Abstract
Tomosynthesis is a decades-old technique for section imaging that has seen a recent upsurge in interest due to its promise to provide three-dimensional information at lower dose and potentially lower cost than CT in certain clinical imaging situations. This renewed interest in tomosynthesis began in the late 1990s as a new generation of flat-panel detectors became available; these detectors were the one missing piece of the picture that had kept tomosynthesis from enjoying significant utilization earlier. In the past decade, tomosynthesis imaging has been investigated in a variety of clinical imaging situations, but the two most prominent have been in breast and chest imaging. Tomosynthesis has the potential to substantially change the way in which breast cancer and pulmonary nodules are detected and managed. Commercial tomosynthesis devices are now available or on the horizon. Many of the remaining research activities with tomosynthesis will be translational in nature and will involve physicist and clinician alike. This overview article provides a forward-looking assessment of the translational questions facing tomosynthesis imaging and anticipates some of the likely research and clinical activities in the next five years.
Keywords: tomosynthesis, breast imaging, chest imaging, tomography
OVERVIEW
Digital tomosynthesis is a simple and relatively inexpensive method of producing section images using conventional digital x-ray equipment. It is a form of limited angle tomography that produces section, or “slice,” images from a series of projection images acquired as the x-ray tube moves over a prescribed path. The total angular range of movement is often less than 40°. Because the projection images are not acquired over a full 360° rotation about the patient, the resolution in the z direction (i.e., in the depth direction perpendicular to the x-y plane of the projection images) is limited, and thus tomosynthesis does not produce the isotropic spatial resolution achievable with computed tomography (CT). However, the resolution of images in the x-y plane of the reconstructed slices is often superior to CT, and the ease of use in conjunction with conventional radiography makes tomosynthesis a potentially quite useful imaging modality.
There has been a high degree of research interest in tomosynthesis imaging in the past decade, and at least two commercial products have recently been approved by the Food and Drug Administration (FDA) and released on the market. It is expected that other approved devices will soon follow. As such, tomosynthesis imaging is in a period of a high rate of change, with an increasing number of investigators and manufacturers nearing completion of projects involving both the physics and clinical aspects of the technique. If one were to compare the current state of tomosynthesis imaging to a metaphorical calendar year, the field is firmly in the middle of spring. It has moved beyond the “winter” of initial research investigation but is not yet in the “summer” as a mature and accepted clinical modality. Just as the rate of change in daylight hours is at its greatest at the equinoxes and slows down near the solstices, so the rate of progress in a new imaging modality is greatest in the middle of its developmental pathway. Tomosynthesis imaging is clearly in March of its allegorical calendar year of development. Change is happening at a rapid pace, which is exciting for investigators, but it also makes the long-term future difficult to predict with certainty.
Although tomosynthesis has not yet passed its pivotal clinical evaluation, it can, to borrow another metaphor, be said to have passed the “light box test.” A clinical colleague has often remarked that he can gauge which new imaging modalities will be clinically successful from an intuitive appraisal of how substantially the new technique appears to improve upon the modality it is vying to replace by viewing the images “on a light box across the room.” In the opinion of this colleague, if improvements from a new modality are only demonstrable by comparing small changes in Az values following an extensive ROC study, then the improvement of the new modality may be statistically significant but the level of its clinical impact may be hard to predict in advance. On the other hand, if the new modality produces images that are so substantially superior to the predecessor technique that the difference is visible “on a light box viewed across the room,” then the clinical impact is likely to be substantial. After having viewed several thousand tomosynthesis images in early clinical studies, most observers would agree that there is a subjective but substantial improvement in the ability to appreciate abnormal anatomy or disease in tomosynthesis images relative to conventional radiography. At the current time there are only a limited number of completed clinical studies to quantify this improvement and to determine how tomosynthesis affects specificity as well as sensitivity. Nonetheless, based purely on the gestalt impression of the images, most would agree that tomosynthesis passes the “light box test” with what appears to be a substantial visual improvement over conventional radiographic imaging.
Notwithstanding the improved visibility of anatomy in tomosynthesis images, the true test of the impact of a new imaging modality is how it affects management of patients and clinical outcomes. Issues of cost, radiation dose, flexibility of use, and clinical workflow must be considered in addition to measures of sensitivity and specificity when determining how important a new modality will be. It is precisely these questions of clinical translation that will be the focus of much of the research effort in tomosynthesis in the next five years.
As with weather forecasting, it is difficult to predict with any certainty the long-range forecast, but one can make some reasonable estimates of near-term effects. So it is with tomosynthesis. In this article, the short-term future of tomosynthesis research and clinical utilization in the next 5 years will be assessed. A brief history of tomosynthesis will be provided so that the reader can have a context for current work in the field. A summary of the current state of the art will be given, and then many of the current questions of clinical translation will be explored in more detail.
A BRIEF HISTORY OF TOMOSYNTHESIS
Tomosynthesis is one of a number of imaging modalities whose conception preceded its feasible implementation by several decades. The basic theoretical framework for limited angle tomography was provided by Ziedses des Plantes1 in the 1930s. However, with the development of digital detectors being decades in the future, the implementation of tomosynthesis was clearly not feasible in those early days. Grant2 coined the term “tomosynthesis” in a landmark paper in 1972 that described the method of simple tomosynthesis reconstruction. A number of variants of tomosynthesis imaging were developed in the 1970s and 1980s, including “ectomography” by Edholm et al.,3 and flashing tomosynthesis4 that provided rapid imaging for vascular applications.
One of the difficulties encountered with these early techniques was the residual blur from objects outside of the plane of interest. As anyone familiar with conventional screen-film based geometrical tomography knows, when the x-ray tube (and sometimes the detector) moves during image acquisition, objects in the fulcrum plane of motion remain in sharp focus but objects outside the fulcrum plane are blurred in relation to their distance from the fulcrum plane. In the case of intravenous pyelograms (IVPs), for example, a zone of relatively sharp focus is centered about the opacified kidneys, ureters, and bladder, and the anatomy outside of this “zone” is relatively blurry. This type of geometrical tomography works well for imaging high contrast opacified structures, as with IVPs, but is not very successful at imaging unopacified soft-tissue anatomy because the residual blur from above and below the plane of interest masks the low-contrast soft-tissue anatomy of interest.
In the mid-1980s, several investigators explored methods to reduce the blur artifacts associated with tomosynthesis imaging. Ghosh Roy et al.,5 Chakraborty et al.,6 Ruttimann et al.,7 Dobbins et al.,8 and others were successful in eliminating all or part of the residual blur from overlying anatomy by solving for the blurring function in different reconstructed tomosynthesis planes. These deblurring algorithms made tomosynthesis suitable for consideration in a wider range of clinical applications
Another more serious challenge to the development of tomosynthesis imaging was the lack of a suitable digital detector for acquisition of the projection images. At the time that much of the work on deblurring algorithms was being done in the 1980s, a suitable large-area, self-scanned digital detector was not available. The earliest evaluation of the matrix inversion tomosynthesis (MITS) algorithm in our laboratory, for example, was tested using a conventional geometric tomography table and screen-film imaging; a series of screen-film projection images was acquired over a range of tube movement and then digitized.8 The digitized images were then reconstructed using the MITS algorithm, and a small phantom comprised of geometric shapes was visible in several reconstructed planes. Needless to say, this approach was extremely time consuming and certainly not clinically feasible. Computed radiography (CR) imaging plates became commercially available in the mid-1980s, and while a substantial improvement over digitizing film, were also not suitable because of the logistical impracticality of acquiring and scanning multiple CR plates during tube movement.
A few investigators used image intensifiers to demonstrate the potential of tomosynthesis imaging. Image intensifiers allowed rapid acquisition of images, thereby resolving the issue of how to acquire multiple images in a clinically realistic time frame. These images demonstrated that the concept of using tomosynthesis imaging could be useful in appreciating disease in the chest and were a substantial breakthrough.9 However, image intensifiers have notable drawbacks, including pincushion distortion and variable geometric uniformity when moving in the earth’s gravitational field, which make them less than ideal for tomosynthesis imaging. A number of corrections are necessary to adequately use image intensifiers for this purpose.
