Abstract
Metabotropic glutamate receptors (mGluRs) modulate glutamatergic and GABAergic neurotransmission. mGluR8 is generally located presynaptically where it regulates neurotransmitter release. Previously we reported that 6-month-old mGluR8-/- male mice show higher measures of anxiety in anxiety tests involving avoidable anxiety-provoking stimuli than age-matched wild-type male mice. In wild-type mice, middle-aged females and males show higher measures of anxiety in such tests and reduced spatial learning than young adults. In this study we evaluated in middle-aged mice the effects of mGluR8 deficiency on measures of anxiety involving avoidable and unavoidable anxiety-provoking stimuli and on cognitive performance and whether these effects are sex-dependent. Female and male mGluR8-/- mice showed increased measures of anxiety in the open field. In contrast, male mGluR8-/- mice showed increased but female male mGluR8-/- mice decreased measures of anxiety in the elevated plus maze and the acoustic startle response. mGluR8 deficiency impaired novel location recognition and spatial memory retention in the water maze. The impairment in spatial memory retention in the water maze, but not in novel location recognition, was more pronounced in female than male mice. Thus, potential sex differences in the therapeutic effects of mGluR8 modulation to reduce measures of anxiety and improve cognitive performance should be carefully considered.
Keywords: water maze, novel location recognition, sex differences, open field, elevated plus maze, acoustic startle
1. Introduction
Metabotropic glutamate receptors (mGluRs), which are coupled to second messenger pathways via G proteins, modulate glutamatergic and GABAergic neurotransmission [1, 2]. Eight different types of mGluRs (mGluR1-mGluR8) have been identified and classified into 3 groups according to their sequence identity, pharmacological profile and signal transduction mechanisms. mGluR8, is a member of group III that also comprises mGluR4, mGluR6 and mGluR7 and are coupled to the inhibition of adenylyl cyclase [3, 4]. Group III receptors are generally located presynaptically, where they regulate neurotransmitter release [5].
Because of their role in modulating neurotransmission, mGluRs are attractive targets for therapies aimed at treating anxiety disorders (reviewed in [6]. Consistent with a role for mGluR8 in the regulation of anxiety, under red-light conditions and on the ICR background, increased measures of anxiety in the elevated plus maze were seen in mGluR8-/- mice at 3 months of age and to a lesser extent at 6 months of age [7]. Under regular light conditions and on the C57BL/6J background, we observed increased measures of anxiety in the open field and elevated plus maze at 6 months of age [8]. In wild-type mice, middle-aged mice show higher measures of anxiety in such tests and reduced spatial learning compared to young adult mice [9]. Here, we examine the effects of mGluR8 deficiency on anxiety-like behavior in one year old mice.
The open field and elevated plus maze involve anxiety-provoking open areas the animal can choose to avoid. However, it is not known whether mGluR8-/- mice show increased measures of anxiety in tests involving unavoidable anxiety-provoking stimuli, such as acoustic stimuli. In this study we analyzed the effects of mGluR8 deficiency on measures of anxiety involving both avoidable and unavoidable anxiety.
The genetic architecture of many human traits is sexually dimorphic [10]. Sex dimorphism in predisposition to psychiatric disorders might involve the influence of sex hormones [11], sex chromosome genes [12], sex differences in epigenetic mechanisms [13], and autosomal genes. For example, the involvement of catechol-O-methyltransferase to predisposition of anxiety phenotypes is sex-dependent [14]. Although most anxiety disorders show a higher incidence in women than men, most animal studies of anxiety-like behaviors use males exclusively [15]. The potential anxiety phenotype of mGluR8-/- female mice is unknown. Altered measures of anxiety might be associated with alterations in learning and memory in a sex-dependent fashion. For example, while both female and male mice lacking the enzyme histidine decarboxylase (HDC) showed enhanced measures of anxiety, they showed sex-dependent alterations in measures of learning and memory [16, 17]. In the current study, the effects of mGluR8 deficiency on cognitive performance in female and male mice were determined. Middle-aged 12-month-old mice were selected for this study as at this age the female mice are acyclic [9], so preventing potential complications with regard to data interpretation due to potential differences in the cycle at the time of testing.
