Abstract
Background
Human oesophageal carcinoma is considered to be one of the most aggressive malignancies and has a very poor prognosis. The incidence of oesophageal cancer shows a gender bias and is higher in males compared with females, the ratio between males and females varying from 3:1 to 7:1. This sex ratio is not entirely attributable to differences in the prevalence of known risk factors between the sexes. The potential role of oestrogen receptors (ER) in oesophageal cancer has been debated for several years but the significance of the receptors in this cancer remains unknown. Most of the work has been based on immunohistochemistry and has not been validated with other available techniques. The inconsistencies in the published literature on the link between ER expression and oesophageal cancer warrant a thorough evaluation of the potential role of ERs in this malignancy. Even the expression of the two ER isoforms, ERα and ERβ, and its implications for outcome of treatments in histological subtypes of oesophageal tumours is ill defined. The aim of this article is to provide updated information from the available literature on the current status of ER expression in oesophageal cancer and to discuss its potential therapeutic role.
Methods and Results
We performed a comprehensive literature search and analysed the results regarding ER expression in oesophageal tumours with special emphasis on expression of different oestrogen receptors and the role of sex hormones in oesophageal cancer. This article also focuses on the significance of the two main ER subtypes and mechanisms underlying the presumed male predominance of this disease.
Conclusion
We postulate that differential oestrogen receptor status may be considered a biomarker of poor clinical outcome based on tissue dedifferentiation or advanced stage of the disease. Further, if we can establish the importance of oestrogen and its receptors in the context of oesophageal cancer, then this may lead to a new future direction in the management of this malignancy.
Introduction
Human oesophageal carcinoma is the eighth most common type of malignancy in the world [1], with approximately half a million people diagnosed annually worldwide [2]. Over the last three decades, the incidence of oesophageal cancer in many parts of the world has risen significantly [3-7]. The prevalence of the two main histological subtypes of oesophageal cancer, adenocarcinoma (AC) and squamous cell carcinoma (SCC) varies depending upon geographical location [8]. The AC is common in Europe and Australia [9] followed by the USA [8,9], while SCC predominates in Asian countries especially in the far East[10]. The incidence of oesophageal AC in the western world has risen rapidly over several years [11-13] whilst that of SCC has decreased[8], although increasing trends have been observed in Denmark and the Netherlands among men [14]. Carcinogens including dioxins, nitrosamines and polycyclic aliphatic hydrocarbons present in tobacco, processed meats and fried foods along with alcohol consumption and gastrooesophageal reflux disease and others have all been identified as risk factors for oesophageal cancer[15] although contribution of aetiological factors varies amongst histological subtypes of the disease. Figures 1 and 2 depict the risk factors for AC and SCC respectively.
Figure 1.
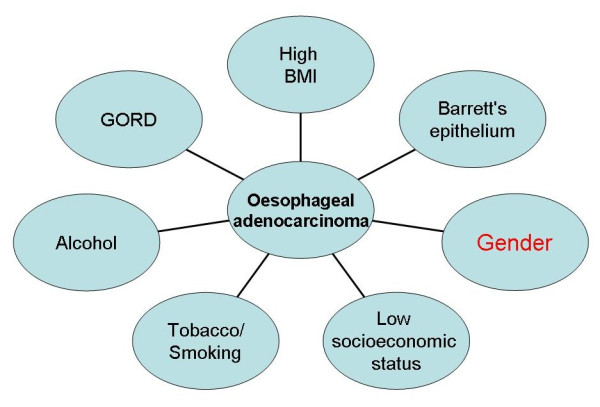
Aetiological factors for oesophageal adenocarcinoma.
Figure 2.
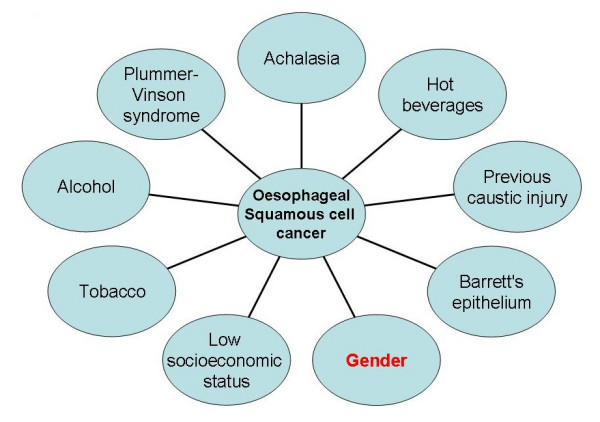
Aetiological factors for oesophageal squamous cell cancer.
Among the different treatment options, surgical resection is used most frequently to obtain locoregional control and long-term survival [16]. However, because of early tumour recurrence and metastasis, the overall five year survival after resection is around 35% [17,18]
The AC of the oesophagus is predominantly a male disease with a male to female ratio of 6-8:1 [14]. It is also reported that both the AC [19] and SCC [20] of the oesophagus are more common in males than females with a male to female ratio exceeding 3-6:1 or higher in some studies [20-22]. Barrett's oesophagus, identified as a potential risk factor for AC, also occurs predominantly in males with a male: female ratio ranging from 2:1 to 4:1 [23,24]. Lofdhal et al have suggested that the sex difference in oesophageal AC does not seem to be explained by differences in risk factor profile of known aetiological agents such as reflux, obesity and tobacco consumption [25].
Badwe et al (1994) studied the impact of age and sex on survival after curative resection for carcinoma of the oesophagus with life stable analysis showing a significantly better 5 year survival for women under 49 years of age (35%, CI 24-48) compared with men of the same age (16%.CI 8-27) (P < 0.008)[26]. The gender of the patient was found to be the second most significant determinant of survival (p = 0.002) after lymph node metastasis. These results of better survival benefit for women provides support for the hypothesis that the endocrine milieu in premenopausal women may prevent the micrometastases of the oesophageal malignancy and the consequent improved prognosis for oesophageal cancer [26].