The advent of spiral CT in the late 1980s, coupled with the lack of a suitable digital radiographic detector, caused much of the research in digital tomosynthesis to come to a halt for about a decade. This author, and others, felt that spiral CT would become the way that all volumetric x-ray imaging would be done in the future and relegated tomosynthesis to the dustheap of research ideas that looked promising at first but just did not seem to pan out. The situation changed substantially in the late 1990s, however, when flat-panel radiographic detectors were introduced. With these new detectors there was finally a high-DQE, stable, low-noise, self-scanned imaging device without geometric distortion that could image at the speeds needed for reasonable use in tomosynthesis. Several investigators, including this author, retrieved tomosynthesis from the shelf of research relics, dusted it off, and picked up again in earnest with tomosynthesis research at that time. At last, there was a detector that made tomosynthesis feasible, and the rate of research exploration in tomosynthesis accelerated considerably.
One of the early applications of tomosynthesis with flat-panel detectors was conducted in our laboratory and was related to chest imaging. Starting in 1998, we developed, optimized, and evaluated tomosynthesis with flat-panel detectors for pulmonary nodule imaging.10, 11, 12, 13, 14, 15, 16 Figure 1 illustrates the advantage of using tomosynthesis for improving the visibility of subtle pulmonary nodules. At about the same time, tomosynthesis with flat-panel detectors was also applied to breast imaging.17, 18, 19
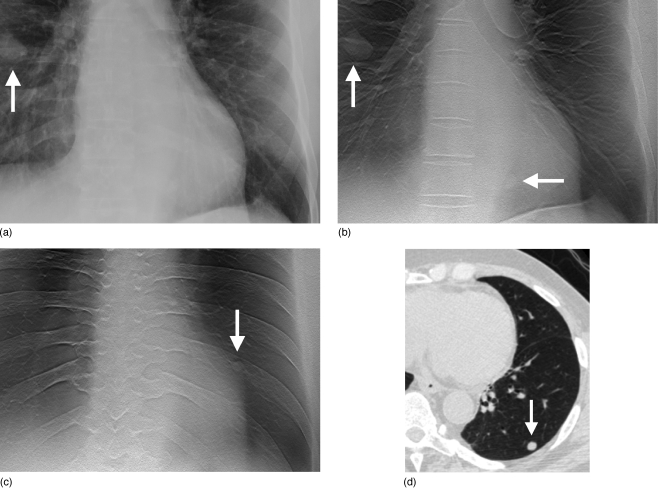
Figure 1.
Images of pulmonary nodules in chest tomosynthesis images of a human subject. (a) Coned view of digital PA radiograph shows one clearly visible right lung nodule (arrow). (b) Tomosynthesis image shows the same nodule (vertical arrow) as seen on the PA radiograph in (a). A second nodule (horizontal arrow) is also visible that was not seen in the PA radiograph in (a). (c) Tomosynthesis image at a more posterior level shows an additional left lung nodule (arrow) not seen in the PA radiograph in (a). (d) CT image (lung window) confirms left lower lobe nodule seen in (c). (Reprinted with permission from Medical Physics, Ref. 16, Copyright © 2008, American Association of Physicists in Medicine (AAPM).)
Tomosynthesis has been applied to a variety of clinical applications over the years, including dental imaging, angiography, and imaging of the chest, breast, and bones (see the review article in Ref. 20 for a more detailed list of citations to these applications). The two that have garnered the most attention since the late 1990s have been breast imaging and pulmonary nodule imaging, which will be described in more detail below.
CURRENT STATE OF THE ART
Geometry of motion
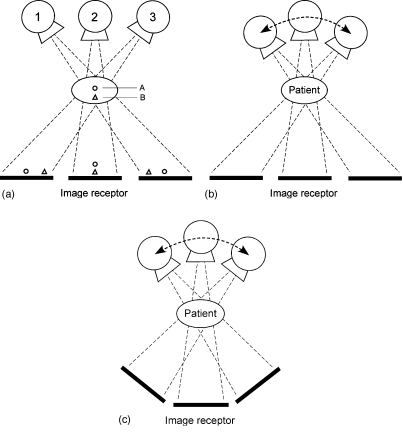
The first item to consider when describing tomosynthesis imaging is the geometry of motion of the tube and∕or detector. There are three basic motion geometries (see Fig. 2): parallel path (where the tube moves in a plane parallel to the detector plane; the detector may also move within its plane), full isocentric motion (where the tube and detector are fixed rigidly with respect to each other and move in tandem in a circular path around the patient), and partial isocentric motion (the detector remains stationary, and the x-ray tube moves in an arc above the detector). (See Ref. 20 for a review article containing more details about these motions.) All three of these motion geometries are used in contemporary tomosynthesis imaging. The parallel-path motion is typically used in chest and abdominal tomosynthesis (in an upright configuration for chest tomosynthesis and a tabletop configuration for IVP and other abdominal applications). Partial isocentric motion is used in virtually all current breast tomosynthesis devices because of the ease of constructing a compact rotational gantry for the x-ray tube; for simplicity, the detector remains fixed beneath the breast. Complete isocentric motion is used in cone-beam CT (which, for reasons of nomenclature, is not included in the family of tomosynthesis techniques because cone-beam CT gives a fully isotropic 3D reconstruction). Full isocentric motion is also being investigated in tomosynthesis applications using C-arm imagers and in radiation oncology applications where the x-ray source and detector are mounted on the linac gantry as it rotates through a limited angle of rotation in order to verify positioning of the patient. Limited-angle tomosynthesis acquisition can be reconstructed using a Feldkamp approach as described by Godfrey et al.21 or through image manipulation described by Kolitsi et al.22
Figure 2.
Geometries of motion for tomosynthesis image acquisition: (a) Parallel-path motion, (b) partial isocentric motion, and (c) full isocentric motion. Parallel-path motion in (a) illustrates how objects in two planes (circle and triangle) are projected onto different locations in the image plane due to parallax as the tube moves. (Reprinted by permission from Physics in Medicine and Biology, Ref. 20, Copyright © 2003, Institute of Physics (IOP) Publishing Ltd.)
Of the three geometries of motion, the parallel-path motion enables the simplest reconstruction algorithm and also maintains uniform magnification at each tube position. The partial isocentric motion leads to variable magnification at different tube orientations, and thus can distort small structures unless care is taken in the backprojection process.23 The full isocentric motion can provide excellent reconstructions but with a more complicated algorithm than the parallel-path motion.
Reconstruction algorithms
The most frequently used reconstruction algorithm for tomosynthesis is commonly referred to as shift and add (SAA). In the case of parallel-path geometry of motion of the tube and∕or detector, SAA involves shifting each of the projection images by a given amount and then adding them together. By selecting the shift amount correctly, objects in a given plane can be brought into sharp focus.
When performing SAA, it is important to align the shifted anatomical information correctly. With most x-ray tube gantries or overhead cranes, there is sufficient mechanical stability such that SAA can be performed adequately based solely on the known positions of the x-ray tube as it travels. However, patient motion must also be taken into account if the desired structures are going to align properly after the SAA image shifting. In the case of breast tomosynthesis, motion is mitigated by the light compression of the breast during image acquisition. In other applications, patient motion can be accounted for by placing fiducial markers on the patient in order to register the final images after taking into account motion of the patient. Webber et al.24 introduced a formalism for incorporating fiducial marker information into the image reconstruction in a method that is a variant of SAA; their method, called tuned aperture computed tomography (TACT), allows images to be acquired at random angles and orientations and then reconstructed in arbitrary planes using the projected locations of the fiducial markers. In our own research laboratory we have adopted a simplified version of the fiducial marker approach of Webber et al. in conjunction with parallel-path geometry to register projection images for chest tomosynthesis. In a research study of chest tomosynthesis, we found that most human subjects shifted vertically or horizontally by less than 2 mm during acquisition of the set of projection images; however, one subject shifted by as much as 8 mm. This motion did not result in blur within individual projection images due to the very short exposure times used. Rather, the subject motion meant that the sharp structures in individual projection images did not align precisely during shift-and-add reconstruction. Despite this motion, most anatomical structures in the subjects were reconstructed quite well with no apparent blur; however, some low-contrast or small objects were reconstructed more poorly unless correction for patient motion was used. As a result, we have developed an automated algorithm to locate the position of a fiducial marker placed on the patient’s back, and we recommend the use of fiducial markers for improved chest tomosynthesis. Such fiducial marker registration is not yet available in commercial tomosynthesis devices.
