreceptor cells from the fate of a synaptic cell to that of a peptidergic neurosecretory cell. Just how complete this conversion is remains unclear, but evidently the normal histamine phenotype of DIMM-transformed R1-R6 is strongly suppressed, as revealed by a lack of histamine immunolabeling. Our results do not let us speculate how DIMM represses this normal transmitter phenotype but do suggest that DIMM-dependent peptidergic differentiation competes with and may outwardly extinguish the differentiation of co-transmitter features. Only a subset of Drosophila peptidergic neurons express DIMM [14]. Thus, while classic neurotransmitters and neuropeptides co-exist in most neurons [39], we speculate that peptidergic differentiation driven by DIMM displays a near exclusive dedication to the accumulation and release of neuropeptides.
The changes induced by DIMM misexpression, finally, are not limited to the presynaptic terminals of photoreceptors, but also appear in their cell bodies. Converted R1-R6 photoreceptors lose their hallmark organelle, the light-transducing rhabdomere while maintaining expression of Rhodopsin 1. In the pupa, rhabdomeres form by membrane accreted through the insertion of secretory vesicles into the rhabdomere terminal web [40]. These observations suggest that the loss of rhabdomeres in dimm-transformed R1-R6 may derive from changes in membrane trafficking. The dimm-dependent phenotypic changes in both presynaptic terminals and rhabdomeres may thus be related to changes in vesicle phenotype.
In summary, the constellation of DIMM-driven cellular properties within transformed photoreceptor neurons greatly resembles those of normal peptidergic neurosecretory cells. The potent and coordinated effects of DIMM in non-peptidergic neurons indicate that DIMM normally promotes a complete program of neurosecretory cell differentiation. This conclusion in turn highlights a need to define DIMM’s normal action mechanisms: in particular, there is a need to define the molecular specification it dictates and at which sub-cellular levels it exerts control over the Regulated Secretory Pathway.
Experimental Procedures
Flies
We misexpressed dimm by itself, or with a neuropeptide precursor (either dFmrf or Pdf), or the “neuropeptide-like” prepro-MII (ppMII) precursor described in Supplemental Data.
Immunohistochemistry
The sources, dilutions and specificities of primary antibodies are described in Supplemental Data. Immunohistochemistry for larval brains were performed as previously described [13] and [41]. The adult heads were embedded in agar and sliced at 80–100 μm thickness using a Vibratome. The slices were incubated in PBS with 1% sodium borohydride for 20min at 22°C to remove eye pigment and washed in PBS. Secondary antibodies conjugated to Alexa Fluor 488 (Molecular Probes) or to Cy3 (Jackson ImmunoResearch) were diluted 1:200.
Functional expression of dFMRFa receptor for quantification of dFMRFa peptide
Larvae were selected by genotype and homogenized in batches of 500 on ice in 900 μl of 90% methanol: 10% acetic acid. After pelleting tissue debris, supernatants were dried under vacuum, resuspended in 0.1% trifluroacetic acid (TFA) and 5% acetonitrile (ACN), then adsorbed to a Bond Elut C18 EWP column (Varian Inc.) and eluted with 0.1% TFA + 60% ACN, lyophilized and then dissolved in 1X PBS (pH 7.4). We studied hEK 293 cells that were stably-transfected with the dFmrf receptor (CG2114) cDNA [42] with additional details described in Supplemental Data.
Mass Spectrometry
Fly heads of the single and double transgenic lines (GMR > ppMII and GMR > ppMII; dimm) respectively were homogenized in acidified acetone (40:6:1, acetone:water:concentrated HCl, by volume). 1 μL of the homogenate was spotted on a stainless steel MALDI target, and co-crystallized with 1 μL of 50 mg/mL 2,5-dihydroxybenzoic acid (Sigma, St. Louis, MO) in a 70/30 (v/v) acetonitrile/water solution. The sample spots were analyzed with an Ultraflex II MALDI-TOF mass spectrometer (Bruker Daltonics). The remaining homogenate was centrifuged at 11,000 rpm for 5 min, and the supernatant was transferred to a vial placed in a SpeedVac (Thermo Electron Co.). Acetone in the extract solution was then evaporated, and an aqueous sample solution was obtained for subsequent LC-MS analysis. The separation was performed with a capillary LC instrument (Waters), and the LC was coupled with either a robotic fraction collector PROTEINEER fc (Bruker Daltonics) for LC-MALDI analysis or an HCTultra ESI ion trap mass spectrometer (Bruker Daltonics) for LC-ESI-MS/MS analysis of the reduced and alkylated peptide. The details on the peptide reduction/alkylation and LC-MS analysis are described in the Supplemental Data.