2. Materials and Methods
2.1. Animals
One-year-old mGluR8-/- mice (n = 30), generated as described (Duvoisin et al, 2005) and age-matched C57BL/6J wild-type mice (n = 27) were used. The number of backcrosses onto the C57Bl6/J background was 10 generations. Het-het breeding was used to generate the wild-type and knockout mice that were used to generate the mice used in the present study using wild-type-wild-type and knockout-knockout breedings (2-3 generations in total). A total of 11 different matings were used to generate the mice for this study. The mice were kept on 12:12 hr light-dark schedule (lights on at 6 AM) with chow (PicoLab Rodent Diet 20, #5053; PMI Nutrition International, St. Louis, MO) and water given ad libitum. All the experiments reported here were conducted in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee at OHSU. All behavioral tests administered, described in detail below, were designed to minimize potential pain or discomfort. To avoid potential effects of circadian variation on behavioral performance, the mice were tested between 9:00 and 13:00 in the open field, elevated plus maze, acoustic startle and rotorod tests. During cognitive testing (object recognition and water maze), mice were tested in the morning and afternoon. The same mice (n = 16 wild-type mice and n = 16 mGluR8-/- mice) were tested in the open field and elevated plus maze (week 1), for object recognition (week 2), in the water maze (week 3), and for rotorod performance (week 4). An independent group of mice (n = 11 wild-type mice and n = 14 mGluR8-/- mice) was tested for the acoustic startle response.
2.2. Open Field
To evaluate exploratory behavior and measures of anxiety in the open field, mice were placed in a 40.6 cm × 40.6 cm brightly lit open arena equipped with infrared photocells interfaced with a computer (Kinder Scientific, Poway, CA). Active times (new beam breaks within 1 sec), distance moved, and rest times (no beam breaks within 1sec) were recorded for a 10-min session. To assess measures of anxiety in the open-field, the more anxiety-provoking center zone (20.3 × 20.3 cm) and the less anxiety-provoking peripheral zone were analyzed separately. The open field and all other enclosures were cleaned with 5% acetic acid between animals.
2.3. Elevated Plus Maze
The elevated plus maze consisted of two open arms and two closed arms equipped with infrared photocells interfaced with a computer (Kinder Scientific, Poway, CA). Rodents avoid the open arms of the plus maze so that decreased time spent in and decreased entries into the open arms are thought to reflect enhanced measures of anxiety. Mice were placed individually in the center of the maze and allowed free access for 10 min. Animals spent time either in a closed, safe, area (closed arms), in an open area (open arms) or in the middle, intermediate zone. Recorded beam brakes were used to calculate path lengths and percent time spent in the open arms of the maze.
2.4. Acoustic Startle
Acoustic startle was tested in Kinder Scientific (Poway, CA) startle chambers in a separate cohort of mice. After a 5-min acclimation, the baseline response was measured. Acoustic pulses where given, increasing from 80 dB to 120 dB using increments of 2 dB within a 500 msec window and the maximum force in newtons (N) was measured. Startle amplitude was defined as the peak force that occurred during the 500 msec record window. White noise was used for the acoustic stimuli. Wideback background noise (72 dB) was used during testing.
2.5. Novel location recognition
Mice were individually habituated to a lit open arena with clear Plexiglas walls (40.6 cm × 40.6 cm; Hamilton-Kinder, Poway, CA) for 5 min for 3 consecutive days. On the fourth day, the mice were trained in 4 consecutive 10 min trials. The first 3 were familiarization trials with 3 objects placed within the arena (one in each of 3 corners). For the novel location recognition test (fourth trial), one object was moved to a novel location in the arena. The same object was moved for all mice. A 4 min inter-trial interval elapsed between all trials. The time spent exploring each object during the familiarization and novel location trials was measured using a video tracking system that can track the nose-point of a mouse ([18]; Ethovision XT, Noldus, Leesburg, VA). Exploration was counted when the nose was in a defined object zone 3 cm surrounding an object. The time spent exploring each object, as a percentage of the total exploration time, was calculated. The difference between the percent time spent exploring the novel location object (trial 4) and the percent time spent exploring the same object in its original location (trial 3) was calculated to measure novel location recognition. The arena was cleaned with 70% ethanol between trials to remove residual odors.
2.6. Water maz
Spatial learning and memory requiring navigation were assessed in the water maze. A circular pool (diameter 140 cm) was filled with water and divided into 4 imaginary quadrants for the purpose of testing and probe trial data analysis (see below). Mice were assigned to 4 testing groups using a randomized block design. Each group was trained to find a platform in a different quadrant of the pool in order to avoid any potential quadrant bias. Mice were first trained to swim to a platform with a visible beacon marking the location of the platform (non-spatial learning, days 1-2) and then trained to swim to a platform hidden beneath opaque water (spatial learning, days 3-5) in 2 daily sessions, spaced 3.5 hours apart and each consisting of 3 sixty-second trials (10 min inter-trial intervals). During visible platform training, the platform was moved to a different quadrant of the pool for each session. During hidden platform training, the platform remained in the same quadrant. Mice were placed into the pool facing the wall in 9 different locations around the pool. The starting location changed from trial to trial. Mice had to rely on spatial cues in the room to find the hidden platform. Mice that failed to find the platform within the 60-second trial were led to the platform and allowed to remain on the platform for 3 seconds.
