Abstract
Hydrogen cyanide polymerizes readily under a variety of conditions and significant prebiotic roles have been suggested for these polymers due to the abundance of HCN in universe. However, the structures of HCN polymers have been more speculative than grounded in experimental data. Here we show that 13C and 15N solid state NMR spectra of polymers formed in neat HCN are inconsistent with the previously proposed structures and suggest instead that the polymers are formed by simple monomer addition, first in head-to-tail fashion to form linear, conjugated chains, and then laterally to form saturated two-dimensional networks. This interpretation of the NMR spectra finds support in other information about the polymerization of neat HCN, including the presence of free radicals. As expected from the literature, formation of the HCN tetramer, diaminomaleonitrile, is also observed, but only when the reaction is catalyzed exclusively by base and then in crystalline form.
INTRODUCTION
Due to the abundance of hydrogen cyanide in the universe,1 there has been much interest in the potential prebiotic role of hydrogen cyanide polymers. However, the structures of these polymers remain unknown despite extensive effort.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18
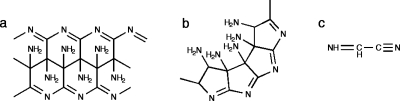
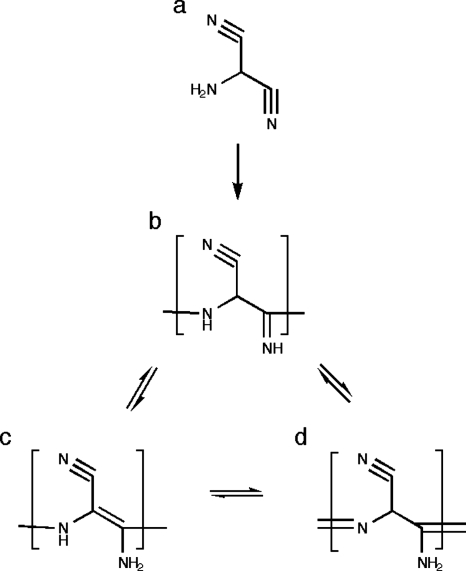
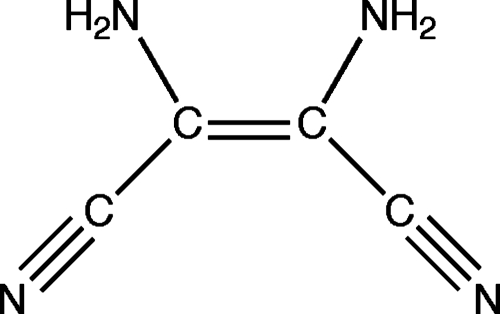
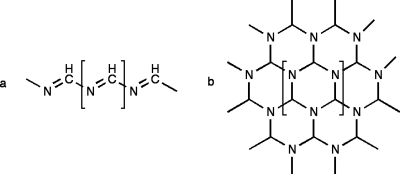
Figures 12 show the proposed polymer structures. The “ladder” structures in Fig. 1 comprise repeats of the HCN dimer; the original trans form was originally suggested by Volker11 [Fig. 1a] and the cis variation was subsequently offered by Umemoto et al.12 [Fig. 1b]. A more provocative model from the standpoint of the origins of life was suggested by Matthews and co-workers16, 17, 18 based on the observation of α-amino acids in polymer hydrolyzates; polymerization is proposed to occur via the HCN trimer aminomalononitrile [Fig. 2a] and results in a structure with a –NCC– backbone [Figs. 2b, 2c, 2d] that would form polypeptide upon contact with water.16, 17, 18 However, Ferris19 has argued that the HCN tetramer, diaminomaleonitrile (DAMN) (Fig. 3), must be the “direct precursor” to HCN polymers since dimers and trimers are not expected to accumulate.
Figure 1.
Ladder structures of (a) Volker (Ref. 11) and (b) Umemoto et al. (Ref. 12) which can be conceived of as products of anti and syn addition of dimer (c), followed by conjugation of the nitriles. Predicted 13C chemical shifts (ppm vs TMS): (a) 46±12 and 188±32; (b) 64±14 and 156±31. Predicted 15N chemical shifts (ppm vs liquid ammonia): (a) 35±7 and 290±39; (b) 37±5 and 298±39.
Figure 2.