Simple SAA reconstruction is basically the same as unfiltered backprojection. SAA forms the basis of most tomosynthesis algorithms today because of its simplicity. It is, however, insufficient to use SAA alone for high-quality tomosynthesis reconstructions due to the overlapping blurry anatomy from outside of the plane of interest. As mentioned earlier, deblurring algorithms are used to correct for the out-of-plane blur and are almost universally adopted in contemporary tomosynthesis imaging. The two deblurring algorithms that have received the most attention in recent years are MITS and filtered backprojection (FBP). MITS, which was developed in our laboratory, solves for the out-of-plane blur using the known blurring functions of all other planes when a given plane is reconstructed.11, 12, 13, 20 Describing the SAA tomosynthesis reconstructions as a sum of blurry components from all planes, the unblurred structures can be obtained by using matrix algebra to solve the set of coupled equations in frequency space. This method is quite fast computationally, and given an object composed of a finite number of planes, can render an exact solution in the absence of noise. Real patients are not merely a set of planes but are three-dimensional structures, so there is some sharing of anatomy from plane to plane with MITS; MITS functions in a well-behaved way for the structures between planes, however.
A technique that is similar to MITS is the iterative restoration approach by Ruttimann et al.7 This method solves for the blur in each of the planes using known blurring functions, but unlike MITS, it solves the equations iteratively in the forward direction rather than by matrix algebra as with MITS. The advantage of image restoration is that it can include all elements of the imaging system, including truncation of structures at the edge of the detector at wide angles. It is, however, an iterative process, and thus is computationally slower than MITS.
The deblurring algorithm that is used today by many tomosynthesis investigators, and by most manufacturers, is filtered backprojection. FBP is well known from decades of work in CT and, like MITS, is a computationally fast algorithm. FBP takes the known sampling density in frequency space to generate an approximate point spread function that is used to correct for the blur of out-of-plane anatomy.25 FBP requires that the Fourier transform of projection images be multiplied by a ramp function to correct for the point spread function of the SAA algorithm. This ramp function tends to produce exaggerated noise in the high frequencies; therefore, a roll-off filter is typically used to suppress high-frequency noise amplification. The main difference between FBP and MITS is in the noise spectrum; FBP has better noise properties at low frequencies than MITS, but MITS has better noise properties at high frequencies (no roll-off filter is required with MITS). Effort is underway in our laboratory to combine FBP and MITS to get the optimum noise properties of each at different parts of the frequency spectrum.
There are also several iterative algorithms that are currently being investigated for tomosynthesis reconstruction. Wu et al.19 published breast tomosynthesis reconstructions using maximum likelihood expectation maximization (ML-EM). An advantage of this approach is that all the components of the imaging system can be modeled, but the downside is that the technique is iterative and quite computationally intensive. In one study, ML was found to be superior to FBP for masses and small calcifications.26 Other investigators have also recently reported on a simultaneous algebraic reconstruction technique (SART) that was found to give results comparable to ML methods but requiring fewer iterations.27, 28
Regardless of the algorithm used to do the basic tomosynthesis reconstruction, it is important when using partial isocentric motion to account for the arc-shaped path of objects projected onto the detector plane. It has been shown by Chen et al.23 that such arc-shaped projections can lead to distortion of the shape of microcalcifications in breast tomosynthesis. It is important for this effect to be included in the geometry of motion in the reconstruction algorithm.
Recent work by Fahrig et al.29 and Pineda et al.30 investigated a novel inverse geometry in tomosynthesis imaging. This inverse geometry uses a scanned source with a small detector element, just the opposite of the geometry of conventional x-ray imaging. Advantages of this device include a reduced level of patient skin exposure, which is potentially advantageous in high-exposure areas such as cardiac imaging.31 The inverse geometry also inherently provides tomosynthesis imaging capabilities.
Acquisition parameters
The optimum image acquisition parameters have been investigated in several laboratories and depend on both the clinical application as well as the reconstruction algorithm used. In the case of chest imaging, research in our own laboratory has determined that 71 projection images (an odd number is preferable) acquired over 20° of tube movement is best for detection of pulmonary nodules with the MITS algorithm.15, 16 A commercial device currently on the market uses about 60 images acquired over 40° for chest imaging. While the number of projection images is fairly consistent between these two implementations, the angle obviously differs substantially. The MITS algorithm used in our laboratory has an impulse response that performs better at midrange angles15 and FBP used with the commercial devices performs better at wider angles,32 thus explaining this difference. In application to breast imaging, anywhere from 11 to 49 projection images have been used,33, 34 with a total angle of tube movement in the range of 15°–50°.33, 35, 36
The spacing of the reconstructed planes also varies with the clinical application and the reconstruction algorithm. In breast imaging, it is common to use 1 mm plane spacing,19, 33 but in chest imaging, 5 mm is more typical.16
Commercial implementation
Commercial manufacturers have recently introduced tomosynthesis products that can be used for chest, abdominal, and musculoskeletal applications. Two such devices are currently on the market and are FDA approved. Devices for breast tomosynthesis are being developed by several manufacturers, and at least one manufacturer has a submission to the FDA for breast imaging applications.37 It is likely that within 1 year there will be an FDA-approved device on the market for breast imaging in the U.S.
TOWARD CLINICAL TRANSLATION
Tomosynthesis research over the past two decades has addressed many of the fundamental physics questions, and the field has reached a level of maturity reminiscent of the first or second generation CT or MR scanners. With the introduction of commercial products, many more investigators and clinicians will have access to tomosynthesis imaging, and a host of new questions will arise regarding its appropriate clinical utilization. Indeed, an increased interest in tomosynthesis has already been seen, as reflected in the exponentially expanding number of papers and presentations on this topic at recent scientific meetings. Many of the questions ahead will largely be translational in nature, which is not to say that physicists will not be actively involved; rather, research and applications in tomosynthesis will increasingly involve the combined efforts of both physicists and clinicians. In this section, some of the likely translational activities will be explored, and a synthesis of where the field is likely to go next will be given.
Continued physics optimization
Reconstruction algorithms
Reconstruction algorithms in tomosynthesis are relatively mature at this point, but continued work on optimizing the deblurring algorithms will occur for the next few years. For example, Chen et al.38 are investigating the combination of MITS and FBP in a technique termed Gaussian frequency blending (GFB). This method combines the mid- and high-frequency components of MITS with the low-frequency components of FBP. Doing so utilizes the excellent high-frequency noise properties of MITS with the excellent low-frequency noise properties of FBP. The combination should enable an improved noise power spectrum and slice profile response across the frequency spectrum. Other investigators are looking at alternative reconstruction algorithms, including SART.27 Various efforts are underway to compare these algorithms to one another26, 27, 39, 40, 41 and to determine the optimum set of acquisition parameters.15, 42, 43, 44, 45
There is also ongoing work to determine the best visual presentation of tomosynthesis images. Subjectively, images processed and reconstructed with deblurring algorithms can have a “high-pass filtered appearance” and may lose some of the overall grayscale range typical of conventional chest or breast imaging. Although it would be ideal to have the equivalent appearance of a sagittal or coronal CT reconstruction, tomosynthesis has a very broad slice profile at low frequencies that can appear somewhat “unnatural” if the image is presented with too much of a high-pass filtered look. For this reason, with MITS imaging, for example, a fraction of the very lowest frequency bins of a conventional tomosynthesis image is added back to the deblurred image. This process produces more of the grayscale range of a conventional chest radiograph, which may make it easier for radiologists to acclimate to the new modality. Likewise, with FBP it may be desirable to add back in a fraction of the lowest frequency grayscale variation to avoid the situation of the raw beam outside the patient having the same average grayscale as the interior anatomy. Again, such change is largely cosmetic but should improve the confidence of radiologists as the technique is gaining acceptance.
Clinical evaluation studies should be performed to determine if these modifications in visual presentation improve radiologists’ performance or make no difference. These projects will involve the input of both physicists and radiologists, and will likely not be completed until tomosynthesis has been commercially available for a while and radiologists have developed some experience with it.