Swimming patterns were recorded using a video tracking system (Noldus, Leesburg, VA), set at 6 samples/seconds. The time to find the platform (latency), distance moved, the distance to the platform summed across all samples during each trial (cumulative distance to the platform, [9, 19]), and the swim speeds during the visible platform training were analyzed. Mice with better spatial learning will swim closer to the platform location during the trial and thus, will have lower cumulative distance scores. Sixty-second probe trials (no platform), designed to examine the extent of spatial discrimination learning, were performed one hour after the last hidden platform training session on days 3-5. For the probe trials, mice were placed in the quadrant opposite from the target quadrant (where the platform was located during hidden platform sessions) and the time spent in each quadrant was analyzed.
2.7. Rotorod
Sensorimotor function was assessed using a rotorod. The rod had a diameter of 7 cm and was placed horizontally 64 cm above the floor of the chamber (Kinder Scientific, Poway, CA). After a 1-min adaptation period, the rod was accelerated by 5 r.p.m. every 15 s, and the length of time the mice remained on the rod (fall latency) was recorded. The mice were tested in three consecutive trials for 3 days.
2.8. Statistical analysis
Data are expressed as mean ± SEM. Differences among means were evaluated by ANOVA, followed by Student's t-test or Tukey-Kramer posthoc tests, as indicated, using GraphPad Prism (San Diego, CA) or SPSS (Chicago, IL) software. Data of the water maze learning curves, rotorod performance, and acoustic startle responses, were analyzed using repeated measures ANOVA with sex and genotype as between-subjects factors and session (water maze), trial (rotorod), or stimulus intensity (acoustic startle) as within-subjects factors. For all analyses, the null hypothesis was rejected at the 0.05 level.
3. Results
3.1. Increased measures of anxiety of female and male mGluR8-/- mice in the open field
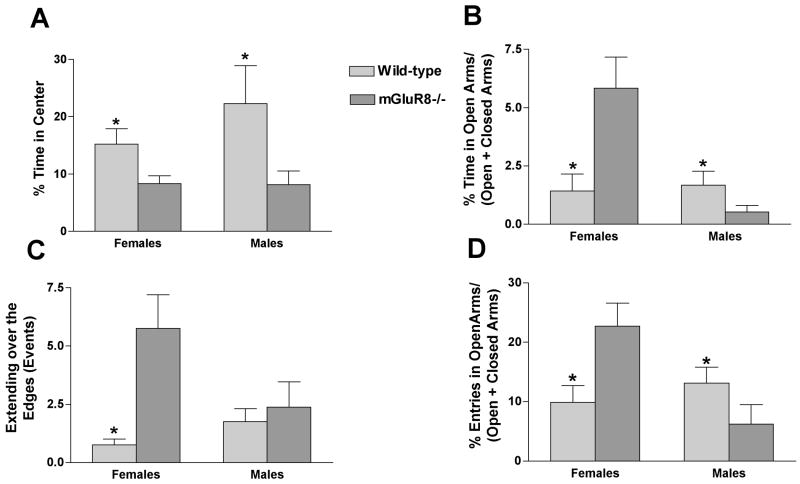
In the open field, mGluR8-/- female and male mice showed higher measures of anxiety compared to wild-type mice. They spent less time in the more anxiety-provoking center of the open field (Fig. 1A) (% time spent in center of the open field; females mGluR8-/-: 8.3 ± 1.4; female wild-types: 15.2 ± 2.7, t(2.264, 14) = 4.002, p = 0.02; males mGluR8-/-: 8.1 ± 2.4; male wild-types: 22.3 ± 6.6, t(1.794, 14) = 9.828, p < 0.05). In contrast to measures of anxiety, there was no genotype difference in activity levels in the open field (females mGluR8-/-: 5846 ± 606 cm; female wild-types: 5363 ± 348 cm; males mGluR8-/-: 4038 ± 205 cm; male wild-types: 4220 ± 358 cm, n = 8 mice/genotype/sex).
Fig. 1.