The protopeptide [(b)–(d)] proposed to result from acid-base addition of trimer (a) (Refs. 16, 17, 18). Predicted 13C chemical shifts (ppm vs TMS) clockwise from the lower right in the bracketed repeat unit: (b) 156±7, 44±12, 112±5; (c) 143±17, 83±35, 106±11; and (d) 153±9, 49±8, 118±3. Predicted 15N chemical shifts (ppm vs liquid ammonia) clockwise from the lower right in the bracketed repeat unit: (b) 199±32, 76±5, 249±7; (c) 113±29, 89±14, 265±7; and (d) 62±5, 326±17, 248±7.
Figure 3.
HCN tetramer DAMN. See Figs. 4b, 5b for the 13C and 15N chemical shifts.
A distinguishing feature of the proposed polymer structures is the proportion of carbons bonded to more than one nitrogen. While the proposed dimer, trimer, and tetramer subunits are all formed from HCN by new homonuclear carbon-carbon bonds, their polymerization involves the formation of new heteronuclear carbon-nitrogen bonds. As a result, the number of carbons bonded to more than one nitrogen varies among the proposed polymers from one in two for a polymer of dimers, to one in three for a polymer of trimers, and one in four for a polymer of tetramers.
Solid state NMR is a powerful tool for studying the structures of molecules not amenable to solution or crystallographic approaches. Chemical shifts help to identify functional groups and dipolar interactions allow mapping of chemical connectivity. Previous solid state NMR studies of base-catalyzed HCN polymerization13, 14, 15 determined that existing C–N bonds were not broken, new C–N bonds were created, and some secondary amides were formed. However, no further conclusions were drawn as to the structures of the polymers. Here we reapply solid state NMR using stronger fields and higher magic angle spinning (MAS) frequencies in conjunction with varied sample preparation schemes. The results indicate that dimer, trimer, and tetramer polymerization products are not present in significant amounts. Rather, the data indicate that polymerization occurs primarily via monomer addition and DAMN is precipitated in crystalline form. We suggest that the polymers normally grow by free-radical addition that is initiated by base-catalyzed formation of the biradical dimer. However, when this pathway is suppressed, slow nucleophilic addition forms similar, albeit more ordered, products.
METHODS
Synthesis: fully-labeled samples were prepared from H13C15N, 15N-enriched samples were prepared from HC15N, and mixed-labeled samples were prepared from a 1:1 mixture of H13CN and HC15N. In each case, the labeled HCN was generated by heating a mixture of correspondingly labeled KCN (Cambridge Isotope Laboratories) and recrystallized stearic acid (1:2 molar ratio) to 80 °C.20 The evolved gas was collected via an evacuated transfer manifold into a dry ice∕acetone-cooled flask containing up to two of the following reagents: triethylamine (Sigma-Aldrich), benzoquinone (Sigma-Aldrich), hydroquinone (Sigma-Aldrich), TEMPO (2,2,6,6-tetramethylpiperidine 1-oxyl, Sigma-Aldrich), rose bengal (Fisher Scientific), and acetaldehyde (Sigma-Aldrich). When HCN collection was complete, the receiving flask was sealed and incubated at 5 °C. When the photoinitiator rose bengal was used, the incubated flask was illuminated by a 50 W halogen lamp. Unlabeled DAMN (Aldrich) was recrystallized from isobutyl alcohol prior to use. When labeled DAMN was required, it was extracted from the triethylamine-catalyzed polymer mixture with hot isopropanol.
Solid state NMR: conventional use was made of cross polarization (CP) from 1H, to enhance 13C and 15N signals and to shorten recycle delays, combined with rapid MAS and high power 1H decoupling, to average anisotropic interactions that contribute to line broadening. In addition, double cross polarization (DCP),21 entailing an additional transfer of polarization from 15N to 13C, was used to assign 13C nuclei bonded to 15N in mixed-labeled samples. All spectra were acquired on a 360 MHz (1H frequency) spectrometer (Cambridge Instruments) using a 4 mm MAS probe (Varian) operated at 10 kHz. Samples (typically 50 mg) were sealed under argon in rotor inserts (Wilmad). The CP∕MAS spectra were obtained with ramped amplitude Hartmann–Hahn CP,22, 23 TPPM 1H decoupling during data acquisition,24 and 9 s recycle delays. Typical rf fields were 50 kHz for CP and 83 kHz for 1H decoupling. CP contact times were 2 ms for 13C and 5 ms for 15N. The usual TPPM parameters were 170° for the pulse angle and 12° for the phase. For DCP, the 15N-to-13C CP was optimized for single bond transfer using a standard glycine sample with a constant amplitude field of 25 kHz accompanied by 83 kHz CW 1H decoupling. The contact time was 5 ms.