Dose optimization
The question of optimum dose for tomosynthesis has not been resolved. The doses reported for a single-view breast tomosynthesis exam are about one to two times that of a single-view full-field digital mammogram (FFDM)33, 46, 47 [doses of 2 mGy (Ref. 47) and 4 mGy (Ref. 33) have been reported; many publications do not state absolute dose values, rather merely citing dose relative to that of single-view FFDM]. Early clinical experience in chest tomosynthesis indicates that exposure equivalent to that of one to three lateral chest exams is about right for reasonable imaging14, 16, 48 (in one study, total tomosynthesis exposures of 68–135 mR were reported,16 and in another study, an effective dose of 0.12 mSv was reported for chest tomosynthesis as compared with 0.04 mSv for a PA∕lateral chest exam48). In both of these clinical applications, however, the issue has not been fully resolved regarding whether to target the dose level to produce an overall “acceptable” image appearance or to address a specific clinical task. For example, in chest imaging, the dose level required solely to identify pulmonary nodules is likely to be perhaps one-half or less of the dose that produces an image suitable for general diagnostic use.49 The reason for this dose disparity is that the clinical task of nodule detection involves looking for larger objects than other general diagnostic tasks in chest imaging. In the case of breast tomosynthesis, the appropriate dose level depends on whether one is looking for both masses and calcifications or just for masses.
One component of dose optimization will be determining the optimum x-ray spectrum to yield the highest signal-to-noise ratio for the diagnostic tasks at hand. Currently, chest tomosynthesis has been performed with spectra equivalent to conventional radiography (125 kVp in one report48 and 120 kVp with 0.2 mm Cu filtration in another16), but further study of whether these spectra are optimum is needed. The spectra reported in breast tomosynthesis have been typical of those used in conventional digital mammography, but the optimum spectrum may not be finally decided upon until it is determined whether breast tomosynthesis will be used for both masses and calcifications or just for masses.
Some initial effort has gone into determining generally acceptable dose levels in tomosynthesis using limited numbers of human subjects or computer simulation and modeling studies.49, 50 Further studies will be needed to first determine the range of diagnostic tasks for which tomosynthesis is useful and then the appropriate dose level for each. These studies will likely take several years as radiologists gain experience with tomosynthesis and develop a sense for its best clinical utilization.
Breast imaging
Breast imaging has seen the greatest number of studies to date in tomosynthesis. Because there is currently no FDA-approved breast tomosynthesis device, all of the experience in breast tomosynthesis has been gained in research studies with human subjects. The total number of human subjects imaged with breast tomosynthesis is currently over 4000 worldwide. Despite the large number of human subjects participating in clinical studies, there is scant published data to date indicating sensitivity and specificity results. One study with 98 subjects found that breast tomosynthesis had image quality (lesion conspicuity and feature analysis) rated as equivalent or superior to diagnostic mammography in 89% of cases.33 Observer studies of computer simulated lesions in a structured 3D breast model found Az scores of 0.93 for tomosynthesis compared with 0.76 for digital mammography.46 Thus, completion of the various ongoing clinical trials will be necessary before definitive sensitivity and specificity data are known in human subjects.
Several important translational questions in breast tomosynthesis need study. First, it will be important to determine whether breast tomosynthesis will be most useful in screening, diagnostic use, or both. Some investigators feel that tomosynthesis will eventually be useful in both screening and diagnostic use, but the case for screening seems stronger at present. Because tomosynthesis may improve sensitivity of detection, it is likely to be most useful in screening where most breast cancer is initially discovered. However, because more potential cancers may be visualized with tomosynthesis, it will be important to carefully evaluate specificity in large clinical trials. It will also be necessary to evaluate recall rate and biopsy rate, as well as whether the positive predictive value for biopsy recommendation improves with tomosynthesis.36 The higher sensitivity of tomosynthesis may mean that more objects are seen that look suspicious, and thus the number of recalls may initially increase; however, there is some evidence that tomosynthesis may allow a better look at potential pathologies, thereby ruling out some false positives, and thus ultimately the number of cases that would be recalled may be reduced compared with conventional mammography.33 The very important translational question of how tomosynthesis affects diagnostic decision making and patient outcomes will need larger clinical studies to resolve.47, 51
A second important question is how many views to take with breast tomosynthesis. Currently, some investigators feel that tomosynthesis should be performed in both mediolateral oblique (MLO) and craniocaudal (CC) views,52 although that opinion is not universally held. There also remains a question about whether or not a static view should also be obtained. The answer to this question depends largely on how effective tomosynthesis is at imaging microcalcifications. There are several challenges with imaging microcalcifications with tomosynthesis, including the reduced resolution of the method compared with conventional single-image mammography and also the fact that the pattern of distribution of microcalcifications is more difficult to appreciate in section imaging unless a relatively thick section is used. The reduced resolution with tomosynthesis relative to a single-view FFDM image is due to the need to shift images by fractional pixel amounts prior to adding during reconstruction. This reduced resolution may impact the ability to appreciate the morphology of small calcifications, the importance of which is open to debate among clinical observers. If it is decided that a static view is needed to adequately evaluate calcifications, then a scenario may develop where an MLO static view mammogram is acquired along with MLO and CC view tomosynthesis exams. Nishikawa et al.53 have proposed a hybrid scheme where a regular MLO or CC view digital mammogram at normal dose is evaluated for microcalcifications, and a reduced dose and reduced resolution tomosynthesis data set is reviewed for masses. Clinical trials will be needed to determine which of these is the most appropriate utilization scheme.
A third translational question for breast tomosynthesis is how tomosynthesis used for screening will impact diagnostic workup procedures. Tomosynthesis used for screening will likely result in fewer diagnostic workups, and traditional methods such as ultrasound and magnification view mammography can still be used to evaluate suspicious opacities noted on screening tomosynthesis. Will tomosynthesis have sufficient specificity that certain patients can go directly to biopsy? And how will the very small number of cancers (e.g., circumscribed) that do not display well on tomosynthesis be handled in screening? These practical questions of utilization will likely be resolved as radiologists gain clinical experience with tomosynthesis in the first few years after FDA-approved devices are available.
There are other new breast imaging approaches under investigation that may impact how tomosynthesis is used. One example is the development of novel x-ray sources using carbon nanotube field-emission cathodes.54 If these devices are successful and reasonably priced, they may enable very rapid collection of tomosynthesis projection images that would eliminate the need for lengthy compression. A second example is the use of iodinated contrast in breast imaging. If injection of contrast is found useful in breast cancer imaging, will it also be advantageous in tomosynthesis imaging? Initial studies indicate that it may be.55 If contrast-enhanced tomosynthesis is found to be advantageous, it may be less costly and more readily available than alternatives such as contrast-enhanced magnetic resonance imaging. A third and larger question is how breast tomosynthesis and cone-beam CT of the breast will compare. CT is advantageous in that it has better depth resolution than tomosynthesis, but it also requires a larger room with more specialized equipment, requires the patient to lie prone, and has difficulty imaging near the chest wall.56 Computer simulation studies indicated that both tomosynthesis and breast CT outperformed digital mammography for detection of masses, but tomosynthesis and CT performed roughly comparably to each other.46 The jury is out on this comparison, and studies comparing these two modalities will be necessary to decide ultimately whether CT or tomosynthesis will prove superior.
Chest imaging
Although chest imaging was one of the first applications evaluated with tomosynthesis, there have not been as many studies in the chest as with breast tomosynthesis. We have investigated detection of pulmonary nodules with tomosynthesis for many years in our laboratory, and there are other investigators entering this area of research now that commercial chest tomosynthesis devices are available.
Imaging of the chest is very complex due to the wide range of disease with thoracic manifestations. Partly due to the wide range of disease in the chest, investigators have traditionally selected pulmonary nodules as one of the principal areas of research in chest imaging. Pulmonary nodules are often subtle and difficult to appreciate with conventional radiography due to the overlying anatomy that reduces their conspicuity. Tomosynthesis is ideally suited for improving detection of pulmonary nodules because it eliminates the visibility of overlying structures while still producing an image that is reminiscent of a posteroanterior (PA) chest radiograph (Fig. 1). Tomosynthesis can be performed with minor modifications to a conventional digital chest imaging room, making it possible to acquire tomosynthesis images at the same time as a conventional chest exam. Thus, although tomosynthesis does not have the depth resolution of CT, it has certain practical advantages and a price and radiation dose likely less than those of CT.