A population-based study by Derakhshan et al, has suggested that the increased incidence of oesophageal cancer in females is age-related and occurs postmenopausally [27]. Measuring and correlating systemic sex steroid hormone levels and their interaction with their receptors in pre- and postmenopausal women may help elucidate the age-related incidence in postmenopausal females.
The observation that females appear to have a survival benefit compared to males [28,29] has led us to consider mechanisms through which oestrogens acting via the oestrogen receptor (ER) are implicated in the gender bias thus raising the possibility of using ER status as a positive or negative biomarker of disease outcome. Our recent work on oesophageal cancer has indicated overexpression of immunoreactive oestrogen receptor beta (ERβ) as compared to oestrogen receptor alpha (ERα) and androgen receptors in oesophageal cancer[30] (Figure 3).
Figure 3.
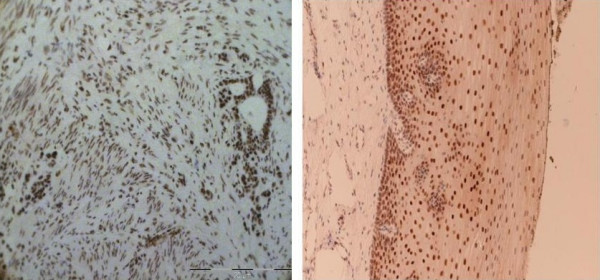
Immunohistochemical overexpression of oestrogen beta receptors.
Oestrogen receptors (ER)
In addition to their well-documented roles in the reproductive tract, diminished ovarian oestrogen levels are implicated in the development of osteoporosis and the raised risk of cardiovascular disease in postmenopausal women [31]. The actions of oestrogens, a group of C-18 steroids, are mediated principally via ERα and ERβ first cloned from rat prostate [32]. This discovery led to a reappraisal and new perspective on the significance of the ER in health and disease. The ERα and ERβ, isoforms are respectively encoded by two distinct genes (ESR1 and ESR2) located on chromosome 6q25.1 and chromosome 14q22-24[33,34]. The two receptors share common functional domains with a conserved (95% sequence homology) central DNA-binding domain thought to be involved in receptor dimerisation [35] (Figure 4). The ER also possesses two activation function domains AF1 and AF2 [36] with the former interacting with non-ER transcription factors while AF2 contains the ligand binding domain (LBD) [37]. Interestingly, AF1 in ER-β lacks functional activity [36]. Of the natural oestrogens that include oestrone and oestriol, oestradiol-17β (E2) has the highest affinity for both ER subtypes.
Figure 4.
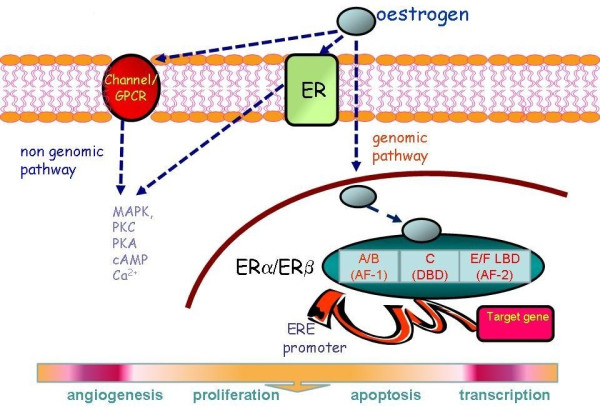
Pathways of oestrogen action. The ERα/ERβ is a simplified schematic of the ER. MAPK mitogen activated protein kinase, PKC protein kinase C, PKA protein kinase A, cAMP 3'-5' cyclic adenosine monophosphate, Ca2+ intracellular calcium, DBD DNA binding domain, LBD ligand binding domain.
The ER belongs to the NR3 steroid receptor class of the nuclear receptor superfamily and consistent with the mechanisms of action of this family, it operates via transcriptional regulation to cause downstream changes in gene expression. This follows dissociation of the intracellular ER from chaperone proteins, principally heat shock proteins (eg HSP90) on binding of ligand, thus releasing the ER-complex for attachment to oestrogen response elements located in the promoter region of target genes [31]. The recruitment of coregulators that either activate or repress gene transcription ultimately determines the cellular response. ER functions may also be mediated by non-genomic mechanisms that are predominantly transduced at the membrane and are acute in nature with the physiological response occurring within minutes as opposed to hours [38].
Expression of ER and other sex hormone receptors in oesophageal cancer
Conflicting data exist on the expression of sex steroid receptors in oesophageal cancer and hence their role in the progression of the disease. Lagergren et al., 1998 [21] suggested that in the absence of other known environmental risk factors with a sex distribution which is sufficiently skewed to explain the imbalance in the risk of adenocarcinoma, male predominance might be due to hormonal factors. Either high oestrogen and/or progesterone levels, low testosterone or a combination of these may be the reason why women are apparently protected from developing oesophageal cancer. If the aforementioned presumption is correct, then any treatment that increases oestrogen levels and/or decreases testosterone levels may potentially reduce the risk of developing adenocarcinoma of the oesophagus [21]. However this is a rather simplistic notion when one considers the multifactorial influences and underlying cellular mechanisms that shape development of this disease.