Electron Microscopy (EM)
We prepared fly heads for conventional EM using previously reported methods [43], and examined ultrathin sections of the lamina innervated by photoreceptor terminals of different genotypes. For immuno-EM by the post-embedding method, we prepared fly heads according to a previous protocol [44], using a secondary antibody conjugated to 10 nm gold particle (1:400; G7402, Sigma). Anti-PDF was used at 1:1,5000. We quantified particle density and sub-cellular morphometry according to procedures described in Supplemental Data.
Supplementary Material
Acknowledgments
We thank Mr. Zhiyuan Lu, Ms. Ripsik Kostyleva, Ms. Dorota Tarnogorska, Dr. Weihua Li, Ms. Jennifer Trigg and Ms. Jane Anne Horne for technical support. We thank the Bloomington Stock Center for fly stocks and the Developmental Studies Hybridoma Bank for antibodies. We thank Dr. Dick Nässel for reading an earlier draft of the manuscript and members of our laboratories for helpful commentary. This work was supported by the National Institute on Drug Abuse under Award No. P30 DA 018310 to the UIUC Neuroproteomics Center, by a P30 Neuroscience Core grant NS057105 to Washington University, and by grants from the NIH (NS 031609 to J.V.S.; EY-03592 to I.A.M.; and NS-21749 to P.H.T.), as well as by a Killam fellowship from the Canada Council (to I.A.M.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burgess TL, Kelly RB. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- 2.Palay S. The fine structure of the neurohypophysis. New York, NY: Hoeber; 1967. [Google Scholar]
- 3.Peters A, Palay SL, Webster HdeF. The fine structure of the nervous system. 3. New York, NY: Oxford University Press; 1991. [Google Scholar]
- 4.De Camilli P, Jahn R. Pathways to regulated exocytosis in neurons. Annu Rev Physiol. 1990;52:625–645. doi: 10.1146/annurev.ph.52.030190.003205. [DOI] [PubMed] [Google Scholar]
- 5.Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J. 1998;332( Pt 3):593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim T, Tao-Cheng JH, Eiden LE, Loh YP. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell. 2001;106:499–509. doi: 10.1016/s0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- 7.Beuret N, Stettler H, Renold A, Rutishauser J, Spiess M. Expression of regulated secretory proteins is sufficient to generate granule-like structures in constitutively secreting cells. J Biol Chem. 2004;279:20242–20249. doi: 10.1074/jbc.M310613200. [DOI] [PubMed] [Google Scholar]
- 8.Arvan P, Chang A. Constitutive protein secretion from the exocrine pancreas of fetal rats. J Biol Chem. 1987;262:3886–3890. [PubMed] [Google Scholar]
- 9.Day R, Benjannet S, Matsuuchi L, Kelly RB, Marcinkiewicz M, Chretien M, Seidah NG. Maintained PC1 and PC2 expression in the AtT-20 variant cell line 6T3 lacking regulated secretion and POMC: restored POMC expression and regulated secretion after cAMP treatment. DNA Cell Biol. 1995;14:175–188. doi: 10.1089/dna.1995.14.175. [DOI] [PubMed] [Google Scholar]
- 10.Grundschober C, Malosio ML, Astolfi L, Giordano T, Nef P, Meldolesi J. Neurosecretion competence. A comprehensive gene expression program identified in PC12 cells. J Biol Chem. 2002;277:36715–36724. doi: 10.1074/jbc.M203777200. [DOI] [PubMed] [Google Scholar]
- 11.Rao S, Lang C, Levitan ES, Deitcher DL. Visualization of neuropeptide expression, transport, and exocytosis in Drosophila melanogaster. J Neurobiol. 2001;49:159–172. doi: 10.1002/neu.1072. [DOI] [PubMed] [Google Scholar]