Early studies have demonstrated that tomosynthesis substantially increases detection sensitivity for pulmonary nodules over conventional PA radiography. In a study of 175 nodules in 21 subjects, we demonstrated that nodule detection improved from 22% with PA radiography to 70% with tomosynthesis; substantial and significant (p<0.004) improvement was noted with tomosynthesis over a range of nodule sizes (3–5, 5–10, and >10 mm).16 In a recent study at the University of Gothenburg, the lesion localization fraction (LLF) increased from 0.16 in PA radiography to 0.56 with tomosynthesis; however, the nonlesion localization fraction (i.e., false positives) was approximately 50% higher with tomosynthesis than with PA radiography.48 In both of these studies, the sensitivity of detection of pulmonary nodules tripled with tomosynthesis relative to conventional PA radiography, indicating the potential for improved clinical detection of lung cancer. However, the increase in false positives may reflect the need for additional clinical training with the new technique. Investigating reasons for the increase in false positives will no doubt be the subject of future work. The primary translational issue for chest tomosynthesis is determining the best clinical utilization strategy. Tomosynthesis is partway between conventional chest radiography (CXR) and CT in its imaging performance. In application to detection of pulmonary nodules, it is much more appropriate to see tomosynthesis not as a replacement for CT but as a vast improvement over CXR. With that perspective, how can tomosynthesis fit into the clinical management of patients along with CXR and CT?
There are four likely scenarios in which tomosynthesis may have clinical utility for pulmonary nodule detection. First, tomosynthesis may function as a problem-solving tool for suspicious opacities noted at CXR; if a nodulelike opacity is noted on conventional radiography, tomosynthesis may enable nodule mimics to be ruled out without the expense of sending the patient to CT. Second, it may be possible to perform tomosynthesis on every patient scheduled for CXR who is at elevated risk for pulmonary malignancies (e.g., smokers over age 50). By acquiring a tomosynthesis exam on all high-risk patients scheduled for CXR, it is likely that some asymptomatic nodules will be detected earlier. Third, it may be possible to follow patients with known nodules using tomosynthesis rather than CT. Such an approach would likely result in monetary savings, but it would need to be demonstrated in clinical trials that such an approach did not miss new small nodules that would be actionable. Fourth, if large-scale screening for lung cancer were adopted in the future, then tomosynthesis might be a lower-dose and lower-cost alternative to CT in such screening.
All four of the above scenarios require additional clinical studies to determine if they are feasible and effective. The larger issue with pulmonary nodule detection is more difficult to solve, and that is whether any of the above procedures results in better patient outcomes. Unlike breast imaging, which has demonstrated reduced mortality from screening mammography, there is still considerable controversy over the role of chest imaging for screening for lung cancer. This controversy is irrespective of the type of chest imaging (i.e., CXR, tomosynthesis, or CT). The problem lies in the biology of lung cancer. Studies have yet to demonstrate that detecting lung cancer earlier through imaging-based screening results in reduced mortality. One recent study found that screening with low-dose CT resulted in lengthened survival,57 while another study found that low-dose CT screening did not change mortality from lung cancer.58 These two prominent papers came to differing conclusions as to whether imaging-based screening is beneficial. It is likely that this controversy will not be resolved until results from the National Lung Screening Trial (NLST) (Ref. 59) are available some time in the future. Tomosynthesis appears to be a promising technique for improving pulmonary nodule detection, but the ultimate benefit to patient outcomes will need to await the conclusion of the NLST. In the meantime, additional clinical studies are needed to address the best way to use tomosynthesis as a problem-solving tool in chest imaging and to determine whether it makes sense to image high-risk patients scheduled for CXR with an additional tomosynthesis exam.
One additional translational question to address is whether or not the lateral chest image can be eliminated when using tomosynthesis. The lateral image is not as important as the PA radiograph for most chest imaging cases but accounts for a majority of the radiation exposure in a conventional PA∕lateral chest exam. The lateral is most commonly used to confirm presence of an anomaly noted in the PA radiograph or to triangulate the location of a noted object. Tomosynthesis is likely to be better at both those tasks, and thus, it raises the question of whether the lateral can be eliminated when using tomosynthesis; doing so would save enough radiation exposure that a composite exam composed of a PA radiograph and tomosynthesis may have comparable dose to a conventional chest exam. Eliminating the lateral would make the clinical acceptance of chest tomosynthesis much easier (due to dose concerns), but a clinical study will be required to satisfactorily answer this question.
Radiation oncology applications
Tomosynthesis also has generated considerable interest recently for application in determining patient positioning in radiation oncology.21, 60, 61, 62, 63, 64, 65, 66 On-board imaging has become an important part of verification of patient positioning, especially with intensity-modulated radiation therapy (IMRT). Tomosynthesis offers a quicker approach to validating positioning than a full-circle cone-beam computed tomography (CBCT) acquisition, although it does not have the full isotropic volumetric information obtainable from CBCT. Tomosynthesis has shown improved localization based upon bony anatomy when compared with traditional orthogonal radiographs (e.g., for head and neck cancers),61 but it has been more difficult to use with soft-tissue localization, although it has been shown subjectively to be good for visualizing thoracic and abdominal soft tissues in breath-hold treatment techniques. Tomosynthesis has also been used for the localization of brachytherapy seeds.67 There continues to be interest from vendors in evaluating tomosynthesis in radiation oncology applications. Further physics development work and clinical studies will be needed to confirm its role in these applications.
Other clinical applications
An additional area of exciting prospect for tomosynthesis is in orthopedic imaging. Studies of joint and bone disease can be difficult with CT due to the limited resolution in sagittal or coronal planes and with conventional radiography because of the inability to distinguish three-dimensional structures. Tomosynthesis naturally addresses both of these concerns. Flynn et al.68 showed approximately threefold improvement in spatial resolution in tomosynthesis images of bone relative to that in CT, and yet with dose considerably below that of CT. Flynn’s group is investigating tomosynthesis in application to knee, femur, shoulder, arm, leg, ankle, and wrist and has found in early results that tomosynthesis is good at demonstrating subtle fractures and at imaging metallic implants that would be difficult to image with CT. Webber’s TACT method has also been used in the evaluation of metallic implants in joint arthroplasty.69
Another application of tomosynthesis to orthopedic use is in evaluation of arthritis. Duryea et al.70 showed that tomosynthesis could demonstrate the joint space in the fingers with better clarity than on plain radiographic imaging. They demonstrated the ability to measure the joint space accurately and speculated that tomosynthesis would also have the potential to evaluate erosions of the joint space; both of these measures can be indicative of arthritic changes, lending support to the idea that tomosynthesis could become part of the screening for and monitoring of arthritic disease. Others are investigating tomosynthesis joint imaging with a C-arm device.71
Tomosynthesis has also been used in dental imaging for indications such as evaluation of implants, and there is a commercial dental tomosynthesis imaging unit on the market. However, the trend today seems to be more toward CBCT for a broader range of dental uses, and so the future of tomosynthesis imaging in dental applications is uncertain.
Tomosynthesis is also being evaluated for use intraoperatively72 or in emergency settings where standard CT imaging would be impractical or too time consuming.
Computer-aided detection and diagnosis
Computer-aided detection (CAD) and computer-aided detection diagnosis (CADx) schemes are often mentioned in connection with tomosynthesis73 and for two primary reasons. First, the reduction of overlying anatomy offers the potential for improved sensitivity and specificity for CAD algorithms. One of the factors that limits the success of some CAD algorithms is the large number of false-positive findings. Using tomosynthesis to eliminate much of the confusing background should theoretically reduce the number of false positives. However, in some cases (such as with chest tomosynthesis), this reduction in false positives from reduction of overlying soft-tissue might be limited by increased false positives from circular cross sections of imaged vessels. Thus, exactly how much tomosynthesis will benefit CAD algorithms remains to be seen. There is ongoing research in several laboratories, including ours, into these issues.
A second reason that tomosynthesis and CAD may make a good marriage is that CAD may reduce some of the overhead of viewing the large number of images in a set of tomosynthesis slices. One concern about tomosynthesis that is sometimes expressed by clinical colleagues is that tomosynthesis produces many more images than conventional projection imaging, and therefore, may slow down workflow. It is the opinion of this author that such reduction in workflow will not scale with the number of images and will ultimately not be too burdensome on the clinical staff. The reasoning behind that opinion is that the stack of images may be viewed at a PACS station dynamically by paging through the set quickly. The entire set of images viewed in such a dynamic fashion will take more time than reading a single conventional image, to be sure, but will be much faster than viewing each of the slice images statically (as was the case long ago with CT images printed side by side on hardcopy film). Furthermore, the human eye is very sensitive to dynamic changes, and subjectively when anomalous objects in tomosynthesis slices appear in a dynamically viewed stack, they are quite apparent. That being said, if CAD algorithms could be developed to review tomosynthesis image stacks, they might be able to speed up the process with which radiologists could review the data. Clearly, ongoing research in this area is needed.