There is limited evidence on progesterone receptor expression in oesophageal cancer although Kalayarasan et al., have shown it is absent in both normal epithelial mucosa and oesophageal tumours [39]. With respect to the androgen receptor (AR), Tiffin et al., [40] demonstrated focal staining of this receptor in only two of ten patients [40] whilst Awan et al., [41] identified AR expression in the stromal component of oesophageal adenocarcinoma [41]. Nuclear and cytosolic AR expression in two newly established human oesophageal carcinoma cell lines (ES-25C and ES-8C) has also been shown [42]. Androgen receptors may be important mediators of oesophageal cancer as shown in studies where oesophageal SCC cell lines underwent enhanced growth when treated with testosterone in vitro studies [43,44]. Shinji Tanaka et al., are of the opinion that androgens too may play a role in the regulation of gene expression associated with malignant transformation [45].
In studies of the ER expression in oesophageal cancer, Nozoe et al., [46] suggested an inverse relationship between ERα and ERβ, in oesophageal squamous cell cancer [46]. Although Kalayarasan et al.,, investigating the expression of ER between oesophageal cancer and normal oesophageal mucosa reported no detectable immunohistochemical expression for ERα, the authors propose a positive correlation of ERβ status with tumour dedifferentiation, type and stage [39]. Interestingly, AC showed a higher mean score for ERβ expression as compared with SCC. Furthermore, ERβ positive immunoreactivity in tumour cells increased with dedifferentiation and increasing tumour stage in both types of oesophageal cancer and has prompted suggestions that ERβ status is a potentially useful marker of worsening disease progression [39]. In contrast, to the findings of Kalayarasan et al., [39] Tiffin et al., [40], identified mild to moderate staining of ER in most of their oesophageal tissue samples but the authors did not discriminate between the ER subtype detected [40]. Given the conflicting evidence on the ERα and ERβ expression in oesophageal cancer, Table 1 summarises the studies performed to date. Cui et al., [47] have also examined ER expression but patients in this study had oesophagogastric carcinomas likely originating from the stomach.
Table 1.
Studies assessing the risk of oesophageal cancer in relation to oestrogen receptors.
| No of patients | M:F | PR | ER-α | ER-β | Histological subtypes of OC | Source & affinity of ER AB | Significance/Dedifferentiation | |
|---|---|---|---|---|---|---|---|---|
| Kalayarasan et al., (India, 2008)[39] | 45 | 3:2 (SCC) 4:1(AC) |
0 | 0 | 45 | AC (n = 15) SCC (n = 30) AC>SCC |
Novacastra- ERα- Clone 6F11- ERβ- Clone EMR02- |
ERβ staining increases with dedifferentiation |
| Boone J et al, (Netherlands,2009)[54] |
108 (Tissue Microarray) |
0 | 0 | 100% s SCC | Dako- M7047 |
No staining found with ERα | ||
| Nozoe T et al.,(Japan, 2007)[46] | 73 | 10:1 | ND | 47 | 21 | 100% SCC | Santa Cruz- HC-20(α) H-150(β) |
ERα expression -unfavourable independent prognostic indicator |
| Tiffin et al., (UK,2003)[40] | 20 | 1:1 | ND | ND | ND | ER= +ve (n = 8 AC) |
Dako- NS |
Oestrogen receptors are more important than androgen receptors-require further investigations |
| Wang L et al., (China,1991)[56] | 48 | 19 | Unknown | Unknown | Unknown | Unknown | Gender & grade of tumour were influencing ER expression | |
| Akgun et al., (USA,2002)[49] | 31 | ND | ND | NA | 23 | AC | NS- MYEB(β) |
AC, BM show higher expression of ERβ |
| Liu et al., (USA, 2004)[55] | 33 | ND | ND | NA | ERβ 1 = 23/27 ERβ 2 = 22/27 ERβ 3 = 27/27 ERβ5 = 27/27 |
ACC>Barrett's metaplasia negative for dysplasia | In house- Human ER-β (amino acids 1-12). |
ERβ subtypes are overexpressed in oesophgael cancer as compared to its precursor lesions |
ND: Not determined; PR progesterone receptor; AB: antibody; AC: adenocarcinoma; BM: Barrett's metaplasia; NS: not specified; OC: oesophageal cancer; NA: not applicable
*Tiffin et al[40] (n = 20), Six patients with metaplasia also had positive ER staining
It has been postulated that in vivo growth of human oesophageal carcinoma cells mediated via sex hormone receptors is influenced by circulating hormone levels and can be manipulated by systemic oestradiol administration [48]. The effects of various doses of E2 on in vitro growth of these cell lines has established that one of the cell lines (ES-25C) showed significant inhibition at concentrations of 10-10 and 10-12 mol/l compared with the control, indicating a role for the oestrogen-ER system in growth inhibition of ER+ oesophageal cancer cell by oestradiol-17β [42,48]
In studies following the development of Barrett's to oesophageal cancer, it was found that of 31 patients who underwent oesophagectomy, more than 50% (23/31) were found to have positive staining for ER-β [49]. Among patients with high grade dysplasia,10 out of 11 (91%) showed positivity and this ratio was found to be 8 out of 11 (83%) in low grade dysplasia and 10 out of 15 in patients with Barrett's metaplasia having no dysplasia. The authors of the study conclude that premalignant Barrett's and oesophageal adenocarcinoma display positive ERβ immunoreactivity in a significantly high proportion [49]. Given the inconclusive data on studies of the ER subtype expression in cancer, the poor specificity of earlier antibodies against ERβ has highlighted the need to validate methods and reagents properly. This is especially applicable to studies designed to produce accurate and meaningful outcomes on which to base future applications of ERβ as a prognostic measure.