Chemical shift predictions: Few compounds have as high a ratio of nitrogen to carbon, or as low a ratio of hydrogen to carbon, as HCN and its products. And still fewer also have reported 13C or, especially, 15N chemical shifts. Thus the chemical shifts expected for HCN products need to be based on the chemical shifts of these few molecules and the nitrogen rich and hydrogen poor fragments of other molecules. To synthesize chemical shift information from fragments, we make use of the v.10.05 CNMR and NNMR packages of Advanced Chemistry Development (ACD, Toronto). As shown in Figs. S1 and S2,25 the databases of these packages include molecules that support our inquiry. In addition, the accuracies of the ACD predictions for nitrogen-bonded carbons and for nitrogen are illustrated in Table S1 (Ref. 25) by several cases in which data are available that are not in ACD’s v10.05 database. The predictions of the v10.05 packages are generally good with the notable exception of the 15N of the eneamine in DAMN, which is expected to be influenced by both hydrogen bonding and tautomeric equilibria and is therefore likely to be very sensitive to the solid state environment of the experimental measurement. For application to polymers, we use predictions for the central repeat unit in an oligomer long enough that the values for the central repeat unit are no longer dependent on length. Significant figures and error bars are as given by the ACD algorithm.
RESULTS
Kinetics: the rate of appearance of the typical dark product varied with the choice of supplementary reagent(s) as shown in Table 1. Without catalytic (0.5 wt %) base (triethylamine) or free-radical initiator (TEMPO or illuminated rose bengal) the reaction was too slow to observe in 7 days. And even with triethylamine, 10 vol % acetaldehyde prevented a normal reaction. In this case, 8 weeks of incubation resulted in a pale gelatinous product. These slowly formed pale polymers, unlike the relatively rapidly formed dark ones, are soluble in water and in methanol.
Table 1.
Appearance of dark product in liquid HCN mixed with various reagents.