Despite the potential advantages of combining CAD and tomosynthesis outlined above, there are considerations that might argue that tomosynthesis would benefit less from CAD than either conventional radiography or CT. Because objects are much more easily detected in tomosynthesis images than in conventional radiography, the need for CAD may well be less in tomosynthesis than in conventional imaging. On the other hand, chest CT, for example, can demonstrate such an abundance of small nodules that are below the actionability threshold of 5 mm (based on the recommendation of the Fleischner Society74) that reader fatigue may make it difficult to find all nodules in a chest CT data set. Tomosynthesis of the chest, on the other hand, does not display as many small nodules, and therefore, may not be as substantially helped by CAD as CT would be.
It is clear that substantial research into CAD algorithms for tomosynthesis using both slice-by-slice evaluation as well as full three-dimensional evaluation will be an important area of research in the future. The impact of using CAD, either as a prereader or a secondary reader, on user workflow will be important things to measure before tomosynthesis makes its full translational move from the bench to the bedside.
CONCLUSION AND SUMMARY
It seems clear from early studies that tomosynthesis offers the potential for superior performance to conventional radiography for several important clinical applications, and the prospect exists that it may become a cost-effective and low-dose imaging strategy to improve earlier detection of disease. Important clinical studies are underway to quantify both the sensitivity and specificity of tomosynthesis in cancer imaging. Further studies are needed to address its full clinical performance.
Tomosynthesis is in somewhat of a chicken and egg situation at the present time with regard to its clinical acceptance. Before tomosynthesis will be widely adopted in clinical use, its benefit to patient outcomes must be demonstrated with further studies and wider clinical experience. However, in order to complete such studies and gain such wider clinical experience, the technique must be used on more patients. This type of impasse is often seen early in the adoption of any new modality, and it is certain that gaining reimbursement for the technique will be an important component of accelerating its clinical utilization.
Tomosynthesis is fundamentally at a translational crossroads at the present time, with many of the remaining questions not ones of physics optimization or even clinical performance but rather pertaining to practical matters. How will tomosynthesis impact workflow, and what will be the consequences of that effect? How will tomosynthesis fit into the paradigm of clinical management as a tool halfway between traditional radiography and CT? Will patients that receive tomosynthesis be sent on to CT (or other standard diagnostic workup) afterwards in most cases anyway, thus ultimately not saving much money for the healthcare enterprise? Or will tomosynthesis find a niche that enables better utilization of dose and imaging dollars than the existing clinical paradigm? This author believes the latter will ultimately be the case, although further trials are needed to demonstrate it. Tomosynthesis is somewhat unique as a new imaging modality in that it is not likely to produce a “revolution” (as CT did) but rather an “evolution” of better clinical management. We are currently in the exciting and uncertain times when the “tipping point” will likely be seen in the next 1–3 years that will determine the future of this interesting imaging modality.
ACKNOWLEDGMENTS
The author gratefully acknowledges the input and suggestions of Joseph Y. Lo, Ph.D., Jay A. Baker, MD, Devon J. Godfrey, Ph.D., Michael J. Flynn, Ph.D., and Donald A. Tyndall, Ph.D., DDS in the preparation of this manuscript. Some of the research results from the author’s laboratory were supported by grants from NIH (Grant No. R01 CA80490) and GE Healthcare. GE and Duke jointly hold a patent related to certain aspects of tube movement in tomosynthesis imaging. Collaborative work on breast tomosynthesis at the author’s institution was supported in part by a grant from Siemens Medical Solutions.
References
- Ziedses des Plantes B. G., “Eine neue methode zur differenzierung in der roentgenographie (planigraphie),” Acta Radiol. 13, 182–192 (1932). 10.3109/00016923209135135 [DOI] [Google Scholar]
- Grant D. G., “Tomosynthesis: A three-dimensional radiographic imaging technique,” IEEE Trans. Biomed. Eng. BME-19, 20–28 (1972). 10.1109/TBME.1972.324154 [DOI] [PubMed] [Google Scholar]
- Edholm P., Granlund G., Knutsson H., and Petersson C., “Ectomography: A new radiographic method for reproducing a selected slice of varying thickness,” Acta Radiol. 21, 433–442 (1980). [DOI] [PubMed] [Google Scholar]
- Becher H., Schluter M., Mathey D. G., Bleifeld W., Klotz E., Haaker P., Linde R., and Weiss H., “Coronary angiography with flashing tomosynthesis,” Eur. Heart J. 6, 399–408 (1985). [DOI] [PubMed] [Google Scholar]
- Ghosh Roy D. N., Kruger R. A., Yih B., and Del Rio P., “Selective plane removal in limited angle tomographic imaging,” Med. Phys. 12, 65–70 (1985). 10.1118/1.595791 [DOI] [PubMed] [Google Scholar]
- Chakraborty D. P., Yester M. V., Barnes G. T., and Lakshminarayanan A. V., “Self-masking subtraction tomosynthesis,” Radiology 150, 225–229 (1984). [DOI] [PubMed] [Google Scholar]
- Ruttimann U. E., Groenhuis R. A. J., and Webber R. L., “Restoration of digital multiplane tomosynthesis by a constrained iteration method,” IEEE Trans. Med. Imaging 3, 141–148 (1984). 10.1109/TMI.1984.4307670 [DOI] [PubMed] [Google Scholar]
- J. T.DobbinsIII, Powell A. O., and Weaver Y. K., “Matrix inversion tomosynthesis: Initial image reconstruction,” in RSNA 73rd Scientific Assembly, Chicago, IL, 1987. (unpublished).
- Sone S., Kasuga T., Sakai F., Oguchi K., Itoh A., Li F., Maruyama Y., Kubo K., Honda T., Haniuda M., and Takemura K., “Digital tomosynthesis imaging of the lung,” Radiat. Med. 14, 53–63 (1996). [PubMed] [Google Scholar]
- J. T.DobbinsIII, Webber R. L., and Hames S. M., “Tomosynthesis for improved pulmonary nodule detection,” in RSNA 84th Scientific Assembly, Chicago, IL, 1998. (unpublished).