ERs and link with other cancers
Mounting evidence supports a role for the ER in halting or promoting cancer progression. Most of our current understanding on the biological significance of ERs in tumourigenesis has emerged from studies of breast cancer and the relationship between the expression of ERα and response to the nonsteroidal antioestrogen, tamoxifen. Oestrogens and ERs also play a vital role in the development or suppression of several other malignancies classified into four main subgroups [50,51] most of which express both ERα and ERβ [50,51]. Specifically, in the Women's Health Initiative study in which a cohort of 16,608 women were randomized into either a hormone replacement therapy (HRT) group or a non-HRT group [52], the risk of colorectal cancer was almost halved in women receiving HRT. Similarly, the association between HRT and risks of oesophageal and gastric cancer was studied in a nested-case control study where a total of 1619563 patients were identified from the General Practitioners Research Database in the UK. The conclusions of this latter study were that HRT leads to a 50% reduction in the risk of gastric adenocarcinoma but there was no relationship between HRT and oesophageal adenocarcinoma [53]. However, only a relatively small number of women with oesophageal cancer (n = 299) [53] were included which may have limited the power of the study. In addition, the lack of such association does not exclude more complex cellular and molecular interactions that are not detectable in this sort of clinical study.
Mechanisms underlying altered ER expression and activity in oesophageal cancer and therapeutic implications
Based on the available yet rather scarce literature on the potential association of ER receptor expression with oesophageal cancer [39,40,46,49,54-56], some of the mechanisms underlying ER and E2 interactions in oesophageal cancer are based on studies of other cancers in which ERs have been implicated and are briefly discussed below:
Differential ERα and ERβ expression and the ERα:ERβ ratio in cancer
In many cancers, ERα appears to be instrumental in promoting cell proliferation. However, recent studies have suggested that both mRNA and protein levels of ERβ may have greater significance in certain cancers. A loss of ERβ expression is observed in colorectal [57,58], prostate [59] and breast [60] carcinoma that all express high levels of ERβ in normal tissues [60,61]. Decreased ERβ levels are associated with improved disease outcomes and longer term disease-free survival in malignant mesothelioma attributed to the antiproliferative effects of ERβ[62]. The beneficial effects of HRT in colon cancer, which expresses very little ERα in normal colon, are likely to be mediated by downregulation of ERβ [58]. Evidence supports a possible protective role for ERβ in prostate cancer where a loss of ERβ expression accompanied the development of prostate cancer [59]. Interestingly, the small number of cancers that continued to express ERβ were positively correlated with a higher rate of relapse [59]. In malignant mesothelioma, attenuated ERβ expression appears to be an independent indicator of improved prognosis and survival [62]. Yet in tissues expressing both isoforms of the ER at comparable levels, the growth inhibitory effects of ERβ are less obvious thought to be due to the lack of AF1 ERβ activity. ERβ is known to bind to and suppress ERα function [63,64] thereby demonstrating inverse biological activity.
Activation of ER-α and ER-β involves the formation of dimers and as the two isoforms are coexpressed in many cell types, receptors may exist as ERα (αα) or ERβ (ββ) homodimers or as an ERαβ (αβ) heterodimer [65]. Homo- and heterodimerisation may also introduce diversity of tissue and cell-specific functions.
In oesophageal cancer, it is likely to be the relative abundance of ERα:ERβ, dominance of one ER dimer over another and their roles in many of oestrogen's nonendocrine functions that likely contribute to disease onset and severity.
Phosphorylation and ligand-independent activation of ER
Gene transcription via ER may proceed indirectly without binding of native ligand to oestrogen response elements involving instead protein-protein interactions. The most prominent for ERα appear to be the transcription factors, specificity protein (SP-1) and nuclear factor kappa b (NFκB), the proinflammatory transcription factor. The activator protein-1 (AP-1) complex of Jun/Fos hetero- or homodimers is a key regulator of cell proliferation with one of the target genes identified as cyclin D1. Depending on the whether ERα or ERβ is activated, the AP-1 complex acts in a reciprocal fashion to stimulate or inhibit cell proliferation. Ligand-independent activation may also determine the phosphorylation status of ERα which occurs principally at serine residues in the AF1 domain (Figure 4) [31]. Phosphorylation of ER may also occur via a plethora of bioactive mediators that include growth factors, cytokines and enzymes and has been linked with hormone-independent growth, loss of cell-cell adhesion and angiogenesis.
Proliferation and apoptosis
The mitogenic and growth-promoting actions of oestrogens in target tissues are well-established and are achieved in part by increased transcription of cell-cycle genes via ERα. However, in certain cancers where ERβ is considered protective, antiproliferative effects are achieved by cell cycle growth arrest for example by down-regulation of the cyclin D1 (CCND1) gene thereby preventing cellular progression from the G1 to S-phase of the cell cycle. ERβ may also inhibit gene transcription induced by ERα. These lines of evidence have led to the suggestion that ERβ acts as a tumour suppressor gene and is supported by findings that show localisation of ERβ to chromosome 14q is shared by other tumour suppressor genes that exert protective effects in prostate and ovarian cancer [61]. Regression of tumours and improved survival may be achieved by inhibition of cell proliferation as discussed or alternatively by increased apoptosis as has been observed in prostate cancer. Apoptosis involves a series of cellular events involving loss of membrane gradients, DNA fragmentation and caspase activity. In cancers, such as malignant mesothelioma [62], ERβ appears to be proapoptotic thus enabling it to destroy malignant cells whilst ERα has antiapoptotic activity which underpins its role in normal and abnormal cell proliferation.
Epigenetic modifications
Tumourigenesis may be triggered by epigenetic changes that involve modifications to chromatin structure including DNA methylation and altered histone acetylation thus causing downstream changes in gene expression [66]. Studies in prostate and breast cancer [67] have demonstrated hypermethylation of the ERβ promoter with subsequent silencing of ERβ expression but no evidence yet exists for altered ER methylation in oesophageal cancer.