| Sample | Time | Reagents |
|---|---|---|
| A | a few hours | 0.5 wt % triethylamine and 0.5 wt % hydroquinone |
| B | overnight | 0.5 wt % triethylamine and 0.5 wt % benzoquinone |
| C | 3 days | 0.5 wt % triethylamine |
| D | 3 days | 0.5 wt % TEMPO |
| E | 3 days | 0.5 wt % illuminated rose bengal |
| F | >7 days | None |
| G | >7 days | 0.5 wt % hydroquinone |
| H | >7 days | 0.5 wt % benzoquinone |
| I | >7 days | 0.5 wt % triethylamine and 10 vol % acetaldehyde |
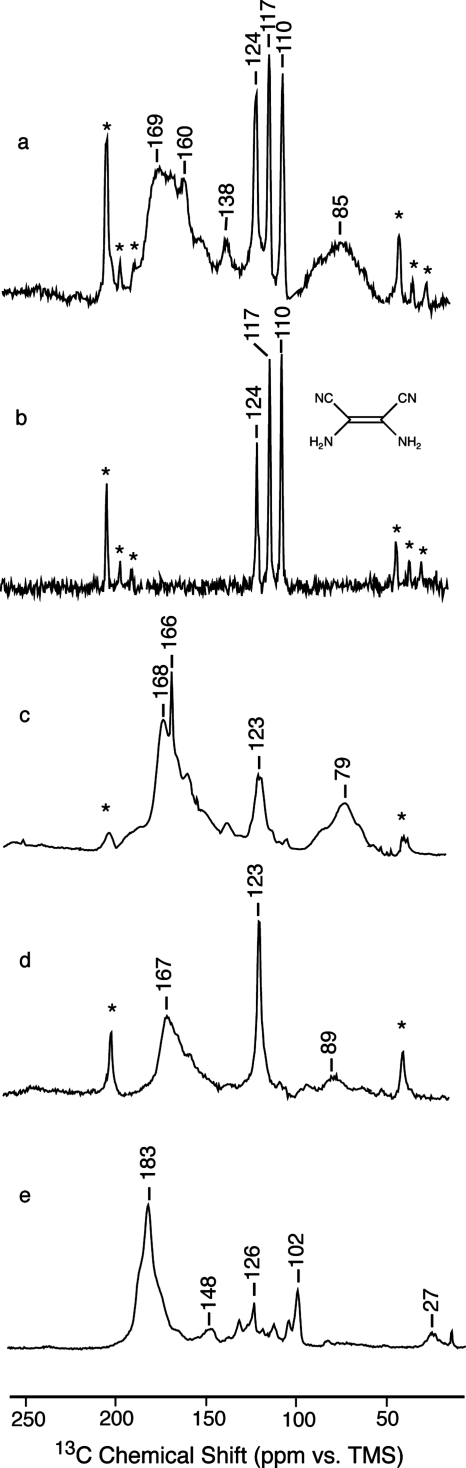
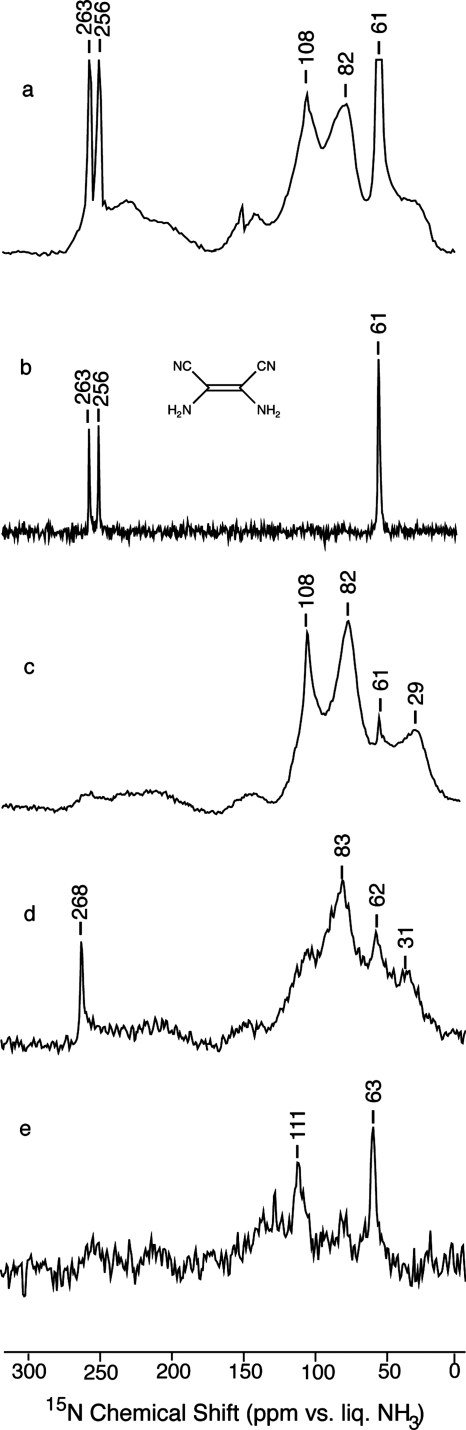
Solid state NMR: Figs. 45 show the 13C and 15N CP spectra of the products obtained in the presence of various reagents. Both sharp and broad features appear in these spectra. The former are expected for small molecules and the latter for disordered and polydisperse polymers. In addition, free radicals26 may contribute to signal broadening to a distance of ∼6 Å.27 The sharp signals illustrate the improved resolution attainable at higher field strengths and MAS frequencies than were previously available.13, 14, 15 Notable features of the 13C spectra (Fig. 4) are the unusually large chemical shifts in the polymer signals (saturated at 70–100 ppm and unsaturated at 140–185 ppm) and the varying amount of residual nitrile in the polymer (isotropic signal at ∼124 ppm flanked by characteristically large spinning sidebands marked with asterisks). The 15N spectra (Fig. 5) also show varying amounts of residual nitrile in the polymer (256–268 ppm).
Figure 4.
13C CPMAS NMR spectra of (a) reaction products of H13C15N in the presence of catalytic triethylamine (sample C, 2178 scans). (b) Natural abundance DAMN (Fig. 3) (4480 scans). (c) Reaction products of H13C15N in the presence of catalytic hydroquinone and triethylamine (sample A, 2048 scans). (d) Reaction products of H13C15N in the presence of catalytic benzoquinone and triethylamine (sample B, 2048 scans). (e) Reaction products of H13C15N mixed with 10 vol % acetaldehyde in the presence of catalytic triethylamine (sample I, 4480 scans). Asterisks mark spinning sidebands.