- Warp R. J., Godfrey D. J., and J. T.DobbinsIII, “Applications of matrix inverse tomosynthesis,” Proc. SPIE 3977, 376–383 (2000). 10.1117/12.384512 [DOI] [Google Scholar]
- Godfrey D. J., Warp R. J., and J. T.DobbinsIII, “Optimization of matrix inverse tomosynthesis,” Proc. SPIE 4320, 696–704 (2001). 10.1117/12.430908 [DOI] [Google Scholar]
- Godfrey D. J., Rader A., and J. T.DobbinsIII, “Practical strategies for the clinical implementation of matrix inversion tomosynthesis (MITS),” Proc. SPIE 5030, 379–390 (2003). 10.1117/12.480352 [DOI] [Google Scholar]
- J. T.DobbinsIII, Godfrey D. J., and McAdams H. P., in Advances in Digital Radiography: RSNA Categorical Course in Digital Radiography, edited by Samei E. and Flynn M. J. (Radiological Society of North America, Oak Brook, 2003). [Google Scholar]
- Godfrey D. J., McAdams H. P., and J. T.DobbinsIII, “Optimization of the matrix inversion tomosynthesis (MITS) impulse response and modulation transfer function characteristics for chest imaging,” Med. Phys. 33, 655–667 (2006). 10.1118/1.2170398 [DOI] [PubMed] [Google Scholar]
- J. T.DobbinsIII, McAdams H. P., Song J. -W., Li C. M., Godfrey D. J., DeLong D. M., Paik S. -H., and Martinez-Jimenez S., “Digital tomosynthesis of the chest for lung nodule detection: Interim sensitivity results from an ongoing NIH-sponsored trial,” Med. Phys. 35, 2554–2557 (2008). 10.1118/1.2937277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklason L. T., Christian B. T., Niklason L. E., Kopans D. B., Castleberry D. E., Opsahl-Ong B. H., Landberg C. E., Slanetz P. J., Giardino A. A., Moore R., Albagli D., DeJule M. C., Fitzgerald P. F., Fobare D. F., Giambattist B. W.a, Kwasnick R. F., Liu J., Lubowski S. J., Possin G. E., Richotte J. F., Wei C. -Y., and Wirth R. F.,, “Digital tomosynthesis in breast imaging,” Radiology 205, 399–406 (1997). [DOI] [PubMed] [Google Scholar]
- Suryanarayanan S., Karellas A., Vedantham S., Glick S. J., D’Orsi C. J., Baker S. P., and Webber R. L., “Comparison of tomosynthesis methods used with digital mammography,” Acad. Radiol. 7, 1085–1097 (2000). 10.1016/S1076-6332(00)80061-6 [DOI] [PubMed] [Google Scholar]
- Wu T., Stewart A., Stanton M., McCauley T., Phillips W., Kopans D. B., Moore R. H., Eberhard J. W., Opsahl-Ong B., Niklason L., and Williams M. B., “Tomographic mammography using a limited number of low-dose cone-beam projection images,” Med. Phys. 30, 365–380 (2003). 10.1118/1.1543934 [DOI] [PubMed] [Google Scholar]
- J. T.DobbinsIII and Godfrey D. J., “Digital x-ray tomosynthesis: Current state of the art and clinical potential,” Phys. Med. Biol. 48, R65–R106 (2003). 10.1088/0031-9155/48/19/R01 [DOI] [PubMed] [Google Scholar]
- Godfrey D. J., Yin F. -F., Oldham M., Yoo S., and Willett C., “Digital tomosynthesis with an on-board kilovoltage imaging device,” Int. J. Radiat. Oncol., Biol., Phys. 65, 8–15 (2006). 10.1016/j.ijrobp.2006.01.025 [DOI] [PubMed] [Google Scholar]
- Kolitsi Z., Anastassopoulos V., Scodras A., and Pallikarakis N., “A multiple projection method for digital tomosynthesis,” Med. Phys. 19, 1045–1050 (1992). 10.1118/1.596822 [DOI] [PubMed] [Google Scholar]
- Chen Y., Lo J. Y., and J. T.DobbinsIII, “Importance of point-by-point back projection correction for isocentric motion in digital breast tomosynthesis: Relevance to morphology of structures such as microcalcifications,” Med. Phys. 34, 3885–3892 (2007). 10.1118/1.2776256 [DOI] [PubMed] [Google Scholar]
- Webber R. L., Horton R. A., Tyndall D. A., and Ludlow J. B., “Tuned-aperture computed tomography (TACT-TM): Theory and application for three-dimensional dento-alveolar imaging,” Dentomaxillofac Radiol. 26, 53–62 (1997). 10.1038/sj.dmfr.4600201 [DOI] [PubMed] [Google Scholar]
- Lauritsch G. and Harer W. H., “A theoretical framework for filtered backprojection in tomosynthesis,” Proc. SPIE 3338, 1127–1137 (1998). 10.1117/12.310839 [DOI] [Google Scholar]
- Wu T., Moore R. H., Rafferty E. A., and Kopans D. B., “A comparison of reconstruction algorithms for breast tomosynthesis,” Med. Phys. 31, 2636–2647 (2004). 10.1118/1.1786692 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chan H. P., Sahiner B., Wei J., Goodsitt M. M., Hadjiiski L. M., Ge J., and Zhou C., “A comparative study of limited-angle cone-beam reconstruction methods for breast tomosynthesis,” Med. Phys. 33, 3781–3795 (2006). 10.1118/1.2237543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chan H. P., Sahiner B., Wu Y. -T., Zhou C., Ge J., Wei J., and Hadjiiski L. M., “Application of boundary detection information in breast tomosynthesis reconstruction,” Med. Phys. 34, 3603–3613 (2007). 10.1118/1.2761968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrig R., Pineda A. R., Solomon E. G., Leung A. N., and Pelc N. J., “Fast tomosynthesis for lung cancer detection using the SBDX geometry,” Proc. SPIE 5030, 371–378 (2003). 10.1117/12.480149 [DOI] [Google Scholar]
- Pineda A. R., Yoon S., Paik D. S., and Fahrig R., “Optimization of a tomosynthesis system for the detection of lung nodules,” Med. Phys. 33, 1372–1379 (2006). 10.1118/1.2190329 [DOI] [PubMed] [Google Scholar]
- Solomon E. G., Van Lysel M. S., Melen R. E., Moorman J. W., and Skillicorn B., “Low-exposure scanning-beam x-ray fluoroscopy system,” Proc. SPIE 2708, 140–149 (1996). 10.1117/12.237777 [DOI] [Google Scholar]
- Li B., Avinash G. B., Eberhard J. W., and Claus B. E. H., “Optimization of slice sensitivity profile for radiographic tomosynthesis,” Med. Phys. 34, 2907–2916 (2007). 10.1118/1.2742499 [DOI] [PubMed] [Google Scholar]
- Poplack S. P., Tosteson T. D., Kogel C. A., and Nagy H. M., “Digital breast tomosynthesis: Initial experience in 98 women with abnormal digital screening mammography,” AJR Am. J. Roentgenol. 189, 616–623 (2007). 10.2214/AJR.07.2231 [DOI] [PubMed] [Google Scholar]
- Zhao W., Zhao B., Fisher P. R., Warmoes P., Mertelmeier T., and Orman J., “Optimization of detector operation and imaging geometry for breast tomosynthesis,” Proc. SPIE 6510, M1–M12 (2007). [Google Scholar]
- Wu T., Moore R. H., and Kopans D. B., “Voting strategy for artifact reduction in digital breast tomosynthesis,” Med. Phys. 33, 2461–2471 (2006). 10.1118/1.2207127 [DOI] [PubMed] [Google Scholar]
- Park J. M., Franken E. A. J., Garg M., Fajardo L. L., and Niklason L. T., “Breast tomosynthesis: Present considerations and future applications,” Radiographics 27, S231–S240 (2007). 10.1148/rg.27si075511 [DOI] [PubMed] [Google Scholar]
- Wallack T., “New dimension in detection,” Boston Globe, June 6, 2008.
- Chen Y., Lo J. Y., Baker J. A., and J. T.DobbinsIII, “Gaussian frequency blending algorithm with matrix inversion tomosynthesis (MITS) and filtered back projection (FBP) for better digital breast tomosynthesis reconstruction,” Proc. SPIE 6142, 0E1–0E9 (2006). [Google Scholar]
- Chen Y., Lo J. Y., and J. T.DobbinsIII, “Impulse response analysis for several digital tomosynthesis mammography reconstruction algorithms,” Proc. SPIE 5745, 541–549 (2005). 10.1117/12.595684 [DOI] [Google Scholar]
- Rakowski J. T. and Dennis M. J., “A comparison of reconstruction algorithms for C-arm mammography tomosynthesis,” Med. Phys. 33, 3018–3032 (2006). 10.1118/1.2219090 [DOI] [PubMed] [Google Scholar]
- Zhou J., Zhao B., and Zhao W., “A computer simulation platform for the optimization of a breast tomosynthesis system,” Med. Phys. 34, 1098–1109 (2007). 10.1118/1.2558160 [DOI] [PubMed] [Google Scholar]
- Gifford H. C., Didier C. S., Das M., and Glick S. J., “Optimizing breast-tomosynthesis acquisition parameters with scanning model observers,” Proc. SPIE 6917, 0S1–0S9 (2008). [Google Scholar]
- Deller T., Jabri K. N., Sabol J. M., Ni X., Avinash G., Saunders R., and Uppaluri R.“Effect of acquisition parameters on image quality in digital tomosynthesis,” Proc. SPIE 6510, 1L1–1L11 (2007). [Google Scholar]
- Reiser I., Lau B. A., and Nishikawa R. M., “Effect of scan angle and reconstruction algorithm on model observer performance in tomosynthesis,” in IWDM 2008, edited by Krupinski E. A. (Springer-Verlag, Berlin, 2008), Vol. LNCS 5116, pp. 606–611.
- Chan H. P., Wei J., Zhang Y., Sahiner B., Hadjiiski L. M., and Helvie M. A., “Detection of massses in digital breast tomosynthesis mammography: Effects of the number of projection views and dose,” in IWDM 2008, edited by Krupinski E. A. (Springer-Verlag, Berlin, 2008), Vol. LNSC5116, pp. 279–285.