Circulating oestrogen levels
In premenopausal women, ovarian E2 levels are high, largely attributable to the aromatisation of testosterone to oestradiol-17β within the ovary. For postmenopausal women in whom ovarian E2 levels are reduced, oestrone is the most abundant oestrogen formed from its precursor, androstenedione. Peripheral aromatization of oestrone from androgens in adipose tissue is one mechanism whereby circulating oestrogen levels may be increased, perhaps explaining in part the gender selectivity of oesophageal cancer. Although it is unlikely, that oestrogen levels will rise to those present in premenopausal women, given the lower affinity of oestrone for ERα, oestrone may still have the capability to confer oestrogenic effects but with less potency. To date, it is not known whether aromatase is produced by the oesophagus but if it is, then it may be factors such as the ratio of local oestrogen to androgen production as well as the form of oestrogen produced (oestrone, oestradiol, oestriol) that may underlie gender bias and the increased risk of postmenopausal women and males to oesophageal cancer compared with their premenopausal females. More scientifically robust studies as proposed by Hogan et al., are needed [68].
Therapeutic relevance of ERs to oesophageal cancer
Therapeutic modalities currently in place for modulation of ER activity include selective oestrogen receptor modulators (SERMs) e.g. tamoxifen which exhibits oestrogenic activity depending upon the target tissue. Tamoxifen, acts as an agonist in bone and the cardiovascular system in postmenopausal women but as an antagonist in the breast of premenopausal women where it has revolutionized breast cancer treatment in the form of adjuvant endocrine therapy [69]. In the same way, another popular SERM, raloxifene is used in osteoporosis to improve bone mineral density where it exhibits greater agonist activity [69]. The variation in the response of a particular SERM either as an agonist or antagonist depends on several factors. Thus, once a SERM attaches to the ER, a specific conformational change in the receptor is induced which determines which corepressors and/or coactivators are recruited to the promoter. Based upon these factors, tamoxifen recruits a coactivator complex to oestrogen regulated genes in endometrial cells whilst it recruits a corepressor complex to the same gene in breast cancer cells [70]. There is a paucity of information in relation to the role of phytooestrogens, albeit of lower potency at the ER but, appear to exhibit greater selectivity for ERβ over ERα [71] An effect of environmental oestrogens and ERs in the pathogenesis of oesophageal cancer has not been reported to date but may introduce another level of complexity in the contribution of ER in the aetiology of this disease.
Potential for future research
Most patients with oesophageal cancer present late with inoperable disease. Despite recent advancement in surgical and oncological treatment, the five year survival after oesophagectomy is about 25% [72,73] hence new means of predicting disease onset and treating oesophageal cancer in order to improve outcomes need to be explored. From the limited number of investigations reported for ER expression in oesophageal cancer, the varied experimental design of these studies, different antibodies used and few other techniques to confirm these findings, it is too early to draw definitive conclusions regarding the future therapeutic utility of ERs in oesophageal cancer. However, evidence presented herein indicates that the presence of ERs appears to have greater significance than other sex steroid receptors while three studies report relatively raised ERβ expression with oesophageal tumour dedifferentiation. Although we are some considerable way off from understanding the apparent paradox of increased ER expression in oesophageal cancer and a seemingly better prognosis in women, a concerted research effort is required in order to determine relative levels of ERα:ERβ according to gender and age, ER expression patterns with disease progression, modulation of oestrogen production and the role of environmental and phytooestrogens, by immunochemical, molecular and functional assays. The use of powerful experimental techniques such as gene microarrays, chromatin immunoprecipitation to investigate transcriptional regulation of ER and silencing of ER subtypes using siRNA methods to tease apart the complexity of the disease process will provide us with deeper insight into underlying mechanisms at play.
Although the two histological subtypes AC and SCC, vary in their origin, aetiology and incidence, the strong male predominance of oesophageal cancer highlights the importance of further investigation regarding oestrogen receptors and ER pathways, as also agreed by a recently published review by Chandanos et al [74]. If conclusive evidence of a role for oestrogen and its receptors s obtained, then this paves the way for the development of a new diagnostic biomarker in early diagnosis and treatment of this disease. Published studies provide only a hint of the possible use of sex hormone therapy for managing oesophageal cancer. Nonetheless, if a role for ERs in oesophageal cancer is proven this could potentially lead to new and revolutionary approaches in the form of hormonal therapy to treat oesophageal cancer.
Search strategy and selection criteria
Information for this personal review was obtained by searches between March 1978 to October 2009 of Pubmed using the following key words: 'oestrogen receptors', 'sex hormones', 'oesophageal cancer' and 'oestrogen'. Papers or abstracts published in English were included. All authors reviewed original articles and reviews for relevance and included all pertinent studies in the preparation of the manuscript. We have also considered the bibliographies of the selected articles for the pertinent citations.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
FR and RNK have reviewed the literature. FR has performed the laboratory based work. RNK and SYI provided the supervision. FR wrote the manuscript. RNK and SYI edited the manuscript. All authors contributed to the manuscript, and all read and approved the final version
Contributor Information
Farhan Rashid, Email: farhan.rashid@nottingham.ac.uk.
Raheela N Khan, Email: Raheela.Khan@nottingham.ac.uk.
Syed Y Iftikhar, Email: syed.iftikhar@btinternet.com.
Acknowledgements
We would like to thank Ms Averil Warren, Mr Andy Lee, Mr Jon Lund and Professor Mike Larvin for their support with this study.