Figure 5.
15N CPMAS NMR spectra of the same products as in Fig. 4 except that in this case the DAMN (panel b) is 15N-labeled: (a) reaction products of H13C15N in the presence of catalytic triethylamine (sample C, 4864 scans); (b) 15N-enriched DAMN (Fig. 3) (4096 scans); (c) reaction products of H13C15N in the presence of catalytic hydroquinone and triethylamine (sample A, 4608 scans); (d) reaction products of H13C15N in the presence of catalytic benzoquinone and triethylamine (sample B, 4408 scans); (e) reaction products of H13C15N mixed with 10 vol % acetaldehyde in the presence of catalytic triethylamine (sample I, 5376 scans). The narrow downfield signal in (d) is probably due to residual cyanide.
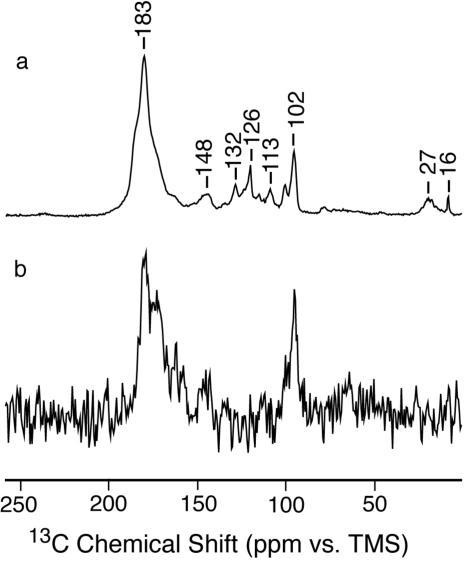
Figure 6 compares the 13C CP spectrum of fully labeled pale polymer with the DCP spectrum of mixed-labeled pale polymer (formed from a mixture of H13CN and HC15N). The latter detects only carbons that have formed at least one new bond with nitrogen because the contact time for the CP from 15N to 13C was optimized for single bond transfer. Previously,14, 15 such experiments have demonstrated the formation of new saturated and unsaturated carbon-nitrogen bonds in the dark polymer. The present results for the pale polymer indicate that the dominant signals at ∼183 and ∼102 ppm are due to carbons that have formed new bonds with nitrogen. Thus most of the carbon atoms in the pale polymer, as in the dark polymer, are bonded to more than the one nitrogen partner they had in HCN.
Figure 6.
13C NMR spectra of the pale polymers formed in the presence of aldehyde. (a) As in Fig. 4e and (b) DCP of a sample made from a 1:1 mixture of H13CN and HC15N (8192 scans).
Chemical shift predictions: the 13C and 15N chemical shifts predicted for various polymer structures are given in the legends of Figs. 127. As expected, saturated sites are relatively shielded (small chemical shifts) and unsaturated sites are relatively deshielded (large chemical shifts). Furthermore, for the carbon atoms within each category, deshielding is greater for those with more nitrogen bonding partners.
Figure 7.
Proposed products of free radical polymerization: (a) chains formed by head-to-tail addition of HCN monomers and (b) the saturated network formed by the further lateral addition of chains. Predicted 13C chemical shifts (ppm vs TMS): (a) 181±19 and (b) 90±11. Predicted 15N chemical shifts (ppm vs liquid ammonia): (a) 106±5 and (b) 50±6.
DISCUSSION
The most abundant small molecule is expected to be the hydrogen cyanide tetramer DAMN. In 1923, Bedel8 argued that the stable oligomer formed by HCN is a tetramer rather than the previously proposed trimer.9, 10 More recently, Ferris and co-workers3, 4, 5 found that (in water) DAMN forms irreversibly from hydrogen cyanide. In fact, we find that all of the narrow signals in our dark, base-catalyzed product [Figs. 4a, 5a] are accounted for by microcrystalline DAMN [Figs. 4b, 5b] due to the splittings that result from the asymmetric environment of the molecule in the crystal.28, 29, 30 Furthermore, washing our samples with hot isopropanol, which is a good solvent for DAMN, removes all of the narrow signals, leaving some residual nitrile and all of the broad signals (spectrum not shown). On the other hand, DAMN is not formed in the presence of hydroquinone [Figs. 4c, 5c], benzoquinone [Figs. 4d, 5d], illuminated rose bengal (spectrum not shown), or acetaldehyde [Figs. 4e, 5e].