Measures of anxiety in mGluR8-/- and wild-type female and male mice in the open field (A) and elevated plus maze (B-D). A. mGluR8-/- female and male mice spent less time in the more anxiety-provoking center of the open field. B. mGluR8-/- female mice spent more time in the anxiety-provoking open arms of the elevated plus maze, while mGluR8-/- male mice spent less time in open arms of the elevated plus maze. C. mGluR8-/- female mice spent more time over the open arms of the elevated plus maze than sex-matched wild-type mice. D. mGluR8-/- female mice entered open arms of the elevated plus maze more often than sex-matched wild-type mice, while mGluR8-/- male entered the open arms of the elevated plus maze less frequently than sex-matched wild-type mice. n = 8 mice/genotype/sex. *p < 0.05 versus sex matched wild-type control.
3.2. Sex-dependent alterations in measures of anxiety of mGluR8-/- mice in the elevated plus maze
Similar to the open field, mGluR8-/- male mice showed higher measures of anxiety than wild-type mice in the elevated plus maze. They spent less time in the more anxiety-provoking open arms (Fig. 1B) and entered the open arms less (Fig. 1D) (% time spent in the open arms of the maze; males mGluR8-/-: 0.52 ± 0.29; male wild-types: 1.68 ± 0.59, t(1.913, 14) = 3.013, p < 0.05). In the males, there was no difference in activity levels that could have contributed to the genotype difference in measures of anxiety (distance moved (cm); males mGluR8-/-: 1077 ± 97; male wild-types: 1172 ± 74, n = 8 mice/genotype).
In contrast to the male mice, mGluR8-/- female mice showed lower measures of anxiety in the elevated plus maze (Fig. 1B) (% time spent in the open arms of the maze; females mGluR8-/-: 5.84 ± 1.33; female wild-types: 1.43 ± 0.73, t(2.912, 14) = 3.286, p < 0.05). Similarly, extending over the edges of the open arms was higher in mGluR8-/- than wild-type female mice (Fig. 1C) (female mGluR8-/-: 5.75 ± 1.45; female wild-types: 0.75 ± 0.25, t(3.402, 14) = 33.57, p = 0.0043). In the females, there was no difference in activity levels that could have contributed to the genotype difference in measures of anxiety (distance moved (cm); females mGluR8-/-: 1542 ± 57; female wild-types: 1360 ± 107, n = 8 mice/genotype).
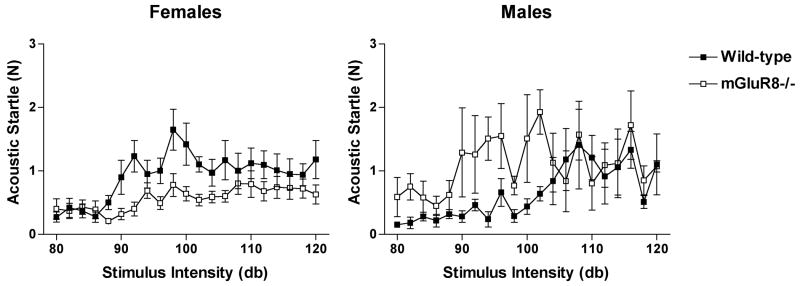
3.3. Sex-dependent alterations in the acoustic startle response of mGluR8-/- mice
In the acoustic startle test, a test involving unavoidable anxiety-provoking stimuli, there was a sex × genotype × sound intensity (F(20, 400) = 2.123, p = 0.003), sex × sound intensity (F(20, 400) = 1.824, p = 0.017), and a sex × genotype interaction (F(1, 20) = 8.311, p = 0.009). While there was no genotype difference in the basal startle response of male mice (basal startle response in N; mGluR8-/-: 0.188 ± 0.066; wild-types: 0.186 ± 0.042), mGluR8-/- male mice showed a larger acoustic startle response than wild-type mice (Fig. 2). There was also a difference in the lowest intensity causing a startle response significantly different from baseline; 82 dB for mGluR8-/- mice and 92 dB for wild-type mice.
Fig. 2.
Acoustic startle response of mGluR8-/- and wild-type female (left) and male (right) mice. Male mGluR8-/- mice had an increased acoustic startle response, while mGluR8-/- female mice showed a decreased acoustic startle response. The acoustic stimulus was increased increments of 2 Db, starting at 80 Db. n = 11 wild-type mice and n = 14 mGluR8-/- mice.