References
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–50. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83(10):2049–53. doi: 10.1002/(SICI)1097-0142(19981115)83:10<2049::AID-CNCR1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Armstrong RW, Borman B. Trends in incidence rates of adenocarcinoma of the oesophagus and gastric cardia in New Zealand, 1978-1992. Int J Epidemiol. 1996;25(5):941–7. doi: 10.1093/ije/25.5.941. [DOI] [PubMed] [Google Scholar]
- Dolan K, Sutton R, Walker SJ, Morris AI, Campbell F, Williams EM. New classification of oesophageal and gastric carcinomas derived from changing patterns in epidemiology. Br J Cancer. 1999;80(5-6):834–42. doi: 10.1038/sj.bjc.6690429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson LE, Sparen P, Nyren O. Increasing incidence of both major histological types of esophageal carcinomas among men in Sweden. Int J Cancer. 1993;54(3):402–7. doi: 10.1002/ijc.2910540309. [DOI] [PubMed] [Google Scholar]
- Gregersen LH, Friis S, Olsen JH. Cancer in esophagus and cardia--incidence trends in Denmark. Ugeskr Laeger. 2008;170(17):1460–4. [PubMed] [Google Scholar]
- Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol. 2009;24(5):729–35. doi: 10.1111/j.1440-1746.2009.05824.x. [DOI] [PubMed] [Google Scholar]
- Bollschweiler E, Wolfgarten E, Gutschow C, Holscher AH. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001;92(3):549–55. doi: 10.1002/1097-0142(20010801)92:3<549::AID-CNCR1354>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Corley DA, Buffler PA. Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the Cancer Incidence in Five Continents database. Int J Epidemiol. 2001;30(6):1415–25. doi: 10.1093/ije/30.6.1415. [DOI] [PubMed] [Google Scholar]
- Lepage C, Rachet B, Jooste V, Faivre J, Coleman MP. Continuing rapid increase in esophageal adenocarcinoma in England and Wales. Am J Gastroenterol. 2008;103(11):2694–9. doi: 10.1111/j.1572-0241.2008.02191.x. [DOI] [PubMed] [Google Scholar]
- Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100(16):1184–7. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97(2):142–6. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- Vizcaino AP, Moreno V, Lambert R, Parkin DM. Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973-1995. Int J Cancer. 2002;99(6):860–8. doi: 10.1002/ijc.10427. [DOI] [PubMed] [Google Scholar]
- Lambert R, Hainaut P. The multidisciplinary management of gastrointestinal cancer. Epidemiology of oesophagogastric cancer. Best Pract Res Clin Gastroenterol. 2007;21(6):921–45. doi: 10.1016/j.bpg.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol. 2007;8(6):545–53. doi: 10.1016/S1470-2045(07)70172-9. [DOI] [PubMed] [Google Scholar]
- Hulscher JB, van Sandick JW, Tijssen JG, Obertop H, van Lanschot JJ. The recurrence pattern of esophageal carcinoma after transhiatal resection. J Am Coll Surg. 2000;191(2):143–8. doi: 10.1016/S1072-7515(00)00349-5. [DOI] [PubMed] [Google Scholar]
- Omloo JM, Lagarde SM, Hulscher JB, Reitsma JB, Fockens P, van Dekken H, Ten Kate FJ, Obertop H, Tilanus HW, van Lanschot JJ. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg. 2007;246(6):992–1000. doi: 10.1097/SLA.0b013e31815c4037. [DOI] [PubMed] [Google Scholar]
- Kubo A, Corley DA. Marked multi-ethnic variation of esophageal and gastric cardia carcinomas within the United States. Am J Gastroenterol. 2004;99(4):582–8. doi: 10.1111/j.1572-0241.2004.04131.x. [DOI] [PubMed] [Google Scholar]
- Muir CS, McKinney PA. Cancer of the oesophagus: a global overview. Eur J Cancer Prev. 1992;1(3):259–64. doi: 10.1097/00008469-199204000-00007. [DOI] [PubMed] [Google Scholar]
- Lagergren J, Nyren O. Do sex hormones play a role in the etiology of esophageal adenocarcinoma? A new hypothesis tested in a population-based cohort of prostate cancer patients. Cancer Epidemiol Biomarkers Prev. 1998;7(10):913–5. [PubMed] [Google Scholar]
- Blot WJ, Devesa SS, Kneller RW, Fraumeni JF Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265(10):1287–9. doi: 10.1001/jama.265.10.1287. [DOI] [PubMed] [Google Scholar]
- Sarr MG, Hamilton SR, Marrone GC, Cameron JL. Barrett's esophagus: its prevalence and association with adenocarcinoma in patients with symptoms of gastroesophageal reflux. Am J Surg. 1985;149(1):187–93. doi: 10.1016/S0002-9610(85)80031-3. [DOI] [PubMed] [Google Scholar]
- Winters C, Spurling TJ Jr, Chobanian SJ, Curtis DJ, Esposito RL, Hacker JF, Johnson DA, Cruess DF, Cotelingam JD, Gurney MS. Barrett's esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology. 1987;92(1):118–24. [PubMed] [Google Scholar]
- Lofdahl HE, Lu Y, Lagergren J. Sex-specific risk factor profile in oesophageal adenocarcinoma. Br J Cancer. 2008;99(9):1506–10. doi: 10.1038/sj.bjc.6604701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badwe RA, Patil PK, Bhansali MS, Mistry RC, Juvekar RR, Desai PB. Impact of age and sex on survival after curative resection for carcinoma of the esophagus. Cancer. 1994;74(9):2425–9. doi: 10.1002/1097-0142(19941101)74:9<2425::AID-CNCR2820740906>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Derakhshan MH, Liptrot S, Paul J, Brown IL, Morrison D, McColl KE. Oesophageal and gastric intestinal-type adenocarcinomas show the same male predominance due to a 17 year delayed development in females. Gut. 2009;58(1):16–23. doi: 10.1136/gut.2008.161331. [DOI] [PubMed] [Google Scholar]