It remains to interpret the broader NMR signals due to polymer in Figs. 45. The fact that most of these signals appear in the DCP spectra of mixed-label samples indicates that most of the carbons form new bonds with nitrogen. This suggests that HCN monomer is the basic unit of the polymer rather than dimer, trimer, or tetramer. Monomer addition polymers are shown in Fig. 7. Figure 7a shows a linear polymer formed by head-to-tail addition, as would be favored by the very strong dipole moment of HCN (2.98 D), and Fig. 7b shows a two-dimensional (2D) network polymer formed by further lateral addition (such as may occur by a Diels–Alder process). The new C–N bonds formed in the linear and network polymers are consistent with the downfield and upfield signals in the DCP spectrum [Fig. 6b]. It is also the case that saturated cyclic structures potentially derivative from the polymer in Fig. 7b have been reported by Minard et al.7
Chemical shifts lead to the same structures. Polymer signals are observed at 180–150 and 100–70 ppm for 13C and 270–180, 108, 82, and 61 ppm for 15N. Strikingly absent are some of the signals predicted for ladder polymers (see legend of Fig. 1) and protopeptides (see legend of Fig. 2). For the ladder polymers, the saturated carbons (at 46±12 and 64±14 ppm) and the unsaturated nitrogens (at 290±39 and 298±39 ppm) are not observed. We can therefore conclude that ladder polymers are not present in significant amounts. The protopeptides can be similarly ruled out. For structures 2b and 2d the saturated carbons (at 44±12 and 49±8) are not observed. For structure 2c, the downfield carbons (at 143±17 and 106±11 ppm) are not significantly represented.
In contrast, the predicted 13C and 15N chemical shifts for monomer addition polymers (see legend of Fig. 7) are consistent with the dominant features in the relatively simple 13C and 15N spectra of the pale gelatinous polymer formed slowly in the presence of acetaldehyde [Figs. 4e, 5e]. In particular, because each of the carbons in the one-dimensional chain is bonded to two nitrogens, the 13C signal is predicted to be well downfield of the signals of more common unsaturated carbons. And because each of the carbons in the 2D network is bonded to three nitrogens, the 13C signal is predicted to be well downfield of the signals of more common saturated carbons.
The dark polymer is clearly more complicated than the pale polymer. However, the dark color is consistent with the alternately nitrogen substituted polyacetylene shown in Fig. 7a.31 That the structures in the dark polymers are largely the same as in the present pale polymer is suggested by the similarity in the dominant features of the spectra for the dark polymer [Figs. 4a, 4c, 4d, 5a, 5c, 5d] and the pale polymer [Figs. 4e, 5e]. However, in the dark polymer, the 13C signals in each of the two dominant features extend further upfield than the corresponding signals in the pale polymers. This is consistent with an expectation of more frequent structural defects in rapidly formed polymers as upfield 13C deviations are predicted for carbons bonded to fewer nitrogens. In addition, the likelihood of longer chains and greater crosslinking in the more rapidly formed dark samples than in the slowly formed pale samples is consistent with the divergence in color and solubility.
The polymers in Fig. 7 can grow by either nucleophilic addition or free-radical addition. The latter seems more likely for the dark polymer because
-
(1)
Direct observation of free radicals has recently been reported in dark HCN polymers.26
-
(2)
Since the dimer of HCN is expected to form a biradical,32, 33, 34 one does not have to look far for a radical initiator.
-
(3)
Seeding a toluene solution of HCN with dark polymer promotes the formation of new dark polymer.35
-
(4)
Gamma radiation of frozen HCN induces polymerization to a degree that depends on the radiation dose and with spectral characteristics suggesting a free-radical polyconjugated product.36, 37
-
(5)
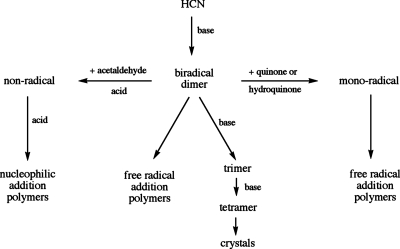
In our own experiments, TEMPO and illuminated rose bengal are each as effective catalysts of polymerization as triethylamine. Of course, reduction and oxidation are also expected to promote free-radical addition and the fact that hydroquinone and benzoquinone significantly accelerate the formation of polymer in the presence of triethylamine but not noticeably in its absence suggests that they are capable of converting the biradical HCN dimer into a monoradical, but not capable of oxidizing or reducing the HCN monomer. The absence of DAMN in the presence of the quinones is consistent with the absence of DAMN in the products of the polymer seeded reaction38 and with the expectation that free-radical addition reactions resulting in polymers compete for HCN with the base-catalyzed addition reactions that form DAMN, as shown in Fig. 8.