In contrast, female mice showed a genotype difference in basal startle response (basal startle response in N; mGluR8-/-: 0.154 ± 0.018; wild-types: 0.291 ± 0.049, (t(2.880, 13) = 4.226, p = 0.0076). Further, in contrast to male mice and similar to the decreased anxiety behaviors of female mGluR8-/- mice in the elevated plus maze data, female mGluR8-/- mice also showed a lower acoustic startle response than wild-type mice (Fig. 2). In addition, similar to male mice, female mice also showed a genotype difference in the lowest intensity causing a startle response significantly different from baseline with a lower threshold in mGluR8-/- than wild-type mice (84 dB for mGluR8-/- mice and 92 dB for wild-type mice).
3.4. Comparable sensorimotor function of mGluR8-/- and wild-type mice on the rotorod
When sensorimotor performance was assessed on a rotarod (3 trials per day for 3 days), the animals improved their performance with training (effect of trial, (F(8, 224) = 5.942, p < 0.001). There was no effect of genotype on rotorod performance or genotype × sex interaction. However, there was an effect of sex (F(1, 28) = 10.721, p = 0.003) with higher fall latencies in female than male mice (mean fall latency in sec over 9 trials; males: mGluR8-/-: 18.20 ± 1.22; wild-types: 20.17 ± 1.54, p = 0.3448; females: mGluR8-/-: 25.84 ± 1.42; wild-types: 32.83 ± 1.78).
3.5. Sex-dependent impairments in spatial memory retention of mGluR8-/- mice
Spatial learning and memory was assessed in the water maze. During the visible platform training, all groups improved their performance with training (effect of session on distance moved, (F(3, 84) = 31.371, p < 0.001) but there were no effects of genotype, sex, or genotype × sex interactions. All groups also improved their performance during hidden platform training (effect of session on distance moved, (F(5, 140) = 2.712, p = 0.023) but there were no effects of genotype, sex, or a genotype × sex interaction. The swim speeds during visible platform training were comparable in the groups (mean swim speeds in cm/sec; males: mGluR8-/-: 13.16 ± 0.61; wild-types: 13.25 ± 0.65; females: mGluR8-/-: 12.21 ± 0.65; wild-types: 12.78 ± 0.61). There were no effects of sex, genotype, or a sex × genotype interaction.
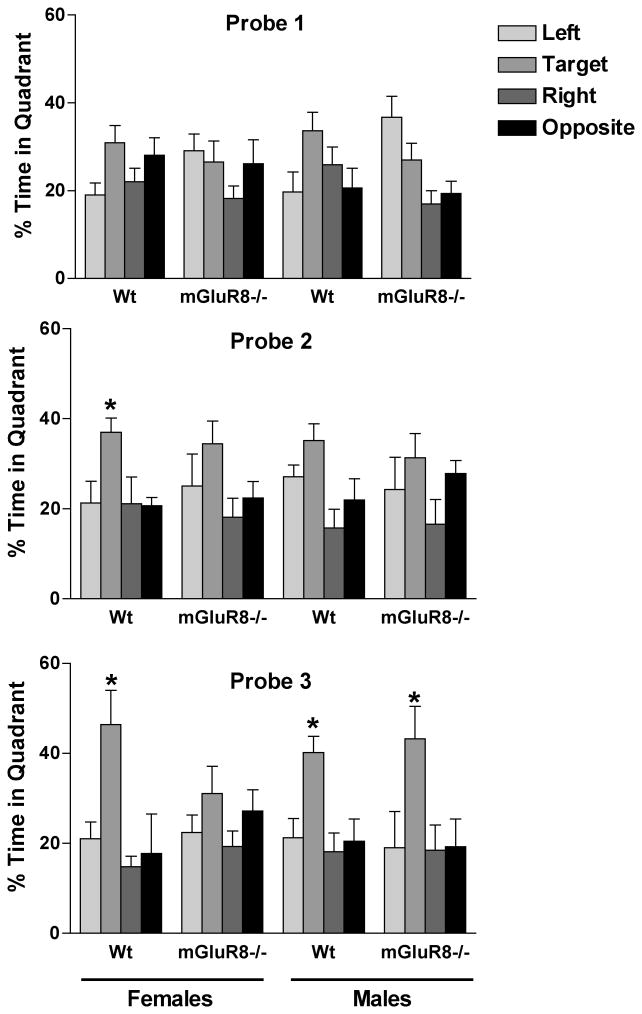
Genotype differences in spatial memory retention in the probe trial were observed in female, but not male, mice (Fig. 3). Female mGluR8-/- mice did not show spatial memory retention in the probe trial following the first, second, or third day of hidden platform training. In contrast, female wild-type mice showed spatial memory retention in the probe trial following the second (F(3, 31) = 3.514, p = 0.028; p < 0.05 target versus any other quadrant) and third (F(3, 31) = 5.468, p = 0.0044; p < 0.05 target versus left and p < 0.001 target versus right and opposite) day of hidden platform training.