- 12.Helfrich-Förster C, Täuber M, Park JH, Mühlig-Versen M, Schneuwly S, Hofbauer A. Ectopic expression of the neuropeptide pigment-dispersing factor alters behavioral rhythms in Drosophila melanogaster. J Neurosci. 2000;20:3339–3353. doi: 10.1523/JNEUROSCI.20-09-03339.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewes RS, Park D, Gauthier SA, Schaefer AM, Taghert PH. The bHLH protein Dimmed controls neuroendocrine cell differentiation in Drosophila. Development. 2003;130:1771–1781. doi: 10.1242/dev.00404. [DOI] [PubMed] [Google Scholar]
- 14.Park D, Veenstra JA, Park JH, Taghert PH. Mapping peptidergic cells in Drosophila: where DIMM fits in. PLoS ONE. 2008;3:e1896. doi: 10.1371/journal.pone.0001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allan DW, Park D, St Pierre SE, Taghert PH, Thor S. Regulators acting in combinatorial codes also act independently in single differentiating neurons. Neuron. 2005;45:689–700. doi: 10.1016/j.neuron.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 16.Gauthier SA, Hewes RS. Transcriptional regulation of neuropeptide and peptide hormone expression by the Drosophila dimmed and cryptocephal genes. J Exp Biol. 2006;209:1803–1815. doi: 10.1242/jeb.02202. [DOI] [PubMed] [Google Scholar]
- 17.Park D, Shafer OT, Shepherd SP, Suh H, Trigg JS, Taghert PH. The Drosophila basic helix-loop-helix protein DIMMED directly activates PHM, a gene encoding a neuropeptide-amidating enzyme. Mol Cell Biol. 2008;28:410–421. doi: 10.1128/MCB.01104-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benveniste RJ, Thor S, Thomas JB, Taghert PH. Cell type-specific regulation of the Drosophila FMRF-NH2 neuropeptide gene by Apterous, a LIM homeodomain transcription factor. Development. 1998;125:4757–4765. doi: 10.1242/dev.125.23.4757. [DOI] [PubMed] [Google Scholar]
- 19.Park D, Han M, Kim YC, Han KA, Taghert PH. Ap-let neurons--a peptidergic circuit potentially controlling ecdysial behavior in Drosophila. Dev Biol. 2004;269:95–108. doi: 10.1016/j.ydbio.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 20.O’Tousa JE, Baehr W, Martin RL, Hirsh J, Pak WL, et al. The Drosophila ninaE gene encodes an opsin. Cell. 1985;40:839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- 21.Moses K, Rubin GM. Glass encodes a site-specific DNA-binding protein that is regulated in response to positional signals in the developing Drosophila eye. Genes Dev. 1991;5:583–593. doi: 10.1101/gad.5.4.583. [DOI] [PubMed] [Google Scholar]
- 22.Hummon AB, Amare A, Sweedler JV. Discovering new invertebrate neuropeptides using mass spectrometry. Mass Spectrom Rev. 2006;25:77–98. doi: 10.1002/mas.20055. [DOI] [PubMed] [Google Scholar]
- 23.Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new alpha-conotoxin which targets alpha3beta2 nicotinic acetylcholine receptors. J Biol Chem. 1996;271:7522–7528. doi: 10.1074/jbc.271.13.7522. [DOI] [PubMed] [Google Scholar]
- 24.Borycz JA, Borycz J, Kubow A, Kostyleva R, Meinertzhagen IA. Histamine compartments of the Drosophila brain with an estimate of the quantum content at the photoreceptor synapse. J Neurophysiol. 2005;93:1611–1619. doi: 10.1152/jn.00894.2004. [DOI] [PubMed] [Google Scholar]
- 25.Pollack I, Hofbauer A. Histamine-like immunoreactivity in the visual system and brain of Drosophila melanogaster. Cell Tissue Res. 1991;266:391–398. doi: 10.1007/BF00318195. [DOI] [PubMed] [Google Scholar]
- 26.Fabian-Fine R, Verstreken P, Hiesinger PR, Horne JA, Kostyleva R, et al. Endophilin promotes a late step in endocytosis at glial invaginations in Drosophila photoreceptor terminals. J Neurosci. 2003;23:10732–10744. doi: 10.1523/JNEUROSCI.23-33-10732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandstätter JH, Shaw SR, Meinertzhagen IA. Invagination of presynaptic ribbons in the fly’s optic lobe following loss of their target neuron. Proc Biol Sci. 1991;245:13–22. doi: 10.1098/rspb.1991.0082. [DOI] [PubMed] [Google Scholar]