- Molife R, Lorigan P, MacNeil S. Gender and survival in malignant tumours. Cancer Treat Rev. 2001;27(4):201–9. doi: 10.1053/ctrv.2001.0220. [DOI] [PubMed] [Google Scholar]
- Sugimachi K, Matsuoka H, Matsufuji H, Maekawa S, Kai H, Okudaira Y. Survival rates of women with carcinoma of the esophagus exceed those of men. Surg Gynecol Obstet. 1987;164(6):541–4. [PubMed] [Google Scholar]
- Rashid F, Warren A, Lee AY, Lund JN, Leeder PC, Khan RN, Larvin M, Iftikhar SY. Potential significance of oestrogen receptors in oesophageal cancer. British Journal of Surgery. [Abstract] 2009;96(S6):1–58. [Google Scholar]
- Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116(3):561–70. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93(12):5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjöld M, Gustafsson JA. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82(12):4258–65. doi: 10.1210/jc.82.12.4258. [DOI] [PubMed] [Google Scholar]
- Menasce LP, White GR, Harrison CJ, Boyle JM. Localization of the estrogen receptor locus (ESR) to chromosome 6q25.1 by FISH and a simple post-FISH banding technique. Genomics. 1993;17(1):263–5. doi: 10.1006/geno.1993.1320. [DOI] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392(1):49–53. doi: 10.1016/0014-5793(96)00782-X. [DOI] [PubMed] [Google Scholar]
- Delaunay F, Pettersson K, Tujague M, Gustafsson JA. Functional differences between the amino-terminal domains of estrogen receptors alpha and beta. Mol Pharmacol. 2000;58(3):584–90. doi: 10.1124/mol.58.3.584. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81(4):1535–65. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19(8):1951–9. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayarasan R, Ananthakrishnan N, Kate V, Basu D. Estrogen and progesterone receptors in esophageal carcinoma. Dis Esophagus. 2008;21(4):298–303. doi: 10.1111/j.1442-2050.2007.00767.x. [DOI] [PubMed] [Google Scholar]
- Tiffin N, Suvarna SK, Trudgill NJ, Riley SA. Sex hormone receptor immunohistochemistry staining in Barrett's oesophagus and adenocarcinoma. Histopathology. 2003;42(1):95–6. doi: 10.1046/j.1365-2559.2003.01513_3.x. [DOI] [PubMed] [Google Scholar]
- Awan AK, Iftikhar SY, Morris TM, Clarke PA, Grabowska AM, Waraich N, Watson SA. Androgen receptors may act in a paracrine manner to regulate oesophageal adenocarcinoma growth. Eur J Surg Oncol. 2007;33(5):561–8. doi: 10.1016/j.ejso.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Utsumi Y, Nakamura T, Nagasue N, Kubota H, Harada T, Morikawa S. Effect of 17 beta-estradiol on the growth of an estrogen receptor-positive human esophageal carcinoma cell line. Cancer. 1991;67(9):2284–9. doi: 10.1002/1097-0142(19910501)67:9<2284::AID-CNCR2820670913>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Tihan T, Harmon JW, Wan X, Younes Z, Nass P, Duncan KL, Duncan MD. Evidence of androgen receptor expression in squamous and adenocarcinoma of the esophagus. Anticancer Res. 2001;21(4B):3107–14. [PubMed] [Google Scholar]
- Yamashita Y, Hirai T, Mukaida H, Kawano K, Toge T, Niimoto M, Hattori T. Detection of androgen receptors in human esophageal cancer. Jpn J Surg. 1989;19(2):195–202. doi: 10.1007/BF02471585. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Ueo H, Mafune K, Mori M, Wands JR, Sugimachi K. A novel isoform of human fibroblast growth factor 8 is induced by androgens and associated with progression of esophageal carcinoma. Dig Dis Sci. 2001;46(5):1016–21. doi: 10.1023/A:1010753826788. [DOI] [PubMed] [Google Scholar]
- Nozoe T, Oyama T, Takenoyama M, Hanagiri T, Sugio K, Yasumoto K. Significance of immunohistochemical expression of estrogen receptors alpha and beta in squamous cell carcinoma of the esophagus. Clin Cancer Res. 2007;13(14):4046–50. doi: 10.1158/1078-0432.CCR-07-0449. [DOI] [PubMed] [Google Scholar]
- Cui G, Yuan A, Qvigstad G, Waldum HL. Estrogen receptors are not expressed in esophagogastric carcinomas that come from a high incidence area of China. Hepatogastroenterology. 2001;48(39):594–5. [PubMed] [Google Scholar]
- Ueo H, Matsuoka H, Sugimachi K, Kuwano H, Mori M, Akiyoshi T. Inhibitory effects of estrogen on the growth of a human esophageal carcinoma cell line. Cancer Res. 1990;50(22):7212–5. [PubMed] [Google Scholar]
- Akgun H, Lechago J, Younes M. Estrogen receptor-beta is expressed in Barrett's metaplasia and associated adenocarcinoma of the esophagus. Anticancer Res. 2002;22(3):1459–61. [PubMed] [Google Scholar]
- Chen GG, Zeng Q, Tse GM. Estrogen and its receptors in cancer. Med Res Rev. 2008;28(6):954–74. doi: 10.1002/med.20131. [DOI] [PubMed] [Google Scholar]