-
(6)
Acetaldehyde has been reported to facilitate DAMN hydrolysis.39, 40 However under the anhydrous conditions of our system, the carbon alpha to the aldehyde is expected to deplete biradical dimers via acid-catalyzed nucleophilic attack and thereby drastically reduce the rate of free radical addition. This could allow time for nucleophilic addition to form a smaller and more uniform product as shown in Fig. 8. An absence of free radicals might also contribute to the sharper signals in these samples.
Figure 8.
Suggested pathways to the products observed by solid state NMR. HCN monomers are added from top to bottom and reactions with substoichiometric reagents are shown horizontally. Acid to catalyze C–N bond formation is supplied by HCN and base to catalyze C–C bond formation is supplied by triethylamine.
CONCLUSIONS
Solid state NMR spectra show that HCN polymers formed in neat HCN grow primarily by monomer addition to form unsaturated chains and saturated sheets. The effects of substoichiometric amounts of various reagents on the rate of polymerization are consistent with their expected modulation of competing free radical and nucleophilic addition pathways. The resulting structures may be precursors of purines and pyrimidines but not reported α-amino acids.
The solid state NMR spectra also show signals from crystalline DAMN, suggesting that DAMN is poorly soluble in HCN. Of the products observed, only DAMN is a likely source of the glycine in hydrolysates, as disproportionation of DAMN to cyanogen and amino acetonitrile would produce oxalic acid and glycine in water.
ACKNOWLEDGMENTS
The NMR spectra were obtained at the MIT-Harvard Center for Magnetic Resonance supported by NIH (Grant No. EB002026). We are also grateful to Barry Snider, Robert Minard, and Robert Griffin for insightful discussions and especially thank Barry Snider for calling our attention to Ref. 20. Financial support was provided by NIH (Grant No. R01EB002175).
References
- Debes J. H., Weinberger A. J., and Schneider G., Astrophys. J. 673, L191 (2008). [Google Scholar]
- Proust J. L., Ann. Chim. Phys. 60, 233 (1807). [Google Scholar]
- Ferris J. P., Joshi P. C., Edelson E. H., and Lawless J. G., J. Mol. Evol. 11, 293 (1978). [DOI] [PubMed] [Google Scholar]
- Ferris J. P., Edelson E. H., Auyeung J. M., and Joshi P. C., J. Mol. Evol. 10.1007/BF01732676 17, 69 (1981). [DOI] [PubMed] [Google Scholar]
- Ferris J. P. and Hagan W. J., Tetrahedron 10.1016/S0040-4020(01)99315-9 40, 1093 (1984). [DOI] [PubMed] [Google Scholar]
- Liebman S. A., Pesce-Rodriguez R. A., and Matthews C. N., Adv. Space Res. 10.1016/S0273-1177(99)80066-0 15, 71 (1995). [DOI] [PubMed] [Google Scholar]
- Minard R. D., Hatcher P. G., Gourley R. C., and Matthews C. N., Origins Life Evol. Biosphere 10.1023/A:1006566125815 28, 461 (1998). [DOI] [PubMed] [Google Scholar]
- Bedel C., C. R. Hebd. Seances Acad. Sci. 176, 168 (1923). [Google Scholar]
- Lange O., Berichte 6, 99 (1863). [Google Scholar]
- Wippermann R., Berichte 7, 767 (1874). [Google Scholar]
- Volker T. H., Angew. Chem., Int. Ed. 72, 379 (1960). [Google Scholar]
- Umemoto K., Takahashi M., and Yokata K., Origins Life Evol. Biosphere 10.1007/BF02386468 17, 283 (1987). [DOI] [Google Scholar]