Fig. 3.
Spatial memory retention of mGluR8-/- and wild-type female and male mice after 1 (top), 2 (middle), and 3 (lower) days of hidden platform training in the water maze. Female mGluR8-/- mice showed reduced spatial memory retention. n = 8 mice/genotype/sex. *p < 0.05 versus any other quadrant.
In male mice, mGluR8-/- mice did not show spatial memory retention in the second probe trial (p = 0.2821). We also did not observe spatial memory retention in wild-type mice in the second probe trial but there was a trend towards memory retention (F(3, 31) = 4.516, p = 0.0105; p < 0.001 target versus right but p > 0.05 target versus left and opposite). In the third probe trial, both mGluR8-/- (F(3, 31) = 3.189, p = 0.0389; p < 0.05 target versus any other quadrant) and wild-type (F(3, 31) = 5.718, p = 0.0054; p < 0.05 versus any other quadrant) male mice showed spatial memory retention.
3.6. Impairments in novel location recognition of female and male mGluR8-/- mice
Spatial learning and memory test was also tested in the novel location recognition test. In this test, mice were exposed to three objects in constant positions for 5 min on three consecutive trials. All groups explored the different objects similarly (p = 0.31). Prior to the 4th trial, one object was moved to a novel location to assess novel location recognition. Wild-type female and male mice spent a higher fraction of their time exploring the object in the novel location in trial 4 than in the old location in trial 3 (Fig. 4). In contrast, female and male mGluR8-/- showed an impairment in novel location recognition and did not show a preference for the object in the novel location (Fig. 4).
Fig. 4.
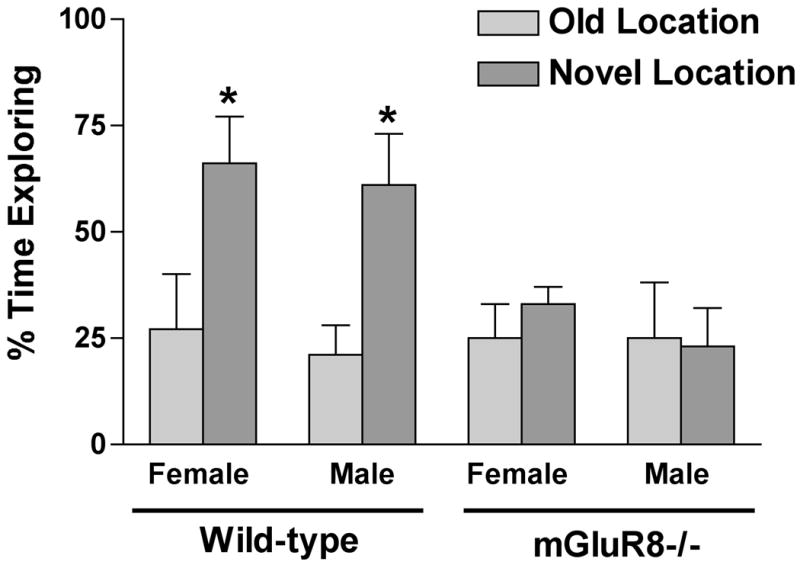
Novel location recognition of mGluR8-/- and wild-type female and male mice. mGluR8-/- mice did not show a preferential exploration of the object in a novel location. *p < 0.05 exploring the object in the old location. n = 8 mice/genotype/sex.
4. Discussion
The current study shows that the effects of mGluR8 deficiency on measures of anxiety and spatial learning and memory are critically modulated by sex. While in the open field, both female and male mGluR8-/- mice showed increased measures of anxiety, the measures of anxiety in the elevated plus maze and acoustic startle response were decreased in female mGluR8-/- mice but increased in male mGluR8-/- mice. There were no genotype differences in activity measures in the open field or elevated plus maze. Thus, potential differences in activity could not account for any of the observed genotype differences in measures of anxiety. The discrepancy between measures of anxiety in the open field and either the elevated plus maze or elevated zero maze is not unique and has also been seen in mice lacking the histamine H3 receptor [20] or impaired in glucocorticoid II receptor function [21]. What is different in this particular case is that this discrepancy in measures of anxiety is seen in female, but not male, mice.