- Bosetti C, Negri E, Franceschi S, Conti E, Levi F, Tomei F, La Vecchia C. Risk factors for oral and pharyngeal cancer in women: a study from Italy and Switzerland. Br J Cancer. 2000;82(1):204–7. doi: 10.1054/bjoc.1999.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, Hubbell FA, Ascensao J, Rodabough RJ, Rosenberg CA, Taylor VM, Harris R, Chen C, Adams-Campbell LL, White E. Women's Health Initiative Investigators. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350(10):991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- Lindblad M, Garcia Rodriguez LA, Chandanos E, Lagergren J. Hormone replacement therapy and risks of oesophageal and gastric adenocarcinomas. Br J Cancer. 2006;94(1):136–41. doi: 10.1038/sj.bjc.6602906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone J, van Hillegersberg R, Offerhaus GJ, van Diest PJ, Borel Rinkes IH, Ten Kate FJ. Targets for molecular therapy in esophageal squamous cell carcinoma: an immunohistochemical analysis. Dis Esophagus. 2009;22(6):496–504. doi: 10.1111/j.1442-2050.2009.00951.x. [DOI] [PubMed] [Google Scholar]
- Liu L, Chirala M, Younes M. Expression of estrogen receptor-beta isoforms in Barrett's metaplasia, dysplasia and esophageal adenocarcinoma. Anticancer Res. 2004;24(5A):2919–24. [PubMed] [Google Scholar]
- Wang LY. Estrogen and progesterone receptors in esophageal carcinoma cells. Zhonghua Zhong Liu Za Zhi. 1991;13(1):23–5. [PubMed] [Google Scholar]
- Cavallini A, Notarnicola M, Giannini R, Montemurro S, Lorusso D, Visconti A, Minervini F, Caruso MG. Oestrogen receptor-related receptor alpha (ERRalpha) and oestrogen receptors (ERalpha and ERbeta) exhibit different gene expression in human colorectal tumour progression. Eur J Cancer. 2005;41(10):1487–94. doi: 10.1016/j.ejca.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Jassam N, Bell SM, Speirs V, Quirke P. Loss of expression of oestrogen receptor beta in colon cancer and its association with Dukes' staging. Oncol Rep. 2005;14(1):17–21. [PubMed] [Google Scholar]
- Horvath LG, Henshall SM, Lee CS, Head DR, Quinn DI, Makela S, Delprado W, Golovsky D, Brenner PC, O'Neill G, Kooner R, Stricker PD, Grygiel JJ, Gustafsson JA, Sutherland RL. Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer Res. 2001;61(14):5331–5. [PubMed] [Google Scholar]
- Jensen EV, Cheng G, Palmieri C, Saji S, Makela S, Van Noorden S, Wahlström T, Warner M, Coombes RC, Gustafsson JA. Estrogen receptors and proliferation markers in primary and recurrent breast cancer. Proc Natl Acad Sci USA. 2001;98(26):15197–202. doi: 10.1073/pnas.211556298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer. 2004;11(3):537–51. doi: 10.1677/erc.1.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton G, Brunelli E, Murer B, Puntoni R, Puntoni M, Fennell DA, Gaudino G, Mutti L, Moro L. Estrogen receptor-beta affects the prognosis of human malignant mesothelioma. Cancer Res. 2009;69(11):4598–604. doi: 10.1158/0008-5472.CAN-08-4523. [DOI] [PubMed] [Google Scholar]
- Hayashi SI, Eguchi H, Tanimoto K, Yoshida T, Omoto Y, Inoue A, Yoshida N, Yamaguchi Y. The expression and function of estrogen receptor alpha and beta in human breast cancer and its clinical application. Endocr Relat Cancer. 2003;10(2):193–202. doi: 10.1677/erc.0.0100193. [DOI] [PubMed] [Google Scholar]
- Liu MM, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, Price RH Jr, Pestell RG, Kushner PJ. Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J Biol Chem. 2002;277(27):24353–60. doi: 10.1074/jbc.M201829200. [DOI] [PubMed] [Google Scholar]
- Li X, Huang J, Yi P, Bambara RA, Hilf R, Muyan M. Single-chain estrogen receptors (ERs) reveal that the ERalpha/beta heterodimer emulates functions of the ERalpha dimer in genomic estrogen signaling pathways. Mol Cell Biol. 2004;24(17):7681–94. doi: 10.1128/MCB.24.17.7681-7694.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe AP, Matzke MA. Epigenetics: regulation through repression. Science. 1999;286(5439):481–6. doi: 10.1126/science.286.5439.481. [DOI] [PubMed] [Google Scholar]
- Zhao C, Lam EW, Sunters A, Enmark E, De Bella MT, Coombes RC, Gustafsson JA, Dahlman-Wright K. Expression of estrogen receptor beta isoforms in normal breast epithelial cells and breast cancer: regulation by methylation. Oncogene. 2003;22(48):7600–6. doi: 10.1038/sj.onc.1207100. [DOI] [PubMed] [Google Scholar]
- Hogan AM, Collins D, Baird AW, Winter DC. Estrogen and gastrointestinal malignancy. Mol Cell Endocrinol. 2009;307(1-2):19–24. doi: 10.1016/j.mce.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Fabian CJ, Kimler BF. Selective estrogen-receptor modulators for primary prevention of breast cancer. J Clin Oncol. 2005;23(8):1644–55. doi: 10.1200/JCO.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295(5564):2465–8. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, Saag PT van der, Burg B van der, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–63. doi: 10.1210/en.139.10.4252. [DOI] [PubMed] [Google Scholar]
- Muller JM, Erasmi H, Stelzner M, Zieren U, Pichlmaier H. Surgical therapy of oesophageal carcinoma. Br J Surg. 1990;77(8):845–57. doi: 10.1002/bjs.1800770804. [DOI] [PubMed] [Google Scholar]
- Lee PC, Port JL, Paul S, Stiles BM, Altorki NK. Predictors of long-term survival after resection of esophageal carcinoma with nonregional nodal metastases. Ann Thorac Surg. 2009;88(1):186–92. doi: 10.1016/j.athoracsur.2009.03.079. [DOI] [PubMed] [Google Scholar]
- Chandanos E, Lagergren J. The mystery of male dominance in oesophageal cancer and the potential protective role of oestrogen. Eur J Cancer. 2009;45(18):3149–55. doi: 10.1016/j.ejca.2009.09.001. [DOI] [PubMed] [Google Scholar]