- Schaefer J., Stejskal E. O., Jacob G. S., and McKay R. A., Appl. Spectrosc. 36, 179 (1982). [Google Scholar]
- McKay R. A., Schaefer J., Stejskal E. O., Ludicky R., and Matthews C. N., Macromolecules 10.1021/ma00136a003 17, 1124 (1984). [DOI] [Google Scholar]
- Garbow J. R., Schaefer J., Ludicky R., and Matthews C. N., Macromolecules 10.1021/ma00168a012 20, 305 (1987). [DOI] [Google Scholar]
- Minard R. D., Yang W., Varma P., Nelson J., and Matthews C. N., Science 10.1126/science.170680 190, 387 (1975). [DOI] [PubMed] [Google Scholar]
- Matthews C. N., Nelson J., Varma P., and Minard R. D., Science 198, 622 (1977). [DOI] [PubMed] [Google Scholar]
- Matthews C. N. and Moser R. E., Proc. Natl. Acad. Sci. U.S.A. 56, 1087 (1966). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris J. P., Science 10.1126/science.218287 203, 1135 (1979). [DOI] [PubMed] [Google Scholar]
- Romanini D. and Lehmann K. K., J. Chem. Phys. 10.1063/1.469462 102, 633 (1995). [DOI] [Google Scholar]
- Schaefer J., McKay R. A., and Stejskal E. O., J. Magn. Reson. 34, 443 (1979). [Google Scholar]
- Hartmann S. R. and Hahn E. L., Phys. Rev. 10.1103/PhysRev.128.2042 128, 2042 (1962). [DOI] [Google Scholar]
- Pines A., Gibby M. G., and Waugh J. S., J. Chem. Phys. 10.1063/1.1680061 59, 569 (1973). [DOI] [Google Scholar]
- Bennett A. E., Rienstra C. M., Auger M., Lakshmi K. V., and Griffin R. G., J. Chem. Phys. 10.1063/1.470372 103, 6951 (1995). [DOI] [Google Scholar]
- See EPAPS Document No. E-JCPSA6-130-021911 for supplemental table and figures. For more information on EPAPS, see http://www.aip.org/pubservs/epaps.html.
- Budil D. E., Roebber J. L., Liebman S. A., and Matthews C. N., Astrobiology 3, 323 (2003). [DOI] [PubMed] [Google Scholar]
- Arnesano F., Banci L., Bertini I., Felli I. C., Luchinat C., and Thompsett A. R., J. Am. Chem. Soc. 10.1021/ja034112c 125, 7200 (2003). [DOI] [PubMed] [Google Scholar]
- Penfold B. R. and Lipscomb W. N., Acta Crystallogr. 14, 589 (1961). [Google Scholar]
- Sidorov A. A., Fomina I. G., Nesterov V. V., Nefedov S. E., Eremenko I. L., and Moiseev I. I., Russ. Chem. Bull. 10.1007/BF02496182 48, 573 (1999). [DOI] [Google Scholar]
- Yamada Y., Nagashimi N., Iwashita Y., Nakamura A., and Kumashir I., Tetrahedron Lett. 9, 4529 (1968). [Google Scholar]
- Lowe J. P. and Kafafi S. A., J. Am. Chem. Soc. 10.1021/ja00332a013 106, 5837 (1984). [DOI] [Google Scholar]
- Kliss R. M. and Matthews C. N., Proc. Natl. Acad. Sci. U.S.A. 48, 1300 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser R. E., Fritsch J. M., Westman T. L., Kliss R. M., and Matthews C. N., J. Am. Chem. Soc. 89, 5673 (1967). [Google Scholar]
- Serre J. and Schneider F., J. Chim. Phys. 61, 1655 (1964). [Google Scholar]
- Minard R., personal communication (15 July 2007).
- Barkalov I. M. and Kiryukhin D. P., Int. Rev. Phys. Chem. 19, 1 (2002). [Google Scholar]
- Mozhaev P. S., Kichigina G. A., Kiryukhin D. P., and Barkalov I. M., High Energy Chem. 29, 15 (1995). [Google Scholar]
- Court R. and Parnell J., personal communication (18 July 2007).
- Eschenmoser A., Chem. Biodivers. 4, 554 (2007). [DOI] [PubMed] [Google Scholar]
- Koch K., Schweizer W. B., and Eschenmoser A., Chem. Biodivers. 4, 541 (2007). [DOI] [PubMed] [Google Scholar]