Novel location recognition, a test thought to measure hippocampus-dependent memory [22], was impaired similarly in female and male mGluR8-/- mice. However, when spatial learning and memory was assessed in the water maze, cognitive impairments were more profound in female mice. While male mGluR8-/- mice showed spatial memory retention following the third day of hidden platform training, female mGluR8-/- mice did not. The increased susceptibility of female mice to hippocampus-dependent impairments in spatial memory retention in the water maze is not limited to mGluR8 deficiency. For example, female mice are also more susceptible to the cognitive hippocampus-dependent detrimental effects of cranial irradiation with 137Cs [23] or 56Fe [24], exposure to methamphetamine during brain development [25], age-related cognitive decline [9], and the expression of apolipoprotein E4 [26], in humans a genetic risk factor for and Alzheimer's disease. Androgens are able to antagonize age- related cognitive decline [27] and apoE-related cognitive impairments [28, 29]. Thus the sex-dependence of impairments in spatial memory retention in mGluR8-/- mice might be linked to sex differences in circulating androgen levels.
The increased measures of anxiety of mGluR8-/- male mice in the open field and elevated plus maze are consistent with the increased measures of anxiety in these tests seen at 6 months of age [8]. In wild-type male mice, the % time in the open arms is relatively low. Therefore, it is striking that we still see lower levels of this measure in the mGluR8-deficient mice. However, as in wild-type male mice the number of events for extending over the edges was also relatively low, we cannot exclude that due to a potential floor effect genotype differences in this measure might not have been revealed in male mice. The current study shows that in male mice the increased measures of anxiety are not limited to anxiety tests involving avoidable anxiety-provoking stimuli. The acoustic startle response was also higher in mGluR8-/- than wild-type male mice. Both mGluR8-/- male and female mice showed a lower threshold of the startle response, but in contrast to the mGluR8-/- male mice, mGluR8-/- female showed a reduced acoustic startle response.
The presentation of the tests was not counterbalanced, as we were concerned that performance in tests assessing measures of anxiety could be influenced by prior testing in tests likely more aversive such as the water maze. Therefore, we cannot exclude that performance in the object recognition, water maze, and rotorod tests could have been different in behaviorally naïve mice.
In principle, alterations in rotorod performance could affect performance in other tests, for example in the water maze. However, no sex differences were observed in the visible or hidden sessions of the water maze. Therefore, it is unlikely that sex differences in the rotorod tests contributed to impairments in spatial memory retention in the water maze probe trials. Similarly, as no sex or genotype differences were detected in the time the mice spent exploring all objects in the first 3 trials during the object recognition test, it is unlikely that altered rotorod performance contributed to impairments in novel location recognition in female and male mGluR8-/- mice.
In summary, mGluR8 deficiency increased measures of anxiety in the open field, elevated plus maze, and the acoustic startle response in male mice. In contrast, mGluR8 deficiency decreased measures of anxiety and the acoustic startle response in female mice. mGluR8 deficiency caused impairments in novel location recognition and in spatial memory retention in the water maze. The detrimental effects of mGluR8 deficiency on spatial memory retention in the water maze were more pronounced in female than male mice. These data underline the need to include female mice in studies to assess mGluR8 as pharmacological target to reduce measures of anxiety and improve cognitive performance.