- Gong X., Glick S. J., Liu B., Vedula A. A., and Thacker S., “A computer simulation study comparing lesion detection accuracy with digital mammography, breast tomosynthesis, and cone-beam CT breast imaging,” Med. Phys. 33, 1041–1052 (2006). 10.1118/1.2174127 [DOI] [PubMed] [Google Scholar]
- Good W. F., Abrams G. S., Catullo V. J., Chough D. M., Ganott M. A., Hakim C. M., and Gur D., “Digital breast tomosynthesis: A pilot observer study,” AJR Am. J. Roentgenol. 190, 865–869 (2008). 10.2214/AJR.07.2841 [DOI] [PubMed] [Google Scholar]
- Vikgren J., Zachrisson S., Svalkvist A., Johnsson Å. A., Boijsen M., Flinck A., Kheddache S., and Båth M., “Comparison of chest tomosynthesis and chest radiography for detection of pulmonary nodules: Human observer study of clinical cases,” Radiology 249, 1034–1041 (2008). 10.1148/radiol.2492080304 [DOI] [PubMed] [Google Scholar]
- Li C. M. and J. T.DobbinsIII, “Methodology for determining dose reduction for chest tomosynthesis,” Proc. SPIE 6510, 2D1–2D10 (2007). [Google Scholar]
- Timberg P., Båth M., Andersson I., Svahn T., Ruschin M., Hemdal B., Mattsson S., and Tingberg A., “Impact of dose on observer performance in breast tomosynthesis using breast specimens,” Proc. SPIE 6913, 4J1–4J10 (2008). [Google Scholar]
- Gur D., “Tomosynthesis: Potential clinical role in breast imaging,” AJR Am. J. Roentgenol. 189, 614–615 (2007). 10.2214/AJR.07.2588 [DOI] [PubMed] [Google Scholar]
- Rafferty E. A., Niklason L. T., and Jameson-Meehan L. A., “Breast tomosynthesis: One view or two?,” in RSNA 92nd Scientific Assembly, Chicago, IL, 2006. (unpublished).
- Nishikawa R. M., Reiser I., and Seifi P., “A new approach to digital breast tomosynthesis for breast cancer screening,” Proc. SPIE 6510, 3C1–3C8 (2007). [Google Scholar]
- Yang G., Rajaram R., Cao G., Sultana S., Liu Z., Lalush D., Lu J., and Zhou O., “Stationary digital breast tomosynthesis system with a multi-beam field emission x-ray source array,” Proc. SPIE 6913, 1A1–1A10 (2008). [Google Scholar]
- Chen S. C., Carton A. -K., Albert M., Conant E. F., Schnall M. D., and Maidment A. D. A., “Initial clinical experience with contrast-enhanced digital breast tomosynthesis,” Acad. Radiol. 14, 229–238 (2007). 10.1016/j.acra.2006.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karellas A., Lo J. Y., and Orton C. G., “Cone beam x-ray CT will be superior to digital x-ray tomosynthesis in imaging the breast and delineating cancer,” Med. Phys. 35, 409–411 (2008). 10.1118/1.2825612 [DOI] [PubMed] [Google Scholar]
- The International Early Lung Cancer Action Program Investigators, “Survival of patients with stage I lung cancer detected on CT screening,” N. Engl. J. Med. 355, 1763–1771 (2006). 10.1056/NEJMoa060476 [DOI] [PubMed] [Google Scholar]
- Bach P. B., Jett J. R., Pastorino U., Tockman M. S., Swensen S. J., and Begg C. B., “Computed tomography screening and lung cancer outcomes,” JAMA, J. Am. Med. Assoc. 297, 953–961 (2007). 10.1001/jama.297.9.953 [DOI] [PubMed] [Google Scholar]
- Aberle D. R., Chiles C., Gatsonis C., Hillman B. J., Johnson C. D., McClennan B. L., Mitchell D. G., Pisano E. D., Schnall M. D., and Sorensen A. G., “Imaging and cancer: Research strategy of the American College of Radiology Imaging Network,” Radiology 235, 741–751 (2005). 10.1148/radiol.2353041760 [DOI] [PubMed] [Google Scholar]
- Godfrey D. J., Ren L., Yan H., Wu Q. J., Yoo S., Oldham M., and Yin F. -F., “Evaluation of three types of reference image data for external beam radiotherapy target localization using digital tomosynthesis (DTS),” Med. Phys. 34, 3374–3384 (2007). 10.1118/1.2756941 [DOI] [PubMed] [Google Scholar]
- Wu Q. J., Godfrey D. J., Wang Z., Zhang J., Zhou S., Yoo S., Brizel D. M., and Yin F. -F., “On-board patient positioning for head-and-neck IMRT: Comparing digital tomosynthesis to kilovoltage radiography and cone-beam computed tomography,” Int. J. Radiat. Oncol., Biol., Phys. 69, 598–606 (2007). 10.1016/j.ijrobp.2007.05.045 [DOI] [PubMed] [Google Scholar]
- Yan H., Ren L., Godfrey D. J., and Yin F. -F., “Accelerating reconstruction of reference digital tomosynthesis using graphics hardware,” Med. Phys. 34, 3768–3776 (2007). 10.1118/1.2779945 [DOI] [PubMed] [Google Scholar]
- Ren L., Godfrey D. J., Yan H., Wu Q. J., and Yin F. -F., “Automatic registration between reference and on-board digital tomosynthesis images for positioning verification,” Med. Phys. 35, 664–672 (2008). 10.1118/1.2831903 [DOI] [PubMed] [Google Scholar]
- Yan H., Godfrey D. J., and Yin F. -F., “Fast reconstruction of digital tomosynthesis using on-board images,” Med. Phys. 35, 2162–2169 (2008). 10.1118/1.2896077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang G., Bani-Hashemi A., Au P., O’Brien P. F., Rowlands J. A., Morton G., Lim T., Cheung P., and Loblaw A., “Megavoltage cone beam digital tomosynthesis (MV-CBDT) for image-guided radiotherapy: A clinical investigational system,” Phys. Med. Biol. 53, 999–1013 (2008). 10.1088/0031-9155/53/4/012 [DOI] [PubMed] [Google Scholar]
- Descovich M., Morin O., Aubry J. F., Aubin M., Chen J., Bani-Hashemi A., and Pouliot J., “Characteristics of megavoltage cone-beam digital tomosynthesis,” Med. Phys. 35, 1310–1316 (2008). 10.1118/1.2868763 [DOI] [PubMed] [Google Scholar]
- Tutar I. B., Managuli R., Shamdasani V., Cho P. S., Pathak S. D., and Kim Y., “Tomosynthesis-based localization of radioactive seeds in prostate brachytherapy,” Med. Phys. 30, 3135–3142 (2003). 10.1118/1.1624755 [DOI] [PubMed] [Google Scholar]
- Flynn M. J., McGee R., and Blechinger J., “Spatial resolution of x-ray tomosynthesis in relation to computed tomography for coronal∕sagittal images of the knee,” Proc. SPIE 6510, 0D1–0D9 (2007). [Google Scholar]
- Fahey F. H., Webber R. L., Chew F. S.-K., and Dickerson B. A., “Application of TACT® to the evaluation of total joint arthroplasty,” Med. Phys. 30, 454–460 (2003). 10.1118/1.1544676 [DOI] [PubMed] [Google Scholar]
- Duryea J., J. T.DobbinsIII, and Lynch J. A., “Digital tomosynthesis of hand joints for arthritis assessment,” Med. Phys. 30, 325–333 (2003). 10.1118/1.1543573 [DOI] [PubMed] [Google Scholar]
- Li S. and Jiang H., “A practical method for three-dimensional reconstruction of joints using a C-arm system and shift-and-add algorithm,” Med. Phys. 32, 1491–1499 (2005). 10.1118/1.1915289 [DOI] [PubMed] [Google Scholar]
- Bachar G., Siewerdsen J. H., Daly M. J., Jaffray D. A., and Irish J. C., “Image quality and localization accuracy in C-arm tomosynthesis-guided head and neck surgery,” Med. Phys. 34, 4664–4677 (2007). 10.1118/1.2799492 [DOI] [PubMed] [Google Scholar]
- Chan H. P., Wei J., Sahiner B., Rafferty E. A., Wu T., Roubidoux M. A., Moore R. H., Kopans D. B., Hadjiiski L. M., and Helvie M. A., “Computer-aided detection system for breast masses on digital tomosynthesis mammograms: Preliminary experience,” Radiology 237, 1075–1080 (2005). 10.1148/radiol.2373041657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMahon H., Austin J. H. M., Gamsu G., Herold C. J., Jett J. R., Naidich D. P., E. F.Patz, Jr., and Swensen S. J., “Guidelines for management of small pulmonary nodules detected on CT scans: A statement from the Fleischner Society,” Radiology 237, 395–400 (2005). 10.1148/radiol.2372041887 [DOI] [PubMed] [Google Scholar]