- 28.Day R, Gorr SU. Secretory granule biogenesis and chromogranin A: master gene, on/off switch or assembly factor? Trends Endocrinol Metab. 2003;14:10–13. doi: 10.1016/s1043-2760(02)00011-5. [DOI] [PubMed] [Google Scholar]
- 29.Pin CL, Rukstalis JM, Johnson C, Konieczny SF. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J Cell Biol. 2001;155:519–530. doi: 10.1083/jcb.200105060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–222. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 31.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tachibana M, Takeda K, Nobukuni Y, Urabe K, Long JE, Meyers KA, Aaronson SA, Miki T. Ectopic expression of MITF, a gene for Waardenburg syndrome type 2, converts fibroblasts to cells with melanocyte characteristics. Nat Genet. 1996;14:50–54. doi: 10.1038/ng0996-50. [DOI] [PubMed] [Google Scholar]
- 33.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 34.Sossin WS, Fisher JM, Scheller RH. Cellular and molecular biology of neuropeptide processing and packaging. Neuron. 1989;2:1407–1417. doi: 10.1016/0896-6273(89)90186-4. [DOI] [PubMed] [Google Scholar]
- 35.Rehfeld JF. The endoproteolytic maturation of progastrin and procholecystokinin. J Mol Med. 2006;84:544–550. doi: 10.1007/s00109-006-0055-3. [DOI] [PubMed] [Google Scholar]
- 36.Jiang N, Kolhekar AS, Jacobs PS, Mains RE, Eipper BA, Taghert PH. PHM is required for normal developmental transitions and for biosynthesis of secretory peptides in Drosophila. Dev Biol. 2000;226:118–136. doi: 10.1006/dbio.2000.9832. [DOI] [PubMed] [Google Scholar]
- 37.Nicol D, Meinertzhagen IA. An analysis of the number and composition of the synaptic populations formed by photoreceptors of the fly. J Comp Neurol. 1982;207:29–44. doi: 10.1002/cne.902070104. [DOI] [PubMed] [Google Scholar]
- 38.Hiesinger PR, Zhai RG, Zhou Y, Koh TW, Mehta SQ, et al. Activity-independent prespecification of synaptic partners in the visual map of Drosophila. Curr Biol. 2006;16:1835–1843. doi: 10.1016/j.cub.2006.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hokfelt T, Millhorn D, Seroogy K, Tsuruo Y, Ceccatelli S, Lindh B, Meister B, Melander T, Schalling M, Bartfai T, et al. Coexistence of peptides with classical neurotransmitters. Experientia. 1987;43:768–780. doi: 10.1007/BF01945354. [DOI] [PubMed] [Google Scholar]
- 40.Li BX, Satoh AK, Ready DF. Myosin V, Rab11, and dRip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. J Cell Biol. 2007;177:659–669. doi: 10.1083/jcb.200610157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamanaka Y, Tanaka S, Numata H, Shiga S. Peptide immunocytochemistry of neurons projecting to the retrocerebral complex in the blow fly, Protophormia terraenovae. Cell Tissue Res. 2007;329:581–593. doi: 10.1007/s00441-007-0433-3. [DOI] [PubMed] [Google Scholar]
- 42.Johnson EC, Bohn LM, Barak LS, Birse RT, Nässel DR, Caron MG, Taghert PH. Identification of Drosophila neuropeptide receptors by G protein-coupled receptors-beta-arrestin2 interactions. J Biol Chem. 2003;278:52172–52178. doi: 10.1074/jbc.M306756200. [DOI] [PubMed] [Google Scholar]
- 43.Meinertzhagen IA. Ultrastructure and quantification of synapses in the insect nervous system. J Neurosci Methods. 1996;69:59–73. doi: 10.1016/S0165-0270(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 44.Hamanaka Y, Yasuyama K, Numata H, Shiga S. Synaptic connections between pigment-dispersing factor-immunoreactive neurons and neurons in the pars lateralis of the blow fly Protophormia terraenovae. J Comp Neurol. 2005;491:390–399. doi: 10.1002/cne.20712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.