Acknowledgments
This work was supported by NIMH R01 MH77647. The authors would like to acknowledge Jacqueline Gayet for help with animal husbandry and genotyping.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 2.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 3.Duvoisin RM, Zhang C, Ramonell K. A novel metabotropic glutamate receptor expressed in the retina and olfactory bulb. J Neurosci. 1995;15:3075–3083. doi: 10.1523/JNEUROSCI.15-04-03075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saugstad JA, Kinzie JM, Shinohara MM, Segerson TP, Westbrook GL. Cloning and expression of rat metabotropic glutamate receptor 8 reveals a distinct pharmacological profile. Mol Pharmacol. 1997;51:119–125. doi: 10.1124/mol.51.1.119. [DOI] [PubMed] [Google Scholar]
- 5.Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- 6.Swanson C, Bures M, Johnson M, Linden AM, Monn J, Schoepp D. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nature Rev Drug Disc. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- 7.Linden AM, Johnson BG, Peters SC, Shannon HE, Tian M, Wang Y, et al. Increased anxiety-related behavior in mice deficient for metabotropic glutamate 8 (mGlu8) receptor. Neuropharmacology. 2002;43:251–9. doi: 10.1016/s0028-3908(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 8.Duvoisin R, Zhang C, Pfankuch T, O'Connor H, Gayet-Primo J, Quraishi S, et al. Increased measures of anxiety and weight gain in mice lacking the group III metabotropic glutamate receptor mGluR8. Eur J Neurosci. 2005:1–13. doi: 10.1111/j.1460-9568.2005.04210.x. [DOI] [PubMed] [Google Scholar]
- 9.Benice T, Rizk A, Pfankuch T, Kohama S, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–423. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 10.Weiss L, Pan L, Abney M, Ober C. The sex-specific genetic arhcitecture of quantitative traits in humans. Nat Genet. 2006;38:218–222. doi: 10.1038/ng1726. [DOI] [PubMed] [Google Scholar]
- 11.Seeman P. Psychopathology in women and men: focus on female hormones. Am J Psychiatr. 1997;154:1641–1647. doi: 10.1176/ajp.154.12.1641. [DOI] [PubMed] [Google Scholar]
- 12.Davies W, Wilkonson L. It is not all hormones: alternative explanations for sexual differentiation of the brain. Brain Res. 2006;1126:36–45. doi: 10.1016/j.brainres.2006.09.105. [DOI] [PubMed] [Google Scholar]
- 13.Kaminsky Z, Wang S, Petronis A. Complex disease, gender, and epigenetics. Ann Med. 2006;38:530–544. doi: 10.1080/07853890600989211. [DOI] [PubMed] [Google Scholar]
- 14.Harrison P, Turnbridge E. Catechol-O-Methyltransferase (COMT): A gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychoparmacology. 2008;33:3037–3045. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- 15.Charney D, Nestler E. Neurobiology of mental illness. Oxford University Press; 2005. p. 555. [Google Scholar]
- 16.Acevedo S, Ohtsu H, Benice T, Rizk-Jackson A, Raber J. Age-dependent effects on measures of anxiety and cognition in male histidine decarboxylase knockout (Hdc-/-) mice. Brain Res. 2006;1071:113–123. doi: 10.1016/j.brainres.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 17.Acevedo S, Pfankuch T, Ohtsu H, Raber J. Anxiety and cognition in female histidine decarboxylase knockout (Hdc-/-) mice. Beh Brain Res. 2006;168:92–99. doi: 10.1016/j.bbr.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Benice T, Bader T, Raber J. Object recognition analysis in mice using nose-point digital video tracking. J Neurosci Method. 2008;168:422–430. doi: 10.1016/j.jneumeth.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:1–16. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 20.Rizk A, Curley J, Robertson J, Raber J. Anxiety and cognition in histamine H3-receptor -/- mice. Eur J Neurosci. 2004;19:1–5. doi: 10.1111/j.1460-9568.2004.03251.x. [DOI] [PubMed] [Google Scholar]
- 21.Rochford J, Beaulieu S, Rousse I, Glowa JR, Barden N. Behavioral reactivity to aversive stimuli in a transgenic mouse model of impaired glucocorticoid (type II) receptor function: effects of diazepam and FG-7142. Psychopharmacology (Berl) 1997;132:145–52. doi: 10.1007/s002130050330. [DOI] [PubMed] [Google Scholar]
- 22.Save E, Poucet B, Foreman N, Buhot M. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav Neurosci. 1992;106:447–456. [PubMed] [Google Scholar]
- 23.Villasana L, Acevedo S, Poage C, Raber J. Sex- and ApoE Isoform-dependent effects of radiation on cognitive function. Rad Res. 2006;166:883–891. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- 24.Villasana L, Rosenberg J, Raber J. Sex-dependent effects of 56Fe Irradiation on contextual fear conditioning in C56BL/6J mice. Hippocampus. 2009 doi: 10.1002/hipo.20659. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acevedo S, de Esch I, Raber J. Sex- and histamine-dependent long-term cognitive effects of methamphetamine exposure. Neuropsychoparmacology. 2007;32:665–672. doi: 10.1038/sj.npp.1301091. [DOI] [PubMed] [Google Scholar]
- 26.Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, et al. Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: Increased susceptibility of females. Proc Natl Acad Sci USA. 1998;95:10914–10919. doi: 10.1073/pnas.95.18.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benice T, Raber J. Testosterone and dihydrotestosterone differentially improve cognition in aged female mice. Learn Mem. 2009 doi: 10.1101/lm.1428209. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. J Neurosci. 2002;22:5204–5209. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acevedo S, Gardell L, Bradley SR, Piu F, Raber J. Selective androgens receptor modulators antagonize apolipoprotein E4-induced cognitive impairments. Lett Drug Design Discovery. 2008;5:271–276. [Google Scholar]